Abstract
Background & objectives:
Survival of patients with multiple myeloma (MM) has improved in the past two decades following use of novel agents and autologous stem cell transplantation. To determine predictors of long-term outcome, data of MM patients who underwent autologous stem cell transplantation (ASCT) at a tertiary care centre in north India were retrospectively analyzed.
Methods:
Between 1995 and 2016, 349 MM patients underwent ASCT. Patients' median age was 52 yr, ranging from 29 to 68 yr, 68.2 per cent were males. Thirty three per cent patients had international staging system (ISS) Stage III and 68.5 per cent had received novel agents-based induction. High-dose melphalan (200 mg/m2) was used for conditioning; patients with renal insufficiency (estimated glomerular filtration rate <40 ml/min) received melphalan 140-150 mg/m2.
Results:
Post-transplant, 317 of 349 (90.8%) patients responded; complete [complete response (CR)] −213 (61%)], very good partial response (VGPR) −62 (17.8%) and PR in 42 (12%)]. Induction with novel agents, pre-transplant chemosensitive disease, transplant in first remission and serum albumin (≥3.5 g/dl) were predictors of significant response. At a median follow up of 73 months, median overall survival (OS) was 90 months [95% confidence interval (CI) 70.8-109.2], and progression-free survival (PFS) was 41 months (95% CI 33.0-49.0). On multivariate analysis, achievement of CR post-transplant, transplant in first remission, ISS Stages I and II (vs. III), absence of extramedullary disease and serum albumin ≥3.5 g/dl were predictors of prolonged OS. For PFS, achievement of post-transplant CR and transplant in first remission were predictors of superior outcome.
Interpretation & conclusions:
Treatment with novel agents, achievement of complete remission post-transplant, ISS Stages I and II, absence of extramedullary disease and transplant in first remission were predictors of long-term survival for patients with MM.
Keywords: Autologous stem cell transplantation, long-term outcome, multiple myeloma, predictors, prognostic factors, response to transplant
Multiple myeloma (MM), a clonal plasma cell malignancy accounts for approximately 10 per cent of haematologic malignancies. Compared to industrialized nations, myeloma occurs a decade earlier in India, at a median age of 55 yr1. Present management of myeloma patients includes novel agents (immunomodulators - thalidomide, lenalidomide, proteasome inhibitors - bortezomib)-based initial (induction) therapy for 4-6 months followed by autologous stem cell transplantation (ASCT) in patients aged ≤65-70 yr without major co-morbidities. This is followed by low-dose maintenance therapy with either lenalidomide or thalidomide or bortezomib for two years. For elderly patients (≥65-70 yr) or those not suitable for ASCT induction, therapy is given for 6-9 months followed by maintenance therapy. The ASCT is an integral component of myeloma management and has contributed to survival improvement in the past two decades2. Initial transplant studies have used conventional chemotherapy before ASCT. A number of randomized studies3,4,5,6,7 and meta-analyses8 have confirmed that ASCT is associated with deepening of response rate and improved progression-free survival (PFS) in most and overall survival (OS) in some studies compared to conventional cytotoxic chemotherapy. Subsequently, these results were confirmed in recent randomized studies9,10,11,12,13,14 using novel agents-based induction before ASCT further augmenting responses with improvement in survival. While enough experience with ASCT for long-term outcome has been reported from developed countries15,16,17,18,19,20, comprehensive information from resource-limited setting like ours, on the long-term outcome following transplant is limited21. We have reported our initial experience for MM patients transplanted till the year 201422. Here we report an updated follow up with long-term outcome on patients with MM transplanted between 1995 and 2016 as well as comprehensive analysis of prognostic factors associated with long-term survival.
Material & Methods
The data on 349 consecutive MM patients, who underwent ASCT at Institute Rotary Cancer Hospital, All India Institute of Medical Sciences (AIIMS), New Delhi, India, between 1995 and December 2016 were analysed. The study was approved by the Ethics Committee of AIIMS. The patients' characteristics are shown in Table I. The median age was 52 yr ranging from 29 to 68 yr; 236 (67.6%) were males, 34.7 per cent had international staging system (ISS) Stage III disease and 24.4 per cent had Stage IIIB by Durie-Salmon staging (DSS). Eighty one (23.6%) patients had light chain myeloma, 251 (71.9%) received novel agents for induction, 75 (21.5%) received [vincristine, adriamycin and dexamethasone (VAD) as continuous infusion] and the remaining 23 (6.6%) received alkylating agents-based induction regimens. One hundred twenty five patients (36%) had received more than one induction regimen before transplant. Median interval from diagnosis to transplant was 10 months, ranging from 2 to 128 months.
Table I.
