Abstract
The US prevalence of nonalcoholic fatty liver disease (NAFLD) is 30.6% and increasing. NAFLD shares some risk factors with periodontitis and dental caries. We explored the association between NAFLD and several oral conditions among US adults, using data from the cross-sectional, nationally representative National Health and Nutrition Examination Survey (NHANES), 1988 to 1994. NAFLD was assessed with ultrasonography (USON), the screening gold standard not available in the more recent NHANES, and the noninvasive Fibrosis Score (FS), Fatty Liver Index (FLI), and US Fatty Liver Index (US-FLI) as other screening alternatives. There were 5,421 eligible dentate adults aged 21 to 74 y with complete relevant data, with transferrin levels ≤50%, without hepatitis B or C, who were not heavy drinkers. Multivariable models were developed to examine the independent effects of moderate-severe periodontitis, untreated dental caries, caries experience, and tooth loss (<20 teeth) on NAFLD while controlling for clinical, biological, and sociodemographic factors. Weighted estimates for odds ratios (ORs) and 95% CIs were calculated with logistic regression. Between 17% and 24% of adults had NAFLD depending on the classification criteria. In adjusted models, as compared with those with better oral health, adults with <20 teeth were more likely to have NAFLD depending on the measure (USON: OR = 1.50, 95% CI = 1.11 to 2.02; FS: OR = 4.36, 95% CI = 3.47 to 5.49; FLI: OR = 1.99, 95% CI = 1.52 to 2.59; US-FLI: OR = 2.32, 95% CI = 1.79 to 3.01). People with moderate-severe periodontitis were more likely to have NAFLD (USON: OR = 1.54, 95% CI = 1.06 to 2.24; FS: OR = 3.10, 95% CI = 2.31 to 4.17; FLI: OR = 1.61, 95% CI = 1.13 to 2.28; US-FLI: OR = 2.21, 95% CI = 1.64 to 2.98). People with any untreated caries were more likely to have NAFLD (USON: OR = 1.51, 95% CI = 1.20 to 1.90; FLI: OR = 1.80, 95% CI = 1.33 to 2.44). NAFLD was associated with tooth loss, periodontitis, and, for some NAFLD measures, untreated dental caries but not overall caries experience after controlling for several key sociodemographic and behavioral factors. Results suggest that further evaluation is needed to better understand this health–oral health interrelationship and potential opportunities for medical-dental integration.
Keywords: oral health, periodontitis, tooth loss, dental caries, NHANES, cross-sectional studies
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the excessive lipid accumulation in the liver not due to excess alcohol consumption. NAFLD is the most common chronic liver disease in the United States; adult prevalence from 2011 to 2012 was estimated to be 30.6% and increasing (Ruhl and Everhart 2015). About 24% of the population worldwide is potentially affected (Younossi et al. 2016). The prevalence is higher among those with type 2 diabetes, obesity, and hyperlipidemia (Younossi et al. 2016; Le et al. 2017). People with NAFLD can progress to nonalcoholic steatohepatitis (NASH) and NASH-related liver cirrhosis.
NAFLD is a risk factor and determinant of metabolic syndrome (Yki-Järvinen 2014; Lonardo et al. 2015). The disease also induces and enhances insulin resistance and increases the risk of type 2 diabetes (Yki-Järvinen 2014; Lonardo et al. 2015). Consumption of simple sugars (glucose and fructose) is a factor leading to NAFLD and dental caries. Chronic fructose exposure (e.g., high-fructose corn syrup) can lead to inflammation, liver fat accumulation, NAFLD, and metabolic syndrome (Lustig 2014; Yki-Järvinen 2014; Lonardo et al. 2015; Softic et al. 2016). A study with a murine model showed that injecting mice with a Streptococcus mutans strain, a causative factor in dental caries, and feeding them a high-fat diet could induce or expedite liver fibrosis and NASH (Naka et al. 2014; Naka et al. 2018).
Several hypotheses link NAFLD and periodontitis, through periodontal pathogens, inflammatory mediators, and oxidative stress (Han et al. 2016). Porphyromonas gingivalis was found in higher frequencies in patients with NAFLD than in non-NAFLD controls (Yoneda et al. 2012). In the population-based cohort Study of Health in Pomerania, NAFLD incidence increased in participants with prior elevated periodontal clinical attachment loss (Akinkugbe et al. 2017). Other investigators found a significant relationship between liver steatosis and periodontitis, especially among people with advanced rather than mild liver disease (Alazawi et al. 2017).
NAFLD and NASH are diagnosed in several ways. A liver biopsy is the most definitive, but it is invasive and costly, can have complications, and is not used for screening purposes. Ultrasonography (USON) has become the standard noninvasive diagnostic method, but this imaging technique is not feasible or available for most population-based field studies. Other approaches have been developed through a combination of demographic and clinical and/or laboratory variables, such as liver enzymes, to construct an algorithm for low-cost screening purposes.
We had 2 aims for this secondary data analysis: first, to explore the association between NAFLD and the prevalence of periodontitis, dental caries, and permanent tooth loss among a nationally representative sample of US adults, controlling for selected potential confounders; second, to determine if the same NAFLD associations with oral health status with USON are found with other noninvasive screening methods of categorizing NAFLD.
