Abstract
Background
The combined effect of transitions of metabolic health and weight on cardiovascular disease (CVD) remains unclear. We aimed to examine the association of concurrent changes of metabolic health and weight on CVD over time.
Methods and Results
The study population consisted of 205 394 from the Korean National Health Insurance Service. Metabolic health was determined by fasting serum glucose, total cholesterol, and blood pressure levels, while obesity was determined by body mass index. All participants were divided into either metabolically healthy nonobese (MHNO), metabolically healthy obese, metabolically unhealthy nonobese, or metabolically unhealthy obese for each of the first (2002–2003) and second (2004–2005) health screening periods, after which participants were followed‐up for CVD from 2006 to 2015. Cox proportional hazards regression was used to determine adjusted hazard ratios (aHRs) and 95% CIs. Among initial MHNO participants, those who became metabolically healthy obese (aHR, 1.25; 95% CI, 1.10–1.41), metabolically unhealthy nonobese (aHR, 1.23; 95% CI, 1.15–1.31), and metabolically unhealthy obese (aHR, 1.34; 95% CI, 1.12–1.61) had elevated risk for CVD compared with those who remained MHNO. Conversely, improving metabolic health and obesity were associated with reduced CVD risk among initially metabolically unhealthy nonobese to secondary MHNO (aHR, 0.79; 95% CI, 0.73–0.84), metabolically unhealthy obese to MHNO (aHR, 0.68; 95% CI, 0.58–0.81), and metabolically unhealthy obese to metabolically healthy obese (aHR, 0.73; 95% CI, 0.66–0.80) participants.
Conclusions
Changes toward metabolically unhealthy or obese states resulted in increased CVD risk. Improving metabolic health along with reducing weight may lead to decreased risk of CVD.
Keywords: cardiovascular disease, high blood pressure, hypercholesterolemia, hyperglycemia, hypertension, obesity
Subject Categories: Cardiovascular Disease, Epidemiology, Obesity, Risk Factors
Clinical Perspective
What Is New?
This large study including 205 394 participants followed up for 9 years is the first longitudinal study to determine the association between combined transitions of metabolic health and weight on cardiovascular disease risk.
What Are the Clinical Implications?
Changes toward metabolically unhealthy or obese status were associated with increased risk of cardiovascular disease and improvements in metabolic health or weight were associated with reduced cardiovascular disease risk.
Metabolically unhealthy or obese patients who improve metabolic health or lose weight may benefit from reduced risk of subsequent cardiovascular disease.
Introduction
Obesity is a well‐known major risk factor for cardiovascular disease (CVD). Moreover, the effects of obesity on CVD are thought to be associated with other metabolic risk factors such as hypertension, hypercholesterolemia, and hyperglycemia.1 However, a certain proportion of obese individuals do not appear to develop metabolic abnormalities,2, 3 resulting in the emergence of the concept of metabolically healthy obesity, which is used to describe an obese phenotype without the burden of any metabolic disorder.4 While there is no definite criteria in defining metabolically healthy obesity,5, 6 it is commonly described as the absence of hypertension, dyslipidemia, and diabetes mellitus among obese patients.7
Recent systematically reviewed and cohort studies demonstrated that metabolically healthy obesity is not a benign condition.8, 9, 10, 11, 12, 13, 14, 15 Previous studies also suggested that the risk is higher than metabolically healthy normal‐weight people but lower than metabolically unhealthy obese individuals.5, 7, 8, 9, 10, 11, 12 While previous studies have focused on the effects of changes in weight13 or metabolic components such as fasting glucose,16, 17 blood pressure,18 and serum cholesterol19 levels on cardiovascular risk, there were only a few studies that investigated the concurrent changes of metabolic health status and weight for individuals at risk of CVD. A few recent studies15, 20, 21 investigated the transition from metabolic healthy to unhealthy phenotypes among the metabolically healthy obese (MHO) population. However, these studies were limited because one study relied on self‐reports of metabolic disorders and were limited to women,21 and none of the studies considered how cardiovascular risk alters upon conversion of metabolically unhealthy to healthy while also considering changes in weight.