Characteristics of the patients included in the study
| Variable | All patients (n=349), n (%) |
|---|---|
| Age (yr) | |
| Median | 52 |
| Range | 29-68 |
| Gender | |
| Male | 236 (67.6) |
| Female | 113 (32.4) |
| International staging system (ISS) | |
| I | 103 (30.0) |
| II | 121 (35.3) |
| III | 119 (34.7) |
| Durie-Salmon staging system (DSS) | |
| ≤IIIA | 263 (75.6) |
| IIIB | 85 (24.4) |
| Ig type (n=342) | |
| IgG | 204 (59.6) |
| IgA | 57 (16.7) |
| Light chain | 81 (23.7) |
| Extramedullary disease (EMD) | |
| Yes | 80 (22.9) |
| No | 229 (77.1) |
| Haemoglobin (g/dl) | |
| ≤10 | 201 (57.6) |
| >10 | 148 (42.4) |
| Serum albumin (g/dl) | |
| <3.5 | 140 (40.1) |
| ≥3.5 | 59.9 (59.9) |
| BM-PC% (n=348) | |
| <40 | 180 (51.7) |
| ≥40 | 168 (48.3) |
| Calcium (mg/dl) (n=324) | |
| ≤11.4 | 296 (91.4) |
| ≥11.5 | 28 (8.6) |
| eGFR (ml/min) | |
| <40 | 86 (24.6) |
| ≥40 | 263 (75.4) |
| Induction treatment | |
| Novel agents | 251 (71.9) |
| VAD | 75 (21.5) |
| Alkylating agents | 23 (6.6) |
| Pre-transplant status | |
| Sensitive (CR+VGPR+PR) | 291 (83.4) |
| Resistant (stable+progressive disease) | 58 (16.6) |
| Interval (months) | |
| ≤12 | 220 (63.0) |
| >12 | 129 (37.0) |
| Induction regimen (n=348) | |
| One line | 223 (64.1) |
| >One line | 125 (35.9) |
BM-PC, bone marrow plasma cell; eGFR, estimated glomerular filtration rate; VAD, vincristine, adriamycin and dexamethasone; Ig, immunoglobulin; CR, complete response; PR, partial response; VGPR, very good partial response
Novel agents-based induction therapy: Among 251 patients, 178 (71.9%) had received two drug combination (thalidomide+dexamethasone, n=92, lenalidomide+dexamethasone, n=54 and bortezomib+dexamethasone, n=32), 71 (20.3%) patients received three-drug combination [VTd (bortezomib+thalidomide+dexamethasone) n=23, VRd (bortezomib+thalidomide+ dexamethasone) n=23, VCd (bortezomib+cyclophosphamide+ dexamethasone) n=21, PAd (liposomal doxorubicin, bortezomib and dexamethasone) n=1 and 3 patients received thalidomide-based combinations]. Two patients received four-drug VTCd (bortezomib+thalidomide+cyclophosphamide+dexamethasone)-based combination.
Transplant protocol: Granulocyte colony stimulating factor (G-CSF) mobilized peripheral blood stem cells (CD34+ ≥2×106/kg) were collected. For conditioning, high-dose melphalan (200 mg/m2) was administered; patients with renal impairment (RI) received melphalan23 in dose of 140-150 mg/m2. This was followed by stem cells infusion. Fifty six patients received stem cells cryopreserved at −80°C, the remaining 293 patients received stem cells stored at 4°C. Transplant response evaluation was done on day 100 ± one week as per European Group for Blood and Marrow Transplant (EBMT) criteria24. Patients were advised maintenance therapy using low-dose thalidomide (50 mg daily) or lenalidomide (5-10 mg/day) for 21 days every month or injection bortezomib 2 mg subcutaneously twice a month. In addition, patients with adequate estimated glomerular filtration rate (eGFR) (≥60 ml/min)23,25 also received injection zoledronic acid once in three months for first two years then once in six months indefinitely along with calcium and vitamin D supplement.
Statistical analysis: An intention-to-treat analysis was done. Descriptive statistics (median and range) were calculated for all variables. Response to transplant was defined as per the EBMT criteria24. The prognostic factors for response to transplant were analyzed by Pearson Chi-square test and binary logistic regression analysis. OS was defined as the time from date of transplant until death or date of censor (December 31, 2017). PFS was calculated from date of transplant to disease progression or death (regardless of the cause of death). Survival curves were plotted according to the method of Kaplan and Meier26 and were compared by the log-rank test. The prognostic factors for survival were analyzed by Cox regression analysis. Analysis was carried out using SPSS-16 statistical software (IBM, Atlanta, USA). The median follow up for the whole group was 73 months (range 12.50-292 months).
Results
A total of 213 (61%) patients achieved complete response (CR), 62 (17.8%) had very good partial response (VGPR), 42 (12.0%) partial response (PR) and 14 (4.0%) patients had stable disease. Eighteen (5.2%) patients died of transplant-related complications (before day 100).
