Abstract
Ionizing radiation (IR) can promote migration and invasion of cancer cells, but the basis for this phenomenon has not been fully elucidated. IR increases expression of glucose-regulated protein 78kDa (GRP78) on the surface of cancer cells (CS-GRP78), and this up-regulation is associated with more aggressive behavior, radioresistance, and recurrence of cancer. Here, using various biochemical and immunological methods, including flow cytometry, cell proliferation and migration assays, Rho activation and quantitative RT-PCR assays, we investigated the mechanism by which CS-GRP78 contributes to radioresistance in pancreatic ductal adenocarcinoma (PDAC) cells. We found that activated α2-Macroglobulin (α2M*) a ligand of the CS-GRP78 receptor, induces formation of the AKT kinase (AKT)/DLC1 Rho-GTPase-activating protein (DLC1) complex and thereby increases Rho activation. Further, CS-GRP78 activated the transcriptional coactivators Yes-associated protein (YAP) and tafazzin (TAZ) in a Rho-dependent manner, promoting motility and invasiveness of PDAC cells. We observed that radiation-induced CS-GRP78 stimulates the nuclear accumulation of YAP/TAZ and increases YAP/TAZ target gene expressions. Remarkably, targeting CS-GRP78 with C38 monoclonal antibody (Mab) enhanced radiosensitivity and increased the efficacy of radiation therapy by curtailing PDAC cell motility and invasion. These findings reveal that CS-GRP78 acts upstream of YAP/TAZ signaling and promote migration and radiation-resistance in PDAC cells. We therefore conclude that, C38 Mab is a promising candidate for use in combination with radiation therapy to manage PDAC.
Keywords: selective internal radiation therapy (SIRT), cell signaling, cell surface receptor, phosphotyrosine signaling, tumor cell biology, 70 kilodalton heat shock protein (Hsp70), C38 mAb, CS-GRP78, pancreatic ductal adenocarcinoma (PDAC), radiation therapy, YAP/TAZ signaling, PDAC cell motility and invasion
Introduction
Pancreatic ductal adenocarcinoma (PDAC)3 is an exceptionally lethal cancer, in part because of its aggressive invasiveness and metastatic properties. Despite combination therapies with surgical resection, chemotherapy, and radiotherapy long-term outcomes for PDAC remain poor. Unfortunately, radiotherapy can trigger invasive migration, which facilitates therapeutic resistance and recurrence (1–3). Understanding the underlying mechanisms of PDAC invasion and metastasis and overcoming radioresistance are key to development of new therapies. Our research focuses on approaches to curtail radiation-induced PDAC motility and invasion. The transformation of a normal cell to an invasive cancer cell occurs through the accumulation of genetic and epigenetic changes (4). Moreover, metastatic tumors are known to actively remodel the surrounding extracellular matrix to facilitate invasion into nearby organs and vessels (5).
GRP78 is a stress-inducible, multifunctional, prosurvival, endoplasmic reticulum chaperone in the HSP70 family. Expression of cell surface GRP78 (CS-GRP78) is associated with increased malignancy and resistance to chemotherapy and radiotherapy in various cancers (6, 7). Recently, we showed that the GRP78 primary amino acid sequence Leu98–Leu115 is the binding site for proteolytically activated α2-macroglobulin (α2M*), which promotes cancer cell proliferation by regulating the transcriptional activation of c-Myc target genes (8). Importantly, targeting the α2M*/CS-GRP78 axis with C38 mAb directed against the C-terminal domain of GRP78 abrogates tumor cell histone acetylation through epigenetic regulation under hypoxic stress (9). Recent studies suggest that radiation induces CS-GRP78 (10), but the molecular mechanism is unclear. Moreover, the mechanism by which CS-GRP78 regulates PDAC cell motility and invasion is also unknown.
The transcriptional coactivators YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif) are integral parts of the Hippo signaling pathway. This pathway is important for organ growth during development, and YAP/TAZ activation is often a hallmark of many human malignancies (11–14). Phosphorylation by the primary mediators of Hippo signaling, the large tumor suppressor kinases 1 and 2 (LATS1/2), or additional unidentified kinases suppresses YAP and TAZ activity through 14–3-3–dependent nuclear export (15–17) with YAP phosphorylation at Ser127 and Ser397 results in cytoplasmic retention and proteasomal degradation, respectively (18–20), whereas TAZ is primarily degraded upon phosphorylation at Ser89 (21). Therefore, a core serine/threonine kinase cascade, including MST1/2 kinases and their substrates LATS1/2 kinases, is responsible for inhibiting YAP/TAZ by inducing their phosphorylation, nuclear exclusion, and degradation (22). YAP/TAZ transduces mechanical and cytoskeletal cues with actin stress fibers promoting nuclear translocation. Upon cell adhesion, nuclear YAP/TAZ activates gene expression by binding to TEAD (TEA domain transcription factors) family transcription factors (23). Growth factors and defects in Hippo signaling decrease phosphorylation and promote nuclear accumulation of YAP and TAZ in human breast cancer (14, 24–26), supporting receptor tyrosine kinase signaling as potential regulator of Hippo and YAP/TAZ activity. Moreover, RhoA is implicated in YAP/TAZ activation (27), suggesting that Rho GTPase may act as an upstream regulator of YAP/TAZ signaling. A rigorous understanding of signaling at the cell surface that leads to inactivation of the Hippo pathway and contributes to enhanced YAP/TAZ activity is still emerging. The mechanism of aberrant YAP/TAZ activation triggered by altered upstream components is unclear. A search for mechanisms activating YAP/TAZ in response to radiation is required to develop therapeutic approaches to reduce radioresistance.
The current study seeks to understand the cellular mechanisms underlying tumor resistance to radiation. This knowledge can guide therapeutic approaches to improve clinical efficacy of cancer therapy. The proposed studies explore mechanistic aspects of CS-GRP78 signaling and their contribution to radiation-induced PDAC cell motility and invasion. Our data indicate that CS-GRP78 is an oncogenic driver, contributing to radioresistance in PDAC. Furthermore we demonstrate an important molecular link between CS-GRP78, Rho activation, YAP/TAZ signaling, and PDAC cell motility and invasion. Here we show that α2M*/CS-GRP78 regulates Rho activation by increased AKT/DLC1 complex formation. We further extend this observation and demonstrate that CS-GRP78 regulates YAP/TAZ activity by modulating their phosphorylation and nuclear localization in a Rho-dependent manner. C38 mAb dramatically reduces the expression of YAP/TAZ targets Ctgf, Cyr61, and Axl in irradiated PDAC cells, suggesting that CS-GRP78 regulates YAP/TAZ transcriptional activity. Finally, we show that targeting CS-GRP78 with C38 mAb enhances the efficacy of radiotherapy by curtailing PDAC cell motility and invasion. These data define a previously unknown mechanism of YAP/TAZ activation by CS-GRP78 and describe a new radiation-dependent role of YAP/TAZ to enhance PDAC cell motility and invasion. Together these studies indicate that targeting CS-GRP78 by C38 mAb might be employed as a potential therapeutic intervention in curtailing PDAC cell motility and enhancing radiosensitization.
Results
The α2M*/CS-GRP78 axis promotes PDAC cell motility and invasion through a Rho-dependent mechanism
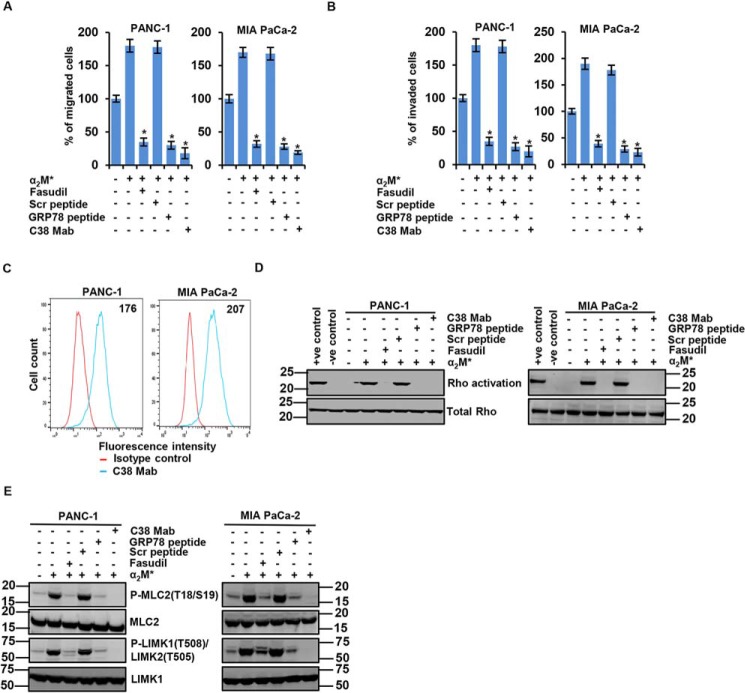
To examine the impact of the α2M*/CS-GRP78 axis on PDAC cell motility and invasion, we targeted CS-GRP78 by utilizing C38 mAb, scrambled (Scr) peptide, and GRP78 peptide derived from the GRP78 primary amino acid sequence (Leu98–Leu115), as described in our previous publication (8). Further, we also determined whether Rho is necessary to activate α2M*/CS-GRP78–mediated PDAC cell motility and invasion. Treatment with C38 mAb or GRP78 peptide potently inhibited α2M*-induced PDAC cell motility and invasion (Fig. 1, A and B and Fig. S1A). We also found that targeting CS-GRP78 inhibits the basal cell motility and invasion. Moreover, CS-GRP78 is a multifunctional receptor and has various ligands in addition to α2M* (7). Therefore these data indicate that C38 mAb and GRP78 peptide will also affect any other CS-GRP78 ligand–mediated cell motility and invasion. Alternatively, the selective Rho inhibitor fasudil similarly inhibited α2M*-induced PDAC cell motility and invasion (Fig. 1, A and B and Fig. S1A). To provide evidence for the role of CS-GRP78 we evaluated the surface expression of GRP78 in PDAC cells (Fig. 1C). Surface expression of GRP78 was found in PDAC cell lines (PANC-1 and MIA PaCa-2). These results suggest that the pro-motility function of CS-GRP78 in PDAC cells is mediated through the Rho GTPase–dependent pathway.
