Abstract
Caries lesions develop when acid production from bacterial metabolism of dietary carbohydrates outweighs the various mechanisms that promote pH homeostasis, including bacterial alkali production. Therapies that provide arginine as a substrate for alkali production in supragingival oral biofilms have strong anticaries potential. The objective of this study was to investigate the metabolic profile of site-specific supragingival plaque in response to the use of arginine (Arg: 1.5% arginine, fluoride-free) or fluoride (F: 1,100 ppm F/NaF) toothpastes. Eighty-three adults of different caries status were recruited and assigned to treatment with Arg or F for 12 wk. Caries lesions were diagnosed using International Caries Detection and Assessment System II, and plaque samples were collected from caries-free and carious tooth surfaces. Taxonomic profiles were obtained by HOMINGS (Human Oral Microbe Identification using Next Generation Sequencing), and plaque metabolism was assessed by the levels of arginine catabolism via the arginine deiminase pathway (ADS), acidogenicity, and global metabolomics. Principal component analysis (PCA), partial least squares–discriminant analysis, analysis of variance, and random forest tests were used to distinguish metabolic profiles. Of the 509 active lesions diagnosed at baseline, 70 (14%) were inactive after 12 wk. Generalized linear model showed that enamel lesions were significantly more likely to become inactive compared to dentin lesions (P < 0.0001), but no difference was found when treatment with Arg was compared to F (P = 0.46). Arg significantly increased plaque ADS activity (P = 0.031) and plaque pH values after incubation with glucose (P = 0.001). F reduced plaque lactate production from endogenous sources (P = 0.02). PCA revealed differences between the metabolic profiles of plaque treated with Arg or F. Arg significantly affected the concentrations of 16 metabolites, including phenethylamine, agmatine, and glucosamine-6-phosphate (P < 0.05), while F affected the concentrations of 9 metabolites, including phenethylamine, N-methyl-glutamate, and agmatine (P < 0.05). The anticaries mechanisms of action of arginine and fluoride are distinct. Arginine metabolism promotes biofilm pH homeostasis, whereas fluoride is thought to enhance resistance of tooth minerals to low pH and reduce acid production by supragingival oral biofilms.
Keywords: biofilm, dental caries, bacteria, metabolomics, metabolite, microbiome
Introduction
Supragingival oral biofilms comprise multispecies microbial communities with complex structural organization and sophisticated metabolic and functional activities. In the caries process, recurrent acid production from bacterial metabolism of dietary carbohydrates favors the emergence of a highly acidogenic and aciduric microflora in biofilms. This selective process alters the pH homeostasis of biofilms while shifting the demineralization-remineralization equilibrium toward loss of tooth minerals (Marsh 2006). Recent evidence supports the hypothesis that protection against caries may be also achieved by increasing the capacity of microbial communities to neutralize biofilm pH. In particular, the amino acid arginine (L-Arg) can be metabolized by the arginine deiminase pathway (ADS) of certain oral bacteria to generate ammonia. Ammonia production via the ADS has a significant pH-raising effect (Kanapka and Kleinberg 1983) and can inhibit tooth demineralization by neutralizing glycolytic acids (Nascimento and Burne 2014). A positive correlation between plaque ADS activity and absence of caries activity has been clinically recognized (Nascimento et al. 2013).
Evidence accumulated from in vitro studies (Kanapka and Kleinberg 1983; Casiano-Colon and Marquis 1988; Margolis et al. 1988; He et al. 2016; Agnello et al. 2017) and clinical observations (Nascimento et al. 2009; Kraivaphan et al. 2013; Nascimento et al. 2013; Nascimento et al. 2014) supports the hypothesis that providing arginine regularly to supragingival biofilms can be an effective approach for caries intervention. In vitro studies have shown that arginine disrupts the formation of biofilm matrix and the microbial interactions associated with cariogenic biofilms (He et al. 2016). In fact, toothpaste formulations containing arginine were shown to have a significant anticaries effect (Acevedo et al. 2005; Acevedo et al. 2008; Kraivaphan et al. 2013). In our previous study, the use of a 1.5% arginine fluoride-free toothpaste for 4 wk significantly increased plaque ADS activity and promoted a favorable change in the plaque microbiome of caries-active individuals (Nascimento et al. 2014). However, much remains to be investigated with respect to the impact on oral microbial ecology of delivery of exogenous arginine from oral health care products.
Comprehensive investigations based on omics approaches play an important role in medical science and are now expanding our knowledge of the etiology of oral diseases. Metabolomics are used to acquire ample biological information about low molecular weight metabolites that are present in biological specimens (Fiehn 2002). Few studies have used metabolomics in dentistry (Barnes et al. 2014; Edlund et al. 2015), but they revealed the tremendous potential for this technology to elucidate key metabolites associated with oral pathogenic pathways. Notably, the metabolic profiling of dental plaque of individuals with different caries status can provide an overview of bacterial metabolism and ultimately reveal novel biomarkers for caries. Metabolomics can also provide insights into plaque metabolic alterations in response to different therapeutic interventions, such as arginine-containing versus fluoride-containing products. This study aimed to investigate the effects of the use of an arginine- or a fluoride-containing toothpaste for 12 wk on 1) arginine catabolism via ADS, 2) acidogenicity, and 3) the metabolic profiles of plaque samples from adults and tooth surfaces with different caries status.