Post-transplant complete response (CR) rate according to pre-transplant status: Among patients with pre-transplant VGPR, 70 (42/60) per cent achieved CR post-transplant, CR rate was 45.5 per cent for those in PR, 23 per cent for those with stable disease and 12.5 per cent for patients with progressive disease pre-transplant (Table II).
Table II.
Response to transplant in patients
| Pre-transplant | Number of patients, n (%) | Post-transplant | ||||
|---|---|---|---|---|---|---|
| CR, n (%) | VGPR | PR | Stable | Died | ||
| CR | 119 (34.1) | 110 (92.4) | 4 | - | 1 | 4 |
| VGPR | 60 (17.2) | 42 (70.0) | 14 | 1 | - | 3 |
| PR | 112 (32.1) | 51 (45.5) | 30 | 20 | 6 | 5 |
| Stable | 26 (7.4) | 6 (23.1) | 10 | 7 | - | 3 |
| Progressive disease | 32 (9.2) | 4 (12.5) | 4 | 14 | 7 | 3 |
| Total, n (%) | 349 | 213 (61.0) | 62 (17.8) | 42 (12.0) | 14 (4.0) | 18 (5.2) |
Abbreviations are as given in Table I
Post-transplant response rate according to primary induction regimen: Overall response rate (CR+VGPR+PR) was higher for patients who received novel agents (92.8%) versus VAD (92.0%) versus alkylating agents (65.2%), P<0.001 [novel agents vs. VAD (P<0.02), novel agents vs. alkylating agents (P<0.001), VAD versus alkylating agents, (P<0.01)]. Corresponding CR rates were 68.1 per cent versus 48.0 per cent versus 26.1 per cent (P<0.001). Among novel agents, there was no significant difference in the response rate between those who received doublet (n=178) versus triplet (n=73) (CR 68.8 vs. 71.2%, P=0.223).
Predictors of transplant response: Patients with pre-transplant chemosensitive disease (CR+VGPR+PR, P<0.001), induction with novel agents (P<0.001), transplant in first remission compared to those who underwent transplant after salvage induction (P<0.001), those who received one line of induction therapy (P<0.001) and serum albumin >3.5 g/dl at diagnosis (P<0.02) had higher probability of response to transplant.
Day 100 transplant-related mortality (TRM): Eighteen (5.2%) patients died before day 100 due to transplant-related complications. Low haemoglobin (Hb) (<10 g/dl) (P<0.05), low serum albumin (<3.5 g/dl) (P<0.005), low eGFR <40 ml/min25, (P<0.01), Durie-Salmon Stage IIIB (P<0.01) and transplant during second or subsequent remission after salvage induction (P<0.05) were predictors of higher mortality. Transplant-related mortality (TRM) was higher for patients transplanted before 2005 compared to those transplanted between 2006-2010 and 2011-2016; 9/81 (11.1%) versus 3/80 (3.8%) versus 6/188 (3.2%), P<0.02 (Table III).
Table III.