Figure 1.
Cell surface GRP78 (CS-GRP78) regulates Rho-dependent PDAC cell motility and invasion. A and B, indicated PDAC cell lines were stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h to analyze migration and invasion by EZCellTM Cell Migration Assay and Matrigel Invasion Assay. Mean ± S.D. of triplicates is shown. C, surface expression of GRP78 was detected in the indicated cancer cell lines by flow cytometric analysis of nonpermeabilized cells. Surface GRP78 was visualized with murine C38 mAb, followed by fluorescently labeled secondary antibody and compared with matched isotype control. Positively stained cells are represented as the area under the respective histogram, and MFI values are shown. D, the indicated PDAC cell lines were stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h to analyze Rho activation. E, immunoblot analysis of the indicated PDAC cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h. *, p values ≤ 0.05.
Next we performed Rho activation assay to determine whether the α2M*/CS-GRP78 axis regulates Rho activation for PDAC cell motility and invasion. We observed that fasudil, GRP78 peptide, and C38 mAb drastically suppressed α2M*-induced Rho activation (Fig. 1D). To confirm that the downstream effector of Rho is activated by the α2M*/CS-GRP78 axis we examined phosphorylation of myosin light chain 2 (MLC2), a known substrate of the ROCK kinase and LIMK (28). Fasudil, GRP78 peptide, and C38 mAb inhibited the α2M*-induced phosphorylation of MLC2 (Thr18/Ser19) and LIMK1 (Thr508)/LIMK2 (Thr505) (Fig. 1E), confirming the ROCK inhibition. Collectively, these results indicate that the α2M*/CS-GRP78 axis promotes PDAC cell motility and invasion in a Rho-dependent manner.
CS-GRP78 regulates interaction of AKT/DLC1 complex to activate RhoA-GTPase and promote PDAC cell motility and invasion
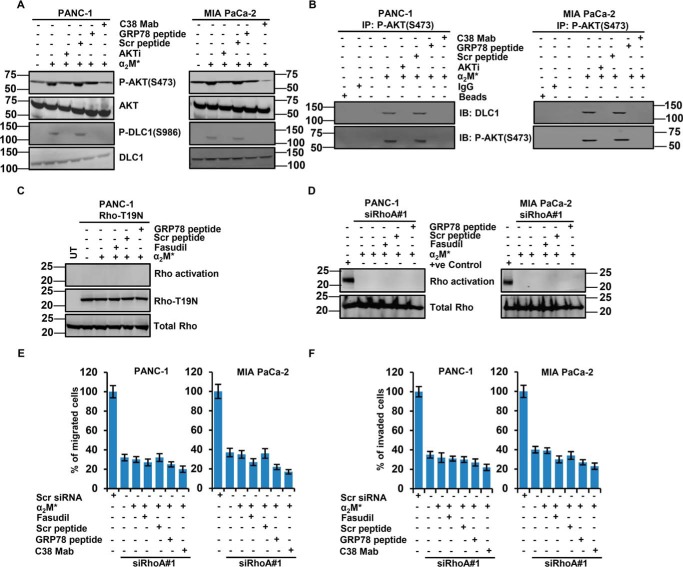
We next examined signaling pathways downstream of the α2M*/CS-GRP78 axis to identify those responsible for Rho activation. Previous studies from our laboratory and others demonstrate that CS-GRP78 is a potent regulator of the PI3-kinase/AKT signaling pathways to promote tumor proliferation and prolong survival (7, 9, 29, 30). Moreover, several receptor tyrosine kinase ligands activate RhoA by regulating AKT-mediated phosphorylation of deleted in liver cancer 1 (DLC1), which controls Rho signaling (31). To test whether α2M*/CS-GRP78 signaling regulates AKT activation to modulate Rho signaling, we treated PDAC cell lines with C38 mAb, Scr, and GRP78 peptide or MK-2206 (AKTi) and then stimulated with α2M*. As expected we observed that α2M* induced the phosphorylation of AKT Ser473 and surprisingly increased the phosphorylation of DLC1 Ser986, whereas AKTi, GRP78 peptide, and C38 mAb suppressed this event (Fig. 2A). These data suggest that α2M*-induced P-DLC1 depends on CS-GRP78/AKT signaling. To determine whether CS-GRP78–mediated AKT activation is required for RhoA-GTPase, we performed Rho activation assay by treating PDAC cell lines with AKTi and then stimulated with α2M*. We found that AKTi suppressed α2M*-induced Rho activation (Fig. S1B). These results demonstrate that α2M*/CS-GRP78–induced AKT activity is required for the Rho signaling. To verify that the observed effect of AKT activity on RhoA–GTP depended on DLC1, we investigated whether AKT binds DLC1 and forms a complex. Through co-immunoprecipitation assay, we demonstrate that α2M* promoted interaction between AKT and DLC1 that is blocked by AKTi, GRP78 peptide and C38 mAb (Fig. S1C). We next examined whether phosphorylated AKT was required for the AKT/DLC1 complex formation, we immunoprecipitated with a phosphorylation-specific antibody against P-AKT Ser473 and immunoblotted for DLC1 (Fig. 2B). These studies are consistent with previous findings which report that AKT/DLC1 complex is required for Rho activation (31). However, our studies are discrepant from them in one aspect, namely that complex formation depends on the enzymatic activity of AKT. Collectively, these data suggest that α2M*/CS-GRP78 axis–mediated Rho activation requires AKT/DLC1 complex formation.
Figure 2.
α2M*/CS-GRP78 axis attenuates Rho activation via an AKT signaling pathway. A, immunoblot analysis of the indicated cancer cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or AKTi (5 μm) for 16 h. B, immunoprecipitation analysis of P-AKT Ser473 and DLC1 in the indicated cancer cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or AKTi (5 μm) for 16 h. C, Rho activation assay was measured in PANC1 cells transduced with Rho-T19N and then stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or fasudil (5 μm) for 16 h. D, Rho activation assay was performed in RhoA-silenced PDAC cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm) or GRP78 peptide (100 pm) for 6 h or fasudil (5 μm) for 16 h. E and F, RhoA-silenced PDAC cell lines were stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h to analyze migration and invasion by EZCellTM cell migration and Matrigel invasion assay. Mean ± S.D. of triplicates is shown. UT, untreated.
Using a complementary genetic approach, CS-GRP78–mediated Rho activation was assessed by transiently transfecting the dominant negative Rho–T19N mutant vector in PANC-1 cells and then treated with fasudil, Scr, or GRP78 peptide. In PANC-1 cells, which overexpress dominant negative Rho mutant, α2M* did not induce the Rho activation which is consistent with pharmacological (fasudil) Rho inhibition. In these cells, fasudil and GRP78 peptide had no effect (Fig. 2C). To further substantiate the role of α2M*/CS-GRP78 signaling in Rho activation, we silenced RhoA gene expression by two independent siRNA in PDAC cells. The efficiency of RhoA silencing is shown in Fig. S1D. siRhoA#1 cells were treated with fasudil, Scr, or GRP78 peptide and then stimulated with α2M*. As expected, α2M* did not induce the Rho activation (Fig. 2D). Together, these data support the role of α2M*/CS-GRP78 signaling in promoting Rho activation. Next we sought to determine the functional role of RhoA-silenced cells. In PDAC cells, two independent siRNAs targeting RhoA significantly suppressed the PDAC motility and invasion compared with cells treated with scrambled siRNA, whereas α2M* did not have any further effect (Fig. 2, E and F and Fig. S2, A–D). Further, scrambled siRNA cells function in a similar manner as naïve PDAC cells (data not shown). Together these results support the findings that the α2M*/CS-GRP78 axis potentiates the AKT/DLC1 complex formation to activate Rho signaling, which promotes PDAC cell motility and invasion.
