Abstract
Background
There has been much variation between epidemiological studies that report the prevalence of ankylosing spondylitis (AS). This study aimed to analyze the diagnostic prevalence rates and treatment patterns of male and female AS patients in the United States adult insured population from 2006 to 2016.
Methods
Trends in AS prevalence were calculated for the 11-year period covering January 1, 2006 to December 31, 2016. Adult (18+ years old) AS patients were included in this retrospective analysis of medical and pharmacy claims data from the IBM Marketscan Commercial, Medicaid and Medicare-Supplemental Claims database. Prevalence was determined as having ≥1 AS diagnostic codes (ICD-9:720.0; ICD-10:M45.x). Trends in treatment patterns were also analyzed and stratified by gender.
Results
The AS prevalence increased from 0.04 to 0.09% from 2006 to 2016. The mean age between 2006 and 2016 ranged from 49.52–50.00 years. In 2006, approximately 40% of AS patients were female, while in 2016 over 47% of AS patients were female. Rates of use of TNF inhibitors and oral glucocorticoids increased, while NSAIDs and non-biologic DMARDs (sulfasalazine & methotrexate) rates decreased. Opioid use rates were stable. In 2016, males were more likely to be prescribed biologics, while females were more likely to be prescribed methotrexate, sulfasalazine, NSAIDs, muscle relaxants, anticonvulsants, opioids, and glucocorticoids.
Conclusions
The prevalence of AS diagnosis codes more than doubled between 2006 and 2016, but the very low prevalence suggests that AS continues to be underdiagnosed and under-addressed in routine clinical practice. Despite the increase in female AS patients, females were less likely to be prescribed biologics compared to male AS patients.
Keywords: Ankylosing spondylitis, Prevalence, Treatment patterns
Background
Ankylosing spondylitis (AS) is a chronic inflammatory disease that affects the spine and sacroiliac joints [1]. AS is associated with pain, impaired health-related quality of life (HRQoL) and disability [2, 3]. Musculoskeletal features as well as extra-articular manifestations such as enthesitis, uveitis, inflammatory bowel disease, and psoriasis can cause a substantial burden to AS patients. There has been much variation between epidemiological studies that report the prevalence of AS [4–6]. Given these discrepancies, the prevalence of AS in the United States (US) has been estimated to be between 0.2 and 1% [7–9].
Treatment options for AS patients include nonsteroidal anti-inflammatory drugs (NSAIDs), nonbiological disease-modifying antirheumatic drugs (DMARDs) such as sulfasalazine and methotrexate, and biologics such as tumor necrosis factor inhibitors (TNFi) and interleukin-17 (IL-17) antagonists [10–16]. The American College of Rheumatology (ACR), Spondylitis Association of America (SAA), and Spondyloarthritis Research and Treatment Network (SPARTAN) recommend NSAIDs as a first-line treatment for AS and biologic therapy for patients that do not respond to NSAIDs [16].
The purpose of this study was to assess the current diagnostic prevalence of AS and trends in treatment patterns among adults in the US that have commercial, Medicaid, or Medicare supplemental insurance. In order to do this, data from administrative insurance claims databases over the period 2006 to 2016 were analyzed.
Methods
Study design
This retrospective cross-sectional study utilized data from the IBM MarketScan® Research database (Ann Arbor, MI, USA). Data were analyzed to assess trends in AS diagnostic prevalence focusing on the 11-year period from January 1, 2006 to December 31, 2016. AS diagnostic prevalence rates were analyzed for the total AS population and stratified by age and gender. Demographic variables and patient characteristics were assessed and the age-adjusted prevalence rate was measured and stratified for 2016 cohort.
IBM Marketscan Commercial, Medicaid and Medicare-Supplemental Claims database contains de-identified patient data including in-patient and outpatient physician visits, emergency room visits, procedures, and pharmacy prescriptions. Study variables were defined in the IBM Marketscan database using patient enrollment records and International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and International Classification of Disease, 10th Revisions, Clinical Modification (ICD-10-CM) codes. In order to protect patient privacy, all data from the IBM Marketscan Research database are compliant to the Health Insurance Portability and Accountability Act (HIPAA).
