Abstract
Understanding the mechanism of how liver ductal cells (cholangiocytes) differentiate into hepatocytes would permit liver-regenerative medicine. Emerging liver ductal organoids provide an ex vivo system to investigate cholangiocyte-to-hepatocyte differentiation. However, as current gene manipulation methods require organoid dissociation into single cells and have only low efficiency, it is difficult to dissect specific gene functions in these organoids. Here we developed the adeno-associated virus (AAV) vector AAV-DJ as a powerful tool to transduce mouse and human liver ductal organoids. Via AAV-DJ–mediated up- or down-regulation of target genes, we successfully manipulated cholangiocyte-to-hepatocyte differentiation. We induced differentiation by overexpressing the hepatocyte-specifying regulator hepatocyte nuclear factor 4α (HNF4α) and blocked differentiation by stimulating Notch signaling or interfering with Smad signaling. Further screening for transcriptional factors critical for cholangiocyte-to-hepatocyte differentiation identified HOP homeobox (HOPX), T-box 15 (TBX15), and transcription factor CP2-like 1 (TFCP2L1) as master regulators. We conclude that this highly efficient and convenient gene manipulation system we developed could facilitate investigation into genes involved in cell lineage transitions and enable application of engineered organoids in regenerative medicine.
Keywords: liver, hepatocyte, differentiation, signaling, gene therapy, adeno-associated virus (AAV) vector, cholangiocyte-to-hepatocyte differentiation, functional screening, gene manipulation, liver ductal organoid
Introduction
The liver is the largest internal organ and controls organism metabolism. It is mainly composed of two types of epithelial cells: hepatocytes and cholangiocytes. Hepatocytes are crucial for protein synthesis, carbohydrate/lipid metabolism, and detoxification (1). The liver exhibits an extraordinary regenerative capacity to rescue hepatic mass loss, which is commonly mediated by hepatocyte compensatory proliferation (2). Cholangiocyte-to-hepatocyte transition/differentiation can be triggered to restore the hepatocyte population, especially in the case of impaired hepatocyte proliferation (3, 4).
In 3D culture systems, bile ducts embedded in Matrigel can self-organize into long-term-expanding liver ductal organoids in defined medium, including the Wnt agonist R-spondin1, EGF,5 FGF10, and hepatocyte growth factor. Notably, cholangiocytes in ductal organoids can differentiate into functional hepatocytes by withdrawing Wnt stimulation and blocking TGF-β and Notch signaling (5, 6), which mimics in vivo cholangiocyte-to-hepatocyte transition. This near-physiological system not only accelerates liver disease modeling and drug discovery (7, 8) but also enables dissection of how hepatocyte specification is regulated by integrated signals.
To date, organoids had to be dissociated into single cells for gene manipulation through Lipofectamine transfection, retroviral/lentiviral transduction, or electroporation (9, 10). In addition to the low primary manipulation efficiency, single-cell dissociation of organoids disrupts the tissue structure and niche, which takes weeks to re-establish (6, 9, 11, 12). Therefore, it would be of great value to establish a convenient and effective gene delivery method to facilitate the study of specific gene functions in liver cell fate determination by employing organoids. Also, manipulating human liver organoids with biosafety would pave the way for transplantation therapy.
Adeno-associated virus (AAV) vectors have good in vivo gene delivery ability and medical safety (13–15). Allende et al. (16) injected AAVrh8 into cerebral organoids to express β-hexosaminidase. However, as AAV serotypes have distinct lineage tropism, it is critical to determine the specific AAV serotype with high cholangiocyte affinity for liver ductal organoid transduction. Besides, an inherently small genome size and simple capsid structure enable evolution to further improve tissue specificity and transduction efficiency (17–20).
In this study, we showed that the AAV-DJ vector transduced mouse and human liver ductal organoid with high efficiency. AAV-DJ capsid mutations (N498S, Y706F, and Y732F) further boosted its transduction capacity. We induced cholangiocyte-to-hepatocyte differentiation by overexpressing the hepatocyte specification factor HNF4α and blocked the same process by stimulating Notch signaling or interfering with Smad signaling. Moreover, screening of 16 hepatocyte-enriched transcriptional factors identified HOPX, TBX15, and TFCP2L1 as master regulators of hepatocyte differentiation. This high-efficiency and convenient gene manipulation method facilitates the investigation of gene functions in liver lineage transition and application of engineered organoids in regenerative medicine.
Results
The AAV-DJ vector transduces intact liver ductal organoids with high efficiency
While searching for an ideal tool to deliver genes into liver ductal organoids, we focused on AAV vectors, which have been recognized for primary tissue affinity and biosafety in gene therapy. For convenient AAV transduction of intact ductal organoids, we designed a Matrigel-supported planar infection (MSPI) method. As shown in Fig. 1A, liver ductal organoids ready for infection were collected, suspended in AAV-containing culture medium, and placed on a flat layer of Matrigel. Following 12-h incubation in a planar manner, the organoids were returned to 3D culture. The organoid architecture was well-preserved during the operation (Fig. 1A), suggesting that the MSPI method enables sufficient AAV exposure while sustaining organoid survival and integrity.
Figure 1.