Predictors of transplant related mortality
| Factor | n | Day 100 mortality, n (%) | P value |
|---|---|---|---|
| Age (yr) | |||
| ≤52 | 177 | 11 (6.2) | 0.254 |
| >52 | 172 | 7 (4.1) | |
| Gender | |||
| Male | 236 | 9 (3.8) | 0.08 |
| Female | 113 | 9 (8.0) | |
| International staging system (ISS) (n=343) | |||
| I | 103 | 2 (1.9) | 0.229 |
| II | 121 | 7 (5.8) | |
| III | 119 | 8 (6.7) | |
| Durie-Salmon staging system (DSS) (n=348) | |||
| ≤IIIA | 263 | 9 (3.4) | 0.01 |
| IIIB | 85 | 9 (10.6) | |
| Extramedullary disease (EMD) | |||
| Yes | 80 | 5 (6.3) | 0.39 |
| No | 269 | 13 (4.8) | |
| Induction therapy | |||
| Novel agents | 251 | 10 (4.0) | 0.13 |
| VAD | 75 | 5 (6.7) | |
| Alkylating agents | 23 | 3 (13.0) | |
| Number of regimens | |||
| One line | 223 | 8 (3.6) | 0.06 |
| >One line | 125 | 10 (8.0) | |
| Myeloma type (n=342) | |||
| IgG | 204 | 15 (7.4) | 0.09 |
| IgA | 57 | 2 (3.5) | |
| K+L | 81 | 1 (1.2) | |
| Interval (months) | |||
| ≤12 | 220 | 10 (4.54) | 0.33 |
| >12 | 129 | 8 (6.2) | |
| Haemoglobin (g/dl) | |||
| ≤10 | 201 | 14 (7.0) | 0.05 |
| >10 | 148 | 4 (2.7) | |
| Serum albumin (g/dl) | |||
| <3.5 | 140 | 13 (9.3) | 0.005 |
| ≥3.5 | 209 | 5 (2.4) | |
| BM-PC% (n=348) | |||
| ≤40 | 180 | 10 (5.6) | 0.46 |
| >40 | 168 | 8 (4.8) | |
| Baseline eGFR (ml/min) | |||
| ≤40 | 86 | 9 (10.5) | 0.01 |
| >40 | 263 | 9 (3.4) | |
| Serum calcium (n=324) (mg/dl) | |||
| ≥11.5 | 28 | 3 (10.7) | 0.13 |
| <11.5 | 296 | 12 (4.1) | |
| Pre-transplant status | |||
| Sensitive (CR+VGPR+PR) | 291 | 13 (4.5) | 0.16 |
| Resistant (stable+progressive disease) | 58 | 5 (8.6) | |
| Transplant in first remission versus during second remission | 245 104 | 9 (3.7) 9 (8.7) | 0.05 |
| Melphalan dose (n=347) (mg/m2) | |||
| ≤140 | 35 | 2 (5.7) | 0.56 |
| >140 | 312 | 16 (5.1) | |
| Year of transplant | |||
| Till 2005 | 81 | 9 (11.1) | 0.02 |
| 2006-2010 | 80 | 3 (3.8) | |
| 2011-2016 | 188 | 6 (3.2) | |
P value calculated by Chi-square test. Abbreviations are as given in Table I
Survival: Median OS and PFS from date of transplant for all patients was 90 months [95% confidence interval (CI) 70.8-109.2] and 41 months (95% CI 33.0-49.03), respectively (Figs. 1 and 2). The estimated OS and PFS at 2, 5, 10 and 15 yr was 81.4 versus 64.6 per cent, 60.8 versus 40.6, 40.4 versus 28.2 per cent and 17.7 versus 15.6 per cent, respectively.
Fig. 1.
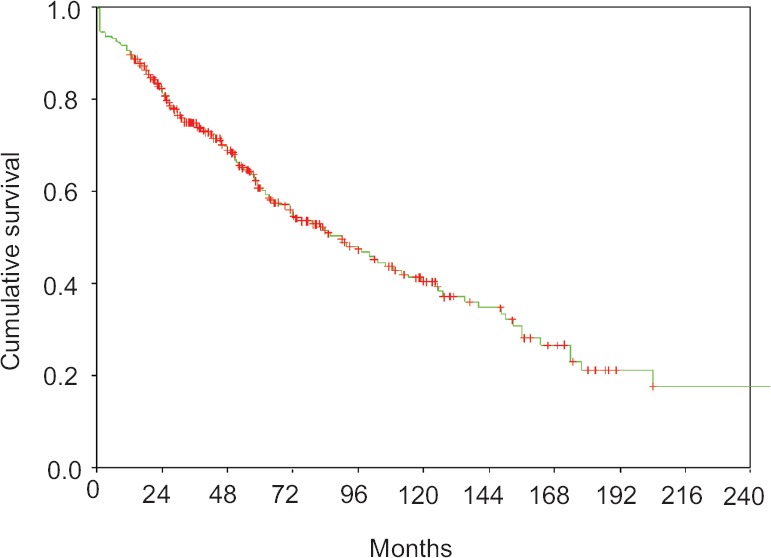
Overall survival for patients from date of transplant.
Fig. 2.
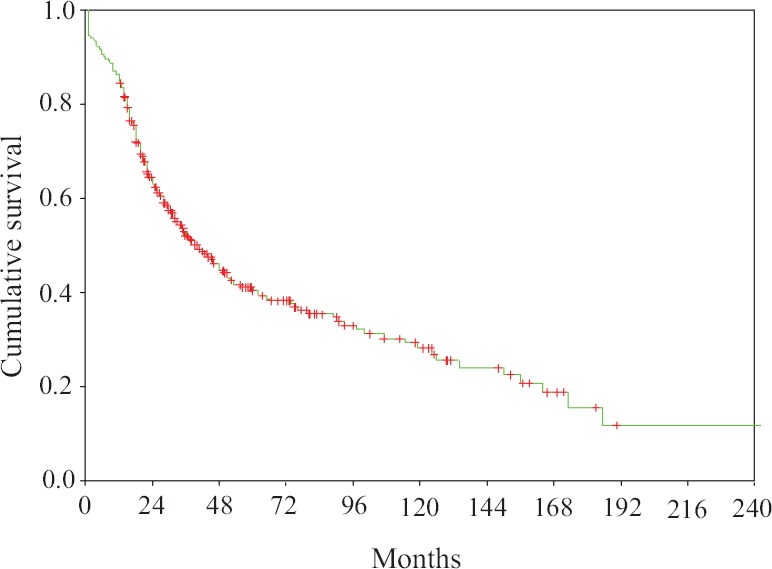
Progression free survival for patients from date of transplant.