Materials and Methods
Study Design
This cohort study had a double-blind, randomized, and parallel-group design in accordance with the STROBE guidelines. The study included a washout period of 1 to 2 wk, followed by a treatment regimen of 12 wk with either the arginine (Arg; 1.5% arginine, fluoride free) or fluoride (F; 1,100 ppm F as NaF) toothpastes. A computer-generated randomization process was used to assign subjects to the treatment groups at a 1.5:1 (Arg/F) ratio. Inclusion of control groups such as “toothpaste without active compounds” or “brushing only” was not possible due to ethical concerns. Oral examination and plaque collection occurred at baseline and 12 wk. During the washout period, subjects were instructed to brush their teeth twice a day for 1 min using F. During the treatment period, subjects were instructed to brush their teeth twice a day for 1 min using either Arg or F. The recommended amount of toothpaste applied to the toothbrush was shown to subjects as being approximately 2 cm in diameter (±1 g).
Study Population
Power analyses (proc power-SAS) performed using data from our previous study (Nascimento et al. 2014) indicated that recruitment of at least 70 subjects was required to address the study aims with a power of 80% (α = 0.05). A total of 83 subjects (ages between 18 and 65 y) were recruited via study advertisements. Of these, 45 were caries active (CA) with at least 2 active, cavitated caries lesions (DT [number of decayed teeth] ≥2, MFT [number of missing and filled teeth due to caries] ≥0), and 38 were caries free (CF) with no history of caries experience (DMFT [number of decayed, missing and filled teeth due to caries] = 0). With regards to treatment, 51 used Arg (29 CA and 22 CF) and 32 used F (16 CF and 16 CA; Appendix).
Sample Collection
Subjects were asked to refrain from oral hygiene and fast for at least 8 h prior to plaque collection. Site-specific supragingival plaque samples were collected at baseline and 12 wk by pooling material from 1 or 2 tooth sites of similar caries status (Nascimento et al. 2013). Samples were collected from 1) caries lesion-free tooth surfaces (PF; International Caries Detection and Assessment System [ICDAS] = 0); 2) active, enamel lesions (PE; ICDAS = 1–3); and 3) active, dentin lesions (PD; ICDAS = 4–6). Collected samples were immediately transferred to and dispersed in sterile microcentrifuge tubes containing 10 mM sodium phosphate buffer (pH 7.0) and transported on ice to the laboratory to be analyzed or, if necessary, snap-frozen and stored at −80 °C until the day of analysis.
Caries Diagnosis
At each study visit, caries lesions were detected and diagnosed using the ICDAS-II visual criteria (Ekstrand et al. 2007) and the Lesion Activity Assessment (LAA) system (Braga et al. 2009). The ICDAS scores range from 0 to 6 (0: caries-free surfaces, 1–3: enamel lesions, and 4–6: dentin lesions). CF presented only ICDAS 0, and CA presented ICDAS 0 to 6, with a minimum of 2 scores ≥5. Lesion activity was determined by clinical appearance, plaque stagnation, and tactile sensation using visual-tactile methods. Lesion progression was recorded as “active,” “inactive,” “progressed,” or “treated” (Appendix).
Plaque ADS Activity
Plaque ADS activity was measured by monitoring citrulline production from arginine using a validated protocol (Nascimento et al. 2019). To accommodate small samples of site-specific plaque, the assays used a nanodrop scale on the Biotek Synergy H4 with microspot quantification. ADS activity was normalized to protein content using the Pierce BCA Protein-Assay-Kit (Thermo-Fisher Scientific) and defined as nmol of citrulline generated [minute × (mg protein)]–1.
Plaque Acidogenicity
Lactate concentration was measured in plaque samples using the L-lactate assay kit (Eton Bioscience; Appendix) and as a function of lactate produced from 1) endogenous cell sources (LPE; e.g., glycogen and exopolysaccharides in the absence of added glucose) and 2) added 55 mM glucose (LPG). Controls included L-lactate standards and overnight cultures of Streptococcus mutans UA159 and Streptococcus gordonii DL-1. Lactate concentration was normalized to protein content (Pierce BCA Protein-Assay-Kit), and defined as µM of lactate [minute × (mg protein)]–1. For the LPG assay, the initial and final plaque pH were recorded before and after the incubation period with glucose, respectively.
Bacterial Community Profiles and Global Metabolomics
A total of 141 supragingival plaque samples collected from 12 CF and 14 CA subjects at baseline and 12 wk after treatment with Arg and F were analyzed by HOMINGS (Human Oral Microbe Identification using Next Generation Sequencing) and global metabolomics. These samples represent a random subset of all samples collected in this study from the different study groups. HOMINGS was used to survey the plaque communities (Appendix, Richards et al. 2017). The metabolomic analyses were performed using a Thermo Q-Exactive high resolution mass spectrometer with a Dionex UHPLC and autosampler (Appendix).
Statistical Analysis
Data management and statistical analyses were performed using Bioestat 3.0 and Stata 15.0 software. For descriptive analysis, the distribution of percentages and means was calculated when appropriate. A normality test was used to determine if the data set was well modeled by a normal distribution. A generalized linear model (GLM) was used to identify differences in caries lesion arrestment at the levels of lesion and treatment adjusting for subjects and lesion severity. One-way analysis of variance (ANOVA), t test, Tukey’s test, and nonparametric Kruskal-Wallis test were used to examine the differences in plaque ADS activity and acidogenicity. Principal component analysis (PCA), partial least squares–discriminant analysis (PLS-DA), and random forest were performed to distinguish metabolic profiles and identify key metabolites differentially expressed in each group. Multivariate techniques employed here included Orthogonal PLS-DA, hierarchical cluster analysis (HCA), and multivariate Bayesian time-series analysis (MEBA).