In this study, we aimed to examine the association of concurrent changes in metabolic health and weight with CVD risk over time. To assess the association of changes in metabolic health and weight on CVD, we used a validated health survey, examination, and claims database from the National Health Insurance Service (NHIS)22 in the Republic of Korea.
Methods
The authors do not have permission to share the data because of restrictions set by data‐sharing policies from the NHIS of Korea.
Study Population
The study population was derived from the National Health Insurance Service–Health Screening Cohort (NHIS‐HEALS). The NHIS provides mandatory health care for all citizens of South Korea, covering nearly all forms of health care.23 Furthermore, for all citizens aged ≥40 years, the NHIS provides biannual health screening examinations, which include a self‐reported questionnaire on health behavior; measurements for height, weight, and blood pressure; and urine and blood tests. On the basis of the claims data, the NHIS provides a cohort for research purposes on participants who underwent health screening examinations in 2002–2003. This cohort, the NHIS‐HEALS, includes information on participant sociodemographics, hospital use, drug prescriptions, and results from health screening examinations from 2002 to 2015.23 The NHIS database has been used previously in a number of epidemiologic studies, and its validity is described in detail elsewhere.24, 25
Our cohort began with 232 045 participants who underwent health screening examinations in both the first (2002–2003) and second (2004–2005) health screening periods without type 2 diabetes mellitus, dyslipidemia, or hypertension medication prescriptions during 2002–2005. Among them, we excluded 14 376 participants who were diagnosed with CVD before the index date of January 1, 2006, as well as 391 participants who died before the index date. Then, 928 participants with missing values on body mass index (BMI), fasting serum glucose (FSG), blood pressure, or total cholesterol were excluded. Finally, 10 956 participants with missing values on covariates were excluded, resulting in a study population of 205 394 participants.
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (Institutional Review Board number: X‐1809‐493‐902). The need for consent was waived, as the NHIS‐HEALS database was anonymized according to strict confidentiality guidelines.
Key Variables
Metabolic health and obesity were determined for each of the first (2002–2003) and second (2004–2005) health screening periods. FSG, blood pressure, and total cholesterol levels were the considered components of metabolic health. Metabolically healthy was defined as having normal FSG (<126.0 mg/dL), blood pressure (<140/90 mm Hg), and total cholesterol (<240.0 mg/dL), while metabolically unhealthy was defined as having high FSG (≥126.0 mg/dL), blood pressure (≥140/90 mm Hg), or total cholesterol (≥240.0 mg/dL). BMI, calculated by dividing the height in meters by weight in kilograms squared, was used to determine obesity. Obese was defined as having BMI of 25.0 kg/m2 or higher in accordance with the World Health Organization guidelines.26
CVD events were defined upon hospitalization of ≥2 days or death caused by coronary heart disease or stroke. Coronary heart disease and stroke were defined using the International Classification of Diseases, Tenth Revision (ICD‐10) codes in accordance with the American Heart Association (I20–I25 for coronary heart disease and I60–I69 for stroke).27
Statistical Analysis
Multivariate Cox proportional hazards regression was used to determine the adjusted hazard ratios (aHRs) and 95% CIs for CVD according to the change in metabolic health and obesity. The considered covariates included age (years, continuous), sex (categorical, men and women), household income (categorical, first, second, third, and fourth quartiles), smoking (categorical, never smoker, quitter, and current smoker), alcohol consumption (categorical, 0, 0–1, 3–4, 5–6, and 7 times per week), physical activity (categorical, 0, 1–2, 3–4, 5–6, and 7 times per week), and Charlson comorbidity index (categorical, 0, 1, and ≥2). Household income was determined according to the insurance premium. The algorithm for calculating Charlson comorbidity index scores from claims data was adapted from another study.28
All participants were divided into either metabolically health nonobese (MHNO), metabolically health obese (MHO), metabolically unhealthy nonobese (MUNO), or metabolically unhealthy obese (MUO) for each of the first and second health screening periods. Starting from the index date of January 1, 2006, participants were followed up until the date of CVD, death date, or December 31, 2015, whichever came earliest. Then, the risk of CVD according to the change in number of metabolically unhealthy components was determined among subgroups of persistent weight groups of <23.0, 23.0 to 24.9, and ≥25.0 kg/m2. Similarly, the risk of CVD according to weight change was determined among subgroups of persistently metabolically healthy and unhealthy participants. Finally, the risk of CVD according to secondary metabolic health status by metabolic components among initially MHNO participants were determined.