Identification of AS
For each calendar year of analysis, a cohort was assembled that consisted of all AS patients that were 18 years or older on January 1st of the calendar year. Patients were required to have continuous enrollment in medical and pharmacy benefits throughout the calendar year, with the exception of an enrollment gap allowance of less than 30 days. Patients with at least one AS diagnostic code (ICD-9:720.0; ICD-10: M45.x) were identified from these cohorts.
Prevalence estimation
Annual AS diagnostic prevalence was estimated using the US adult population in the IBM MarketScan® Research database during the 11-year period of 2006 to 2016. For each calendar year, a cohort was created and the AS case identification was applied. The numerator in the AS diagnostic prevalence estimation was the number of patients that met the AS definition described in the previous section. The denominator was the number of all patients over the age of 18 with continuous enrollment (during the calendar year) in the cohort.
Statistical analyses
AS diagnostic prevalence was estimated and stratified by gender (male and female) and age (< 25, 25–34, 35–44, 45–54, 55–64, and ≥ 65) for each calendar year from 2006 to 2016. Trends in treatments patterns of tumor necrosis factor inhibitors (TNFi) (adalimumab, infliximab, etanercept, golimumab, certolizumab pegol), secukinumab, Cox-2 inhibitor (celecoxib), sulfasalazine, methotrexate, acetaminophen, NSAIDs (aspirin, ibuprofen, meloxicam, nabumetone, diclofenac, naproxen, diflunisal, etodolac, fenoprofen, fluribiprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, mefanamic, meprobamate, oxaprozin, piroxicam, sulindac, tolmetin, salsalate), muscle relaxants (cyclobenzaprine, orphenadrine, chlorzoxazone, methocarbamol, carisoprodol, metaxalone, dantrolene, baclofen, tizanidine), anticonvulsant (gabapentin, pregabalin, carbamazepine, topiramate, oxcarbazepine), opioids (codeine, oxycodone, hydrocodone, propoxyphene, dihydrocodeine, fentanyl, hydromorphone, levorphanol, methadone, morphine, oxymorphone, tramadol, tapentadol, meperidine, butorphanol, buprenorphine, nalbuphine, pentazocine), and oral and injectable glucocorticoids (betamethasone, cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisolone, prednisone, triamcinolone). Descriptive statistics of patient demographics and treatment patterns were conducted for the total AS cohort and male and female subgroups. Continuous variables were analyzed by means and standard deviations (SD) while categorical variables were analyzed by frequency counts and percentages (%).
Results
AS patient characteristics in 2016
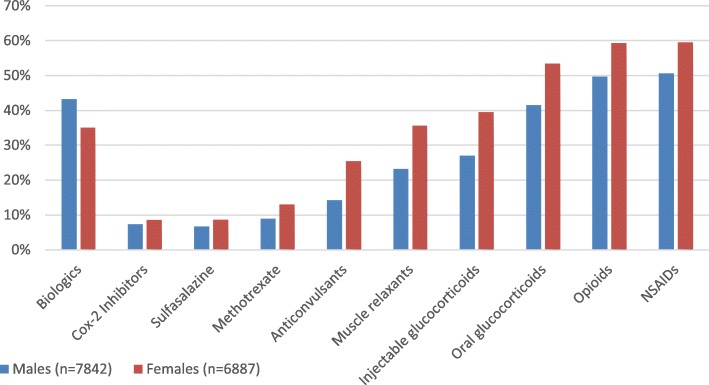
In 2016, out of a total of 16,097,378 adult patients that had commercial insurance, Medicaid, or supplemental Medicare with continuous enrollment in the IBM MarketScan® Research database, there were 14,729 (0.09%) patients with AS. Of these 14,729 patients, 7842 (53.24%) were male and 6887 (46.76%) were female. Mean age for overall AS population was 50.00 years (SD = 14.36). A majority of patients were commercially insured and the most frequent geographic location was the Southern US region. In 2016, males were more likely to be prescribed biologics, while females were more likely to be prescribed methotrexate, sulfasalazine, NSAIDs, muscle relaxants, anticonvulsants, opioids, and glucocorticoids (Fig. 1). The patients’ demographic information is presented in Table 1.