Predictors of overall survival (OS): Univariate analysis: Patients with ISS Stages I and II (P<0.001), Durie-Salmon Stages IIIA (P<0.001), absence of extramedullary disease (P<0.001), serum albumin (≥3.5 g/dl) (P<0.001), eGFR at diagnosis (≥40 ml/min) (P<0.001), pre-transplant chemosensitive disease (P<0.001), novel agents-based induction (P<0.004), achievement of CR post-transplant (P<0.001), transplant within 12 months of diagnosis (P<0.001) and those who had received one line of induction therapy (P<0.001) and patients with transplant in first remission had better OS (Table IV).
Table IV.
Predictors of overall survival: Univariate analysis
| Factor | Variable | No. of patients | Median OS | 95% CI | P value |
|---|---|---|---|---|---|
| Age (yr) | ≤52 | 177 | 90.0 | 52.2-127.8 | 0.897 |
| >52 | 172 | 85.5 | 65.3-105.7 | ||
| Gender | M | 236 | 91 | 65.5116.4 | 0.229 |
| F | 113 | 85.5 | 33.5-137.5 | ||
| ISS (n=343) | I | 103 | 127 | 73.9-180.0 | 0.001 |
| II | 121 | 91.50 | 76.8-106.2 | I Vs II=0.31 | |
| III | 119 | 59.0 | 44.8-73.2 | II Vs III-0.006 I Vs III=0.0002 | |
| DSS (n=348) | ≤IIIA | 263 | 97.0 | 69.3-125.0 | 0.001 |
| IIIB | 85 | 60.5 | 43.7-77.3 | ||
| EMD | Yes | 80 | 42.5 | 19.5-65.5 | 0.001 |
| No | 269 | 102.0 | 80.6-123.4 | ||
| Albumin (g/dl) | ≥3.5 | 140 | 59.0 | 37.6-80.4 | 0.001 |
| <3.5 | 209 | 114.5 | 75.1-154.0 | ||
| Hb (g/dl) | ≤10 | 201 | 71.5 | 41.2-101.8 | 0.07 |
| >10 | 148 | 97.0 | 58.7-135.3 | ||
| BM-PC% (n=348) | ≤40 | 180 | 100.0 | 57.9-142.1 | 0.260 |
| >40 | 168 | 79.0 | 56.4-101.6 | ||
| Ig type (n=342) | IgG | 204 | 96.0 | 66.8-125.2 | 0.48 |
| IgA | 57 | 79.0 | 51.2-106.8 | ||
| K + L | 81 | 83.5 | 44.2-122.7 | ||
| Induction | Novel | 251 | 91.5 | 67.4-115.6 | 0.004 |
| VAD | 75 | 85.5 | 54.4-116.6 | ||
| Alkylating agents | 23 | 24.0 | 9.1-39.0 | ||
| Regimen (n=348) | One line | 223 | 124.5 | 87.7-161.3 | 0.001 |
| >One line | 125 | 50.5 | 35.8-65.2 | ||
| eGFR (ml/min) | <40 | 86 | 60.5 | 43.5-77.5 | 0.001 |
| ≥40 | 263 | 97.0 | 69.93-124.1 | ||
| Pre-transplant status | Sensitive (CR+VGPR+PR) | 291 | 102.0 | 82.9-121.1 | 0.001 |
| Resistant (stable+progressive disease) | 58 | 48.0 | 29.2-66.8 | ||
| Interval diagnosis-transplant (months) | ≤12 | 220 | 106.0 | 78.9-133.0 | 0.001 |
| >12 | 129 | 59.0 | 41.9-76.0 | ||
| CD34+ cells (×106/kg) | ≤4.0 | 240 | 91.5 | 68.9-114.1 | 0.10 |
| >4 | 68 | 106.0 | 58.1-153.9 | ||
| Post-transplant response | CR | 213 | 150.0 | 123.5-184.8 | 0.001 |
| Others | 136 | 32.0 | 22.6-41.4 | ||
| Transplant in remission | 1st remission | 245 | 125 | 96.6-154.4 | 0.001 |
| Post-salvage | 104 | 37 | 23.0-51.0 |
ISS, international staging system; DSS, Durie-Salmon staging system; BM-PC, bone marrow plasma cell; EMD, extra-medullary disease; Hb, haemoglobin; VAD, vincristine, adriamycin and dexamethasone; Ig, immunoglobulin; OS, overall survival. Abbreviations are as given in Table I
Predictors of progression-free survival (PFS): Patients with DSS Stage IIIA (P<0.02), transplant within 12 months of diagnosis (P<0.03), novel agents-based induction (P<0.001), pre-transplant chemosensitive disease (P<0.001) and achievement of CR post-transplant (P<0.001) were associated with superior PFS. The presence of extramedullary disease (P<0.001), transplant in second or subsequent remission post-salvage therapy (P<0.001), Hb ≤10 g/dl (P<0.05) and albumin <3.5 g/dl (P<0.001) were predictors of inferior PFS (Table V).