Results
Of 83 subjects enrolled at baseline, 7 dropped out during the study (retention rate: 92%). Appendix Table 1 shows their demographic characteristics and DMFT scores. Of the 509 active caries lesions diagnosed at baseline, 70 (14%) lesions were inactive after 12 wk (Table 1). GLM analysis showed that enamel lesions were significantly more likely to become inactive (χ2: P < 0.0001; odds ratio [OR] = 23.04; P < 0.0001) compared to dentin lesions. Among the CA presenting inactive lesions (44% of all CA), 68% used Arg and 32% used F, but GLM analysis showed no difference between the treatments (OR = 1.46; P = 0.457). Of note, there was no difference in regards to the distribution of active enamel lesions assigned to treatment with Arg (n = 154) or F (n = 157; χ2: P > 0.05), but there were significantly more dentin lesions assigned to Arg (n = 130) than F (n = 68; χ2: P < 0.0001).
Table 1.
Number of Active Caries Lesion Diagnosed at Baseline and Their Progression State after 12 wk of Treatment.
| Baseline, n (Mean ± SD per Subject) (%) | 12 wk, n (%) | |||||
|---|---|---|---|---|---|---|
| ICDAS-II Scores | Toothpaste | Active | Active | Inactive | Progressed | Treated |
| 1 | Arg | 17 (0.6 ± 1.46) (100) | 6 (35) | 10 (59) | 1 (6) | 0 |
| F | 1 (0.06 ± 0.32) (100) | 0 | 1 (100) | 0 | 0 | |
| 2 | Arg | 58 (2.0 ± 3.3) (100) | 48 (83) | 10 (17) | 0 | 0 |
| F | 95 (5.9 ± 21.9) (100) | 78 (82) | 16 (17) | 1 (1) | 0 | |
| 3 | Arg | 79 (2.7 ± 2.0) (100) | 55 (70) | 24 (30) | 0 | 0 |
| F | 61 (3.8 ± 13.9) (100) | 54 (89) | 6 (10) | 1 (1) | 0 | |
| 4 | Arg | 63 (2.2 ± 2.0) (100) | 62 (98) | 0 | 0 | 1 (2) |
| F | 40 (2.5 ± 9.2) (100) | 38 (95) | 2 (5) | 0 | 0 | |
| 5 | Arg | 40 (1.4 ± 1.7) (100) | 38 (96) | 1 (2) | 0 | 1 (2) |
| F | 15 (0.9 ± 3.6) (100) | 14 (93) | 0 | 0 | 1 (7) | |
| 6 | Arg | 27 (0.9 ± 1.9) (100) | 24 (89) | 0 | 0 | 3 (11) |
| F | 13 (0.8 ± 3.4) (100) | 12 (92) | 0 | 0 | 1 (8) | |
| Total | 509 (100) | 429 (84) | 70 (14) | 3 (0.6) | 7 (1.4) | |
Percentages are within rows for each ICDAS score and toothpaste. Active enamel lesions (ICDAS 1–3) presented loss of luster, “chalky” appearance, rough surface upon gently probing, and, in some cases, localized shallow defects or microcavitations. Inactive enamel lesions presented shiny and smooth surfaces upon gentle probing (despite the presence of microcavities). Active cavitated lesions (ICDAS 5–6) presented surface breakdown with exposed soft or leathery dentinal carious tissues. Inactive cavitated lesions were shiny and felt hard upon gentle probing. Progressed: carious lesions progressed to a higher ICDAS score; Treated: teeth/tooth surfaces received endodontic therapy, had restorative treatment, or were extracted. No new lesions were diagnosed during the study period.
Arg, arginine; F, fluoride; ICDAS-II, International Caries Detection and Assessment System II.
At baseline, plaque ADS activity levels from CF (14.4 ± 5.5) were significantly higher than those of CA (10.8 ± 4.6; P < 0.0001), and ADS activity of PF (13.07 ± 4.8) was higher compared to PE (11.8 ± 4.9) and significantly higher compared to PD (9.41 ± 5.0; P < 0.0001). Regardless of subjects’ plaque caries status, Arg significantly increased ADS activity from baseline (11.7 ± 5.3) to 12 wk (13.5 ± 5.4; P = 0.031; Table 2). At 12 wk, ADS activity of subjects who used Arg (13.5 ± 5.4) was significantly higher compared to those who used F (11.4 ± 4.3; P = 0.022). Arg significantly increased ADS activity of CA from baseline (10.9 ± 4.9) to 12 wk (13.1 ± 5.7; P = 0.031), and ADS activity of CA who used Arg (13.1 ± 5.7) was significantly higher compared to CA who used F (10.0 ± 3.5; P = 0.006). PD samples of CA subjects who used Arg showed a significant increase in ADS activity from baseline (9.7 ± 5.3) to 12 wk (13.2 ± 6.3; P = 0.045). In addition, ADS activity of PF samples exposed to Arg (14.0 ± 4.8) was significantly higher compared to those exposed to F (12.8 ± 4.3; P = 0.006) at 12 wk.