Methods
Study Population
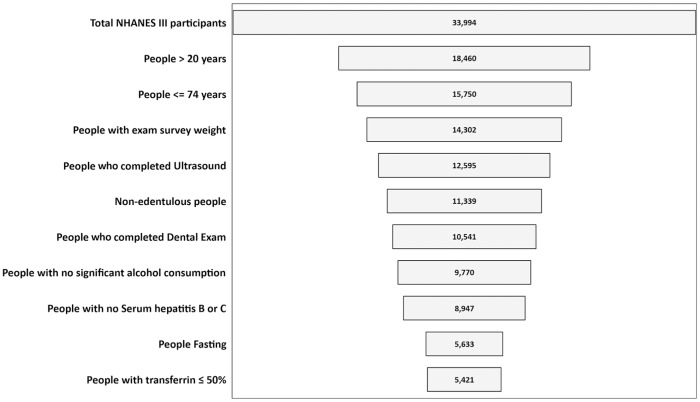
Data from the cross-sectional National Health and Nutrition Examination Survey, 1988 to 1994 (NHANES III), were analyzed because USON information is only available for these years. In our study, we included dentate persons aged 21 to 74 y with USON rated as “confident” or “absolute” based on NHANES III criteria (Centers for Disease Control and Prevention 2011). Participants were excluded if they had an incomplete dental examination, had not been fasting (required for some laboratory measurements), or had other etiologies for liver disease (e.g., classified with “significant alcohol consumption” [Chalasani et al. 2012], or had hepatitis B/C). People who had transferrin >50% were not part of the study sample, because excessive iron from hereditary hemochromatosis can lead to liver dysfunction and fibrosis (Cheng et al. 2009). See Figure 1 for selection details. Survey participants provided written informed consent, and additional details regarding NHANES III are located elsewhere (Centers for Disease Control and Prevention 2017b).
Figure 1.
Number of individuals meeting eligibility criteria for analytic sample. NHANES III, National Health and Nutrition Examination Survey (1988 to 1994).
Sociodemographic Variables
Demographic characteristics, such as age, sex, education, race/ethnicity, and marital status, were obtained from the NHANES III household interview. Categorizations of these variables are shown in Table 1.
Table 1.
Distribution of Demographic, Behavioral, Clinical, and Oral Health Characteristics in Analytic Sample: NHANES III (1988 to 1994).
| % (SE) or Mean ± SE |
|||||
|---|---|---|---|---|---|
| All(N = 5,421) | USON Positive(n = 1,030, 17%) | FS > –1.455(n = 1,337, 24%) | FLI > 30(n = 1,089, 24%) | US-FLI > 30(n = 794, 22%) | |
| Demographic characteristics | |||||
| Age, y | |||||
| 21 to 39 | 56.56 (1.25) | 12.44 (1.20) | 6.67 (0.75) | 17.85 (1.44) | 12.89 (1.44) |
| 40 to 59 | 31.68 (1.13) | 21.93 (1.80) | 36.16 (1.49) | 30.30 (2.26) | 29.08 (2.07) |
| 60 to 74 | 11.76 (0.75) | 23.84 (2.20) | 72.98 (2.29) | 32.18 (1.85) | 39.66 (2.73) |
| Sex | |||||
| Female | 52.14 (0.99) | 20.56 (1.72) | 22.49 (1.17) | 29.41 (2.11) | 28.38 (1.78) |
| Male | 47.86 (0.99) | 13.32 (1.04) | 25.68 (1.37) | 18.11 (1.03) | 16.52 (1.54) |
| Race/ethnicity | |||||
| Non-Hispanic White | 75.41 (1.36) | 16.44 (1.12) | 25.08 (1.02) | 23.39 (1.63) | 21.28 (1.66) |
| Non-Hispanic Black | 11.19 (0.75) | 12.17 (1.24) | 26.86 (1.90) | 26.40 (1.44) | 13.59 (1.25) |
| Mexican American | 6.17 (0.56) | 24.63 (2.25) | 15.10 (1.16) | 28.02 (1.59) | 41.60 (2.77) |
| Other racea | 7.23 (1.01) | 20.86 (4.22) | 18.12 (3.02) | 18.21 (2.78) | 26.64 (4.74) |
| Education | |||||
| Less than high school | 18.54 (1.19) | 21.48 (2.11) | 30.99 (2.03) | 31.19 (1.70) | 30.34 (2.14) |
| High school/GED or equivalent | 34.26 (0.96) | 17.75 (1.69) | 26.37 (1.85) | 25.02 (1.99) | 26.19 (2.03) |
| More than high school | 47.2 (1.36) | 14.25 (1.43) | 19.82 (1.34) | 19.52 (1.43) | 16.09 (1.63) |
| Marital status: married | 64.88 (1.09) | 19.18 (1.31) | 25.89 (1.10) | 26.06 (1.74) | 24.61 (1.68) |
| Poverty status, FPG | |||||
| <100% | 11.31 (0.86) | 16.41 (1.88) | 22.69 (2.30) | 26.43 (2.50) | 23.49 (2.55) |
| 100% to 199% | 20.13 (1.24) | 17.69 (2.14) | 24.45 (2.43) | 26.62 (1.94) | 26.72 (2.36) |
| ≥200% | 68.56 (1.50) | 16.80 (1.25) | 23.88 (1.25) | 22.31 (1.78) | 20.38 (2.01) |
| Behavioral and clinical characteristics | |||||