In a sensitivity analysis, we determined the association of changes in obesity and metabolic health on the risk of CVD using BMI of ≥30 kg/m2 as the cutoff value for obesity. Moreover, we conducted another sensitivity analysis with the definition for being metabolically unhealthy as having abnormal laboratory results or the prescription of diabetes mellitus, dyslipidemia, or hypertension medications. In addition, we demonstrated an additional analysis that the association of changes in metabolic health and weight with CVD using secondary metabolic health and weight measurements during 2008–2009 to reflect a long‐term exposure period. Finally, to reflect the comorbidities that can effect unintentional weight loss, we conducted a stratified analysis according to subgroups of Charlson comorbidity index of 0 and ≥1, as well as a sensitivity analysis by excluding those with liver disease, kidney disease, or cancer.
Statistical significance was defined as having a P value of <0.05 in a 2‐sided manner. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Table 1 shows the descriptive characteristics of the study population. The number of MHNO, MHO, MUNO, and MUO participants during the second health screening period were 104 924, 34 639, 41 156, and 24 675, respectively. The mean (SD) age for MHNO, MHO, MUNO, and MUO participants were 52.2 (8.2), 52.0 (7.5), 54.7 (9.0), and 53.2 (8.1) years, respectively. Compared with MHNO participants, MUO participants tended to be men, have lower household income, be current smokers, consume alcohol, exercise more, and have fewer comorbidities.
Table 1.
Descriptive Characteristics of the Study Population
| Secondary Metabolic Health and Obesity | ||||
|---|---|---|---|---|
| MHNO | MHO | MUNO | MUO | |
| Number of participants | 104 924 | 34 639 | 41 156 | 24 675 |
| Initial metabolic health and obesity, N (%) | ||||
| MHNO | 77 055 (75.8) | 4995 (4.9) | 17 688 (17.4) | 1910 (1.9) |
| MHO | 5335 (15.6) | 19 374 (56.7) | 1521 (4.5) | 7955 (23.3) |
| MUNO | 20 165 (46.7) | 1490 (3.5) | 19 407 (44.9) | 2152 (5.0) |
| MUO | 2369 (9.0) | 8780 (33.3) | 2540 (9.6) | 12 658 (48.0) |
| Initial metabolic health components and obesity, mean (SD) | ||||
| Fasting serum glucose, mg/dL | 90.9 (22.7) | 92.4 (20.4) | 95.9 (29.6) | 97.0 (27.3) |
| Systolic blood pressure, mm Hg | 118.4 (13.9) | 122.0 (13.6) | 128.3 (16.2) | 130.9 (15.7) |
| Diastolic blood pressure, mm Hg | 74.8 (9.8) | 77.3 (9.7) | 80.9 (10.8) | 82.9 (10.6) |
| Total cholesterol, mg/dL | 189.5 (33.1) | 196.8 (32.8) | 206.2 (39.9) | 212.3 (38.9) |
| Body mass index, kg/m2 | 22.3 (2.0) | 26.5 (2.0) | 22.6 (2.1) | 26.8 (2.1) |
| Secondary metabolic health components and obesity, mean (SD) | ||||
| Fasting serum glucose, mg/dL | 89.9 (11.7) | 91.9 (12.2) | 99.6 (31.7) | 100.9 (30.3) |
| Systolic blood pressure, mm Hg | 116.3 (11.3) | 119.6 (10.5) | 134.7 (16.1) | 136.8 (15.3) |
| Diastolic blood pressure, mm Hg | 73.0 (8.0) | 75.1 (7.3) | 85.0 (10.6) | 86.8 (10.3) |