CS-GRP78 activates YAP and TAZ in a Rho-dependent mechanism
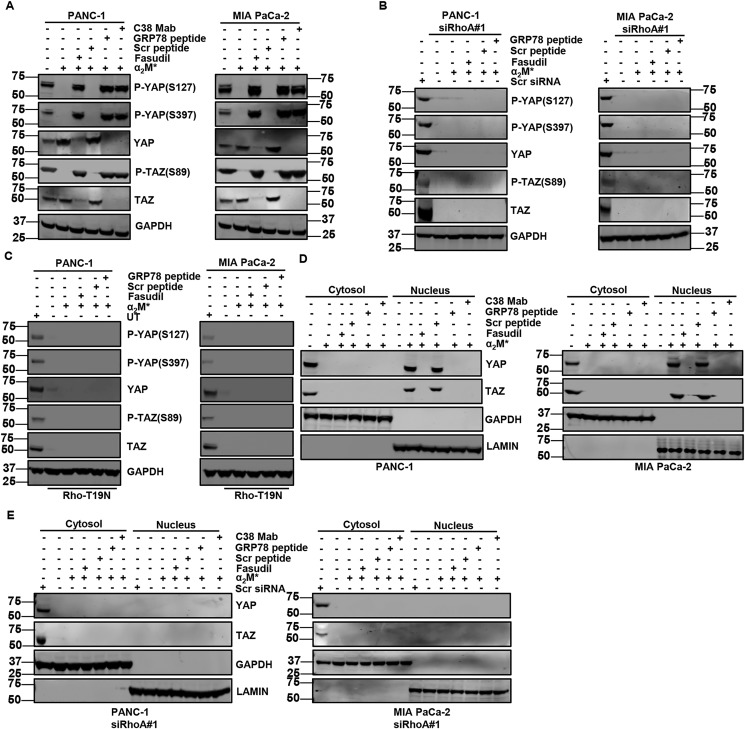
Although Rho activation can induce cancer cell motility and invasion (32), the mechanism has not been elucidated. Because RhoA can activate the transcriptional cofactors YAP and TAZ (27, 33), we reasoned that increased PDAC cell motility and invasion induced by α2M*/CS-GRP78 axis could be mediated by RhoA-dependent YAP and TAZ activation. To determine whether the α2M*/CS-GRP78 axis activates YAP/TAZ, we stimulated PDAC cells with α2M* in the presence or absence of C38 mAb, fasudil, Scr, or GRP78 peptide. α2M* increased the expression of YAP and TAZ, whereas targeting CS-GRP78 and RhoA suppressed this event (Fig. 3A). In the same condition as in Fig. 3A, we further investigated whether α2M*-induced YAP and TAZ expression is associated with gene transcription. We found increased mRNA expression of YAP and TAZ with α2M* stimulation, whereas fasudil, GRP78 peptide, and C38 mAb suppressed these events (Fig. S3A). Moreover, two independent siRNAs targeting RhoA drastically reduced both protein and transcript levels of YAP/TAZ in PDAC cells, whereas α2M*, fasudil, GRP78 peptide, and C38 mAb did not have any further effect (Fig. 3B and Fig. S3, B–E). These results demonstrate that α2M*/CS-GRP78 signaling promotes the expression of YAP and TAZ at both protein as well as transcript level in a RhoA-dependent manner. Phosphorylation of YAP and TAZ is associated with their sequestration in the cytoplasm (34). Therefore, we assessed the phosphorylation status of YAP and TAZ, which are required for its activity. Fasudil-, GRP78 peptide–, and C38 mAb-treated cells exhibited greater phosphorylation of YAP (Ser397 and Ser127) and TAZ (Ser89), as well as reduced total YAP/TAZ protein and transcript levels (Fig. 3A and Fig. S3A). Surprisingly, RhoA silenced cells did not exhibit any phosphorylation of YAP and TAZ, but they had reduced both protein and transcript levels of YAP/TAZ expression (Fig. 3B and Fig. S3, C–E). To further confirm the role of RhoA, we transiently transfected PDAC cells with dominant negative Rho–T19N mutant vector and then stimulated the cells with α2M* in the presence or absence of fasudil, Scr, or GRP78 peptide. Surprisingly, dominant negative Rho mutant cells suppressed the expression of YAP/TAZ consistent with pharmacological Rho inhibition. Like RhoA silenced cells, α2M* did not increase the phosphorylation of YAP (Ser397 and Ser127) and TAZ (Ser89) in the overexpression of the dominant negative Rho mutant cells, whereas targeting CS-GRP78 and RhoA did not have any further effect (Fig. 3C). Together, these findings support the role of RhoA signaling in promoting α2M*/CS-GRP78–mediated YAP/TAZ activation.
Figure 3.
α2M*/CS-GRP78 axis regulates Rho signaling to activate YAP and TAZ. A, PDAC cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h and probed for indicated proteins. B, RhoA-silenced PDAC cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm) or GRP78 peptide (100 pm) for 6 h or fasudil (5 μm) for 16 h and probed for indicated proteins. C, PDAC cell lines were transduced with Rho-T19N and then stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm) or GRP78 peptide (100 pm) for 6 h or fasudil (5 μm) for 16 h and probed for indicated proteins. D, cytoplasmic and nuclear localization of YAP/TAZ were analyzed in the indicated cancer cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h. E, cytoplasmic and nuclear localization of YAP/TAZ were analyzed in the RhoA-silenced PDAC cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h. UT, untransfected.
Next, we assessed how the downstream signaling of the α2M*/CS-GRP78 axis contributes to YAP/TAZ cytoplasmic sequestration and nuclear localization. We treated PDAC cells with C38 mAb, fasudil, Scr, or GRP78 peptide and then stimulated with α2M* to determine the subcellular localization of YAP/TAZ by immunoblot. In control PDAC cells, YAP/TAZ localized to the cytosol, but α2M* stimulation resulted in nuclear localization of YAP/TAZ. By contrast, fasudil, GRP78 peptide, and C38 mAb treatment caused reduced nuclear localization of YAP/TAZ but not cytoplasmic retention, suggesting the degradation of total YAP/TAZ protein (Fig. 3D). These results suggests that YAP/TAZ accumulate in the nucleus in response to α2M*/CS-GRP78 signaling. To determine the role of RhoA in an α2M*/CS-GRP78–mediated nuclear localization of YAP/TAZ, we treated the RhoA silenced PDAC cells with C38 mAb, fasudil, Scr, or GRP78 peptide and then stimulated with α2M*. Similar to the treatment with fasudil, GRP78 peptide or C38 mAb led to reduced total YAP/TAZ protein in RhoA silenced cells, suggesting that α2M* did not have any significant changes in the subcellular localization (Fig. 3E). Collectively, these results support the mechanism that YAP/TAZ activated by α2M*/CS-GRP78 axis in a RhoA-dependent manner.
CS-GRP78–YAP/TAZ signaling axis is required for PDAC cell motility and invasion
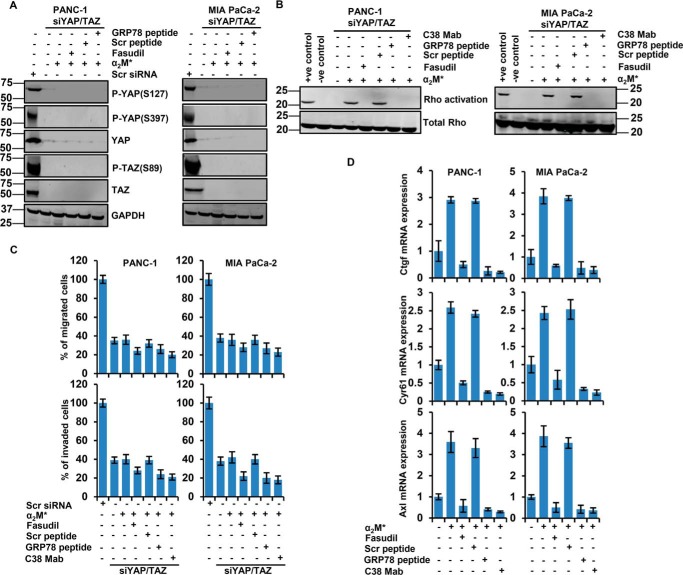
To determine whether YAP and TAZ mediate CS-GRP78–dependent PDAC cell motility and invasion, YAP/TAZ was knocked down in PDAC cells by siRNA-mediated gene silencing. The efficiency of YAP/TAZ silencing is shown in Fig. S4, A and B. siYAP/TAZ cells were stimulated with α2M* in the presence or absence of C38 mAb, fasudil, Scr, or GRP78 peptide. α2M* did not induce the YAP/TAZ expression or phosphorylation of YAP (Ser381 and Ser127) and TAZ (Ser89), and targeting RhoA and CS-GRP78 did not have any further effect (Fig. 4A and Fig. S4B). These results are consistent with aforementioned results (Fig. 3, A and B), suggesting that CS-GRP78 signaling is required for the YAP/TAZ activation. Next we assessed the role of RhoA–GTPase in the YAP/TAZ silenced cells by Rho activation assay. Similar to the results obtained from Fig. 1D, we observed in YAP/TAZ silenced cells that fasudil, GRP78 peptide, and C38 mAb drastically suppressed α2M*-induced Rho activation (Fig. 4B). These results confirm that RhoA–GTPase is upstream of YAP/TAZ, which is regulated by the α2M*/CS-GRP78 axis. To determine whether CS-GRP78–YAP/TAZ signaling axis is relevant to PDAC cell motility and invasion, we stimulated siYAP/TAZ cells with α2M* in the presence or absence of C38 mAb, fasudil, Scr, or GRP78 peptide. YAP/TAZ silenced cells significantly reduced PDAC cell motility and invasion, whereas α2M*, C38 mAb, fasudil, Scr, or GRP78 peptide do not have any further effect, indicating a role for YAP/TAZ in regulating CS-GRP78–mediated PDAC cell motility and invasion (Fig. 4C and Fig. S4C). We also found that, scrambled siRNA cells functions in a similar manner as naïve PDAC cells (data not shown). Further, the impact of YAP/TAZ on PDAC cell motility did not stem from off-target effects because three independent siRNAs targeting YAP/TAZ were pooled together. Furthermore, this SMARTpool of siRNAs has been verified in multiple cancers (33). Together, these results support the finding that CS-GRP78 activates YAP/TAZ complex to promote PDAC cell motility and invasion in a RhoA-dependent manner.