Table 2.
ADS Activity Levels of Site-Specific Dental Plaque Collected from Subjects and Tooth Sites of Different Caries Status.
| Caries Group | Plaque Group | Toothpaste | Baseline | 12 wk | P Value |
|---|---|---|---|---|---|
| CF + CA | PF + PE + PD | Arg | 11.7 ± 5.3a | 13.5 ± 5.4a,b | 0.03 |
| CF + CA | PF + PE + PD | F | 11.8 ± 4.6 | 11.4 ± 4.3b | 0.71 |
| CA | PF + PE + PD | Arg | 10.9 ± 4.9a | 13.1 ± 5.7a,b | 0.02 |
| CA | PF + PE + PD | F | 10.6 ± 4.0 | 10.0 ± 3.5b | 0.52 |
| CA | PF | Arg | 11.9 ± 3.9 | 13.4 ± 5.3 | 0.24 |
| CA | PF | F | 12.0 ± 4.0 | 10.8 ± 3.5 | 0.37 |
| CF | PF | Arg | 14.4 ± 5.7 | 14.7 ± 4.3 | 0.83 |
| CF | PF | F | 14.5 ± 5.5 | 14.7 ± 4.2 | 0.93 |
| CF + CA | PF | Arg | 12.9 ± 4.9 | 14.0 ± 4.8b | 0.28 |
| CF + CA | PF | F | 13.3 ± 4.9 | 12.8 ± 4.3b | 0.69 |
| CA | PE | Arg | 11.2 ± 5.6 | 13.0 ± 4.3 | 0.39 |
| CA | PE | F | 12.1 ± 4.4 | 10.2 ± 5.4 | 0.41 |
| CA | PD | Arg | 9.7 ± 5.3a | 13.2 ± 6.3a | 0.05 |
| CA | PD | F | 8.8 ± 3.7 | 10.3 ± 3.5 | 0.32 |
ADS activity is expressed as nmol of citrulline generated [minute × (mg of protein)]–1 and shown as mean ± SD. P values are shown in rows for comparisons of ADS activity between study visits within the same toothpaste group.
ADS, arginine deiminase pathway; Arg, 1.5% arginine toothpaste; CA, caries-active subjects; CF, caries-free subjects; F, 1,100 ppm fluoride as NaF; PD, plaque from active, dentin carious lesions; PE, plaque from active, enamel carious lesion; PF, plaque from carious lesion-free tooth surfaces.
Significant P values (P < 0.05) for comparisons of ADS activity between baseline and 12 wk within the same type of toothpaste.
Significant P values (P < 0.05) for comparisons of ADS activity between toothpaste Arg and F within the same study visit.
F significantly reduced the levels of LPE in all plaque groups from baseline (0.17 ± 0.14) to 12 wk (0.11 ± 0.10; P = 0.02), especially in plaque from CA (0.17 ± 0.12 vs. 0.11 ± 0.09; P = 0.01; Table 3). Although not statistically significant for any of the comparisons, in general, the use of F reduced the levels of LPG from baseline to 12 wk. Appendix Table 2 shows the initial and final pH values of plaque samples after incubation with glucose. Higher pH values were achieved when plaque samples from subjects treated with Arg (5.32 ± 0.60) were incubated in excess glucose compared to samples from subjects treated with F (4.99 ± 0.40; P = 0.001), and this observation was particularly significant for PD samples (5.74 ± 0.66 vs. 5.09 ± 0.19; P = 0.028). The final plaque pH of CF who used Arg was significantly higher at baseline (Arg: 5.23 ± 0.52 vs. F: 5.00 ± 0.39; P = 0.045) and at 12 wk (5.36 ± 0.66 vs. F: 4.99 ± 0.34; P = 0.007) compared to those who used F.
Table 3.
Lactate Production by Site-Specific Dental Plaque Collected from Subjects and Tooth Sites of Different Caries Status.