Fig. 1.
Medication use among male and female as patients in 2016
Table 1.
Baseline characteristics for patients with ankylosing spondylitis (2016)
| Variable | Overall Population (N = 14,729) |
Males (N = 7842) |
Females (N = 6887) |
|---|---|---|---|
| Mean Age (SD) | 50.00 (14.36) | 50.69 (14.62) | 49.21 (14.02) |
| Age (Years) | |||
| < 25 | 672 (4.6%) | 332 (4.2%) | 340 (4.9%) |
| 25–34 | 1530 (10.4%) | 843 (10.7%) | 687 (10.0%) |
| 35–44 | 2930 (19.9%) | 1482 (18.9%) | 1448 (21.0%) |
| 45–54 | 3806 (25.8%) | 1883 (24.0%) | 1923 (27.9%) |
| 55–64 | 3909 (26.5%) | 2160 (27.5%) | 1749 (25.4%) |
| ≥ 65 | 1882 (12.8%) | 1142 (14.6%) | 740 (10.7%) |
| Insurance | |||
| Commercial | 12,687 (86.1%) | 6605 (84.2%) | 6082 (88.3%) |
| Medicare | 2149 (14.6%) | 1307 (16.7%) | 842 (12.2%) |
| Geographic Region | |||
| Northeast | 2423 (16.5%) | 1348 (17.2%) | 1075 (15.6%) |
| North Central | 2953 (20.0%) | 1693 (21.6%) | 1260 (18.3%) |
| South | 6400 (43.5%) | 3244 (41.4%) | 3156 (45.8%) |
| West | 2895 (19.7%) | 1528 (19.5%) | 1367 (19.8%) |
| Unknown | 58 (0.4%) | 29 (0.4%) | 29 (0.4%) |
| Medication | |||
| Biologics | 5795 (39.3%) | 3384 (43.2%) | 2411 (35.0%) |
| Cox-2 inhibitor | 1154 (7.8%) | 569 (7.3%) | 585 (8.5%) |
| Sulfasalazine | 1122 (7.6%) | 528 (6.7%) | 594 (8.6%) |
| Methotrexate | 1591 (10.8%) | 695 (8.9%) | 896 (13.0%) |
| Acetaminophen | 425 (2.9%) | 118 (1.5%) | 307 (4.5%) |
| NSAIDs | 8065 (54.8%) | 3970 (50.6%) | 4095 (59.5%) |
| Muscle Relaxants | 4273 (29.0%) | 1823 (23.2%) | 2450 (35.6%) |
| Anticonvulsants | 2859 (19.4%) | 1110 (14.2%) | 1749 (25.4%) |
| Opioids | 7980 (54.2%) | 3897 (49.7%) | 4083 (59.3%) |
| Oral Glucocorticoids | 6928 (47.0%) | 3251 (41.5%) | 3677 (53.4%) |
| Injectable Glucocorticoids | 4832 (32.8%) | 2115 (27.0%) | 2717 (39.5%) |
Ankylosing spondylitis prevalence and treatment patterns: 2006–2016
Annual AS diagnostic prevalence rates increased from 0.04 to 0.09% from 2006 to 2016 for adult US patients in the IBM MarketScan® Research database. The diagnostic prevalence rate varied by age and gender during each year and increased gradually across the years for males and females. Overall diagnostic prevalence in males gradually increased from 0.06% in 2006 to 0.10% in 2016, while the overall diagnostic prevalence among females gradually increased from 0.03% in 2006 to 0.08% in 2016 (Fig. 2). In 2006, approximately 40% of AS patients were female, while in 2016 over 47% of AS patients were female. Mean age was stable across the years with an overall mean age of 49.52 in 2006 and 50.00 in 2016.
Fig. 2.