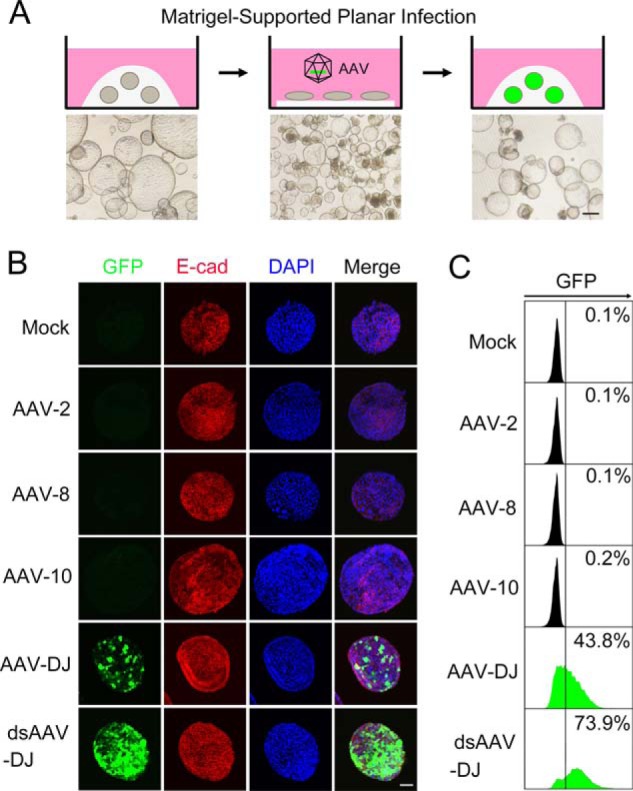
The AAV-DJ vector transduces liver ductal organoids with high-efficiency. A, illustration of the experimental procedure for liver ductal organoid transduction through MSPI and representative bright-field images of organoids at the indicated stage. Organoids in 3D culture were collected and mixed with the AAV and then incubated on precoated Matrigel in a planar manner overnight. Infected organoids were returned to 3D culture. Scale bar = 300 μm. B, liver ductal organoids were transduced with the indicated AAV vectors expressing GFP (green). 48 h post-infection, organoids were stained for E-cadherin (E-cad, red) and DAPI (blue) and subjected to confocal cross-sectioning. ds, double-stranded. A representative result of three independent experiments is shown. Scale bar = 100 μm. C, liver ductal organoids were transduced with the indicated AAV vectors expressing GFP (green). 48 h post-infection, organoids were dissociated and subjected to flow cytometry to quantify the transduction efficiency.
Given that AAV serotypes show distinguished lineage tropism, we screened widely employed serotypes, including AAV-2, AAV-8, AAV-DJ, and AAV-10, to locate an appropriate AAV vector for liver ductal organoid transduction. Confocal cross-sectioning and flow cytometry analysis revealed that AAV-DJ–GFP achieved 43.8% transduction efficiency on liver ductal organoids 2 days after infection, whereas rare transduced cells could be detected in the presence of AAV-2–GFP, AAV-8–GFP, and AAV-10–GFP (Fig. 1, B and C). Interestingly, different from the performance in liver ductal organoids, both AAV-2 and AAV-DJ showed a superior ability to infect Huh7 hepatocarcinoma cells (Fig. S1), suggesting that cholangiocytes and hepatocytes have distinct preferences for AAV serotypes.
Genome DNA conversion from single-stranded to double-stranded is a rate-limiting step for AAV-mediated gene expression. As expected, the self-complementary AAV-DJ, which has a double-stranded DNA genome because of an inverted terminal repeat mutation (21), dramatically increased the transduction efficiency to 73.9% (Fig. 1, B and C).
AAV-DJ capsid mutations further boost transduction efficiency
To further improve the transduction capacity of AAV-DJ, we engineered an AAV-DJ capsid through site-directed mutations. Tyrosine residues on the AAV capsid can be phosphorylated by epidermal growth factor receptor protein tyrosine kinase, which leads to ubiquitin/proteasome-mediated degradation of the AAV (22, 23). We designed Y706F and Y732F mutations through homologous sequence alignment with the Y704F and Y730F mutations on the AAV-2 capsid, which have been reported to increase the transduction efficiency of AAV-2 (22, 23). AAV-8 capsid random mutation library screening identified that the N498S mutation could enhance packaged gene expression. We found that combination of three point mutations (N498S, Y706F, and Y732F) further enhanced the transduction efficiency of double-stranded AAV-DJ–GFP in liver ductal organoids (80.5%) (Fig. 2, A–C). Quantitative RT-PCR revealed that GFP expression capacity was also significantly enhanced (Fig. 2D).
Figure 2.
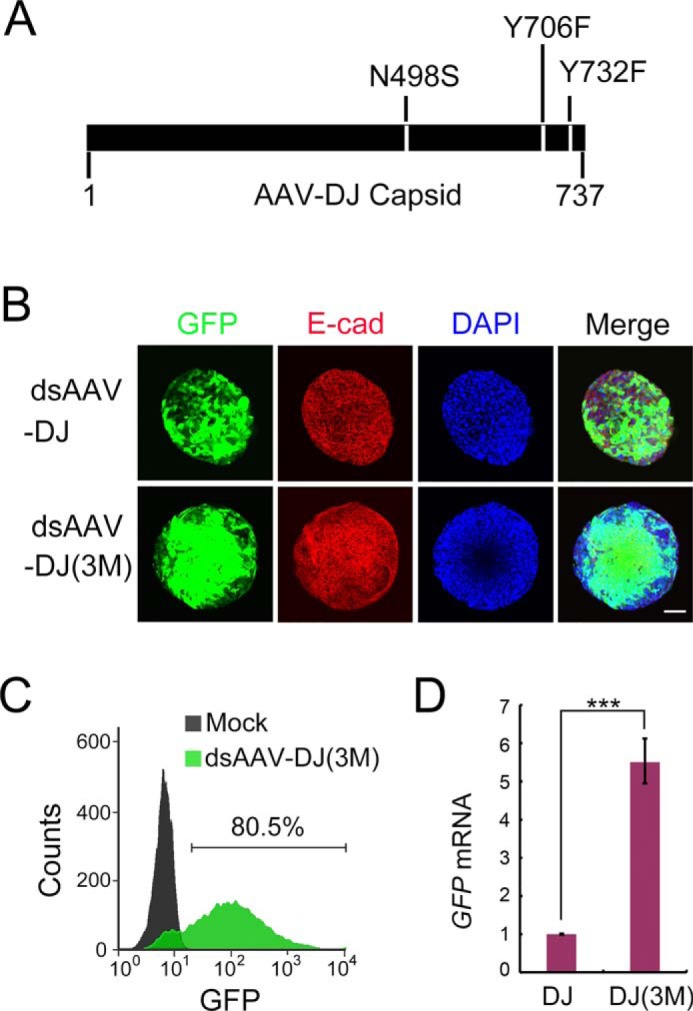
AAV-DJ capsid mutations further boost the transduction efficiency. A, schematic of the engineered AAV-DJ capsid. Three point mutations (N498S, Y706F, and Y732F) on AAV-DJ generate AAV-DJ(3M). B, liver ductal organoids were transduced with AAV-DJ(3M) expressing GFP (green). 48 h post-infection, organoids were stained for E-cadherin (E-cad, red) and DAPI (blue) and subjected to confocal cross-sectioning. A representative result of three independent experiments is shown. Scale bar = 100 μm. An experiment identical to that shown in Fig. 1B was performed. The dsAAV-DJ panel from Fig. 1B is shown again, with an identical description for ease of comparison. ds, double-stranded. C, organoids transduced with the indicated AAVs were dissociated and subjected to flow cytometry to quantify the transduction efficiency. D, qRT-PCR examination of the GFP expression level in organoids transduced with AAV-DJ–GFP (DJ) and AAV-DJ(3M)–GFP (DJ(3M)). Histone H3 was used as an internal control. The statistical data represent mean ± S.D. (n = 3). ***, p < 0.001.