| Lactate Background (LPE) | Lactate Production (LPG) | |||||||
|---|---|---|---|---|---|---|---|---|
| Caries Group | Plaque Group | Toothpaste | Baseline | 12 wk | P Value | Baseline | 12 wk | P Value |
| CF + CA | PF + PE + PD | Arg | 0.14 ± 0.12 | 0.15 ± 0.17 | 0.88 | 0.18 ± 0.13 | 0.22 ± 0.18 | 0.31 |
| CF + CA | PF + PE + PD | F | 0.17 ± 0.14a | 0.11 ± 0.10a | 0.02 | 0.24 ± 0.19 | 0.19 ± 0.11 | 0.07 |
| CA | PF + PE + PD | Arg | 0.16 ± 0.13 | 0.15 ± 0.19 | 0.90 | 0.18 ± 0.14 | 0.22 ± 0.17 | 0.35 |
| CA | PF + PE + PD | F | 0.17 ± 0.12a | 0.11 ± 0.09a | 0.01 | 0.25 ± 0.18 | 0.18 ± 0.11 | 0.06 |
| CA | PF | Arg | 0.16 ± 0.14 | 0.12 ± 0.09 | 0.43 | 0.20 ± 0.14 | 0.21 ± 0.16 | 0.97 |
| CA | PF | F | 0.18 ± 0.12 | 0.12 ± 0.12 | 0.15 | 0.26 ± 0.19 | 0.18 ± 0.14 | 0.17 |
| CF | PF | Arg | 0.09 ± 0.08 | 0.13 ± 0.11 | 0.39 | 0.18 ± 0.12 | 0.21 ± 0.19 | 0.67 |
| CF | PF | F | 0.18 ± 0.23 | 0.13 ± 0.11 | 0.55 | 0.24 ± 0.23 | 0.20 ± 0.12 | 0.70 |
| CF + CA | PF | Arg | 0.13 ± 0.12 | 0.12 ± 0.10 | 0.94 | 0.19 ± 0.13 | 0.21 ± 0.17 | 0.74 |
| CF + CA | PF | F | 0.18 ± 0.16 | 0.13 ± 0.11 | 0.15 | 0.25 ± 0.20 | 0.19 ± 0.13 | 0.18 |
| CA | PE | Arg | 0.19 ± 0.13 | 0.11 ± 0.08 | 0.19 | 0.17 ± 0.16 | 0.20 ± 0.13 | 0.78 |
| CA | PE | F | 0.19 ± 0.15 | 0.12 ± 0.05 | 0.32 | 0.29 ± 0.25 | 0.21 ± 0.10 | 0.11 |
| CA | PD | Arg | 0.13 ± 0.14 | 0.24 ± 0.31 | 0.39 | 0.16 ± 0.12 | 0.26 ± 0.24 | 0.31 |
| CA | PD | F | 0.15 ± 0.08 | 0.09 ± 0.08 | 0.07 | 0.21 ± 0.14 | 0.17 ± 0.09 | 0.33 |
Lactate concentration was expressed as µM of lactate generated [minute × (mg of protein)]–1 and shown as mean ± SD. P values are shown in rows for comparisons between study visits within the same toothpaste group.
Arg, 1.5% arginine toothpaste; CA, caries-active subjects; CF, caries-free subjects; F, 1,100 ppm fluoride as NaF; LPE, lactate production from endogenous sources; LPG, lactate production from exogenous sources; PD, plaque from active, dentinal carious lesions; PE, plaque from active, enamel carious lesion; PF, plaque from carious lesion-free tooth surfaces.
Significant P values (P < 0.05) for comparisons between baseline and 12-wk within the same type of toothpaste.
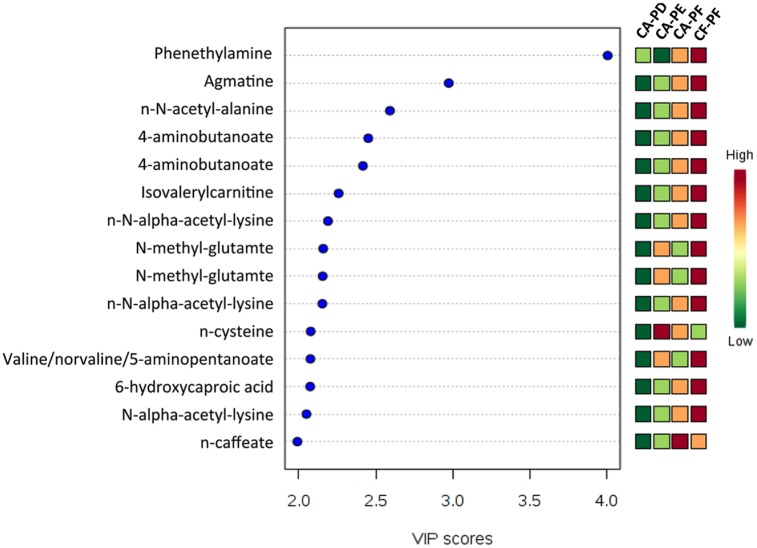
Permutational multivariate analysis of variance (PERMANOVA) tests and distance-based redundancy analysis (db-RDA) showed no significant difference in microbial composition when the groups were compared at baseline and 12 wk after treatment with Arg (P = 0.64) or F (P = 0.09). Appendix Table 3 shows the most prevalent taxa detected when the CF-PF and CA-PD groups were compared after treatment with Arg or F. With regards to the metabolomic analysis, a total of 3,447 metabolic features, including 208 known metabolites, were detected from all metabolomic measurements. At baseline, 27 unknown and known metabolites were differently expressed among the CF-PF, CA-PF, CA-PE, and CA-PD groups (P < 0.05). However, PLS-DA of known metabolites showed minimal separation between the metabolic profiles of these groups at baseline (Appendix Fig. 1). Variable importance of projection (VIP) demonstrated that 20 known metabolites were differently expressed among the groups (Fig. 1). The concentration levels of 159 known and unknown metabolites were found to be significantly different (P < 0.05) when CF-PF and CA-PD were compared at baseline. Of these, 12 were known metabolites (Appendix Table 4). Metabolites with elevated concentrations in CF-PF at baseline included phenethylamine, agmatine, N-α-acetyl-lysine, 4-aminobutanoate (GABA), N-methyl-glutamate, guanidinoacetate, stachydrine, and 1,3-diaminopropane (P < 0.05). ANOVA revealed significant differences in the levels of phenethylamine when the study groups were compared at baseline (P < 0.05). Metabolites from the tricarboxylic acid (TCA) cycle with elevated concentrations in CA-PD at baseline included fumarate, malate, and also aspartate (P < 0.05).
Figure 1.