| Hypertension | 27.45 (1.13) | 27.50 (2.22) | 44.29 (2.30) | 41.93 (1.94) | 38.33 (2.19) |
| Diagnosed diabetes | 15.86 (0.85) | 34.96 (2.63) | 73.72 (2.09) | 50.54 (3.22) | 62.09 (3.11) |
| Abnormal HDL | 40.11 (1.41) | 24.30 (1.96) | 29.57 (1.73) | 40.09 (2.37) | 36.13 (2.62) |
| Elevated triglycerides | 25.39 (1.32) | 34.19 (2.32) | 33.08 (2.43) | 56.52 (2.89) | 49.47 (2.96) |
| Elevated fasting glucose | 24.10 (0.98) | 30.66 (2.32) | 58.18 (1.90) | 43.65 (2.90) | 49.63 (2.85) |
| Elevated AST | 3.72 (0.48) | 42.19 (4.66) | 27.02 (3.63) | 48.81 (3.45) | 50.78 (4.96) |
| Elevated ALT | 5.18 (0.56) | 46.05 (4.65) | 17.82 (3.07) | 50.92 (4.81) | 65.82 (6.04) |
| AST/ALT | 1.40 ± 0.03 | 1.13 ± 0.03 | 1.62 ± 0.06 | 1.12 ± 0.03 | 1.07 ± 0.03 |
| Platelet count | 268.70 ± 2.70 | 267.78 ± 5.90 | 232.79 ± 2.74 | 266.63 ± 3.24 | 262 ± 3.86 |
| Serum albumin, g/dL | 4.19 ± 0.02 | 4.18 ± 0.03 | 4.02 ± 0.02 | 4.10 ± 0.02 | 4.09 ± 0.02 |
| Smoker | |||||
| Current | 26.49 (1.03) | 15.45 (1.84) | 18.64 (1.84) | 23.52 (2.25) | 18.25 (2.33) |
| Former | 23.48 (0.81) | 21.86 (2.06) | 34.53 (1.44) | 32.41 (3.23) | 35.46 (3.14) |
| Never | 50.03 (1.18) | 15.11 (1.04) | 22.09 (1.57) | 19.35 (1.20) | 17.02 (1.24) |
| BMI | |||||
| <25 | 45.37 (1.21) | 6.83 (0.85) | 13.14 (1.53) | 0.42 (0.09) | 2.47 (0.53) |
| ≥25 to <30 | 33.43 (0.90) | 16.21 (1.29) | 25.87 (1.70) | 19.27 (1.63) | 21.46 (2.05) |
| ≥30 | 21.21 (1.03) | 39.00 (2.55) | 43.93 (2.91) | 78.21 (2.05) | 63.83 (2.69) |
| Central obesityb | 32.51 (1.06) | 32.54 (1.95) | 43.70 (2.08) | 57.54 (2.04) | 49.34 (2.34) |
| Oral health characteristics | |||||
| Caries experience | 94.54 (0.62) | 16.87 (1.08) | 24.45 (0.89) | 23.37 (1.40) | 22.07 (1.50) |
| Untreated dental caries | 27.38 (1.24) | 20.45 (1.52) | 27.42 (2.05) | 32.90 (2.25) | 28.32 (2.29) |
| DMFT | 11.96 ± 0.18 | 13.21 ± 0.39 | 16.01 ± 0.26 | 13.24 ± 0.31 | 14.32 ± 0.36 |
| DFT | 8.75 ± 0.18 | 8.62 ± 0.30 | 9.61 ± 0.29 | 8.38 ± 0.21 | 8.85 ± 0.27 |
| DMFS | 36.96 ± 0.75 | 43.86 ± 1.86 | 57.41 ± 1.34 | 44.69 ± 1.6 | 50.30 ± 1.78 |
| DFS | 21.48 ± 0.58 | 21.88 ± 1.16 | 26.76 ± 0.94 | 21.38 ± 0.69 | 24.08 ± 1.12 |
| DS | 1.66 ± 0.11 | 2.18 ± 0.31 | 1.74 ± 0.18 | 2.34 ± 0.26 | 2.01 ± 0.24 |
| DS/DFS | 13.03 (0.79) | 14.77 (1.61) | 13.49 (1.45) | 18.34 (1.32) | 16.20 (1.31) |
| No. of permanent teeth | 24.36 ± 0.11 | 22.96 ± 0.31 | 21.30 ± 0.34 | 22.77 ± 0.32 | 22.22 ± 0.28 |
| <20 permanent teeth | 12.85 (0.66) | 26.06 (2.68) | 54.41 (2.41) | 39.75 (3.28) | 41.08 (3.21) |
| Gingival recession, mm | 0.21 ± 0.01 | 0.33 ± 0.04 | 0.46 ± 0.03 | 0.31 ± 0.04 | 0.37 ± 0.03 |
| Pocket depth, mm | 1.45 ± 0.02 | 1.54 ± 0.03 | 1.49 ± 0.03 | 1.52 ± 0.03 | 1.51 ± 0.03 |
| Loss of attachment, mm | 1.00 ± 0.03 | 1.23 ± 0.07 | 1.51 ± 0.05 | 1.24 ± 0.06 | 1.35 ± 0.06 |
| Periodontal disease | 12.26 (1.01) | 25.08 (2.39) | 38.63 (2.62) | 37.28 (2.77) | 34.42 (3.01) |
| Moderate or severe periodontitis | 8.82 (0.52) | 27.32 (4.29) | 45.88 (2.92) | 36.42 (3.99) | 39.85 (3.13) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DFS, decayed and filled surfaces; DFT, decayed and filled teeth; DMFS, decayed, missing and filled surfaces; DMFT, decayed, missing and filled teeth; DS, decayed surfaces; FLI, Fatty Liver Index; FPG, federal poverty guideline; FS, Fibrosis Score; GED, General Education Diploma; HDL, high-density lipoprotein; NHANES, National Health and Nutrition Examination Survey; US-FLI, US Fatty Liver Index; USON, ultrasonography.Column % in “All” column; row % in others.