AAV-DJ vector–mediated gene manipulation enables dissection of gene function in cholangiocyte-to-hepatocyte differentiation
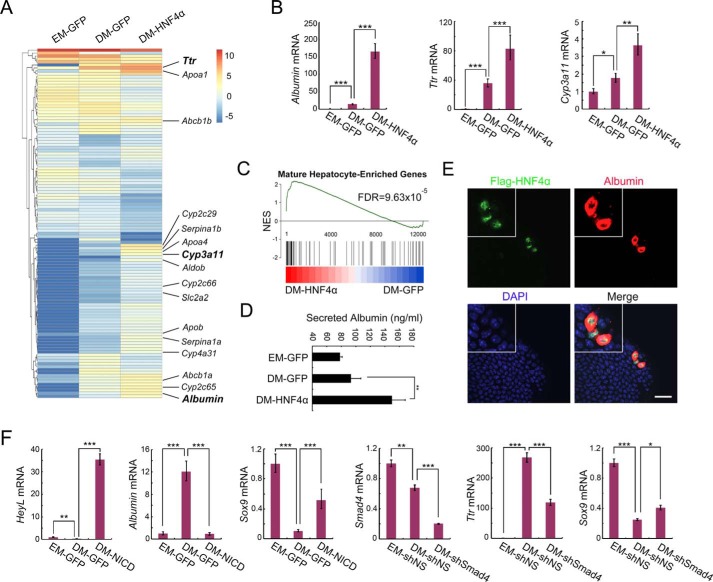
To verify that this system can be adopted to dissect the roles of specific genes in cholangiocyte-to-hepatocyte differentiation, we packaged AAV-DJ vector–expressing human HNF4α, which is a master transcription factor guiding hepatocyte specification (24). Liver ductal organoids were infected with AAV-DJ–GFP or AAV-DJ–HNF4α and then maintained in differentiation medium (DM) for 5 days to induce hepatocyte differentiation before being subjected to transcriptome examination. RNA-Seq data revealed that forced expression of HNF4α led to up-regulation of hepatocyte marker genes (Fig. 3A), represented by Albumin, Ttr, and Cyp3a11 (validated by qRT-PCR, Fig. 3B). Particularly, the cytochrome P450 (CYP450) genes Cyp2c29, Cyp2c65, Cyp2c66, Cyp3a11, and Cyp4a31, which are specifically expressed in mature hepatocytes, were significantly boosted by HNF4α transduction (Fig. 3A). GSEA revealed that HNF4α induced global cell fate transition from embryonic hepatocytes toward adult hepatocytes (Fig. 3C). These data indicate that AAV-DJ–delivered HNF4α greatly promotes cholangiocyte-to-hepatocyte differentiation following hepatocyte maturation.
Figure 3.
AAV-DJ vector–mediated gene manipulation enables dissection of gene function in cholangiocyte-to-hepatocyte differentiation. A, liver ductal organoids were transduced with AAV-DJ–HNF4α or AAV-DJ–GFP and then subjected to hepatocyte differentiation induction. On day 5 of differentiation, organoids were collected for bulk RNA-Seq analysis. A clustered heatmap of log2-transformed reads per kilobase million shows differentially expressed genes. Hepatocyte marker genes are indicated on the right. EM, expansion medium. B, qRT-PCR validation of Albumin, Ttr, and Cyp3a11 expression in A. C, GSEA plot of DM-HNF4α and DM-GFP compared with a gene list containing the top 300 expressing genes in adult liver compared with embryonic liver. NES, normalized enrichment score. D, organoid culture medium was collected for an albumin ELISA assay to detect secreted albumin. E, organoids infected with low-dose AAV-DJ–HNF4α were subjected to differentiation. Confocal images show staining of FLAG-HNF4α (green), albumin (red), and DAPI (blue). Magnification, ×2. A representative result of three independent experiments is shown. Scale bar = 20 μm. F, organoids were transduced with AAV-DJ–NICD or AAV-DJ–shSmad4, subjected to differentiation, and then harvested to examine expression of the indicated genes using qRT-PCR. shNS, nonspecific shRNA; shSmad4, Smad4 shRNA. For qRT-PCR, histone H3 was used as an internal control. The statistical data represent mean ± S.D. (n = 3). ***, p < 0.001; **, p < 0.01; *, p < 0.05.
We then set out to explore less time-intense methods to permit large-scale screening for hepatocyte differentiation regulators. Albumin is a secretory protein produced only by functional hepatocytes. We investigated whether the albumin abundance in organoid culture medium could reflect the cholangiocyte-to-hepatocyte differentiation status. Indeed, ELISA quantitation revealed that ductal organoids transduced with HNF4α secreted significantly more albumin into the culture supernatant (Fig. 3D). Moreover, as it costs recourse in laboratory to prepare high-dose AAV-DJ vector, we examined whether a low-dose AAV-DJ vector could be employed to study liver lineage transition. Immunofluorescence data showed that a small number of cholangiocytes transduced by lose-dose AAV-DJ-HNF4α (nucleus-localized) specifically flooded albumin (cytoplasm-localized) upon differentiation induction (Fig. 3E). Together, these results demonstrate that HNF4α plays a dominant role in driving the generation of functional hepatocytes. Taking HNF4α as an example, we demonstrate multiple easy-to-use strategies for application of the AAV-DJ vector in liver ductal organoids.