Ankylosing spondylitis prevalence trends stratified by gender (2006–2016)
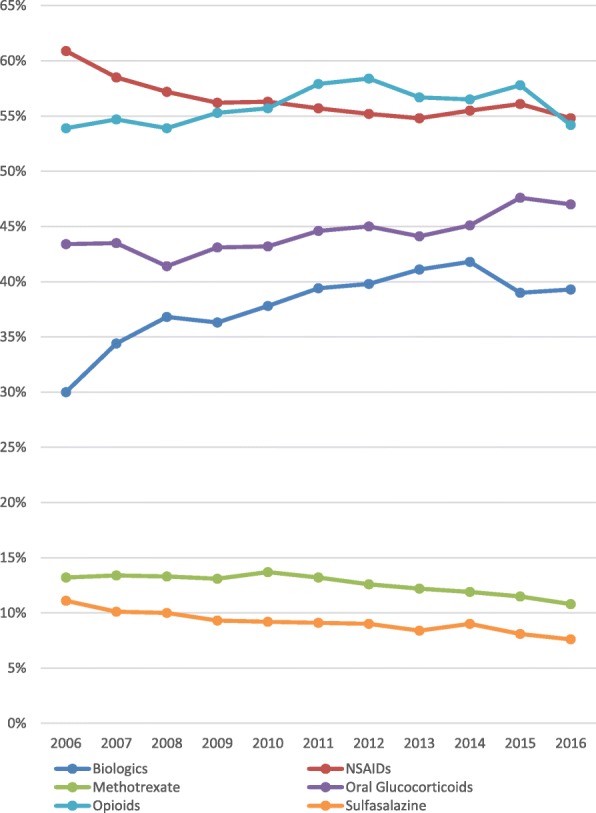
Rates of use of TNFi (30.00 to 39.30%) and oral glucocorticoids (43.40 to 47.00%) increased, while NSAIDs (60.90 to 54.80%) and non-biologic DMARDs (sulfasalazine (11.10 to 7.50%) & methotrexate (13.20 to 10.80%)) rates decreased from 2006 to 2016. Opioid use rates were stable from 2006 to 2016 (Fig. 3).
Fig. 3.
Trends in treatment patterns among as patients, 2006–2016
Discussion
This study evaluated recent trends in diagnostic prevalence of AS and treatment patterns among AS patients in the U.S. Results from this study indicate that the overall diagnostic prevalence of AS in the U.S. ranged from 0.04 to 0.09% and steadily increased from 2006 to 2016 in a U.S. administrative claims database.
When analyzing 1996–2009 computerized health care data from Kaiser Permanente Northern California, Curtis and colleagues reported a point prevalence of AS standardized to the 2000 US census of 2.26 per 1000 (~ 0.23%) [8]. These findings were higher than the rates reported during our study, but lower than previously reported prevalence rates in the U.S. For example, based on the 2009–2010 NHANES data, axSpA (AS and nr-axSpA) prevalence was approximately 1.4% in the U.S. [7]. In addition Strand and colleagues [9] estimated that AS affects nearly 0.3% of the U.S. population ages 18–44 years in a retrospective chart review of at-risk patients in U.S. rheumatology practice. While the Reveille [7] and Strand [9] studies have been frequently referenced, it is important to note that these studies were not calculating diagnostic code prevalence using an administrative claims database. Differences in patient identification, data collection methods, and study design may contribute to the variability in results between studies. The inclusion of previously undiagnosed patients and nr-axSpA patients contributed to the particularly higher prevalence in the Reveille study [7].
Our study found that AS trends in the U.S. have gradually increased from 2006 to 2016 in both males (0.06 to 0.10%) and females (0.03 to 0.08%). Although males had greater prevalence of AS throughout the study, females went from accounting for 40% of the total AS population in 2006 to accounting for 47% of the total AS population in 2016. These findings are similar to the results reported in Ontario, Canada which saw a twofold increase among males (0.10 to 0.24%) and a threefold increase among females (0.06 to 0.19%) from 1995 to 2010 [17]. Increased awareness of AS and changing perceptions about male predominance of AS may have contributed to the increase in rates of diagnostic prevalence.
Rates of use of TNFi and oral glucocorticoids increased, while NSAIDs and non-biologic DMARDs (sulfasalazine & methotrexate) rates decreased from 2006 to 2016. Over the past decade, there has been an increase in TNFi options and a plethora of data on the safety and efficacy of TNFi, which has resulted in physicians becoming more comfortable with prescribing these medications [10–16]. The ACR, SAA, and SPARTAN recommend against the use of non-biologic DMARDs in most patients with AS, that has remained active despite NSAID use, and recommends the use of TNFi; which could also contribute to these changes in treatment patterns [16]. While NSAIDs can alleviate symptoms, the decreasing rate of NSAID use may be attributed to the associated risks with long-term administration and the potential cardiovascular, gastrointestinal, and renal complications [16].