Figure 4.
YAP/TAZ signaling promotes PDAC cell motility and invasion by α2M*/CS-GRP78 axis. A, immunoblot analysis of indicated proteins in YAP/TAZ-silenced PDAC cell lines were stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm) or GRP78 peptide (100 pm) for 6 h or fasudil (5 μm) for 16 h. B, Rho activation assay was measured in YAP/TAZ-silenced PDAC cell lines, stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h. C, YAP/TAZ-silenced PDAC cell lines were stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h to analyze migration and invasion by EZCellTM cell migration and Matrigel invasion assay. Mean ± S.D. of triplicates is shown. D, quantitative RT-PCR analysis was performed in PDAC cell lines stimulated with α2M* (100 pm) for 30 min in the absence or presence of Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h or fasudil (5 μm) for 16 h to quantify the transcript levels of the YAP/TAZ target genes Ctgf, Cyr61, and Axl.
Several previous studies revealed that YAP/TAZ regulates many transcriptional targets which play essential roles in cancer (11, 33, 35, 36). To better define downstream signaling events of YAP/TAZ activation, we examined the expression of YAP/TAZ targets in PDAC cells stimulated with α2M* in the presence or absence of fasudil, Scr, or GRP78 peptide and C38 mAb. Our findings demonstrate, that α2M* up-regulates the YAP/TAZ target genes Ctgf, Cyr61, and Axl, whereas targeting CS-GRP78 and RhoA suppressed these events (Fig. 4D). In YAP/TAZ silenced cells, YAP/TAZ targets Ctgf, Cyr61, and Axl showed dramatically reduced expression, whereas α2M*, C38 mAb, fasudil, Scr, or GRP78 peptide had no further effect (Fig. S4D), suggesting that CS-GRP78 regulates YAP/TAZ transcription activity.
Targeting CS-GRP78 enhances the efficacy of radiation by modulating irradiated PDAC cell motility and invasion
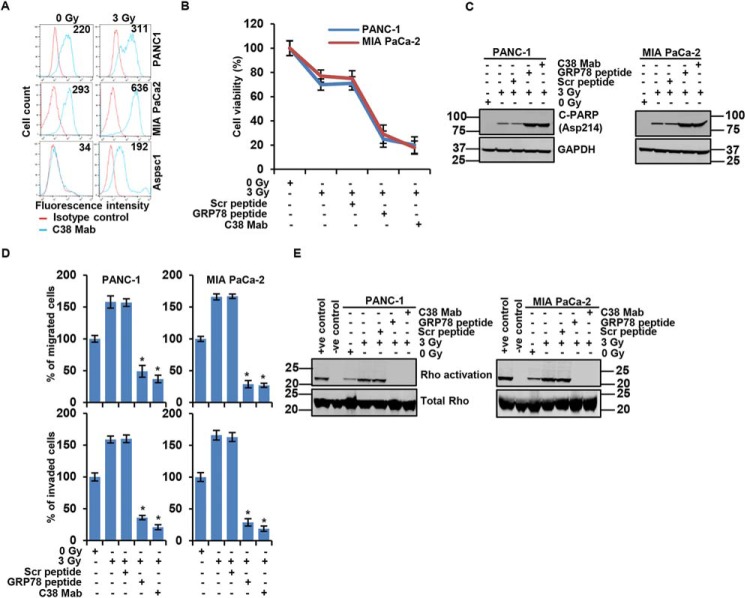
Recently, we showed that hypoxic stress abrogates CS-GRP78 signaling to modulate acetyl-CoA production (9). Despite the emerging role of CS-GRP78 signaling in cancer development nothing is known about its potential function in response to radiation therapy. To determine the effect of radiotherapy on CS-GRP78, we evaluated the surface expression of GRP78 in PDAC cells exposed to 0 Gy versus 3 Gy radiation. Surface expression of GRP78 was elevated in irradiated PDAC cell lines (PANC-1, MIA PaCa-2, and AsPC-1) compared with nonradiated cells (Fig. 5A). Interestingly, we noted low surface expression of GRP78 in AsPC1 cell line whereas radiation significantly increased CS-GRP78, suggesting that irradiated cells may preferentially translocate GRP78 to the cell surface. Our data implicate that radiotherapy induces CS-GRP78 expression in PDAC. To evaluate the efficacy of targeting CS-GRP78 with radiotherapy, we performed proliferation assays (XTT). We found that radiation alone reduced proliferation in PDAC cell lines whereas a combination of radiation with GRP78 peptide or C38 mAb drastically reduced proliferation (Fig. 5B). These findings demonstrate that targeting CS-GRP78 enhances the efficacy of radiotherapy. Next we evaluated the role of CS-GRP78 in irradiated cancer cell survival. As expected, radiation alone induced apoptosis whereas a combination of radiation with GRP78 peptide or C38 mAb resulted in massive apoptosis as evidenced by strong detection of poly (ADP-ribose) polymerase cleavage (C-PARP (Asp214)) (Fig. 5C). Therefore, targeting CS-GRP78 enhances radiation-induced cancer cell death. These results further support the role of CS-GRP78 in irradiated PDAC cell proliferation and malignant progression.
Figure 5.
C38 mAb curtails PDAC motility and invasion to enhance the efficacy of radiation. A, surface expression of GRP78 was detected in the indicated irradiated PDAC cell lines (0 or 3 Gy) by flow cytometric analysis of nonpermeabilized cells. Surface GRP78 was visualized with murine C38 mAb, followed by fluorescently labeled secondary antibody and shown relative to matched isotype control. Positively stained cells are represented as the area under the respective histogram, and MFI values are shown. B, cell viability assay showing the irradiated PDAC cell lines with 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h. C, apoptosis of the indicated PDAC cell lines irradiated with 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h was examined by immunoblotting analysis. D, PDAC cell lines were irradiated with 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h to analyze migration and invasion by EZCellTM cell migration and Matrigel invasion assay. Mean ± S.D. of triplicates is shown. E, Rho activation assay was performed in the PDAC cell lines receiving 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h and then probed for indicated proteins. *, p values ≤ 0.05.
We then examined the molecular mechanism by which C38 mAb modulates cell survival in irradiated PDAC cells. One of the major drawbacks of radiation therapy is that it has been associated with increased PDAC cell motility and invasion, which facilitate radiation resistance and tumor recurrence (1). Therefore, we investigated the role of CS-GRP78 signaling in radiation-mediated effects on PDAC cell motility and invasion. To achieve this goal, we irradiated PDAC cells with 0 or 3 Gy and then treated with C38 mAb, Scr, or GRP78 peptide. We found that targeting CS-GRP78 with GRP78 peptide or C38 mAb significantly reduced irradiated PDAC cell motility and invasion (Fig. 5D and Fig. S5A). To understand the mechanism by which CS-GRP78 regulates irradiated PDAC cell motility and invasion, we examined the downstream signaling of CS-GRP78 in irradiated PDAC cells. A robust increase in the phosphorylation of AKT (Ser473) and DLC1 (Ser986), as well as AKT/DLC1 complex formation, was observed in the irradiated PDAC cells whereas C38 mAb or GRP78 peptide drastically suppressed these events (Fig. S5, B and C). These data suggest that Rho is necessary to induce irradiated PDAC cell motility and invasion, to confirm that we performed motility and invasion assays. Consistent with aforementioned results (Fig. 5D) AKTi and fasudil significantly reduced irradiated PDAC cell motility and invasion (Fig. S5, D and E). To determine whether CS-GRP78 regulates Rho activation in irradiated PDAC cells to promote motility and invasion, we performed Rho activation assays. We observed that GRP78 peptide and C38 mAb drastically suppressed radiation-induced Rho activation in irradiated PDAC cells (Fig. 5E). Together, these results suggest that CS-GRP78 regulates irradiated PDAC cell motility and invasion in a Rho-dependent manner.