Besides key hepatocyte specifiers, signaling pathways integrate to balance cholangiocyte-to-hepatocyte transition (2). To verify that AAV-DJ–mediated transduction can manipulate key signaling pathways to regulate hepatocyte differentiation, we activated Notch signaling by overexpressing the Notch intercellular domain (NICD) and suppressed TGF-β/BMP signaling by interfering with Smad4. As shown in Fig. 3F, AAV-DJ–NICD transduction led to dramatic up-regulation of HeyL (a Notch target gene) and Sox9 (a cholangiocyte marker gene) and down-regulation of Albumin (a hepatocyte marker gene), indicating that Notch activation efficiently abolished the hepatocyte differentiation, thus retaining liver ductal organoids at the cholangiocyte stage. We also achieved efficient Smad4 knockdown using AAV-DJ shRNA and found that the consequent inhibition of both TGF-β and BMP attenuated hepatocyte differentiation (Fig. 3E and Fig. S2). Because the TGF-β and BMP pathways have been reported to play opposite roles in balancing fate determination between ductal cells and hepatocytes (25, 26), our data suggest that BMP could outweigh TGF-β in guiding cholangiocyte-to-hepatocyte differentiation. Of special note is that, as the AAV-DJ vector transduces intact liver ductal organoids with high efficiency, it takes only 7 days (2 days of virus infection and target expression and 5 days of differentiation induction) to address the function of a particular gene or signaling pathway in regulating liver lineage transition.
Screening of 16 hepatocyte-enriched transcriptional factors identifies novel master regulators of hepatocyte differentiation
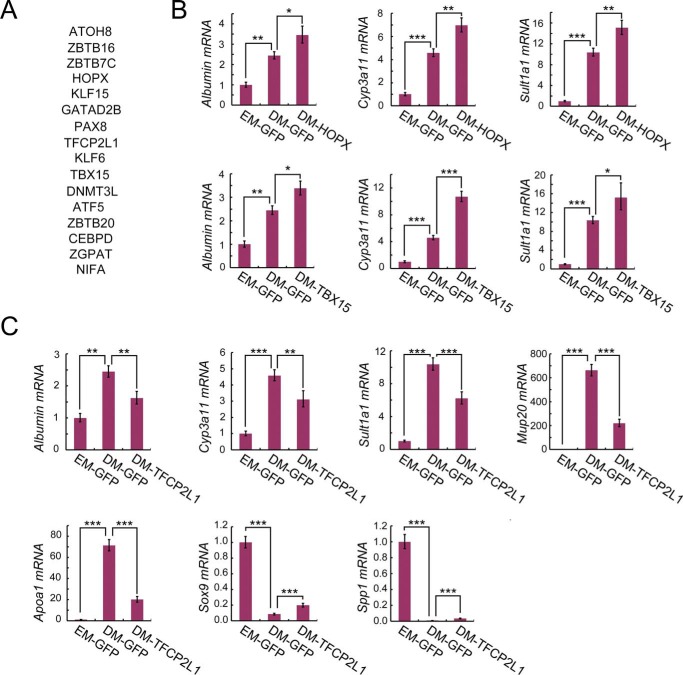
We employed the AAV-DJ vector–mediated gene manipulation system to identify novel master regulators of cholangiocyte-to-hepatocyte differentiation. Examining the differentially expressed genes in ductal organoids upon differentiation induction, we found 16 hepatocyte-enriched transcriptional factors (Fig. 4A) whose function has not been reported in liver lineage transition.
Figure 4.
Screening of 16 transcription factors identifies HOPX, TBX15, and TFCP2L1 as master regulators of hepatocyte differentiation. A, gene list of the 16 hepatocyte-enriched transcription factors subjected to screening. B and C, ductal organoids transduced with the indicated gene-expressing AAV-DJ vectors were subjected to hepatocyte differentiation induction. Then qRT-PCR was performed to examine the expression of hepatocyte signatures (Alb, Cyp3a11, Sult1a1, Mup20, and Apoa1) and cholangiocyte signatures (Sox9 and Spp1). Histone H3 was used as an internal control. The statistical data represent mean ± S.D. (n = 3). ***, p < 0.001; **, p < 0.01; *, p < 0.05.
To address the potential roles of these transcription factors in cholangiocyte-to-hepatocyte differentiation, we packaged gene-expressing AAV-DJ vectors, transduced ductal organoids, and performed a hepatocyte differentiation assay. Represented by KLF6 and DNMT3L, forced expression of most transcriptional factors showed comparable changes of hepatocyte signatures (Alb, Cyp3a11, and Sult1a1) and cholangiocyte signatures (Sox9 and Spp1) (Fig. S3), indicating that these candidates had no obvious effect on hepatocyte differentiation. Of note, we found that HOPX (27–29) and TBX15 (30) significantly promoted cholangiocyte-to-hepatocyte differentiation, as indicated by enhanced elevation of Alb, Cyp3a11, and Sult1a1 (Fig. 4B). In contrast, TFCP2L1 (31, 32) significantly inhibited hepatocyte differentiation by suppressing the expression of Alb, Cyp3a11, Sult1a1, Mup20, and Apoa1 and retaining the expression of Sox9 and Spp1 (Fig. 4C).
In summary, by screening 16 hepatocyte-enriched transcriptional factors, we identified HOPX, TBX15, and TFCP2L1 as master regulators of hepatocyte differentiation. These results further demonstrate that AAV-DJ vector–mediated gene manipulation in ductal organoids could greatly facilitate the discovery of novel liver lineage specifiers.