| Total cholesterol, mg/dL | 185.7 (27.5) | 191.9 (26.4) | 211.4 (42.2) | 217.0 (41.0) |
| Body mass index, kg/m2 | 22.1 (1.9) | 26.8 (1.6) | 22.5 (1.8) | 27.1 (1.8) |
| Age, y, mean (SD) | 52.2 (8.2) | 52.0 (7.5) | 54.7 (9.0) | 53.2 (8.1) |
| Sex, N (%) | ||||
| Men | 56 783 (46.8) | 20 773 (17.1) | 26 908 (22.2) | 16 822 (13.9) |
| Women | 48 141 (57.2) | 13 866 (16.5) | 14 248 (16.9) | 7853 (9.3) |
| Household income, quartiles, N (%) | ||||
| First (highest) | 40 519 (52.6) | 13 618 (17.7) | 13 909 (18.1) | 9014 (11.7) |
| Second | 29 110 (50.2) | 9936 (17.1) | 11 721 (20.2) | 7236 (12.5) |
| Third | 21 748 (51.1) | 6721 (15.8) | 9212 (21.6) | 4905 (11.5) |
| Fourth (lowest) | 13 548 (48.8) | 4364 (15.7) | 6314 (22.8) | 3520 (12.7) |
| Smoking, N (%) | ||||
| Never smoker | 71 962 (53.1) | 23 125 (17.1) | 25 213 (18.6) | 15 224 (11.2) |
| Former smoker | 8952 (44.8) | 3848 (19.3) | 4168 (20.9) | 3010 (15.1) |
| Current smoker | 24 010 (48.1) | 7666 (15.4) | 11 775 (23.6) | 6441 (12.9) |
| Alcohol consumption, times per week, N (%) | ||||
| 0 | 60 671 (54.4) | 18 638 (16.7) | 20 610 (18.5) | 11 667 (10.5) |
| 0–1 | 17 587 (51.4) | 6009 (17.6) | 6526 (19.1) | 4122 (12.0) |
| 1–2 | 17 215 (45.7) | 6687 (17.7) | 8213 (21.8) | 5587 (14.8) |
| 3–4 | 6029 (42.7) | 2318 (16.4) | 3522 (24.9) | 2254 (16.0) |
| ≥5 | 3422 (44.2) | 987 (12.8) | 2285 (29.5) | 1045 (13.5) |
| Physical activity, times per week, N (%) | ||||
| 0 | 53 346 (51.8) | 16 397 (15.9) | 21 491 (20.9) | 11 820 (11.5) |
| 1–2 | 29 320 (50.5) | 10 179 (17.5) | 11 346 (19.5) | 7269 (12.5) |
| 3–4 | 12 649 (50.9) | 4544 (18.3) | 4518 (18.2) | 3130 (12.6) |
| 5–6 | 3134 (51.3) | 1109 (18.2) | 1121 (18.4) | 743 (12.2) |
| 7 | 6475 (48.8) | 2410 (18.2) | 2680 (20.2) | 1713 (12.9) |
| Charlson comorbidity index, N (%) | ||||
| 0 | 54 470 (49.9) | 18 127 (16.6) | 22 576 (20.7) | 13 932 (12.8) |
| 1 | 32 676 (52.1) | 10 704 (17.1) | 12 230 (19.5) | 7119 (11.4) |
| ≥2 | 17 778 (53.0) | 5808 (17.3) | 6350 (18.9) | 3624 (10.8) |
MHNO indicates metabolically healthy nonobese; MHO, metabolically healthy obese; MUNO, metabolically unhealthy nonobese; MUO, metabolically unhealthy obese.
The association of change in metabolic health and weight on CVD is shown in Table 2. Among initial MHNO participants, those who became MHO (aHR 1.25, 95% CI 1.10–1.41), MUNO (aHR, 1.23; 95% CI, 1.15–1.31), and MUO (aHR, 1.34; 95% CI, 1.12–1.61) during the second screening period had elevated risk for CVD compared with those who remained MHNO. Similarly, participants who became MUNO (aHR, 1.41; 95% CI, 1.17–1.70) and MUO (aHR, 1.19; 95% CI, 1.08–1.33) had elevated CVD risk compared with those who remained MHO among initial MHO participants. Among initially MUNO participants, those whose metabolic health improved and became MHNO had reduced risk for CVD (aHR, 0.79; 95% CI, 0.73–0.84). Finally, among initially MUO participants, those who became MHNO (aHR, 0.68; 95% CI, 0.58–0.81) and MHO (aHR, 0.73; 95% CI, 0.66–0.80) had reduced risk for CVD compared with those who remained MUO.