Despite the increase in TNFi use among AS patients, there were still different rates of use among males and females. In 2016, males were more likely than females to be prescribed TNFi. These findings may indicate that female patients with AS are not being prescribed advanced therapy at the same rate as their male counterparts. This may be due to prescribers being more reluctant to treat women with “more aggressive” therapy since there are still misconceptions that AS is a male dominated disease however reasons for not receiving TNFi were not analyzed in this study.
While this study provides insight into the diagnostic prevalence rate and trends in treatment patterns of AS patients in the United States, it is important to note that the primary purpose of insurance claims data is for administrative and billing purposes. Since our study analyzed the diagnostic prevalence of AS patients by identifying patients with ICD-9 and/or ICD-10 codes, our findings could be an under-representation of the overall AS population. As reported by Curtis et al., using different case definitions based on the same ICD codes may lead to variation in the prevalence estimates [8]. Published reports have indicated a delay in diagnosis of up to 10 years for AS patients [18, 19]. which means that many patients with AS may not currently be diagnosed. Diagnostic prevalence rates would not include these patients in the calculation and could thus be an underestimation of the total AS prevalence in the US. Since our analyses were based on ICD-9 and ICD-10 codes, we are not able to assess if these calculations include patients that may have nonradiographic axial spondyloarthritis, but received an AS diagnostic code..
Additionally, since the IBM MarketScan® Research database consists of patients with commercial, Medicaid, and/or Medicare supplement insurance, this may inadvertently exclude some populations from the analyses such as the uninsured population or patients with other insurances. Given the limitations of the database used in this study, all AS patients may not be included in our analyses, thus resulting in a conservative estimate of AS diagnostic prevalence in the U.S.
Conclusion
The large sample size and geographic representation of our study enhances the validity of generalizing our AS diagnostic prevalence estimates to the general U.S. adult population that are insured by commercial insurance, Medicaid, or supplemental Medicare. The prevalence of AS diagnosis codes more than doubled between 2006 and 2016, but the very low prevalence suggests that AS continues to be underdiagnosed and under-addressed in routine clinical practice. Despite the increase in female AS patients, females were less likely to be prescribed biologics compared to male AS patients. The reasons why female patients were less likely to be prescribed biologics were not investigated in this study and additional research is needed to understand the difference in the treatment patterns between male and female AS patients.
Acknowledgements
Not applicable.
Abbreviations
- ACR
American College of Rheumatology
- AS
Ankylosing Spondylitis
- AxSpA
Axial Spondyloarthritis
- DMARD
Disease-modifying antirheumatic drug
- HIPPA
Health Insurance Portability and Accountability Act
- HRQoL
Health related quality of life
- ICD-10-CM
International Classification of Disease, 10th Revisions, Clinical Modification
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- NSAID
Nonsteroidal anti-inflammatory drug
- SAA
Spondylitis Association of America
- SD
Standard deviation
- SPARTAN
Spondyloarthritis Research and Treatment Network
- TNFi
Tumor necrosis factor inhibitor
- U.S
United States
Authors’ contributions
Manuscript design/conception: JW, TH, KS, DS, and RB. Drafting of manuscript: JW, TH, DS, and RB. Critical revision: all authors. Approval of manuscript: all authors read and approved the final manuscript.
Funding
This study was funded by Eli Lilly and Company. Employees of Eli Lilly and Company contributed design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data used in this study was de-identified. Request for data may be made to the corresponding author.
Ethics approval and consent to participate
The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and later amendments. This study was a retrospective analysis of administrative claims data and does not contain any human participants or animals. Thus, permission from a research ethics committee was not required and formal informed consent was not obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jessica Walsh, Email: Jessica.Walsh@hsc.utah.edu.
Theresa Hunter, Email: hunter_theresa_marie@lilly.com.