Partial least squares–discriminant analysis of top 15 known metabolites differently expressed among the different study groups at baseline. Typically, a VIP of 2 or greater is highly significant. Red indicates high expression and green indicates low expression. CA-PD, plaque from dentin carious lesions of caries-active subjects; CA-PE, plaque from enamel lesion of caries-active subjects; CA-PF, plaque from carious-lesion free tooth surfaces of caries-active subjects; CF-PF, plaque from carious-lesion free tooth surfaces of caries-free subjects.
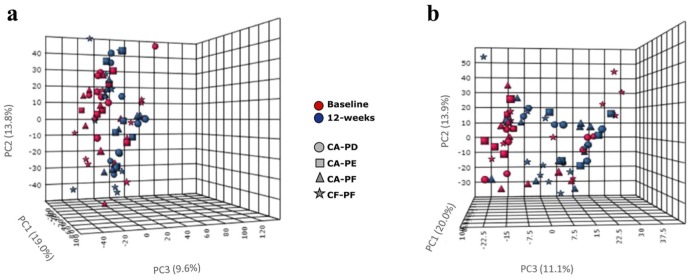
When comparing before and after treatment with Arg or F, PCA revealed minimal separation between the metabolic profiles of the study groups (Fig. 2). MEBA revealed that Arg significantly affected the concentrations of 14 known metabolites (P < 0.05), with phenethylamine, α-hydroxyisobutyric acid, agmatine, and glucosamine-6-phosphate showing the most significant change (Appendix Fig. 2). After Arg use, phenethylamine levels were decreased in CF-PF and CA-PF but not changed in CA-PE and CA-PD, whereas α-hydroxyisobutyric acid levels were decreased in CA-PF and CF-PF and increased in CA-PE and CA-PD. MEBA revealed that F significantly affected the expression of 11 known metabolites (P < 0.05; Appendix Fig. 3). Phenethylamine levels were decreased in all groups as did the levels of α-aminoadipate/methyl-L-glutamate (except for CF-PF). Agmatine levels were increased in CF-PF and CA-PF and slightly decreased in CA-PE and CA-PD after F use. The detection levels of certain metabolites from the Embden-Meyerhof-Parnas (EMP) pathway, pentose-phosphate, and TCA cycle pathways are shown in Appendix Table 5.
Figure 2.
Principal component analysis of known and unknown metabolites that were differently expressed before and after treatment with arginine and fluoride. Treatment with (a) the arginine toothpaste (A), and (b) the fluoride toothpaste (F). CA, caries-active subjects; CF, caries-free subjects; PF, plaque from carious-lesion free tooth surfaces; PE, plaque from active, enamel carious lesion; PD, plaque from active, dentin caries lesions. Note that some metabolites may appear twice because of positive and negative ion analyses.
Discussion
The arginine toothpaste enhanced the capacity of plaque bacteria to metabolize arginine via ADS, likely due to an increased availability of arginine in oral biofilms, and the fluoride toothpaste reduced the capacity of plaque bacteria to produce lactate from bacterial endogenous sources after 12 wk. Both observations were more evident in caries-active subjects. The effects of arginine on ADS activity of supragingival plaque were consistent with those previously reported (Nascimento et al. 2014). The glucose-induced pH drop assay revealed that higher pH values were reached by plaque treated with arginine as compared to those treated with fluoride, and this observation was particularly significant in plaque from dentin lesions. Irrespective of the toothpaste used, there was an overall improvement of the oral health status of the participating subjects likely due to the frequent dental visits and orientation on oral hygiene as part of the study protocol. Certainly, inclusion of “placebo toothpaste” or “brushing only” groups would have allowed us to distinguish the effects of fluoride and arginine from that of mechanical plaque removal. The subjects’ healthier oral status was also demonstrated by the arrestment of 14% of the initial caries lesions. Although not statistically significant, caries arrestment was more pronounced among subjects who used the arginine toothpaste as compared to the fluoride toothpaste. Ammonia production from ADS can counteract the effects of acidification from sugar metabolism on plaque pH, thereby functioning as a caries-inhibiting factor and promoting remineralization. Increased plaque ADS activity favorably impacts the pH profiles of resting and carbohydrate-challenged plaque and, therefore, reduces the risk for caries progression.
L-arginine has been used as a dietary supplement to manage a wide variety of health conditions, such as cardiovascular diseases. This is so because arginine is a precursor of nitric oxide (NO), which plays important roles in vasodilatation, bacterial challenge and cytokine stimulation, and platelet aggregation (Morris 2016). The incorporation of arginine into oral care products has been proven safe and cost-effective to reduce caries incidence. Clinical trials have been conducted to evaluate the anticaries efficacy of arginine formulations with or without fluoride (Kraivaphan et al. 2013; Li et al. 2015). Caries onset was also reduced in children who used mints containing 1.5% arginine (Acevedo et al., 2008). Moreover, a 1.5% arginine and 1,450 ppm of fluoride toothpaste was also shown to reduce caries increments and to have superior caries benefits compared to a regular fluoride toothpaste (Kraivaphan et al. 2013; Li et al. 2015).