Table 2.
Hazard Ratios for Cardiovascular Disease According to the Change in Metabolic Health and Body Mass Index
| Initial Metabolic Health and Obesity | Secondary Metabolic Health and Obesity | |||
|---|---|---|---|---|
| MHNO | MHO | MUNO | MUO | |
| Initial MHNO | ||||
| Events | 3450 | 270 | 1179 | 125 |
| Person‐years | 744 028 | 48 210 | 167 100 | 18 166 |
| aHR (95% CI) | 1.00 (reference) | 1.25 (1.10–1.41) | 1.23 (1.15–1.31) | 1.34 (1.12–1.61) |
| Initial MHO | ||||
| Events | 251 | 1001 | 126 | 536 |
| Person‐years | 51 654 | 187 494 | 14 444 | 75 862 |
| aHR (95% CI) | 0.90 (0.78–1.03) | 1.00 (reference) | 1.41 (1.17–1.70) | 1.19 (1.08–1.33) |
| Initial MUNO | ||||
| Events | 1443 | 130 | 1860 | 191 |
| Person‐years | 190 185 | 14 036 | 179 555 | 20 299 |
| aHR (95% CI) | 0.79 (0.73–0.84) | 1.04 (0.87–1.24) | 1.00 (reference) | 0.98 (0.84–1.14) |
| Initial MUO | ||||
| Events | 163 | 583 | 232 | 1180 |
| Person‐years | 22 502 | 84 027 | 23 773 | 118 858 |
| aHR (95% CI) | 0.68 (0.58–0.81) | 0.73 (0.66–0.80) | 0.89 (0.78–1.03) | 1.00 (reference) |
Hazard ratio calculated by Cox proportional hazards regression after adjustments for age, sex, household income, smoking, alcohol consumption, physical activity, and Charlson comorbidity index. aHR indicates adjusted hazard ratio; MHNO, metabolically healthy nonobese; MHO, metabolically healthy obese; MUNO, metabolically unhealthy nonobese; MUO, metabolically unhealthy obese.
Table 3 depicts the risk of CVD according to the change in number of metabolically unhealthy components among persistently normal weight (BMI, <23.0 kg/m2), overweight (BMI, 23.0–24.9 kg/m2), and obese (BMI, ≥25.0 kg/m2) participants. Among participants who were persistently normal weight and had no metabolically unhealthy components during the first screening period, those who had 1 or >2 metabolically unhealthy components during the second screening period had elevated risk for CVD (aHR, 1.18; 95% CI, 1.07–1.29; aHR, 1.34; 95% CI, 1.03–1.75, respectively). In contrast, among participants who initially had 1 metabolically unhealthy component with persistent normal weight, those whose metabolic health improved to having no metabolically unhealthy components had reduced risk for CVD (aHR, 0.80; 95% CI, 0.72–0.89). Similar associations were observed among participants who were persistently overweight and obese.
Table 3.