Radiation attenuates CS-GRP78 signaling to activate transcriptional coactivators of YAP/TAZ
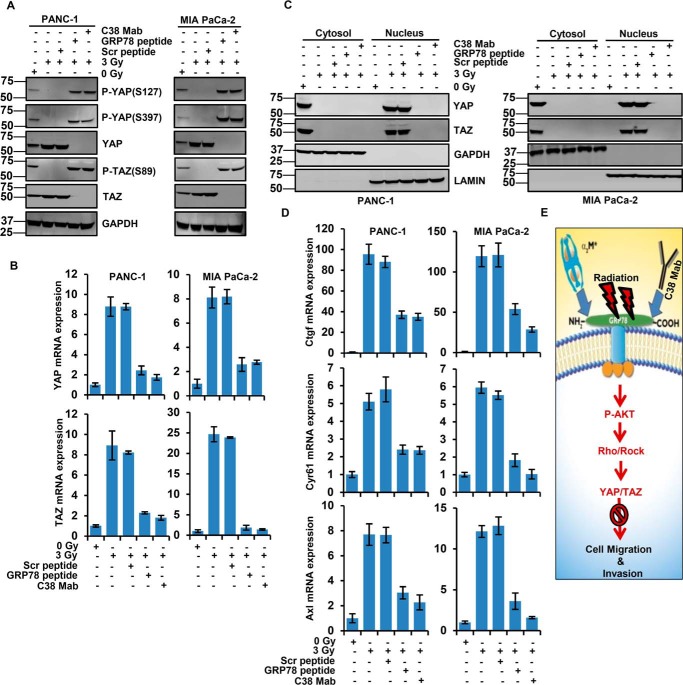
We next examined the mechanisms by which irradiation induces PDAC cell motility and invasion. To determine whether CS-GRP78 increases the YAP/TAZ activity in radiotherapy, we irradiated PDAC cells and then treated with C38 mAb, Scr, and GRP78 peptide. When compared with control cells, targeting CS-GRP78 increased phosphorylation of YAP (Ser397 and Ser127) and TAZ (Ser89) as well as reduced total YAP and TAZ protein and its transcript level (Fig. 6, A and B) in irradiated PDAC cells. Next we assessed the subcellular localization of YAP/TAZ. We found that in control cells, YAP/TAZ were localized in cytoplasmic lysates but irradiated PDAC cells resulted in nuclear lysates, suggesting the YAP/TAZ activity. However, C38 mAb and GRP78 peptide reduced total YAP/TAZ protein (Fig. 6C) consistent with phosphorylation of YAP/TAZ (Fig. 6A). These data support that CS-GRP78 mediates nuclear accumulation of YAP/TAZ. Next, we sought to determine whether CS-GRP78 mediated nuclear localization of YAP/TAZ reflected a functional activation for the transcriptional coactivators. We examined the ability of CS-GRP78 to enhance the transcription of known YAP/TAZ target genes, Ctgf, Cyr61, and Axl, in irradiated PDAC cells. Irradiation of PDAC cells increased the transcript level of Ctgf, Cyr61, and Axl whereas C38 mAb and GRP78 peptide suppressed this event (Fig. 6D). These findings support our hypotheses that CS-GRP78 acts as a positive regulator of the transcriptional coactivators YAP/TAZ. Collectively, these results support the mechanism that YAP and TAZ are activated by CS-GRP78 in a Rho-dependent manner.
Figure 6.
Radiation attenuates CS-GRP78 to regulate YAP/TAZ transcriptional activity. A, immunoblot analysis of the PDAC cell lines were irradiated with 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h and then probed for indicated proteins. B, quantitative RT-PCR analysis of YAP and TAZ gene expression in the PDAC cell lines receiving 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h. C, cytoplasmic and nuclear localization of YAP/TAZ were analyzed in the irradiated PDAC cell lines receiving 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h and then probed for indicated proteins. D, quantitative RT-PCR analysis was performed in PDAC cell lines receiving with 0 or 3 Gy and then treated with Scr peptide (100 pm), GRP78 peptide (100 pm), or C38 mAb (50 μg) for 6 h to quantify the transcript levels of the YAP/TAZ target genes Ctgf, Cyr61, and Axl. E, a model is shown for radiation-induced CS-GRP78 and downstream mechanisms that promote PDAC cell motility and invasion. In addition the red arrow indicates that targeting CS-GRP78 with C38 mAb curtails irradiated PDAC cell motility and invasion.
Discussion
Ionizing radiation therapy is commonly used to treat cancer to improve local control, but it can also contribute to tumor recurrence by promoting migratory and invasive properties of cancer cells (3, 35). However, the underlying molecular mechanisms responsible for PDAC cell motility, invasion, and its radioresistance have not been completely elucidated. Here, we demonstrate that targeting CS-GRP78 with C38 mAb enhances radiosensitivity and curtails PDAC cell motility and invasion. Remarkably, the α2M*/CS-GRP78 axis induces AKT/DLC1 complex formation to increase Rho activation. We also found that CS-GRP78 stimulates YAP/TAZ activity in a Rho-dependent manner to promote PDAC cell motility and invasion. Furthermore, CS-GRP78 regulates YAP/TAZ nuclear accumulation and the expression of its target genes (Ctgf, Cyr61, and Axl), demonstrating that CS-GRP78 functionally stimulates YAP/TAZ signaling. The present finding of CS-GRP78–YAP/TAZ signaling is clinically relevant, because the combination effect of C38 mAb with radiotherapy enhances radiosensitization, which suggests a new therapeutic opportunity (Fig. 6E).
CS-GRP78 plays a key role in hypoxic stress, drug resistance, and stem cell function by modulating pro-proliferative, pro-migratory, and anti-apoptotic signaling pathways (6, 7, 9), although the underlying mechanism remains unknown. What role CS-GRP78 may have in PDAC cell motility and invasion and its differential role in radiation therapy are largely unexplored. Recently, we showed that the GRP78 primary amino acid sequence Leu98–Leu115 is the binding site for α2M*, a crucial ligand for CS-GRP78 which promotes cancer cell proliferation by regulating the transcriptional activation of c-Myc target genes (8). Although α2M*/CS-GRP78 signaling facilitates the metastatic phenotype of cancer cells (37), our results further demonstrate that this signaling axis regulates PDAC cell motility and invasion in a Rho-dependent manner. These data are in line with our previous studies (8, 9), which show that targeting the GRP78 amino acid sequence Leu98–Leu115 with GRP78 peptide or C-terminal domain of GRP78 with C38 mAb are potent agents for targeting CS-GRP78 and modulating PDAC phenotype to abrogate invasive behavior.
Aberrant activation of the Rho signaling pathway is implicated in the pathogenesis of human cancers (38); however, the upstream mechanisms for activating RhoA remain unclear. We previously showed that the α2M*/CS-GRP78 axis is an upstream regulator of PI3K/AKT signaling (8, 30). Our current study uncovered that AKT-dependent phosphorylation of DLC1 regulates PDAC cell motility and invasion. Additionally, our data support that the α2M*/CS-GRP78 axis promotes the interaction between the AKT/DLC1 complex which is critical for RhoA activity. Our results are consistent with Tripathi et al. (31) but differ from Ko et al. (39), most likely because DLC1 has different phosphorylation sites as well as the differential expression of DLC1 in various cancer cell lines (40, 41), which might respond differentially to activate Rho GTPase. Cumulatively, the results of our current study advances understanding of the regulatory mechanisms of RhoA activity. Our findings identify α2M*/CS-GRP78 axis as a novel upstream activator of the AKT/DLC1 complex which regulates RhoA activity to promote PDAC cell motility and invasion. How the remaining Rho family members contribute to CS-GRP78-mediated migration and invasiveness of PDAC cells needs further investigation.
Rho signaling regulates YAP/TAZ activity which is crucial for inducing cell plasticity and modulating cytoskeletal dynamics to enable cell motility (42, 43). Importantly, dysregulation of the upstream signaling that regulates the YAP/TAZ pathway is poorly understood. Here, we identify the α2M*/CS-GRP78 axis as an upstream signaling mechanism that stimulates YAP/TAZ activity. We found that targeting CS-GRP78 abrogates RhoA signaling to induce phosphorylation of YAP/TAZ, leading to reduced YAP/TAZ protein levels through degradation consistent with previous data (33). Notably, YAP/TAZ depletion through CS-GRP78–RhoA inhibition consistently reduced the migration and invasiveness of PDAC cells. Although Rho promotes YAP/TAZ activity independently of Hippo signaling (27, 44), previous studies also show that Rho promotes YAP/TAZ activity indirectly by altering cytoskeletal dynamics (27) and directly by inhibiting LATS phosphorylation (45). How CS-GRP78–RhoA signaling regulates the Hippo pathway either directly or indirectly needs further investigation. Although aspects of this signaling network had been established previously, the current study highlights the novel finding that CS-GRP78 orchestrates this network. We also found that CS-GRP78–mediated YAP/TAZ activation requires intact Rho signaling because targeting CS-GRP78 inhibits Rho/ROCK signaling, which prevents nuclear accumulation through protein destabilization. A recent study shows that Rho inhibition reduces YAP/TAZ nuclear localization and increases YAP/TAZ cytoplasmic localization (33), but we found that CS-GRP78 inhibition and RhoA depletion reduced YAP/TAZ expression as well as its subcellular localization. These divergent results may be because of the cell specificity and also require further investigation. In addition to enhancement of YAP/TAZ nuclear translocation, CS-GRP78 appears to increase the expression of YAP/TAZ at the protein as well as transcript level in PDAC cells. Our data suggest that CS-GRP78 activates YAP/TAZ through decreased phosphorylation, stabilization, and nuclear translocation to promote migration and invasiveness of PDAC cells. Although YAP/TAZ are commonly overexpressed and/or hyperactivated in human cancers, few mutations in these genes have been observed (24). Our data suggest that the CS-GRP78 may contribute to a previously unknown mechanism of YAP/TAZ dysregulation in human cancers. A central theme that has emerged from these studies is the critical role of the Rho GTPases in mediating the signaling events initiated by CS-GRP78 to activate YAP/TAZ.