In this study, the capacity of plaque to produce lactate and to lower the pH was measured after plaque samples were incubated with glucose or equal volume of water (endogenous sources). In general, plaque treated with fluoride had a reduced capacity to produce lactate from endogenous sources as compared to those treated with arginine. Since the differences observed here are fairly small, they may not be biologically relevant from a caries perspective. However, early in vitro studies showed that fluoride inhibits the growth of and acid production by various oral bacteria, including S. mutans and Streptococcus sanguinis and certain Actinomyces spp. (Hamada and Slade 1980; Hamilton and Ellwood 1983). Subsequent studies showed that fluoride inhibits bacterial enolase, an enzyme in the EMP pathway, and subsequently repressed acid production in vitro (Kaufmann and Bartholmes 1992; Guha-Chowdhury et al. 1997) and in vivo (Takahashi and Washio 2011). Other proposed mechanisms of action of fluoride in plaque metabolism include the disruption of intracellular pH regulation due to effects on bacterial membranes (Marquis et al. 2003) and inhibition of extracellular polysaccharide production (Koo et al. 2006) and S. mutans glucosyltransferases (Cai et al. 2015).
Metabolomics is now disclosing the importance of central carbon metabolism pathways in supragingival plaque, including the EMP, pentose-phosphate, and TCA cycle pathways. Major pathways for amino acid metabolism have also been explored in the context of biofilm pH (Washio et al. 2016). Plaque samples exposed clinically to a glucose rinse followed by a fluoride rinse revealed that fluoride significantly inhibited plaque lactate production, increased the concentrations of 3-phosphoglycerate, and decreased phosphoenolpyruvate (Takahashi and Washio 2011). The changes in the plaque levels of phenethylamine observed here are more likely due to bacterial compositional changes of the organisms able to produce this compound (although not significant, there were increased levels of lactobacilli, clostridia, and others), as well as environmental changes that induce metabolic shifts may account for the differences; and less likely due to production of this compound by the host. The increased levels of agmatine may have been influenced by conversion of the arginine provided from the arginine toothpaste into agmatine by arginine decarboxylase enzyme-positive bacteria. The agmatine deiminase system (AgDS) is an ammonia-generating system identified in oral bacteria such as S. mutans (Griswold et al. 2004). Ammonia production via AgDS enhances bacterial acid tolerance by raising cytoplasmic pH, but it seems insufficient to impact biofilm pH (Griswold et al. 2009). The molecular and ecological basis for the changes in phenethylamine, agmatine, and other metabolites identified here needs to be explored in the context of the transcription profiling of the different types of plaque.
This study provides evidence that the mechanisms of action of arginine and fluoride are distinct, but they may be complementary and/or synergistic. Arginine metabolism positively affects pH homeostasis and bacterial ecology and pathogenicity, whereas fluoride is thought to enhance resistance of tooth minerals to low pH and reduce acid production by supragingival oral biofilms. The increased biofilm pH, as a consequence of the acid production inhibition by fluoride, could also be repressive for ADS expression in certain oral organisms. Importantly, future metatranscriptomic and functional analyses will be needed before any specific metabolite can be used to determine caries risk or track beneficial or detrimental biochemical behaviors of oral biofilms, thereby allowing for rapid assessment of the effects of arginine and fluoride.
Author Contributions
M.M. Nascimento, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; A.J. Alvarez, X. Huang, C. Browngardt, contributed to data analysis, critically revised the manuscript; R. Jenkins, D.A. Dilbone, V.P. Richards, contributed to data acquisition, critically revised the manuscript; M.C. Sinhoreti, A.P.D. Ribeiro, T.J. Garrett, R.A. Burne, contributed to data analysis and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519869906 for Metabolic Profile of Supragingival Plaque Exposed to Arginine and Fluoride by M.M. Nascimento, A.J. Alvarez, X. Huang, C. Browngardt, R. Jenkins, M.C. Sinhoreti, A.P.D. Ribeiro, D.A. Dilbone, V.P. Richards, T.J. Garrett and R.A. Burne in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported in part by the National Institutes of Health and National Institute of Dental and Craniofacial Research (DE023579 and DE25832), and by Colgate-Palmolive, which manufactures the toothpastes tested on this study.