Hazard Ratios for Cardiovascular Disease According to Change in Metabolic Health Among Persistently Normal Weight, Overweight, and Obese Participants
| Secondary Number of Metabolically Unhealthy Components | P for Trend | |||
|---|---|---|---|---|
| 0 | 1 | ≥2 | ||
| Persistent BMI <23.0 kg/m2 a | ||||
| Initial 0, events | 2018 | 554 | 56 | |
| Person‐years | 446 952 | 81 386 | 6142 | |
| aHR (95% CI) | 1.00 (reference) | 1.18 (1.07–1.29) | 1.34 (1.03–1.75) | <0.001 |
| Initial 1, events | 701 | 664 | 102 | |
| Person‐years | 92 869 | 64 784 | 7851 | |
| aHR (95% CI) | 0.80 (0.72–0.89) | 1.00 (reference) | 1.18 (0.96–1.46) | <0.001 |
| Initial ≥2, events | 95 | 110 | 64 | |
| Person‐years | 7608 | 8276 | 3502 | |
| aHR (95% CI) | 0.71 (0.52–0.97) | 0.71 (0.52–0.97) | 1.00 (reference) | 0.058 |
| Persistent BMI 23.0 to 24.9 kg/m2 b | ||||
| Initial 0, events | 864 | 306 | 29 | |
| Person‐years | 175 293 | 44 582 | 4235 | |
| aHR (95% CI) | 1.00 (reference) | 1.20 (1.05–1.36) | 1.07 (0.74–1.55) | 0.022 |
| Initial 1, events | 338 | 380 | 65 | |
| Person‐years | 50 656 | 42 906 | 6849 | |
| aHR (95% CI) | 0.79 (0.69–0.92) | 1.00 (reference) | 1.00 (0.77–1.30) | 0.004 |
| Initial ≥2, events | 50 | 83 | 54 | |
| Person‐years | 4711 | 7182 | 3723 | |
| aHR (95% CI) | 0.73 (0.50–1.08) | 0.76 (0.54–1.07) | 1.00 (reference) | 0.114 |
| Persistent BMI ≥25.0 kg/m2 c | ||||
| Initial 0, events | 1001 | 454 | 82 | |
| Person‐years | 187 494 | 67 821 | 8042 | |
| aHR (95% CI) | 1.00 (reference) | 1.14 (1.02–1.27) | 1.67 (1.33–2.09) | <0.001 |
| Initial 1, events | 502 | 712 | 153 | |
| Person‐years | 74 662 | 79 220 | 14 722 | |
| aHR (95% CI) | 0.77 (0.69–0.87) | 1.00 (reference) | 1.15 (0.97–1.37) | <0.001 |
| Initial ≥2, events | 81 | 183 | 132 | |
| Person‐years | 9365 | 15 378 | 9538 | |
| aHR (95% CI) | 0.63 (0.48–0.83) | 0.83 (0.67–1.04) | 1.00 (reference) | 0.001 |
Hazard ratio calculated by Cox proportional hazards regression after adjustments for age, sex, household income, smoking, alcohol consumption, physical activity, and Charlson comorbidity index. aHR indicates adjusted hazard ratio; BMI, body mass index.
Participants who had BMI <23.0 kg/m2 during the first and second health screening periods.
Participants who had BMI 23.0 to 24.9 kg/m2 during the first and second health screening periods.
Participants who had BMI ≥25.0 kg/m2 during the first and second health screening periods.
Figure shows the hazard ratios for CVD according to secondary metabolic health and obesity status by metabolic component and obesity among initially MHNO participants. Compared with participants who maintained their blood pressure at normal levels, those with high blood pressure (>140/90 mm Hg) had increased risk for CVD (aHR, 1.35; 95% CI, 1.24–1.47). Similarly, participants who gained weight to obese levels among initially MHNO participants had elevated risk for CVD (aHR, 1.21; 95% CI, 1.09–1.34). There was a tendency toward increased risk for CVD upon higher total cholesterol levels among initially MHNO participants (P trend <0.001).
Figure 1.