Table V.
Predictors of progression-free survival: Univariate analysis
| Factor | Variable | Median PFS | 95% CI | P value |
|---|---|---|---|---|
| Age (yr) | ≤52 | 36.0 | 25.5-46.4 | 0.817 |
| >52 | 44.0 | 29.8-58.1 | ||
| Gender | Male | 45.5 | 35.4-55.6 | 0.179 |
| Female | 35.0 | 24.2-45.8 | ||
| ISS | I | 53.0 | 16.7-89.3 | 0.070 |
| II | 44.0 | 34.8-53.1 | ||
| III | 30.0 | 20.3-39.5 | ||
| DSS | ≤IIIA | 44.0 | 33.8-54.2 | 0.02 |
| IIIB | 30.0 | 15.9-44.1 | ||
| EMD | Yes | 24.0 | 15.5-22.5 | 0.001 |
| No | 46.0 | 36.0-56.0 | ||
| Albumin (g/dl) | ≥3.5 | 28.0 | 18.3-37.7 | 0.001 |
| <3.5 | 52.0 | 27.5-76.5 | ||
| Hb (g/dl) | ≤10 | 34.0 | 24.9-43.1 | 0.05 |
| >10 | 51.0 | 38.2-64.0 | ||
| BM PC% | ≤40 | 50.0 | 21.8-78.2 | 0.211 |
| >40 | 35.0 | 26.2-43.8 | ||
| Ig type | IgG | 41.0 | 28.2-53.8 | 0.571 |
| IgA | 41.0 | 33.1-48.9 | ||
| K + L | 38.0 | 0.44-75.6 | ||
| Induction | Novel | 50.0 | 35.1-64.8 | 0.001 |
| VAD | 31.0 | 16.9-45.1 | ||
| Alkylating agents | 18.0 | 11.7-24.3 | ||
| Regimen | One line | 62.0 | 34.0-90.0 | 0.001 |
| >One line | 22.0 | 18.2-25.2 | ||
| Pre-transplant status | Sensitive (CR+VGPR+PR) | 51.0 | 38.5-63.5 | 0.001 |
| Resistant (stable+progressive disease) | 18.0 | 12.8-23.2 | ||
| Interval diagnosis-transplant (months) | ≤12 | 48.0 | 38.3-57.7 | 0.03 |
| >12 | 28.0 | 19.7-36.3 | ||
| CD34 + cells (×106/kg) | ≤4 | 39.0 | 31.2-46.7 | 0.24 |
| >4 | 51.0 | 30.1-71.9 | ||
| Post-transplant response | CR | 91.0 | 60.9-121.0 | 0.001 |
| Others | 16.0 | 14.5-17.5 | ||
| Transplant remission | 1st remission | 62.0 | 41.0-82.9 | 0.001 |
| Post-salvage | 20.0 | 14.8-25.2 |
Abbreviations are as given in Table IV
Multivariate analysis for overall and progression-free survival: Serum albumin (<3.5 g/dl), presence of extramedullary disease and ISS Stage III were predictors for inferior OS. Achievement of CR post-transplant and transplant in first remission were predictors for superior OS. Achievement of CR post-transplant and transplant in first remission were predictors for superior PFS (Table VI).
Table VI.
Multivariate analysis for overall and progression free survival
| Variable | P value | Hazard | 95% CI |
|---|---|---|---|
| OS | |||
| Serum albumin | 0.01 | 1.620 | 1.12-2.336 |
| EMD | 0.005 | 1.835 | 1.207-2.79 |
| Stage ISS I + II versus III | 0.009 | 0.598 | 0.405-0.881 |
| Primary versus post-salvage transplant | 0.001 | 0.502 | 0.342-0.737 |
| Post-transplant CR | 0.001 | 0.382 | 0.262-0.556 |
| PFS | |||
| Primary versus post-salvage transplant | 0.017 | 0.652 | 0.459-0.926 |
| Post-transplant CR | 0.001 | 0.245 | 0.180-0.334 |
Abbreviations are as given in Table IV
Current status: In a follow up of patients done in 2018, 184 of 349 patients (52.7%) were alive; 134 (38.4%) progression free, 25 (7.2%) with disease and were on salvage therapy, 17 (4.9%), were in second CR after salvage therapy and eight (2.3%) patients had low level serum M spike, <1 g/dl (MGUS like). A total of 164 (47.1%) patients died; these included – 18 (5.2%) deaths before day 100 (TRM), 129 (37.0%) due to progressive disease and its complications and 17 patients (4.9%) due to unrelated reasons. Causes included second malignancy in five (myelodysplastic syndrome – 1, acute myeloid leukaemia – 1, renal cell cancer – 1, hepatocellular carcinoma – 1, carcinoma tongue – 1), dengue fever in two, coronary artery disease in six, cerebral haemorrhage, Alzheimer's disease, acute graft versus host disease and ventilator-associated complications in one patient each. Status was unknown for one patient.