YAP/TAZ transcriptional coactivators are the key effectors of the Hippo pathway to regulate cancer cell proliferation, migration, and invasion (35, 46). The α2M*/CS-GRP78 axis not only induces YAP/TAZ nuclear accumulation but also regulates the expression of YAP/TAZ target genes (Ctgf, Cyr61, and Axl) through Rho signaling, demonstrating that CS-GRP78 functionally stimulates YAP/TAZ activity. Furthermore, depletion of YAP/TAZ by siRNAs down-regulates Ctgf, Cyr61, and Axl expression, respectively like targeting CS-GRP78. Although, the present study focuses on genes Ctgf, Cyr61, and Axl, which are critical for migration and invasiveness of various cancer cells (35, 47–49), the possibility remains that CS-GRP78 may promote the expression of other YAP/TAZ target genes. Herein, we present evidence indicating that CS-GRP78 promotes PDAC cell migration and invasion by regulating YAP/TAZ transcriptional activity.
Radiation therapy is one of the major treatment modalities for the management of PDAC (1, 3). Despite recent advancements in radiation technologies and multidisciplinary approaches, locoregional pancreatic cancer recurrence remains a common mode of failure after definitive treatment, suggesting radioresistance of PDAC. Therefore, we examined the effect of targeting CS-GRP78 on irradiated PDAC cells. Treatment with C38 mAb or GRP78 peptide has several effects on irradiated PDAC cells, including inhibition of proliferation, cell motility, and invasiveness, as well as increasing apoptosis, thus enhancing the radiosensitivity of PDAC cells. A previous study had documented similar effects of targeting CS-GRP78 on the radiation sensitivity of glioblastoma and non–small cell lung cancer cell lines (10). Radiation-induced CS-GRP78 increases AKT/DLC1 complex formation to promote Rho activation and YAP/TAZ total and nuclear localization. We found that radiation promotes expression YAP/TAZ target genes Ctgf, Cyr61, and Axl are consistent with Minata et al. (35), which was published when our work was under review and discrepant from Zhang et al. (50), likely because of different experimental conditions. The radiosensitizing effect of C38 mAb was increased by inhibiting YAP/TAZ activity and PDAC cell motility and invasion in a Rho-dependent manner. Thus C38 mAb is a promising candidate for a combination with radiation therapy for pancreatic cancer. Targeting CS-GRP78 enhanced the radiosensitivity of PDAC cells and has been found by others to be highly effective to eradicate radioresistant tumors (10), suggesting that C38 mAb is a useful therapeutic agent to enhance the efficacy of radiotherapy for such tumors.
We and others have demonstrated that expression of CS-GRP78 occurs specifically on cancer cells, with expression lacking on normal cells (6, 7). Although radiotherapy is used to target and kill tumor cells, adjacent normal tissue can also be damaged. In this context, we speculate that combination of C38 mAb with radiotherapy will precisely target cancer cells and will not increase normal tissue radiation toxicity. Furthermore, we have previously demonstrated that antibodies to the C-terminal domain of GRP78, such as C38 mAb, have anti-tumor effects even in the absence of radiotherapy. In particular, antibodies targeting the C-terminal domain of GRP78 may counteract the effect of GRP78 autoantibodies, which are present in the serum of some cancer patients and have been associated with poor prognosis (51, 52). We have previously shown that autoantibodies against GRP78 in cancer patients are always directed against its N-terminal domain and trigger a pro-proliferative, anti-apoptotic, and pro-regulatory signaling cascade similar to CS-GRP78's natural ligands (52). Further, we demonstrated that ligation of the C-terminal domain of GRP78 always overrides ligation of its N-terminal domain (53). Thus, antibodies to the C-terminal domain of GRP78 have significant therapeutic potential because of their anti-proliferative, anti-migratory, and pro-apoptotic effects on tumor cells in addition to their radiosensitizing effect.
Based on our work and others (10) it is evident that irradiating multiple types of cancer up-regulates CS-GRP78, whose presence on the cell surface is coupled to pro-proliferative, pro-migratory, and anti-apoptotic signaling pathways. Thus, the treatment itself partially counteracts the beneficial effects of radiation. This unsatisfactory consequence of irradiating cancers can be overcome by treating with monoclonal antibodies such as C38, which target the C-terminal domain of GRP78 to suppress cancer growth both in vivo and in vitro (54). The results of our findings may form the basis for clinical trial design and may benefit radiotherapy patients. Our results collectively indicate that the oncogenic effects of CS-GRP78 play a significant role in radiation biology, potentially offering new perspectives on how to select patients who are more likely to benefit from C38 mAb, alone or in combination with radiotherapy.
Experimental procedures
Cell culture
PANC-1, MIA PaCa-2, and AsPC-1 pancreatic cancer cells were from the Duke Cell Culture Facility. AsPC-1 cells were maintained in RPMI 1640 medium (Sigma) containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin at 37 °C in a 5% CO2-humidified atmosphere. PANC-1 and MIA PaCa-2 cells were in DMEM (high glucose, Gibco-Life Technologies) containing 10% FBS, 1% penicillin/streptomycin at 37 °C in a 5% CO2-humidified atmosphere.
Antibodies and reagents
Antibodies recognizing YAP, TAZ, P-YAP (Ser397), P-YAP (Ser127), P-TAZ (Ser89), P-Akt (Ser473), AKT, P-MLC2 (Thr18/Ser19), MLC2, P-LIMK1 (Thr508)/LIMK2(Thr505), LIMK1, and cleaved PARP (Asp214) were purchased from Cell Signaling Technologies. P-DLC1 (Ser986) antibody was purchased from Thermo Fisher Scientific. DLC1 antibody was purchased from BD Biosciences. Lamin antibody was purchased from Sigma-Aldrich. GAPDH antibody was purchased from GenScript. Secondary antibodies conjugated with Alexa Fluor 680, Alexa Fluor 790, and Alexa Fluor 647 were purchased from Invitrogen. IR dye 800 CW was purchased from Rockland. Rho Activation Assay Kit was purchased from Millipore. Rho inhibitor fasudil and AKT inhibitor MK-2206 were purchased from Selleck Chemicals. α2M* was prepared as described previously (55). α2M* stimulation was performed in serum-free condition unless otherwise specified. The GRP78 murine mAb (C38) was produced in our laboratory (56).
Peptides
GRP78 peptide LIGRTWNDPSVQQDIKFL (Leu98–Leu115) and a scrambled peptide GTNKSQDLWIPQLRDVFI were purchased from Genemed Synthesis, Inc.
Radiation treatment
When indicated, PANC-1, MIA PaCa-2, and AsPC-1 cells were irradiated at 0 or 3 Gy with an X-RAD 160 kV X-ray irradiator using F1 filter. After irradiation the medium was replaced with fresh complete medium and then incubated for 16 h. Irradiated cells were treated with 50 μg/ml C38 mAb for 6 h, Scr peptide, and GRP78 peptide (100 pm for 1 h).
Flow cytometry
CS-GRP78 was analyzed by flow cytometry as described previously (8). The mean fluorescence intensity (MFI) of the signal was calculated by FlowJo software, and signal obtained from GRP78 was normalized with that obtained from isotype controls.
Cell proliferation assay
Cells were plated in 96-well plates at 10,000 cells per well in 0.1 ml of growth medium containing Scr and GRP78 peptide or C38 mAb for 72 h. Cell viability was measured as per manufacturer's instruction protocol by using Roche Cell Proliferation Kit (XTT) assay. Absorbance was read at λ = 450 nm.
Rho activation assay
Rho activation assays were performed according to the manufacturer's instructions by using Millipore Rho Activation Assay Kit.
Cell migration and invasion assays
For analysis of directional cell motility, chemotactic cell migration was carried out as per manufacturer's instructions by using EZCellTM Cell Migration/Chemotaxis Assay Kit (BioVision). Absorbance was read at Ex/Em = 530/590 nm. The mean value was calculated from three independent experiments. Invasion assays were performed with 8-μm 24-well Matrigel-coated Transwell inserts (BD Biosciences). Inserts were rehydrated with medium for 2 h at 37 °C. Prior to plating, cells were serum starved for 16 h, and 4 × 105 cells were subsequently plated in 0.1% serum containing medium. After incubation at 37 °C for 16 h cells were fixed and stained with 0.5% crystal violet. For all motility and invasion experiments, mitomycin C (5 μg/ml) (Sigma) was added at the time of plating to suppress proliferation. Cells migrating through both the Matrigel and the filter pores were counted from five random fields from three wells and represented as a mean (± S.D.) of three replicates.