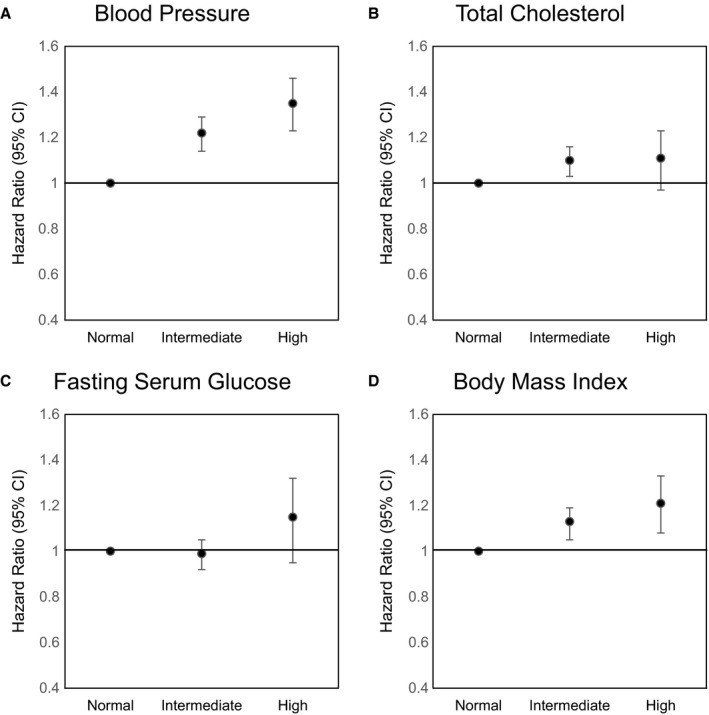
Cardiovascular disease risk according to secondary metabolic health and obesity status by metabolic component among initially metabolically healthy nonobese participants. Hazard ratio calculated by Cox proportional hazards regression after adjustments for age, sex, household income, smoking, alcohol consumption, physical activity, and Charlson comorbidity index. Normal: fasting serum glucose (FSG), <100.0 mg/dL; blood pressure (BP), <120/80 mm Hg; total cholesterol, <200.0 mg/dL; body mass index (BMI), <23.0 kg/m2. Intermediate: FSG, 100.0 to 125.9 mg/dL; BP, 120/80 to 139/89 mm Hg; total cholesterol, 200.0 to 239.9 mg/dL; BMI, 23.0 to 24.9 kg/m2. High: FSG, ≥126.0 mg/dL; BP, ≥140/90 mm Hg; total cholesterol, ≥240.0 mg/dL; BMI, ≥25.0 kg/m2. A, For blood pressure, secondary FSG, secondary total cholesterol, and secondary BMI were additionally adjusted. (B) For total cholesterol, secondary systolic blood pressure, secondary FSG, and secondary BMI were additionally adjusted. (C) For FSG, secondary systolic blood pressure, secondary total cholesterol, and secondary BMI were additionally adjusted. (D) For BMI, secondary systolic blood pressure, secondary total cholesterol, and secondary FSG were additionally adjusted.
Table S1 shows the descriptive characteristics in accordance to cases ending up with or without CVD. Survival curves are presented to display the survival rate at each point of follow‐up (Figure S1). Additional analyses showed that the association of change in metabolic health and weight on CVD is consistent with main result when obesity was defined as having a BMI of ≥30 kg/m2 (Table S2) and metabolically unhealthy participants as having abnormal laboratory results or were prescribed medications for diabetes mellitus, dyslipidemia, or hypertension (Table S3). Additional sensitivity analysis to reflect the long‐term exposure duration (Table S4) shows findings consistent with our main results. Stratified analyses according to subgroups of Charlson comorbidity index of 0 or ≥1 (Table S5) and sensitivity analysis after excluding those with liver disease, kidney disease, or cancer (Table S6) showed results consistent with the primary analysis. Table S7 indicates the effect of BMI change on CVD among persistently metabolically healthy and unhealthy participants. Participants who gained weight tended to have elevated risk for CVD and those with reduced weight have decreased risk, but the results were not statistically significant.
Discussion
In this nationally representative longitudinal study, we found that both changes from metabolically healthy or nonobese to unhealthy or obese were associated with elevated CVD risk. Likewise, improvements in metabolic health or weight were associated with reduced CVD risk. To our knowledge, this is the first study to investigate the association of concurrent changes in metabolic health and weight on CVD risk in a large population of men and women.
There has been a growing interest in whether the MHO phenotype has a protective effect on CVD risk or mortality. However, data from previous studies show that the MHO phenotype is not a benign condition and is associated higher risk of CVD. Recent meta‐analysis8, 10, 11 and cohort studies12, 14, 15 have evaluated the separate and combined associations of obesity and metabolic health with CVD. Previous literature identified that MUO individuals were at higher risk compared with MHNO but had lower risk compared with that of MUO participants. Furthermore, previous studies on the effects of change in metabolic syndrome components such as serum glucose,29 cholesterol,30 blood pressure,31 or weight32 and CVD risk have been performed extensively, and it is well known that the elevation of each component is associated with higher CVD risk. However, only a few studies have considered the change of metabolic health and weight status concurrently. One study by Eckel et al21 identified CVD risk according to transitions from metabolically healthy to unhealthy phenotypes according to BMI categories, but their study was limited to women and did not consider the change of BMI during the follow‐up period. Mongraw‐Chaffin et al20 investigated that the conversion of MHO to MUO but did not evaluate CVD risk with weight change or the conversion of unhealthy to healthy metabolic status.