Including multiracial.
Waist circumference.
Poverty status categories were determined by comparing the ratio of family income with the US Department of Health and Human Services’ federal poverty guidelines (Dye et al. 2017).
Clinical and Behavioral Variables
Based on the average of 3 measures, hypertension was diagnosed if systolic blood pressure was ≥130 mm Hg or diastolic blood pressure was ≥85 mm Hg. A person was considered diabetic if diagnosed as diabetic, if she or he was taking insulin or medication for diabetes, if the fasting plasma glucose was >126 mg/dL, or if the nonfasting plasma glucose (first or second venipuncture) was >200 mg/dL. Abnormal HDL (high-density lipoprotein) cholesterol was <40 mg/dL for men or <50 mg/dL for women. Serum triglycerides level was considered elevated if ≥150 mg/dL. AST (aspartate aminotransferase) >37 U/L for men or >31 U/L for women was treated as elevated AST; similarly, ALT (alanine aminotransferase) >40 U/L for men or >31 U/L for women was considered elevated. Cigarette smoking was categorized as current, never, and former. Body mass index (BMI; kg/m2) was grouped into 3 categories: normal, overweight, and obese (Centers for Disease Control and Prevention 2017a), and central obesity was defined as waist circumference >102 cm for men and >88 cm for women.
NAFLD Definitions
NAFLD was assessed with 4 criteria: USON and 3 algorithms with noninvasive measures (Appendix Table 1).
The gold standard was based on the USON examination. NAFLD was deemed present if the hepatic steatosis assessment was moderate-severe. The technical and clinical methods are documented elsewhere (Westat Inc. 1988).
The Fibrosis Score (FS; Angulo et al. 2007) was calculated with an algorithm that includes 6 variables (age, BMI, hyperglycemia as a measure of diabetes, platelet count, the liver enzymes AST/ALT ratio, and albumin). A value < –1.455 is considered the absence of significant fibrosis and >0.676 the presence of significant fibrosis. The cut point ≥ –1.455 was used in this analysis to categorize intermediate and advanced fibrosis. The FS is designed to detect advanced fibrosis and has an indeterminate area between cut points.
The Fatty Liver Index (FLI) was developed in Italy as a simple way to use routine clinical and laboratory measures to identify patients at greater risk for fatty liver for further evaluation and lifestyle counseling (Bedogni et al. 2006). The algorithm uses 4 variables: BMI, waist circumference, triglycerides, and gamma-glutamyl-transferase. The FLI varies between 0 and 100, and the cut point of <30 is used to rule out fatty liver and ≥60 to rule it in (Bedogni et al. 2006). We used the <30 cut point in this analysis.
The US Fatty Liver Index (US-FLI; Ruhl and Everhart 2015) was developed for better prediction than the FLI in a multiethnic population such as the United States. It incorporates race/ethnicity and age, which are not included in the FLI, and a different set of biomarkers. A score ≥30 is considered NAFLD for the US-FLI.
Oral Health Variables
Dental caries experience was defined as ≥1 decayed or filled permanent tooth surfaces and untreated dental caries as having any decayed permanent tooth surfaces. Permanent tooth loss experience, excluding third molars, was categorized as <20 teeth versus ≥20 teeth, as this number of teeth has been considered necessary for a functional occlusion (Gotfredsen and Walls 2007). The prevalence of moderate periodontitis was defined as ≥2 teeth with loss of attachment ≥4 mm at interproximal sites or ≥2 teeth with pocket depth ≥5 mm at interproximal sites. Severe periodontitis was defined as ≥2 teeth with loss of attachment ≥6 mm at interproximal sites and ≥1 teeth with pocket depth ≥5 mm at interproximal sites. For this study, moderate and severe were combined. Details of the dental caries and periodontal measurements can be found elsewhere (Drury et al. 1996; Page and Eke 2007).
Statistical Analysis
Descriptive analyses were conducted to calculate estimates and standard errors with Taylor series linearization. Indicator variables were used for coding hypertension, diabetes, and other elevated markers; most laboratory measures were analyzed as continuous variables. Unadjusted and adjusted multivariable logistic regression models were developed to examine the relationship among oral health variables (moderate-severe periodontitis, untreated caries, caries experience, and tooth loss) while controlling for sociodemographic factors, with the NAFLD condition as the outcome. Odds ratios (ORs) and 95% CIs were calculated with logistic regression.