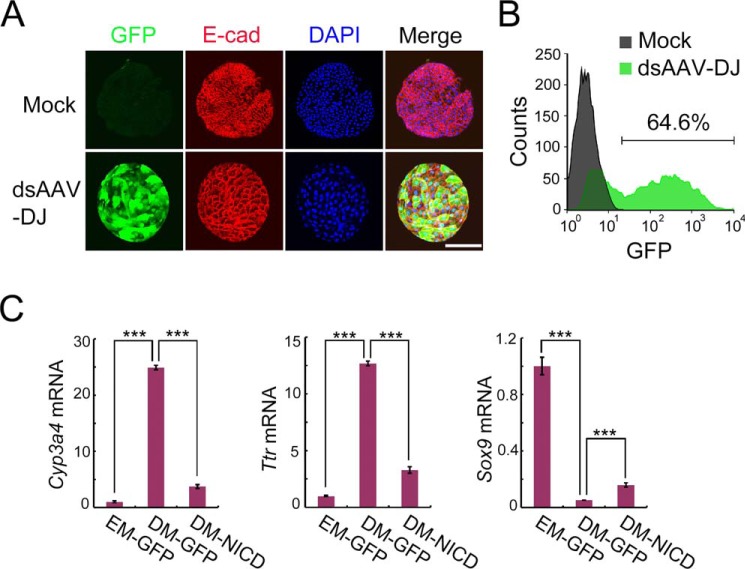
The AAV-DJ vector achieves high transduction efficiency in human liver ductal organoids
Compared with other viral vectors, recombinant AAV is preferred for gene therapy because of low immunogenicity and rare genome integration in human tissue. Therefore, we applied AAV-DJ to human liver ductal organoid engineering. Confocal and flow cytometry data revealed that the double-stranded AAV-DJ vector exhibited high transduction efficiency (64.6%) on human liver ductal organoids (Fig. 5, A and B). Furthermore, we infected human ductal organoids with AAV-DJ–NICD to interfere with human hepatocyte differentiation. Conserved with the mouse, human ductal organoids transduced with AAV-DJ–NICD showed dramatically decreased expression of hepatic genes (Cyp3a4 and Ttr) and increased expression of the Sox9 gene, suggesting that hepatocyte differentiation was blocked by Notch signaling activation (Fig. 5C). These results suggest that AAV-DJ–mediated gene manipulation in human liver ductal organoids can identify critical factors for regulating hepatocyte specification.
Figure 5.
The AAV-DJ vector achieves high transduction efficiency in human liver ductal organoids. A, human liver ductal organoids were transduced with the AAV-DJ vector expressing GFP (green). 48 h after infection, organoids were stained for E-cadherin (E-cad, red) and DAPI (blue) and subjected to confocal cross-sectioning. A representative result of three independent experiments is shown. Scale bar = 100 μm. ds, double-stranded. B, flow cytometry shows the transduction efficiency of the AAV-DJ vector. C, human liver ductal organoids were transduced with AAV-DJ–NICD and subjected to differentiation. On day 7 of differentiation, organoids were harvested to examine the relative RNA levels of the indicated genes by qRT-PCR. Histone H3 was used as an internal control. The statistical data represent mean ± S.D. (n = 3). ***, p < 0.001.
Discussion
Elucidating the molecular mechanisms by which cholangiocytes differentiate into hepatocytes will facilitate liver-regenerative medicine. Compared with in vivo lineage-tracing studies, the liver ductal organoid expansion and differentiation system attracts more attention, as it mimics cholangiocyte-to-hepatocyte transition ex vivo (6). However, previous gene manipulation methods had to dissociate liver ductal organoids into single cells (6, 11, 12), which requires abundant starting material, and it takes weeks to reestablish organoid architecture and tissue behaviors. This greatly restricts the investigation of specific gene functions in liver cell fate determination, especially in cases of valuable and limited materials, such as patient biopsies; studies requiring organoid integrality, such as lineage specification, tissue patterning, cell adhesion, and cell–cell communication; and transient events within a short time window, such as signaling transduction.
Liver ductal organoids are embedded in Matrigel, which supports 3D tissue culture survival but prevents virus–organoid contact. To employ an AAV vector in liver ductal organoid transduction, we designed Matrigel-supported planar infection, which enables sufficient AAV-organoid contact while maintaining the survival of organoids. We found that incubation on a flat layer of Matrigel for 12 h did not affect the original structure and expansion capacity of liver ductal organoids. Consistently, Liu et al. (33) and Thorne et al. (34) reported that intestinal epithelium could be cultured as a 2D monolayer on a flat layer of Matrigel. This MSPI method might be applied to virus-mediated transduction of organoids derived from other endoderm organs, such as the intestine, colon, pancreas, and lung.
We developed AAV-DJ(3M) by mutating Y706F, Y732F, and N498S on AAV-DJ's capsid, which achieved significantly enhanced transduction efficiency and protein expression level. A previous study showed that abolishing tyrosine phosphorylation stabilized the AAV-2 capsid by inhibiting ubiquitination-mediated degradation (35). Therefore, Y706F and Y732F might contribute to slowing the decay of the AAV-DJ vector. The N498 site is located on top of the capsid 3-fold protrusion and loop 5, which might affect vector–host interaction. The molecular mechanism of how these mutations affect the entrance and cellular trafficking of the AAV-DJ vector needs further examination.
HNF4α plays a vital role in hepatocyte specification by mastering the transcription of abundant hepatic genes (24, 36, 37). As expected, HNF4α overexpression in liver ductal organoids by AAV-DJ–mediated gene delivery efficiently promoted cholangiocyte-to-hepatocyte differentiation. Notably, detection of hepatic metabolite secreted into the culture medium provided a simple way to monitor the hepatocyte differentiation process, which also permits high-throughput functional screening for genes involved in liver cell fate determination using AAV libraries. Moreover, as AAV-DJ–HNF4α transduction did not modify the genome, these highly differentiated hepatocytes induced from cholangiocytes bring light to liver-regenerative medicine, whereas hepatocytes differentiated from induced pluripotent stem cells are challenged by the risk of genetic aberrations (38).
In summary, employing the AAV-DJ vector and MSPI system, we achieved a high transduction efficiency (80.5% maximum) on intact liver ductal organoids. We demonstrated that controlling master specifier expression or critical signaling activity using the AAV-DJ vector could manipulate cholangiocyte-to-hepatocyte differentiation. We also developed multiple easy-to-use strategies and screened out HOPX, TBX15, and TFCP2L1 as master transcription factors in regulating liver lineage transition. Moreover, AAV-DJ–mediated gene delivery to human liver ductal organoids may be a potential therapeutic application for engineering human liver organoids for transplantation.