Plasmid and siRNA transfections
PANC-1 and MIA PaCa-2 cells were transiently transfected using the Lipofectamine RNAiMAX Reagent (Invitrogen) with 20 nm SMARTpool human YAP (M-012200-00-0005, Dharmacon), SMARTpool human TAZ (M-016083-00-0005, Dharmacon), siRNA Universal negative control or one of two siRNA sequences targeting Rho: siRhoA#1 (sense, 5′-GAACUAUGUG GCAGAUAUCUU-3′) or siRhoA#2 (sense, 5′-GACAUGCUUGCUCAUAGUCUUC-3′) were purchased from Sigma. The plasmid of pcDNA3.1RhoA-N19 was a kind gift from Prof. Keith Burridge and was transfected into cells using Lipofectamine 2000 according to the manufacturer's protocol. Transfected cells were collected 48 h after transfection for immunoblotting and quantitative PCR.
Cytoplasmic and nuclear protein extraction
Cytosolic and nuclear proteins were extracted from PANC-1 and MIA PaCa-2 cells by incubating cells for 15 min in cytosolic lysis buffer (10 mm HEPES, 10 mm KCl, 15 mm MgCl2, 0.5% Nonidet P-40, and Roche protease inhibitor mixture). The cells were scraped and vortexed for 1 min and then centrifuged for 5 min at maximum speed in a microcentrifuge. The supernatant (cytoplasmic extract) was immediately transferred to a clean prechilled tube. The pellet was washed with cytosolic lysis buffer three times and then the pellet suspended in nuclear lysis buffer (1% SDS, 50 mm Tris, pH 7.5, ≥250 units Benzonase) incubated for 30 min, then centrifuged at maximum speed in a microcentrifuge.
Immunoblotting and immunoprecipitation
Protein extracts, immunoblotting, and immunoprecipitate analysis were performed as described previously (8), and all blots are representative of a minimum two independent experiments.
Quantitative real-time PCR and PCR array
Total RNA was prepared from cells using the Quick-RNA Miniprep (Zymo Research) and cDNAs were generated using the iScript cDNA synthesis kit (Bio-Rad). SYBR Green reactions were performed using a Bio-Rad CFX96 quantitative real-time PCR system. For data analysis, raw counts were normalized to the housekeeping gene averaged for the same time point and condition (ΔCt). Counts are reported as -fold change relative to the untreated control (2−ΔΔCt). All primers were designed and synthesized by Eurofins MWG Operon. Primer sequences were as follows: YAP forward primer 5′-GCT ACA GTG TCC CTC GAA CC-3′; YAP reverse primer 5′-CCG GTG CAT GTG TCT CCT TA-3′; TAZ forward primer 5′-ATC CCC AAC AGA CCC GTT TC-3′; TAZ reverse primer 5′-GAA CGC AGG CTT GCA GAA AA-3′; Ctgf forward primer 5′-ACC GAC TGG AAG ACA CGT TTG-3′; Ctgf reverse primer 5′-CCA GGT CAG CTT CGC AAG G-3′; Cyr61 forward primer 5′-TGA AGC GGC TCC CTG TTT T-3′; Cyr61 reverse primer 5′-CGG GTT TCT TTC ACA AGG CG-3′; Axl forward primer 5′-CGG GTT TCT TTC ACA AGG CG-3′; Axl reverse primer 5′-GTA CTG TCC CGT GTC GGA AAG-3′; β-actin forward primer 5′-GGA CTT CGA GCA AGA GAT GG-3′; β-actin reverse primer 5′-AGC ACT GTG TTG GCG TAC AG-3′.
Statistical analysis
Data are presented as mean ± S.D., unless otherwise stated. A Student's t test was used to compare two groups for statistical significance, P-value ≤ 0.05 was considered as significant.
Author contributions
U. G. and S. V. P. conceptualization; U. G. and S. V. P. resources; U. G. and S. V. P. data curation; U. G. and S. V. P. software; U. G. and S. V. P. formal analysis; U. G. and S. V. P. supervision; U. G. and S. V. P. validation; U. G. and S. V. P. investigation; U. G. and S. V. P. visualization; U. G., Y. M., K. Y., and S. V. P. methodology; U. G. writing-original draft; U. G. and S. V. P. project administration; U. G., Y. M., and S. V. P. writing-review and editing; S. V. P. funding acquisition.
Supplementary Material
The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S5.
- PDAC
- pancreatic ductal adenocarcinoma
- GRP78
- glucose-regulated protein 78kDa
- CS
- cell surface
- α2M*
- α2-macroglobulin
- Scr
- scrambled
- YAP
- Yes-associated protein
- TAZ
- tafazzin
- Gy
- gray
- MFI
- mean fluorescence intensity.
References
- 1. Neoptolemos J. P., Kleeff J., Michl P., Costello E., Greenhalf W., and Palmer D. H. (2018) Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 15, 333–348 10.1038/s41575-018-0005-x [DOI] [PubMed] [Google Scholar]
- 2. Qiang L., Cao H., Chen J., Weller S. G., Krueger E. W., Zhang L., Razidlo G. L., and McNiven M. A. (2019) Pancreatic tumor cell metastasis is restricted by MT1-MMP binding protein MTCBP-1. J. Cell Biol. 218, 317–332 10.1083/jcb.201802032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Ruysscher D., Niedermann G., Burnet N. G., Siva S., Lee A. W. M., and Hegi-Johnson F. (2019) Radiotherapy toxicity. Nat. Rev. Dis. Primers 5, 13 10.1038/s41572-019-0064-5 [DOI] [PubMed] [Google Scholar]
- 4. Vogelstein B., Papadopoulos N., Velculescu V. E., Zhou S., Diaz L. A. Jr., and Kinzler K. W. (2013) Cancer genome landscapes. Science 339, 1546–1558 10.1126/science.1235122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ridley A. J. (2011) Life at the leading edge. Cell 145, 1012–1022 10.1016/j.cell.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 6. Lee A. S. (2014) Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 14, 263–276 10.1038/nrc3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gopal U., and Pizzo S. V. (2018) The endoplasmic reticulum chaperone GRP78 also functions as a cell surface signaling receptor. in Cell Surface GRP78, a New Paradigm in Signal Transduction Biology (Pizzo S. V., ed) pp. 9–40, Academic Press (Elsevier), Cambridge, MA: 10.1016/B978-0-12-812351-5.00009-X [DOI] [Google Scholar]
- 8. Gopal U., Gonzalez-Gronow M., and Pizzo S. V. (2016) Activated α2-macroglobulin regulates transcriptional activation of c-MYC target genes through cell surface GRP78 protein. J. Biol. Chem. 291, 10904–10915 10.1074/jbc.M115.708131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gopal U., and Pizzo S. V. (2017) Cell surface GRP78 promotes tumor cell histone acetylation through metabolic reprogramming: A mechanism which modulates the Warburg effect. Oncotarget 8, 107947–107963 10.18632/oncotarget.22431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dadey D. Y. A., Kapoor V., Hoye K., Khudanyan A., Collins A., Thotala D., and Hallahan D. E. (2017) Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non-small cell lung cancer cell lines and tumor models. Clin. Cancer Res. 23, 2556–2564 10.1158/1078-0432.CCR-16-1935 [DOI] [PubMed] [Google Scholar]
- 11. Zanconato F., Forcato M., Battilana G., Azzolin L., Quaranta E., Bodega B., Rosato A., Bicciato S., Cordenonsi M., and Piccolo S. (2015) Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 10.1038/ncb3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanconato F., Cordenonsi M., and Piccolo S. (2016) YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 10.1016/j.ccell.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panciera T., Azzolin L., Fujimura A., Di Biagio D., Frasson C., Bresolin S., Soligo S., Basso G., Bicciato S., Rosato A., Cordenonsi M., and Piccolo S. (2016) Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19, 725–737 10.1016/j.stem.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson R., and Halder G. (2014) The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 10.1038/nrd4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan S. W., Lim C. J., Loo L. S., Chong Y. F., Huang C., and Hong W. (2009) TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J. Biol. Chem. 284, 14347–14358 10.1074/jbc.M901568200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanai F., Marignani P. A., Sarbassova D., Yagi R., Hall R. A., Donowitz M., Hisaminato A., Fujiwara T., Ito Y., Cantley L. C., and Yaffe M. B. (2000) TAZ: A novel transcriptional co-activator regulated by interactions with 14–3-3 and PDZ domain proteins. EMBO J. 19, 6778–6791 10.1093/emboj/19.24.6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S. A., Gayyed M. F., Anders R. A., Maitra A., and Pan D. (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., and Guan K. L. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 10.1101/gad.1602907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong X., Nguyen H. T., Chen Q., Zhang R., Hagman Z., Voorhoeve P. M., and Cohen S. M. (2014) Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. EMBO J. 33, 2447–2457 10.15252/embj.201489385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao B., Li L., Tumaneng K., Wang C. Y., and Guan K. L. (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TRCP. Genes Dev. 24, 72–85 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu C. Y., Zha Z. Y., Zhou X., Zhang H., Huang W., Zhao D., Li T., Chan S. W., Lim C. J., Hong W., Zhao S., Xiong Y., Lei Q. Y., and Guan K. L. (2010) The Hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFβ-TrCP E3 ligase. J. Biol. Chem. 285, 37159–37169 10.1074/jbc.M110.152942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu F. X., Zhao B., and Guan K. L. (2015) Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halder G., Dupont S., and Piccolo S. (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13, 591–600 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- 24. Harvey K. F., Zhang X., and Thomas D. M. (2013) The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 10.1038/nrc3458 [DOI] [PubMed] [Google Scholar]