Our modeling approach began with developing unadjusted models, followed by including all the covariates in multivariable models (full models). Last, we used a backward selection procedure to produce final models containing only significant covariates (P ≤ 0.05) associated with the 4 NAFLD measures. Receiver operating characteristic (ROC) curves were calculated to the 3 NAFLD algorithms to the USON findings. The ROC curve graphs the true-positive (sensitivity) and false-positive (1 – specificity) rates on the y- and x-axes, respectively, with area under the curve calculated. All the analyses were conducted in SAS 9.4 software with the Survey Procedures (SAS Institute Inc.) and STATA 15.1 (StataCorp) incorporating survey design and sample weight variables as appropriate.
Results
Population Characteristics
The characteristics of the population studied (N = 5,421) are shown in Table 1. Just over half (57%) were 21 to 39 y old; 32%, 40 to 59 y; and 12%, 60 to 74 y. About half (52%) were women. The majority (75%) were non-Hispanic White; 11%, non-Hispanic Black; 6%, Mexican American; and 7%, “other.” The majority (81%) had at least a high school education. Sixty-eight percent were ≥200% of federal poverty guidelines. About a fourth of the population was diagnosed with hypertension, elevated triglycerides, and elevated fasting glucose and was a current smoker. Among participants, 16% had diabetes, 21% were considered obese; and 33% had excessive waist circumference.
Twenty-seven percent had untreated dental caries, and 13% had <20 permanent teeth. The mean numbers of permanent teeth and decayed and filled teeth were 24.36 and 8.75, respectively. The prevalence of moderate or severe periodontal disease was 9%. The USON prevalence of NAFLD was 17% but was higher when assessed by the algorithms, ranging from 22% (US-FLI) to 24% (FLI and FS).
Relationships between NAFLD and Each Oral Health Measure
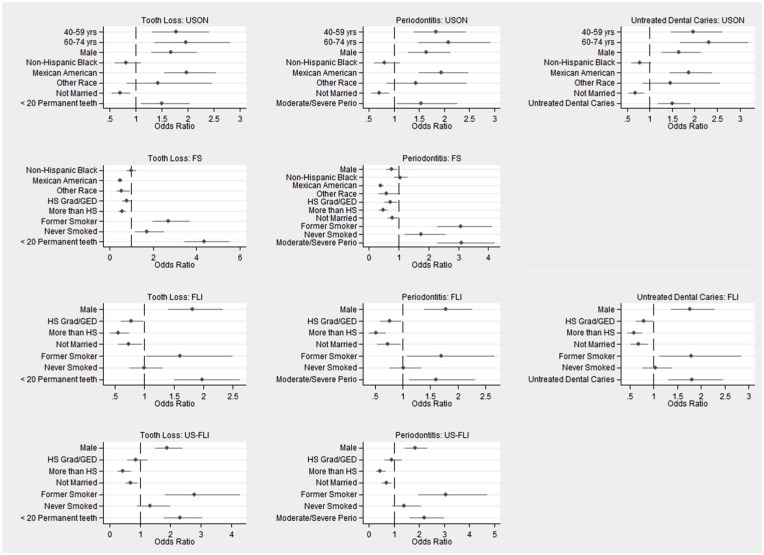
Figure 2 shows the ORs and 95% CIs for the oral health measures and covariates in the final regression models for the NAFLD assessments, graphed as forest plots. The significant ORs for the models are summarized in Table 2. In the United States, NAFLD as determined by USON was associated with untreated dental caries (OR = 1.51, 95% CI = 1.20 to 1.90), moderate or severe periodontitis (OR = 1.54, 95% CI = 1.06 to 2.24), and having <20 teeth (OR = 1.50, 95% CI = 1.11 to 2.02) but not overall caries experience. The findings were similar per the FLI, with slightly higher ORs and wider CIs. When NAFLD was determined with the US-FLI and FS, there were significant relationships with moderate or severe periodontitis (OR = 2.21, 95% CI = 1.64 to 2.98, and OR = 3.10, 95% CI = 2.31 to 4.17, respectively) and with <20 teeth present (OR = 2.32, 95% CI = 1.79 to 3.01, and OR = 4.36, 95% CI = 3.47 to 5.49, respectively) but not with dental caries (caries experience or untreated caries).
Figure 2.
Odds ratios and 95% CIs for variables in final logistic regression models for tooth loss, periodontitis, and untreated dental caries with the use of different nonalcoholic fatty liver disease measures. FLI, Fatty Liver Index; FS, Fibrosis Score; GED, General Education Diploma; HS, high school; US-FLI, US Fatty Liver Index; USON, ultrasonography.
Table 2.
NAFLD Final Models and Oral Health Indicators.
| Oral Health Status |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <20 Permanent Teeth |
Prevalence of Moderate or Severe
Periodontitis |
Prevalence of Untreated Caries (≥1 DS) |
|||||||
| NAFLD Assessments | OR | LCI | UCI | OR | LCI | UCI | OR | LCI | UCI |
| USON | 1.50 | 1.11 | 2.02 | 1.54 | 1.06 | 2.24 | 1.51 | 1.20 | 1.90 |
| FS | 4.36 | 3.47 | 5.49 | 3.10 | 2.31 | 4.17 | — | — | — |
| FLI | 1.99 | 1.52 | 2.59 | 1.61 | 1.13 | 2.28 | 1.80 | 1.33 | 2.44 |
| US-FLI | 2.32 | 1.79 | 3.01 | 2.21 | 1.64 | 2.98 | — | — | — |
Dashes (—) indicate not statistically significant at P < 0.05.