Discussion
Post-transplant high CR rate, higher median OS (90 months) and PFS (41 months) with 10 yr survival rate of 40.4 and 28.2 per cent, respectively, are important findings in the present study. More than one-third of patients had high-risk disease at diagnosis. Post-transplant overall response rate (90.8%) was high in the present study; this was similar to earlier observations13,14,22. Conversion to post-transplant CR from pre-transplant response - very good PR (70%), PR (45.5%) and 23 per cent CR in those with stable disease reflected contribution by transplant in augmenting the response already achieved with pre-transplant therapy. Post-transplant CR rate was not significantly different between doublet versus triplet regimen. At present, it is recommended to use triplet (three-drug combinations)2,13; however, there is no direct comparison between different triplets being used currently (VTD vs. VRD vs. VCD). There is also suggestion that four cycles of induction may be adequate and more may not be better27.
A median OS and PFS of 90 and 41 months\in our study is similar to earlier studies15,16,17,18,19,20 reporting long-term transplant results. An estimated OS at 10 and 15 yr (40.4 and 17.7%, respectively) indicated prolonged survival in some patients. Similarly, PFS of 15.6 per cent at 15 yr was indicative of a functional cure in a subgroup of patients. A long-term follow up is still needed in the absence of a plateau in survival curve28. Achievement of CR post-transplant and transplant in first remission were important predictors of OS and PFS. For those who achieved CR, median OS was 150 months (95% CI 123.5-184.8), significantly higher to those with VGPR and PR. These findings were similar to earlier studies29. Achievement of CR post-transplant has been identified as an important marker of long-term survival and is considered to be a desirable goal. Recent studies have suggested that achievement of 'nil' minimal residual disease (MRD) status on multiparameter flow cytometry is a better surrogate marker of long-term survival30.
In the present study, 'day +100' TRM was 5.2 per cent; this was higher than the current standard of one per cent or less13. Important predictive factors of higher mortality were low serum albumin (<3.5 g/dl) and low estimated GFR (<40 ml/min) at diagnosis and transplant in second or subsequent remission. A reduction in TRM could be due to a combined effect of better case selection, better supportive care and use of novel agents leading to higher response rates including CR which resulted in better depth of response post-transplant and better PFS and OS. Five patients (5/18) had graft failure; three of these had CD34+ stem cells <2 million and two patients had 3.38 and 6.70 million, respectively. In the present study, 56 patients received stem cells cryopreserved at −80°C, the remaining 293 patients received stem cells kept at 4°C. There was no difference in outcome OS and PFS in the two groups. This was consistent with earlier observations from our centre22 and those reported recently31. No difference was observed in outcome of patients who received ≤4 million CD34+ stem cells or more.
Lack of cytogenetic/FISH (florescent in situ hybridization) data was an important limitation of the present study for most patients.
In conclusion, the findings of the present study showed higher response rate to transplant translating into improved progression free and overall survival. Reducing TRM to <1 per cent and further improvement in CR rates and long-term survival remain desirable goals in future studies.
Acknowledgment
Authors acknowledge the quality care provided by the team of residents and nurses, and help by technical staff for stem cell harvest, enumeration and cryopreservation of stem cells, and appreciate the timely help provided by Nephrology department for dialysis and clinical review of many patients in the study.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Kumar L, Verma R, Radhakrishnan VR. Recent advances in the management of multiple myeloma. Nat Med J India. 2010;23:210–8. [PubMed] [Google Scholar]
- 2.Gandolfi S, Prada CP, Richardson PG. How I treat the young patient with multiple myeloma. Blood. 2018;132:1114–24. doi: 10.1182/blood-2017-05-693606. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe français du myélome. N Engl J Med. 1996;335:91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 4.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 5.Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: Long-term results of a randomized control trial from the group myelome-autogreffe. J Clin Oncol. 2005;23:9227–33. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 6.Bladé J, Rosiñol L, Sureda A, Ribera JM, Díaz-Mediavilla J, García-Laraña J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: Long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–9. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 7.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: Final results of phase III US intergroup trial S9321. J Clin Oncol. 2006;24:929–36. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 8.Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13:183–96. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavo M, Palumbo A, Zweegman S, Dimopoulos MA, Hajek R, Pantani L, et al. Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95MM trial) J Clin Oncol. 2016;34:8000. [Google Scholar]