- 25. Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C., Inui M., Montagner M., Parenti A. R., Poletti A., Daidone M. G., Dupont S., Basso G., Bicciato S., and Piccolo S. (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 10.1016/j.cell.2011.09.048 [DOI] [PubMed] [Google Scholar]
- 26. Fan R., Kim N. G., and Gumbiner B. M. (2013) Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. U.S.A. 110, 2569–2574 10.1073/pnas.1216462110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., and Piccolo S. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 28. Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., and Kaibuchi K. (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246–20249 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- 29. Lin Y. G., Shen J., Yoo E., Liu R., Yen H. Y., Mehta A., Rajaei A., Yang W., Mhawech-Fauceglia P., DeMayo F. J., Lydon J., Gill P., and Lee A. S. (2015) Targeting the glucose-regulated protein-78 abrogates Pten-null driven AKT activation and endometrioid tumorigenesis. Oncogene 34, 5418–5426 10.1038/onc.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Misra U. K., and Pizzo S. V. (2010) Ligation of cell surface GRP78 with antibody directed against the COOH-terminal domain of GRP78 suppresses Ras/MAPK and PI 3-kinase/AKT signaling while promoting caspase activation in human prostate cancer cells. Cancer Biol. Ther. 9, 142–152 10.4161/cbt.9.2.10422 [DOI] [PubMed] [Google Scholar]
- 31. Tripathi B. K., Grant T., Qian X., Zhou M., Mertins P., Wang D., Papageorge A. G., Tarasov S. G., Hunter K. W., Carr S. A., and Lowy D. R. (2017) Receptor tyrosine kinase activation of RhoA is mediated by AKT phosphorylation of DLC1. J. Cell Biol. 216, 4255–4270 10.1083/jcb.201703105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haga R. B., and Ridley A. J. (2016) Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 7, 207–221 10.1080/21541248.2016.1232583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Edwards D. N., Ngwa V. M., Wang S., Shiuan E., Brantley-Sieders D. M., Kim L. C., Reynolds A. B., and Chen J. (2017) The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci. Signal. 10, eaan4667 10.1126/scisignal.aan4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basu S., Totty N. F., Irwin M. S., Sudol M., and Downward J. (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14–3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 11, 11–23 10.1016/S1097-2765(02)00776-1 [DOI] [PubMed] [Google Scholar]
- 35. Minata M., Audia A., Shi J., Lu S., Bernstock J., Pavlyukov M. S., Das A., Kim S. H., Shin Y. J., Lee Y., Koo H., Snigdha K., Waghmare I., Guo X., Mohyeldin A., et al. (2019) Phenotypic plasticity of invasive edge glioma stem-like cells in response to ionizing radiation. Cell Rep. 26, 1893–1905.e1897 10.1016/j.celrep.2019.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu M. Z., Chan S. W., Liu A. M., Wong K. F., Fan S. T., Chen J., Poon R. T., Zender L., Lowe S. W., Hong W., and Luk J. M. (2011) AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene 30, 1229–1240 10.1038/onc.2010.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Misra U. K., Deedwania R., and Pizzo S. V. (2005) Binding of activated α2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J. Biol. Chem. 280, 26278–26286 10.1074/jbc.M414467200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chin V. T., Nagrial A. M., Chou A., Biankin A. V., Gill A. J., Timpson P., and Pajic M. (2015) Rho-associated kinase signalling and the cancer microenvironment: Novel biological implications and therapeutic opportunities. Expert Rev. Mol. Med. 17, e17 10.1017/erm.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ko F. C., Chan L. K., Tung E. K., Lowe S. W., Ng I. O., and Yam J. W. (2010) Akt phosphorylation of deleted in liver cancer 1 abrogates its suppression of liver cancer tumorigenesis and metastasis. Gastroenterology 139, 1397–1407 10.1053/j.gastro.2010.06.051 [DOI] [PubMed] [Google Scholar]
- 40. Cao X., Voss C., Zhao B., Kaneko T., and Li S. S. (2012) Differential regulation of the activity of deleted in liver cancer 1 (DLC1) by tensins controls cell migration and transformation. Proc. Natl. Acad. Sci. U.S.A. 109, 1455–1460 10.1073/pnas.1114368109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ravi A., Kaushik S., Ravichandran A., Pan C. Q., and Low B. C. (2015) Epidermal growth factor activates the Rho GTPase-activating protein (GAP) deleted in liver cancer 1 via focal adhesion kinase and protein phosphatase 2A. J. Biol. Chem. 290, 4149–4162 10.1074/jbc.M114.616839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R. P., Chaudhry S. I., Harrington K., Williamson P., Moeendarbary E., Charras G., and Sahai E. (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637–646 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mason D. E., Collins J. M., Dawahare J. H., Nguyen T. D., Lin Y., Voytik-Harbin S. L., Zorlutuna P., Yoder M. C., and Boerckel J. D. (2019) YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility. J. Cell Biol. 218, 1369–1389 10.1083/jcb.201806065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., and Piccolo S. (2013) A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 45. Plouffe S. W., Meng Z., Lin K. C., Lin B., Hong A. W., Chun J. V., and Guan K. L. (2016) Characterization of Hippo pathway components by gene inactivation. Mol. Cell 64, 993–1008 10.1016/j.molcel.2016.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moya I. M., and Halder G. (2019) Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 10.1038/s41580-018-0086-y [DOI] [PubMed] [Google Scholar]
- 47. Aguiar D. P., de Farias G. C., de Sousa E. B., de Mattos Coelho-Aguiar J., Lobo J. C., Casado P. L., Duarte M. E., and Abreu J. G. Jr. (2014) New strategy to control cell migration and metastasis regulated by CCN2/CTGF. Cancer Cell Int. 14, 61 10.1186/1475-2867-14-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang F., Hao F., An D., Zeng L., Wang Y., Xu X., and Cui M. Z. (2015) The matricellular protein Cyr61 is a key mediator of platelet-derived growth factor-induced cell migration. J. Biol. Chem. 290, 8232–8242 10.1074/jbc.M114.623074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leconet W., Chentouf M., du Manoir S., Chevalier C., Sirvent A., Aït-Arsa I., Busson M., Jarlier M., Radosevic-Robin N., Theillet C., Chalbos D., Pasquet J. M., Pèlegrin A., Larbouret C., and Robert B. (2017) Therapeutic activity of anti-AXL antibody against triple-negative breast cancer patient-derived xenografts and metastasis. Clin. Cancer Res. 23, 2806–2816 10.1158/1078-0432.CCR-16-1316 [DOI] [PubMed] [Google Scholar]
- 50. Zhang L., Cheng F., Wei Y., Zhang L., Guo D., Wang B., and Li W. (2019) Inhibition of TAZ contributes radiation-induced senescence and growth arrest in glioma cells. Oncogene 38, 2788–2799 10.1038/s41388-018-0626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arap M. A., Lahdenranta J., Mintz P. J., Hajitou A., Sarkis A. S., Arap W., and Pasqualini R. (2004) Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 6, 275–284 10.1016/j.ccr.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 52. Gonzalez-Gronow M., Cuchacovich M., Llanos C., Urzua C., Gawdi G., and Pizzo S. V. (2006) Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 66, 11424–11431 10.1158/0008-5472.CAN-06-1721 [DOI] [PubMed] [Google Scholar]
- 53. Misra U. K., Mowery Y., Kaczowka S., and Pizzo S. V. (2009) Ligation of cancer cell surface GRP78 with antibodies directed against its COOH-terminal domain up-regulates p53 activity and promotes apoptosis. Mol. Cancer Ther. 8, 1350–1362 10.1158/1535-7163.MCT-08-0990 [DOI] [PubMed] [Google Scholar]
- 54. Mo L., Bachelder R. E., Kennedy M., Chen P. H., Chi J. T., Berchuck A., Cianciolo G., and Pizzo S. V. (2015) Syngeneic murine ovarian cancer model reveals that ascites enriches for ovarian cancer stem-like cells expressing membrane GRP78. Mol. Cancer Ther. 14, 747–756 10.1158/1535-7163.MCT-14-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Misra U. K., Payne S., and Pizzo S. V. (2011) Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: A role for secreted prostate-specific antigen. J. Biol. Chem. 286, 1248–1259 10.1074/jbc.M110.129767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Ridder G. G., Ray R., and Pizzo S. V. (2012) A murine monoclonal antibody directed against the carboxyl-terminal domain of GRP78 suppresses melanoma growth in mice. Melanoma Res. 22, 225–235 10.1097/CMR.0b013e32835312fd [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.