DS, decayed surfaces; FLI, Fatty Liver Index; FS, Fibrosis Score; LCI, lower 95% CI; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; UCI, upper 95% CI; US-FLI, US Fatty Liver Index; USON, ultrasonography.
Relationships between Demographic Covariates and NAFLD Measures
The Appendix Tables show the progression from unadjusted models to full models, including all covariates and then removal of insignificant covariates to produce the final models. For USON (Appendix Table 2), smoking was associated with NAFLD in 4 of 5 unadjusted models, but the association disappeared in those full model groups, remaining insignificant in final models. Being non-Hispanic Black was also associated with NAFLD but in the opposite direction as smoking. Both followed similar patterns: significant in unadjusted models, not significant in multivariate models. Generally, being older, male, Mexican American, and unmarried was associated with NAFLD (USON) in unadjusted models and remained significant in the final multivariate models for all 4 groups. For 3 algorithm-derived NAFLD screening tools, low education, unmarried status, and smoking remained significant in the final models; however, being Mexican American remained significant in the final FS model, whereas being male remained associated with NAFLD in the FLI and US-FLI final models.
Relationship among NAFLD Measures
ROC curves are shown in the Appendix Figure. The area under the ROC curve (Table 3) was least for the FS (0.57), greater for the FLI (0.69), and greatest for the US-FLI (0.72). The FLI and US-FLI areas under the curve were significantly different from that of the FS. Thus, if USON is not available, the US-FLI provides the best NAFLD diagnostic estimate of these 3 other noninvasive assessments in the US population.
Table 3.
ROC Analysis Comparing Ultrasonography Assessment with Other NAFLD Assessments.
| Criteria | AUROC | SE | 95% CI | Sensitivity, % | Specificity, % | Correctly Classified, % | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|---|
| FS | 0.57 | 0.01 | 0.56 to 0.59 | 39.18 | 76.12 | 69.12 | 27.75 | 84.25 |
| FLI | 0.69 | 0.01 | 0.68 to 0.72 | 59.68 | 79.81 | 75.94 | 41.32 | 89.26 |
| US-FLI | 0.72 | 0.01 | 0.71 to 0.75 | 64.31 | 81.55 | 78.18 | 45.84 | 90.39 |
AUROC, area under the receiver operating characteristic; FLI, Fatty Liver Index; FS, Fibrosis Score; NAFLD, nonalcoholic fatty liver disease; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic; US-FLI, US Fatty Liver Index.
Discussion
We found significant associations between moderate-severe periodontitis and tooth loss with all 4 NAFLD assessments, though only 2 assessments were associated with untreated dental caries. More important, the best performing of the 3 screening alternatives to USON, the US-FLI, was unable to show an association with untreated dental caries. This raises an important question for public health researchers: the selection of the screening tool and how that may affect any potential predictive explanatory variables being assessed—in this case, oral health variables. Our findings suggest that choosing the best screening assessment (US-FLI) would result in an inability to explore a potential relationship between NAFLD and dental caries, when we know that an association exists with the standard USON. Choosing a slightly less predictive screening assessment (e.g., FLI) would enhance the researcher’s ability to ascertain an association between NAFLD and dental caries. There may be more misclassification with the algorithmic screening tools than with ultrasound, as more variability is introduced with each risk marker.
The analytic sample was limited to those 21 to 74 y old with complete data for key explanatory variables. The greatest number of people were excluded because an USON assessment was not available. A comparison of this age group in the analytic sample with those excluded indicated no substantial differences by sex, but the mean age of the analytic sample was older, and there were some differences by race/ethnicity, with more non-Hispanic Blacks and Mexican Americans in the sample. The US-FLI is the only NAFLD assessment that includes race/ethnicity in the algorithm. This factor may explain the better fit of this measure.
In a systematic review, there was a significant association between NAFLD and clinical microbial periodontal parameters in 11 of 12 studies (Alakhali et al. 2018). Qiao and colleagues (2018) found a positive association between NAFLD and self-reported categories of tooth loss in males but not females. In our study, there was a greater odds of tooth loss positively associated with NAFLD in men than women with 3 of the 4 NAFLD measures.
Other investigators have noted the higher fatty liver prevalence among men as compared with women and among Mexican Americans as compared with non-Hispanic Whites, with a lower prevalence among non-Hispanic Blacks as compared with non-Hispanic Whites in the United States (Ruhl and Everhart 2015). However, the prevalence among Hispanics varies with country of origin (Fleischman et al. 2014). Risk can also vary by disease severity—for example, male sex and Mexican American ethnicity have been identified as risk factors for NAFLD but not advanced fibrosis (Le et al. 2017).