Experimental procedures
Mice and human biopsies
C57BL/6 mice were purchased from the Shanghai Research Center for Model Organisms. All animal studies were performed in accordance with the relevant guidelines and with approval from the Institutional Animal Care and Use Committee of Fudan University.
Human liver biopsies were obtained and used for research purposes with approval from the Medical Ethical Council of Zhongshan Hospital. The study abides by the Declaration of Helsinki principles.
Recombinant AAV vector production
Highly purified stocks of recombinant AAV vectors were generated by triple-plasmid transfection as described previously (39). Briefly, HEK293 cells were cotransfected with three plasmids using polyethylenimine (Polysciences) according to the manufacturer's instructions. 72 h post-transfection, cells were harvested and subjected to three rounds of freezing–thawing and then digested with 50 units/ml Benzonase (Merck). Viral vectors were purified with iodixanol (Sigma-Aldrich) gradient ultracentrifugation followed by ion exchange chromatography using HiTrap SP/Q HP columns (GE Healthcare), washed with PBS, and concentrated by centrifugal spin concentrators with a 150,000 molecular weight cutoff (Merck). The physical genomic titers of recombinant vector stocks were determined by quantitative RT-PCR.
Liver ductal organoid transduction and differentiation
Matrix coating for planer infection
25 μl of Matrigel was dropped onto a 24-well plate, spread by tips to form a flat layer, and incubated at 37 °C for polymerization.
Organoid preparation and infection
Organoids embedded in Matrigel were hand-picked and washed with cold PBS. After centrifugation (1 min at 300 × g), the medium was discarded, and the organoid pellet was suspended with expansion medium containing 10 μm Y-27632 (Sigma-Aldrich). The pretitrated AAV was added to the medium with gentle mixing, and then the organoid–AAV mixture was transferred to a plate precoated with Matrigel and incubate at 37 °C for 12 h.
After infection
Organoids on the Matrigel surface were collected by gentle pipetting and transferred to a tube. After centrifugation (1 min at 300 × g), the medium was discarded, the organoids were washed with PBS followed by centrifugation (1 min at 300 × g), and the supernatant was discarded. The organoids were embedded with 50 μl of Matrigel and then seeded on a 24-well plate. After polymerization, culture medium, such as expansion medium or differentiation medium, was supplied.
For mouse hepatocyte differentiation, organoids were supplied with differentiation medium (basal medium containing 50 ng/ml EGF (Invitrogen), 100 ng/ml FGF10 (PeproTech), 10 nm gastrin (Sigma-Aldrich), 50 nm A83-01 (Tocris Bioscience), and 10 μm N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (Sigma-Aldrich)) and cultured for 4 days. Then 3 μm dexamethasone (Sigma-Aldrich) was added for 1 day.
For human hepatocyte differentiation, organoids were cultured in human expansion medium containing 25 ng/ml BMP7 (PeproTech) for 2 days and then in human differentiation medium (basal medium containing 50 ng/ml EGF, 25 ng/ml hepatocyte growth factor, 10 nm gastrin, 0.5 μm A83-01, 10 μm N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester, 25 ng/ml BMP7, 25 ng/ml FGF19 (PeproTech), and 3 μm dexamethasone) for 5 days.
Flow cytometry analysis
The organoids were suspended in cold PBS after the medium was discarded, pelleted by centrifugation (1 min at 300 × g), and then incubated in TrypLE (Invitrogen) for 15 min at 37 °C to obtain a single-cell suspension. The disassociated cells were passed through a 40-μm cell strainer (BD Biosciences), and then single GFP-positive cells were analyzed by flow cytometry (Attune NxT, Thermo). Cells were gated as GFP-positive and GFP-negative populations and analyzed with FlowJo software.
Immunofluorescence and ELISA
The organoids were collected and washed with cold PBS after the medium was discarded, pelleted by centrifugation (1 min at 300 × g), and then fixed in 4% paraformaldehyde for 15 min. The fixed organoids were washed with PBS in the tube three times, permeabilized with 0.25% Triton X-100 for 15 min, and then blocked in PBST solution (0.1% Triton X-100 in PBS) containing 1% donkey serum (SolarBio) for 1 h at room temperature. The samples were then incubated overnight with a primary antibody at 4 °C. The following primary antibodies were used: rabbit anti-E-cadherin (Cell Signaling Technology, 3195, 1:400), rabbit anti-FLAG (Cell Signaling Technology, 14793, 1:400), and goat anti-albumin (Bethyl, A90-134A, 1:200). Fluorescein-labeled secondary antibodies (Thermo Fisher Scientific, 1:200) and 4′,6-diamidino-2-phenylindole (DAPI) were applied for 1 h at room temperature. Confocal laser scanning was done on an Olympus FV3000 laser-scanning microscope. All staining samples were plated on glasses and imaged using a ×20 objective lens and a 405-nm laser, a 488-nm laser, a 561-nm laser, and a 640-nm laser. Albumin ELISA was performed according to the manufacturer's instructions (Abcam, ab108792).
qRT-PCR
Total RNA was extracted with the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Complementary DNA was synthesized with the GoScript Reverse Transcription System (Promega). qRT-PCR reactions were performed with GoTaq® qPCR Master Mix (Promega) in triplicates on the CFX96 Touch System (Bio-Rad). Primer pairs are listed in Table S1.
RNA-Seq
RNA from freshly isolated liver ductal organoids was converted into complementary DNA libraries using the Ovation® RNA-Seq System V2 Kit (NuGEN). High-throughput sequencing was performed using Illumina HiSeq 2000. The RNA-Seq data were uniquely mapped to the mm10 genome by TopHat v1.4.1 (40). Expression values were assigned to gene level by Cufflinks v1.3.0 (41). Genes with absolute log2-transformed -fold changes of more than 1.7 were regarded as differentially expressed genes, and a threshold p value of less than 0.01 was used. Hierarchical clustering of log2-transformed fragments per kilobase million was generated by R. Gene set enrichment analysis was performed with GSEA v3.0 software (available from the Broad Institute).