The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Acevedo AM, Machado C, Rivera LE, Wolff M, Kleinberg I. 2005. The inhibitory effect of an arginine bicarbonate/calcium carbonate cavistat-containing dentifrice on the development of dental caries in Venezuelan school children. J Clin Dent. 16(3):63–70. [PubMed] [Google Scholar]
- Acevedo AM, Montero M, Rojas-Sanchez F, Machado C, Rivera LE, Wolff M, Kleinberg I. 2008. Clinical evaluation of the ability of cavistat in a mint confection to inhibit the development of dental caries in children. J Clin Dent. 19(1):1–8. [PubMed] [Google Scholar]
- Agnello M, Cen L, Tran NC, Shi W, McLean JS, He X. 2017. Arginine improves pH homeostasis via metabolism and microbiome modulation. J Dent Res. 96(8):924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes VM, Kennedy AD, Panagakos F, Devizio W, Trivedi HM, Jonsson T, Guo L, Cervi S, Scannapieco FA. 2014. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS One. 9(8):e105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MM, Mendes FM, Martignon S, Ricketts DN, Ekstrand KR. 2009. In vitro comparison of NYVAD’s system and ICDAS-II with lesion activity assessment for evaluation of severity and activity of occlusal caries lesions in primary teeth. Caries Res. 43(5):405–412. [DOI] [PubMed] [Google Scholar]
- Cai JN, Kim MA, Jung JE, Pandit S, Song KY, Jeon JG. 2015. Effects of combined oleic acid and fluoride at sub-mic levels on eps formation and viability of Streptococcus mutans ua159 biofilms. Biofouling. 31(7):555–563. [DOI] [PubMed] [Google Scholar]
- Casiano-Colon A, Marquis RE. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol. 54(6):1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund A, Yang Y, Yooseph S, Hall AP, Nguyen DD, Dorrestein PC, Nelson KE, He X, Lux R, Shi Wet al. 2015. Meta-omics uncover temporal regulation of pathways across oral microbiome genera during in vitro sugar metabolism. ISME J. 9(12):2605–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand KR, Martignon S, Ricketts DJ, Qvist V. 2007. Detection and activity assessment of primary coronal caries lesions: a methodologic study. Oper Dent. 32(3):225–235. [DOI] [PubMed] [Google Scholar]
- Fiehn O. 2002. Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol. 48(1–2):155–171. [PubMed] [Google Scholar]
- Griswold AR, Chen YY, Burne RA. 2004. Analysis of an agmatine deiminase gene cluster in Streptococcus mutans ua159. J Bacteriol. 186(6):1902–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold AR, Nascimento MM, Burne RA. 2009. Distribution, regulation and role of the agmatine deiminase system in mutans streptococci. Oral Microbiol Immunol. 24(1):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha-Chowdhury N, Clark AG, Sissons CH. 1997. Inhibition of purified enolases from oral bacteria by fluoride. Oral Microbiol Immunol. 12(2):91–97. [DOI] [PubMed] [Google Scholar]
- Hamada S, Slade HD. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 44(2):331–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton IR, Ellwood DC. 1983. Carbohydrate metabolism by Actinomyces viscosus growing in continuous culture. Infect Immun. 42(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Hwang G, Liu Y, Gao L, Kilpatrick-Liverman L, Santarpia P, Zhou X, Koo H. 2016. L-arginine modifies the exopolysaccharides matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 198(19):2651–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapka JA, Kleinberg I. 1983. Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol. 28(11):1007–1015. [DOI] [PubMed] [Google Scholar]
- Kaufmann M, Bartholmes P. 1992. Purification, characterization and inhibition by fluoride of enolase from Streptococcus mutans dsm 320523. Caries Res. 26(2):110–116. [DOI] [PubMed] [Google Scholar]
- Koo H, Sheng J, Nguyen PT, Marquis RE. 2006. Co-operative inhibition by fluoride and zinc of glucosyl transferase production and polysaccharide synthesis by mutans streptococci in suspension cultures and biofilms. FEMS Microbiol Lett. 254(1):134–140. [DOI] [PubMed] [Google Scholar]
- Kraivaphan P, Amornchat C, Triratana T, Mateo LR, Ellwood R, Cummins D, Devizio W, Zhang YP. 2013. Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res. 47(6):582–590. [DOI] [PubMed] [Google Scholar]
- Li J, Huang Z, Mei L, Li G, Li H. 2015. Anti-caries effect of arginine-containing formulations in vivo: a systematic review and meta-analysis. Caries Res. 49(6):606–617. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Duckworth JH, Moreno EC. 1988. Composition of pooled resting plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 67(12):1468-1475. [DOI] [PubMed] [Google Scholar]
- Marquis RE, Clock SA, Mota-Meira M. 2003. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev. 26(5):493–510. [DOI] [PubMed] [Google Scholar]
- Marsh PD. 2006. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health. 6(Suppl 1):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM., Jr. 2016. Arginine metabolism revisited. J Nutr. 146(12):2579S–2586S. [DOI] [PubMed] [Google Scholar]
- Nascimento MM, Alvarez AJ, Huang X, Hanway S, Perry S, Luce A, Richards VP, Burne RA. 2019. Arginine metabolism in supragingival oral biofilms as a potential predictor of caries risk. JDR Clin Trans Res 4(3): 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. 2014. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 29(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Burne RA. 2014. Caries prevention by arginine metabolism in oral biofilms: translating science into clinical success. Curr Oral Health Rep. 1(1):79–85. [Google Scholar]
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. 2009. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 24(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Liu Y, Kalra R, Perry S, Adewumi A, Xu X, Primosch RE, Burne RA. 2013. Oral arginine metabolism may decrease the risk for dental caries in children. J Dent Res. 92(7):604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards VP, Alvarez AJ, Luce AR, Bedenbaugh M, Mitchell ML, Burne RA, Nascimento MM. 2017. Microbiomes of site-specific dental plaques from children with different caries status. Infect Immun. 85(8). pii: e00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Washio J. 2011. Metabolomic effects of xylitol and fluoride on plaque biofilm in vivo. J Dent Res. 90(12):1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio J, Ogawa T, Suzuki K, Tsukiboshi Y, Watanabe M, Takahashi N. 2016. Amino acid composition and amino acid-metabolic network in supragingival plaque. Biomed Res. 37(4):251–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519869906 for Metabolic Profile of Supragingival Plaque Exposed to Arginine and Fluoride by M.M. Nascimento, A.J. Alvarez, X. Huang, C. Browngardt, R. Jenkins, M.C. Sinhoreti, A.P.D. Ribeiro, D.A. Dilbone, V.P. Richards, T.J. Garrett and R.A. Burne in Journal of Dental Research