Although USON is a well-accepted technique, the sensitivity and specificity can vary depending on the operator, the presence of obesity, and the extent of steatosis (Ballestri et al. 2017). Because USON is limited in its ability to detect inflammation (Li et al. 2018), there are newer diagnostic imaging tests available, such as transient elastography, magnetic resonance elastography, resonance-based fat quantitative techniques, and ultrasound elastography, though each has limitations, including cost and clinical availability (Li et al. 2018; Younossi et al. 2018). Comparisons of diagnostic performance of some of the different noninvasive NAFLD methods have been made (Shah et al. 2009; McPherson et al. 2010; Aykut et al. 2014; Cui et al. 2015) with results varying depending on the stage of the disease process (Aykut et al. 2014). Another panel of biomarkers, the Fibrosis-4 score (Sterling et al. 2006), had better areas under the ROC as compared with other noninvasive fibrosis panels but was not significantly different from the FS (Shah et al. 2009). More important, the noninvasive tests generally have a higher negative predictive value than positive predictive value and are better at screening to exclude people with advanced fibrosis among those with NAFLD (McPherson et al. 2010). Since effective therapeutic treatment is lacking, general screening for people without other NAFLD risk factors, such as type 2 diabetes or metabolic syndrome, is not recommended (Chalasani et al. 2012; European Association for the Study of the Liver et al. 2016).
The associations found have implications for health care, as oral conditions may be part of the constellation of either risk factors or consequences of NAFLD, or both. Many people do not know if they have NAFLD. As dentists gain access to medical records with the demographic and laboratory information needed for input into algorithmic NAFLD screening tools, they will have the potential means to evaluate NAFLD risk factors and refer patients for medical assessment as necessary. Conversely, our findings suggest that primary care providers whose patients have NAFLD may want to advise them to see their dentist and hygienist because of NAFLD associations with poor oral health status.
Strengths and Limitations
Our study has several strengths. NHANES III is a large, nationally representative study. People are selected randomly, not on the basis of any preexisting disease or condition, from a variety of geographic areas. Many clinical and biological variables were available, including NAFLD and clinically determined oral health measures, a rare combination. The dental caries data are high quality, since the examination is comprehensive, and dental examiners are trained and calibrated. There was masking in the sense that no examiners knew who had NAFLD.
The gold standard used was USON; thus, “fibrosis” was not confirmed by liver biopsy. We used 3 widely reported assessment algorithms for NAFLD, which are noninvasive screening tools with differing diagnostic utility. We explored how using these screening tools would affect associations between NAFLD and oral conditions when imaging or other more expensive methods were not available for epidemiologic studies, and we found some differences regarding caries. We used older data since USON measurements were not obtained in the more recent NHANES.
Children and adults aged ≥75 y did not receive USON, which limits the generalizability of our analysis with respect to our youngest and oldest populations. The oral conditions studied and NAFLD prevalence all increase with age (Dye et al. 2007; Chalasani et al. 2012). NHANES III used only a partial mouth design for periodontal assessments. We know that this substantially underestimates the prevalence of periodontal disease (Eke et al. 2010). Although NAFLD and oral diseases share common risk factors, it was not the primary aim of this study to tease out specific predictors or pathways to better explain causality or directionality. Variables known to be associated with periodontal disease, such as diabetes, were included in ≥1 algorithms and thus could not be used as independent variables. Many potential explanatory factors were not included, such as cariogenic or periodontal pathogens or sugar consumption, because information was not available, limited, or not useful.
Conclusion
In this study based on a large, representative US population, NAFLD was significantly associated with tooth loss, moderate to severe periodontitis, and, for some NAFLD measures, untreated caries, after adjusting for several key sociodemographic factors. The US-FLI provided information in greater agreement with the USON measure than the FS or FLI. Our findings emphasize the importance of understanding the connections between diseases in the oral cavity and other organ systems.
Given that NAFLD prevalence is increasing, studies with contemporary data are needed to better understand its relationship to oral health. Results suggest another oral-systemic link and potential opportunities for medical-dental integration.
Author Contributions
J.A. Weintraub, B.A. Dye, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; G. Lopez Mitnik, contributed to design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519866442 for Oral Diseases Associated with Nonalcoholic Fatty Liver Disease in the United States by J.A. Weintraub, G. Lopez Mitnik and B.A. Dye in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This research was partially supported by the National Institute of Dental and Craniofacial Research, and 2 of the authors were paid a salary by the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: J.A. Weintraub  https://orcid.org/0000-0001-7178-2028
https://orcid.org/0000-0001-7178-2028
References
- Akinkugbe AA, Barritt AS, Cai J, Offenbacher S, Thyagarajan B, Khambaty T, Singer R, Kallwitz E, Heiss G, Slade GD. 2017. Periodontitis and non-alcoholic fatty liver disease, a population-based cohort investigation in the Study of Health in Pomerania. J Clin Periodontol. 44(11):1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakhali MS, Al-Maweri SA, Al-Shamiri HM, Al-Haddad K, Halboub E. 2018. The potential association between periodontitis and non-alcoholic fatty liver disease: a systematic review. Clin Oral Investig. 22(9):2965–2974. [DOI] [PubMed] [Google Scholar]
- Alazawi W, Bernabe E, Tai D, Janicki T, Kemos P, Samsuddin S, Syn WK, Gillam D, Turner W. 2017. Periodontitis is associated with significant hepatic fibrosis in patients with non-alcoholic fatty liver disease. PLoS One. 12(12):e0185902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et al. 2007. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 45(4):846–854. [DOI] [PubMed] [Google Scholar]
- Aykut UE, Akyuz U, Yesil A, Eren F, Gerin F, Ergelen R, Celikel CA, Yilmaz Y. 2014. A comparison of FibroMeter NAFLD Score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Scand J Gastroenterol. 49(11):1343–1348. [DOI] [PubMed] [Google Scholar]
- Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Targher G, Lonardo A. 2017. Ultrasonographic fatty liver indicator detects mild steatosis and correlates with metabolic/histological parameters in various liver diseases. Metab Clin Exp. 72:57–65. [DOI] [PubMed] [Google Scholar]
- Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. 2006. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2011. National Health and Nutrition Examination Survey, 1988–1994. Data documentation, codebook, and frequencies. Hepatic/gallbladder ultrasound and hepatic steatosis (HGUHS); [accessed 2018 Aug 22]. https://wwwn.cdc.gov/nchs/data/nhanes3/34a/HGUHS.htm#GUPCHSC.