Statistical analysis
All values are represented as mean ± S.D. Student's t test and two-way analysis of variance were used to compare differences between two groups as indicated. Statistical analysis was performed with the SPSS software.
Author contributions
J. W., G. R., X. W., N. J., and J. L. investigation; X. L., C. L., and B. Z. supervision; X. L., C. L., and B. Z. project administration; X. L. and C. L. writing-review and editing; B. Z. conceptualization; B. Z. funding acquisition; B. Z. writing-original draft.
Supplementary Material
Acknowledgment
We thank Yilin Xie for technical assistance.
This work was supported by National Key Research and Development Program of China Grant 2018YFA0109400 and the National Natural Science Foundation of China Grant 31771614. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Fig. S1–S3 and Table S1.
The raw NGS data were deposited into the NCBI SRA database under accession number SRP201534.
- EGF
- epidermal growth factor
- FGF
- fibroblast growth factor
- TGF
- transforming growth factor
- BMP
- bone morphogenetic protein
- AAV
- adeno-associated virus
- MSPI
- Matrigel-supported planar infection
- DM
- differentiation medium
- qRT-PCR
- quantitative RT-PCR
- NICD
- Notch intercellular domain
- DAPI
- 4′,6-diamidino-2-phenylindole
- GSEA
- gene set enrichment analysis.
References
- 1. Itoh T. (2016) Stem/progenitor cells in liver regeneration. Hepatology 64, 663–668 10.1002/hep.28661 [DOI] [PubMed] [Google Scholar]
- 2. Michalopoulos G. K. (2018) The regenerative altruism of hepatocytes and cholangiocytes. Cell Stem Cell 23, 11–12 10.1016/j.stem.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 3. Michalopoulos G. K., and DeFrances M. C. (1997) Liver regeneration. Science 276, 60–66 10.1126/science.276.5309.60 [DOI] [PubMed] [Google Scholar]
- 4. Deng X., Zhang X., Li W., Feng R. X., Li L., Yi G. R., Zhang X. N., Yin C., Yu H. Y., Zhang J. P., Lu B., Hui L., and Xie W. F. (2018) Chronic liver injury induces conversion of biliary epithelial cells into hepatocytes. Cell Stem Cell 23, 114–122.e3 10.1016/j.stem.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 5. Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M. M., Ellis E., van Wenum M., Fuchs S. A., de Ligt J., van de Wetering M., Sasaki N., Boers S. J., Kemperman H., de Jonge J., et al. (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 10.1016/j.cell.2014.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broutier L., Andersson-Rolf A., Hindley C. J., Boj S. F., Clevers H., Koo B. K., and Huch M. (2016) Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 10.1038/nprot.2016.097 [DOI] [PubMed] [Google Scholar]
- 7. Broutier L., Mastrogiovanni G., Verstegen M. M., Francies H. E., Gavarró L. M., Bradshaw C. R., Allen G. E., Arnes-Benito R., Sidorova O., Gaspersz M. P., Georgakopoulos N., Koo B. K., Dietmann S., Davies S. E., Praseedom R. K., et al. (2017) Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 10.1038/nm.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nuciforo S., Fofana I., Matter M. S., Blumer T., Calabrese D., Boldanova T., Piscuoglio S., Wieland S., Ringnalda F., Schwank G., Terracciano L. M., Ng C. K. Y., and Heim M. H. (2018) Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 24, 1363–1376 10.1016/j.celrep.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwank G., Andersson-Rolf A., Koo B. K., Sasaki N., and Clevers H. (2013) Generation of BAC transgenic epithelial organoids. PLoS ONE 8, e76871 10.1371/journal.pone.0076871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwank G., Koo B. K., Sasselli V., Dekkers J. F., Heo I., Demircan T., Sasaki N., Boymans S., Cuppen E., van der Ent C. K., Nieuwenhuis E. E., Beekman J. M., and Clevers H. (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 10.1016/j.stem.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 11. Fujii M., Matano M., Nanki K., and Sato T. (2015) Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 10.1038/nprot.2015.088 [DOI] [PubMed] [Google Scholar]
- 12. Koo B. K., Stange D. E., Sato T., Karthaus W., Farin H. F., Huch M., van Es J. H., and Clevers H. (2011) Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83 [DOI] [PubMed] [Google Scholar]
- 13. Bennett J., Ashtari M., Wellman J., Marshall K. A., Cyckowski L. L., Chung D. C., McCague S., Pierce E. A., Chen Y., Bennicelli J. L., Zhu X., Ying G. S., Sun J., Wright J. F., Auricchio A., et al. (2012) AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 4, 120ra15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennett J., Wellman J., Marshall K. A., McCague S., Ashtari M., DiStefano-Pappas J., Elci O. U., Chung D. C., Sun J., Wright J. F., Cross D. R., Aravand P., Cyckowski L. L., Bennicelli J. L., Mingozzi F., et al. (2016) Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet 388, 661–672 10.1016/S0140-6736(16)30371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nathwani A. C., Reiss U. M., Tuddenham E. G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D., Riddell A., Pie J., Rangarajan S., Bevan D., Recht M., et al. (2014) Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 371, 1994–2004 10.1056/NEJMoa1407309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allende M. L., Cook E. K., Larman B. C., Nugent A., Brady J. M., Golebiowski D., Sena-Esteves M., Tifft C. J., and Proia R. L. (2018) Cerebral organoids derived from Sandhoff disease-induced pluripotent stem cells exhibit impaired neurodifferentiation. J. Lipid Res. 59, 550–563 10.1194/jlr.M081323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agbandje-McKenna M., and Kleinschmidt J. (2011) AAV capsid structure and cell interactions. Methods Mol. Biol. 807, 47–92 [DOI] [PubMed] [Google Scholar]