Krista Schroeder, Email: Schroeder_krista_marie@lilly.com.
David Sandoval, Email: Sandoval_david@lilly.com.
Rebecca Bolce, Email: Rebecca.bolce@lilly.com.
References
- 1.Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Primers. 2015;1:15013. doi: 10.1038/nrdp.2015.13. [DOI] [PubMed] [Google Scholar]
- 2.Boonen A, Brinkhuizen T, Landewé R, et al. Impact of ankylosing spondylitis on sick leave, presenteeism, and unpaid productivity, and estimation of the societal cost. Ann Rheum Dis. 2010;69:1123–1128. doi: 10.1136/ard.2009.116764. [DOI] [PubMed] [Google Scholar]
- 3.Kotsis K, Voulgari PV, Dross AA, Carvalho AF, Hyphantis T. Health-related quality of life in patients with ankylosing spondylitis: a comprehensive review. Expert Rev Pharmacoeconomic Outcomes Res. 2014;14:857–872. doi: 10.1586/14737167.2014.957679. [DOI] [PubMed] [Google Scholar]
- 4.Carter ET, McKenna CH, Brian DD, et al. Epidemiology of ankylosing spondylitis in Rochester, Minnesota, 1935-1973. Arthritis Rheum. 1979;22:365–370. doi: 10.1002/art.1780220408. [DOI] [PubMed] [Google Scholar]
- 5.Carbone LD, Cooper C, Michet CJ, et al. Ankylosing spondylitis in Rochester, Minnesota, 1935-1989. Is the epidemiology changing? Arthritis Rheum. 1992;35:1476–1482. doi: 10.1002/art.1780351211. [DOI] [PubMed] [Google Scholar]
- 6.Dean LE, Jones GT, MacDonald AG, et al. Overestimation of the prevalence of ankylosing spondylitis in the Berlin study: comment on the article by Braun et al. Arthritis Rheum. 2005;52:4048–4049. doi: 10.1002/art.21492. [DOI] [PubMed] [Google Scholar]
- 7.Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci. 2011;341:284–286. doi: 10.1097/MAJ.0b013e31820f8c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis JR, Harrold LR, Asgari MM, et al. Diagnostic prevalence of ankylosing spondylitis using computerized health care database, 1996 to 2009: under recognition in a US health care setting. Perm J. 2016;20:4–10. doi: 10.7812/TPP/15-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strand V, Rao SA, Shillington AC, Cifaldi MA, McGuire M, Ruderman EM. Prevalence of axial spondyloarthritis in United States rheumatology practices: assessment of SpondyloArthritis international society criteria versus rheumatology expert clinical diagnosis. Arthritis Care (Hoboken) 2013;65:1299–1306. doi: 10.1002/acr.21994. [DOI] [PubMed] [Google Scholar]
- 10.Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomized controlled multicenter trial. Lancet. 2002;359:1187–1193. doi: 10.1016/S0140-6736(02)08215-6. [DOI] [PubMed] [Google Scholar]
- 11.Davis JC, Jr, van der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230–3236. doi: 10.1002/art.11325. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–2146. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 13.Inman RD, Davis JC, Jr, van der Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402–3412. doi: 10.1002/art.23969. [DOI] [PubMed] [Google Scholar]
- 14.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomized placebo-controlled phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17a inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 16.Ward MM, Deodhar A, Akl EA, et al. American college of rheumatology/spondylitis association of America/Spondyloarthritis research and treatment network 2015 recommendation for the treatment of ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Care Res (Hoboken) 2016;68:151–166. doi: 10.1002/acr.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroon NN, Paterson JM, Li P, Haroon N. Increasing proportion of female patients with ankylosing spondylitis: a population-based study of trends in the incidence and prevalence of AS. BMJ Open. 2014;4(12):e006634. doi: 10.1136/bmjopen-2014-006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieper J, Rudwaleit M. Early referral recommendations for ankylosing spondylitis in primary care. Ann Rheum Dis. 2005;64:659–663. doi: 10.1136/ard.2004.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol. 2012;8:262–268. doi: 10.1038/nrrheum.2012.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study was de-identified. Request for data may be made to the corresponding author.