- Centers for Disease Control and Prevention. 2017. a. About adult BMI. Atlanta, GA; Centers for Disease Control and Prevention; [accessed 2018 Oct 3]. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. [Google Scholar]
- Centers for Disease Control and Prevention. 2017. b. National Health and Nutrition Examination Survey, 1988–1994. Questionnaires, datasets, and related documentation; [accessed 18 Oct 2018]. https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx.
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. 2012. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 55(6):2005–2023. [DOI] [PubMed] [Google Scholar]
- Cheng R, Barton JC, Morrison ED, Phatak PD, Krawitt EL, Gordon SC, Kowdley KV. 2009. Differences in hepatic phenotype between hemochromatosis patients with HFE C282Y homozygosity and other HFE genotypes. J Clin Gastroenterol. 43(6):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, Loomba R. 2015. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 41(12):1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury TF, Winn DM, Snowden CB, Kingman A, Kleinman DV, Lewis B. 1996. An overview of the oral health component of the 1988–1991 National Health and Nutrition Examination Survey (NHANES III–Phase 1). J Dent Res. 75 Spec No.:620–630. [DOI] [PubMed] [Google Scholar]
- Dye BA, Mitnik GL, Iafolla TJ, Vargas CM. 2017. Trends in dental caries in children and adolescents according to poverty status in the United States from 1999 through 2004 and from 2011 through 2014. J Am Dent Assoc. 148(8):550–565.e7. [DOI] [PubMed] [Google Scholar]
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltran-Aguilar ED, Horowitz AM, Li CH. 2007. Trends in oral health status: United States, 1988–1994 and 1999–2004. National Center for Health Statistics. Vital Health Stat. 11(248):1–92. [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. 2010. Accuracy of NHANES periodontal examination protocols. J Dent Res. 89(11):1208–1213. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. 2016. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 9(2):65–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman MW, Budoff M, Zeb I, Li D, Foster T. 2014. NAFLD prevalence differs among Hispanic subgroups: the Multi-ethnic Study of Atherosclerosis. World J Gastroenterol. 20(17):4987–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotfredsen K, Walls AWG. 2007. What dentition assures oral function? Clin Oral Implants Res. 18 Suppl 3:34–45. [DOI] [PubMed] [Google Scholar]
- Han P, Sun D, Yang J. 2016. Interaction between periodontitis and liver diseases. Biomed Rep. 5(3):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, Nguyen MH. 2017. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 12(3):e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. 2018. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 10(8):530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. 2015. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 47(3):181–190. [DOI] [PubMed] [Google Scholar]
- Lustig RH. 2014. Fat chance: beating the odds against sugar, processed food, obesity and disease. New York (NY): PLUME, Penguin Group. [Google Scholar]
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. 2010. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 59(9):1265–1269. [DOI] [PubMed] [Google Scholar]
- Naka S, Nomura R, Takashima Y, Okawa R, Ooshima T, Nakano K. 2014. A specific Streptococcus mutans strain aggravates non-alcoholic fatty liver disease. Oral Dis. 20(7):700–706. [DOI] [PubMed] [Google Scholar]
- Naka S, Wato K, Hatakeyama R, Okawa R, Nomura R, Nakano K. 2018. Longitudinal comparison of Streptococcus mutans–induced aggravation of non-alcoholic steatohepatitis in mice. J Oral Microbiol. 10(1):1428005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Eke PI. 2007. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 78(7 Suppl):1387–1339. [DOI] [PubMed] [Google Scholar]
- Qiao F, Fu K, Zhang Q, Liu L, Meng G, Wu H, Xia Y, Bao X, Gu Y, Shi H, et al. 2018. The association between missing teeth and non-alcoholic fatty liver disease in adults. J Clin Periodontol. 45(8):941–951. [DOI] [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE. 2015. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 41(1):65–76. [DOI] [PubMed] [Google Scholar]
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. 2009. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 7(10):1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Softic S, Cohen DE, Kahn CR. 2016. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 61(5):1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski MS, Torriani FJ, Dieterich DT, Thomas DL, et al. ; APRICOT Clinical Investigators. 2006. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- Westat Inc. 1988. National Health and Nutrition Examination Survey. Gallbladder ultrasonography procedure manual; [accessed 2018 Oct 18]. https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/gallblad.pdf.
- Yki-Järvinen H. 2014. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2(11):901–910. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, et al. 2012. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. 2016. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 64(1):73–84. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, et al. 2018. Diagnostic modalities for non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) and associated fibrosis. Hepatology. 68(1):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519866442 for Oral Diseases Associated with Nonalcoholic Fatty Liver Disease in the United States by J.A. Weintraub, G. Lopez Mitnik and B.A. Dye in Journal of Dental Research