- 18. Kotterman M. A., and Schaffer D. V. (2014) Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445–451 10.1038/nrg3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sen D. (2014) Improving clinical efficacy of adeno-associated vectors by rational capsid bioengineering. J. Biomed. Sci. 21, 103 10.1186/s12929-014-0103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z., Ma H. I., Li J., Sun L., Zhang J., and Xiao X. (2003) Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 10, 2105–2111 10.1038/sj.gt.3302133 [DOI] [PubMed] [Google Scholar]
- 21. McCarty D. M. (2008) Self-complementary AAV vectors: advances and applications. Mol. Ther. 16, 1648–1656 10.1038/mt.2008.171 [DOI] [PubMed] [Google Scholar]
- 22. Zhong L., Zhao W., Wu J., Li B., Zolotukhin S., Govindasamy L., Agbandje-McKenna M., and Srivastava A. (2007) A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol. Ther. 15, 1323–1330 10.1038/sj.mt.6300170 [DOI] [PubMed] [Google Scholar]
- 23. Zhong L., Li B., Jayandharan G., Mah C. S., Govindasamy L., Agbandje-McKenna M., Herzog R. W., Weigel-Van Aken K. A., Hobbs J. A., Zolotukhin S., Muzyczka N., and Srivastava A. (2008) Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 381, 194–202 10.1016/j.virol.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeLaForest A., Nagaoka M., Si-Tayeb K., Noto F. K., Konopka G., Battle M. A., and Duncan S. A. (2011) HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development 138, 4143–4153 10.1242/dev.062547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clotman F., Jacquemin P., Plumb-Rudewiez N., Pierreux C. E., Van der Smissen P., Dietz H. C., Courtoy P. J., Rousseau G. G., and Lemaigre F. P. (2005) Control of liver cell fate decision by a gradient of TGFβ signaling modulated by Onecut transcription factors. Genes Dev. 19, 1849–1854 10.1101/gad.340305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi T. Y., Khaliq M., Tsurusaki S., Ninov N., Stainier D. Y. R., Tanaka M., and Shin D. (2017) Bone morphogenetic protein signaling governs biliary-driven liver regeneration in zebrafish through tbx2b and id2a. Hepatology 66, 1616–1630 10.1002/hep.29309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kee H. J., Kim J. R., Nam K. I., Park H. Y., Shin S., Kim J. C., Shimono Y., Takahashi M., Jeong M. H., Kim N., Kim K. K., and Kook H. (2007) Enhancer of polycomb1, a novel homeodomain only protein-binding partner, induces skeletal muscle differentiation. J. Biol. Chem. 282, 7700–7709 10.1074/jbc.M611198200 [DOI] [PubMed] [Google Scholar]
- 28. Yin Z., Gonzales L., Kolla V., Rath N., Zhang Y., Lu M. M., Kimura S., Ballard P. L., Beers M. F., Epstein J. A., and Morrisey E. E. (2006) Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L191–199 10.1152/ajplung.00385.2005 [DOI] [PubMed] [Google Scholar]
- 29. Friedman C. E., Nguyen Q., Lukowski S. W., Helfer A., Chiu H. S., Miklas J., Levy S., Suo S., Han J. J., Osteil P., Peng G., Jing N., Baillie G. J., Senabouth A., Christ A. N., et al. (2018) Single-cell transcriptomic analysis of cardiac differentiation from human PSCs reveals HOPX-dependent cardiomyocyte maturation. Cell Stem Cell 23, 586–598.e8 10.1016/j.stem.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee K. Y., Singh M. K., Ussar S., Wetzel P., Hirshman M. F., Goodyear L. J., Kispert A., and Kahn C. R. (2015) Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism. Nat. Commun. 6, 8054 10.1038/ncomms9054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun H., You Y., Guo M., Wang X., Zhang Y., and Ye S. (2018) Tfcp2l1 safeguards the maintenance of human embryonic stem cell self-renewal. J. Cell Physiol. 233, 6944–6951 10.1002/jcp.26483 [DOI] [PubMed] [Google Scholar]
- 32. Wang X., Wang X., Zhang S., Sun H., Li S., Ding H., You Y., Zhang X., and Ye S. D. (2019) The transcription factor TFCP2L1 induces expression of distinct target genes and promotes self-renewal of mouse and human embryonic stem cells. J. Biol. Chem. 294, 6007–6016 10.1074/jbc.RA118.006341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y., Qi Z., Li X., Du Y., and Chen Y. G. (2018) Monolayer culture of intestinal epithelium sustains Lgr5+ intestinal stem cells. Cell Discov. 4, 32 10.1038/s41421-018-0036-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thorne C. A., Chen I. W., Sanman L. E., Cobb M. H., Wu L. F., and Altschuler S. J. (2018) Enteroid monolayers reveal an autonomous WNT and BMP circuit controlling intestinal epithelial growth and organization. Dev. Cell 44, 624–633.e4 10.1016/j.devcel.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhong L., Li B., Mah C. S., Govindasamy L., Agbandje-McKenna M., Cooper M., Herzog R. W., Zolotukhin I., Warrington K. H. Jr., Weigel-Van Aken K. A., Hobbs J. A., Zolotukhin S., Muzyczka N., and Srivastava A. (2008) Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. U.S.A. 105, 7827–7832 10.1073/pnas.0802866105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Odom D. T., Zizlsperger N., Gordon D. B., Bell G. W., Rinaldi N. J., Murray H. L., Volkert T. L., Schreiber J., Rolfe P. A., Gifford D. K., Fraenkel E., Bell G. I., and Young R. A. (2004) Control of pancreas and liver gene expression by HNF transcription factors. Science 303, 1378–1381 10.1126/science.1089769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang P., Zhang L., Gao Y., He Z., Yao D., Wu Z., Cen J., Chen X., Liu C., Hu Y., Lai D., Hu Z., Chen L., Zhang Y., Cheng X., et al. (2014) Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 14, 370–384 10.1016/j.stem.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 38. Pera M. F. (2011) Stem cells: The dark side of induced pluripotency. Nature 471, 46–47 10.1038/471046a [DOI] [PubMed] [Google Scholar]
- 39. Ling C., Lu Y., Kalsi J. K., Jayandharan G. R., Li B., Ma W., Cheng B., Gee S. W., McGoogan K. E., Govindasamy L., Zhong L., Agbandje-McKenna M., and Srivastava A. (2010) Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum. Gene Ther. 21, 1741–1747 10.1089/hum.2010.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trapnell C., Pachter L., and Salzberg S. L. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., Pimentel H., Salzberg S. L., Rinn J. L., and Pachter L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.