- 11.Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: A randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29. doi: 10.1016/S1470-2045(15)00389-7. [DOI] [PubMed] [Google Scholar]
- 12.Palumbo A, Cavallo F, Gay F, Di Raimondo DB, Yehuda MT, Petrucci S, et al. Autologous transplantation and maintenance therapy in multiplemyeloma. N Engl J Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 13.Stadtmauer EA, Pasquini MC, Blackwell B, Knust K, Bashy A, Devine SM, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len Maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiplemyeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical TrialsNetwork (BMT CTN 0702 – StaMINA Trial) Blood. 2016;128:LBA–1. [Google Scholar]
- 14.Dhakal B, Szabo A, Chhabra S, Hamadani M, D'Souza A, Usmani SZ, et al. Autologous transplantation for newly diagnosed multiple myeloma in the era of novel agent induction: A systematic review and meta-analysis. JAMA Oncol. 2018;4:343–50. doi: 10.1001/jamaoncol.2017.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehners N, Becker N, Benner A, Pritsch M, Löpprich M, Mai EK, et al. Analysis of long-term survival in multiple myeloma after first-line autologous stem cell transplantation: Impact of clinical risk factors and sustained response. Cancer Med. 2018;7:307–16. doi: 10.1002/cam4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munker R, Baghian A, Koleva Y, Andrews P, Matharoo GS, Wright AE, et al. Long-term follow-up of patients with multiple myeloma treated with total body irradiation-melphalan conditioning. Eur J Haematol. 2017;99:56–9. doi: 10.1111/ejh.12890. [DOI] [PubMed] [Google Scholar]
- 17.Gassiot S, Motlló C, Llombart I, Morgades M, González Y, Garcia-Caro M, et al. Impact of induction treatment before autologous stem cell transplantation on long-term outcome in patients with newly diagnosed multiple myeloma. Eur J Haematol. 2017;98:569–76. doi: 10.1111/ejh.12869. [DOI] [PubMed] [Google Scholar]
- 18.González-Calle V, Cerdá S, Labrador J, Sobejano E, González-Mena B, Aguilera C, et al. Recovery of polyclonal immunoglobulins one year after autologous stem cell transplantation as a long-term predictor marker of progression and survival in multiple myeloma. Haematologica. 2017;102:922–31. doi: 10.3324/haematol.2016.158345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gahrton G, Iacobelli S, Björkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs. autologous transplantation in multiple myeloma: Long-term results of the EBMT-NMAM2000 study. Blood. 2013;121:5055–63. doi: 10.1182/blood-2012-11-469452. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Lopez J, Blade J, Mateos MV, Grande C, Alegre A, García-Laraña J, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118:529–34. doi: 10.1182/blood-2011-01-332320. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni U, Devasia AJ, Korula A, Fouzia NA, Nisham PN, Samoon YJ, et al. Use of non-cryopreserved peripheral blood stem cells is associated with adequate engraftment in patients with multiple myeloma undergoing an autologous transplant. Biol Blood Marrow Transplant. 2018;24:e31–5. doi: 10.1016/j.bbmt.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Kumar L, Boya RR, Pai R, Harish P, Mookerjee A, Sainath B, et al. Autologous stem cell transplantation for multiple myeloma: Long-term results. Natl Med J India. 2016;29:192–9. [PubMed] [Google Scholar]
- 23.Dimopoulos MA, Sonneveld P, Leung N, Merlini G, Ludwig H, Kastritis E, et al. International myeloma working group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34:1544–57. doi: 10.1200/JCO.2015.65.0044. [DOI] [PubMed] [Google Scholar]
- 24.Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma subcommittee of the EBMT. European group for blood and marrow transplant. Br J Haematol. 1998;102:1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 25.MD+ CALC. MDRD GFR Equation. [accessed on August 20, 2018]. Available from: https://www.mdcalc.com/mdrd-gfr-equation .
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 27.Kumar L, Ganesan P. Induction therapy for multiple myeloma: More is not necessarily better! Br J Haematol. 2018;182:7–8. doi: 10.1111/bjh.15242. [DOI] [PubMed] [Google Scholar]
- 28.Ravi P, Kumar SK, Cerhan JR, Maurer MJ, Dingli D, Ansell SM, et al. Defining cure in multiple myeloma: A comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018;8:26. doi: 10.1038/s41408-018-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahuerta JJ, Paiva B, Vidriales MB, Cordón L, Cedena MT, Puig N, et al. Depth of response in multiple myeloma: A pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10. doi: 10.1200/JCO.2016.69.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: A meta-analysis. JAMA Oncol. 2017;3:28–35. doi: 10.1001/jamaoncol.2016.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bittencourt MCB, Mariano L, Moreira F, Schmidt-Filho J, Mendrone A, Jr, Rocha V. Cryopreserved versus non-cryopreserved peripheral blood stem cells for autologous transplantation after high-dose melphalan in multiple myeloma: Comparative analysis. Bone Marrow Transplant. 2019;54:138–41. doi: 10.1038/s41409-018-0250-1. [DOI] [PubMed] [Google Scholar]