The main difference between our analyses and previous studies is that we defined shifts in metabolic health status and weight over time. To assess the transition from metabolically unhealthy to healthy status, we constructed the final cohort with an exclusion of those who were diagnosed with hypertension, diabetes mellitus, or dyslipidemia before the index date. Therefore, this study could show the increased CVD risk with the change toward metabolic unhealthy and obese direction, as well as the protective association with the change toward healthy and nonobese status. Moreover, in an analysis to identify each change of metabolic health or weight on CVD risk, we performed subgroup analyses in which metabolic health or BMI remained persistently constant in both the first and second screening periods. Our results support those of the previous studies,14, 20, 33 which showed that MHO became substantially cardiometabolically unhealthy over time and those who had metabolic abnormalities or obese showed higher risk compared with metabolically healthy and nonobese individuals.
Our analysis additionally demonstrated the reduced CVD risk, with that as the number of metabolic abnormalities decreased or shift toward improvements in metabolic health or weight. We explored the longitudinal effect of each risk factor, including weight change among MHNO participants, and concluded that having glycemic dysfunction, high blood pressure, dyslipidemia, or weight gain are each likely to independently influence CVD risk. In our analysis, blood pressure appeared to be the most important contributor (aHR, 1.35; 95% CI, 1.24–1.47). This is consistent with previous large‐scale pooled analysis,1 which demonstrated the effect of a metabolic mediator on coronary heart disease. Blood pressure was the most important mediator, followed by BMI. Although glucose and cholesterol also increased the risk, the effect was not greater than blood pressure or BMI.
The prevalence of obesity was 28.9%, and the metabolically unhealthy was 32.1% during the second health examination period in our analysis. Similarly, the prevalence of metabolic syndrome was reported to be 30.52% in 2013 from the NHIS database in Korea.34 It has been reported to be prevalent in one fourth to one third of adults in the United States and 34.7% from the National Health and Nutrition Examination Survey from 2011 to 2012.35 Thus, our study participants tended to be healthier compared with the Western population. Recently, the prevalence of metabolic syndrome is continuously increasing with the increase in the proportion of obese individuals. To accomplish primary prevention against CVD, maintenance of both metabolic health and proper weight is important even among relatively healthy individuals. Meanwhile, Asian people have on average higher visceral fat at the same level of BMI as other groups, with a high risk of type 2 diabetes mellitus or metabolic syndrome, so the World Health Organization consultation group recommends a cutoff point for obesity lower than that for the Western population.26 Indeed, morbidity of CVD has been rapidly increasing in Korea as the prevalence of obesity, hypertension, and dyslipidemia are increasing as well. In fact, the prevalence of CVD in adults aged 20 or over was 36.6% in 2011–2014 in the United States.36 In addition, 11% of adults were diagnosed with heart disease in 2017 on the basis of National Health and Nutrition Examination Survey data.36 In our study, the overall incidence rate of CVD was 6.2%, and it seemed to be less compared with the Western population. However, further study is needed to support the CVD risk in a similar obese or metabolic health group with different ethnic populations.
Our results suggest that a public health strategy regarding maintenance of both metabolic health and proper weight is crucial to prevent CVD. Clinical and public health interventions that control metabolic abnormalities and obesity could be provided to all adults. For example, national biennial health screening examinations are furnished for all citizens over 40 years old in Korea. When an individual is suspected to have impaired fasting glucose, high blood pressure, or dyslipidemia through the screening, he or she receives an advisory opinion via a letter from a doctor regarding a suggestion for maintaining a healthy lifestyle and a recommendation for follow‐up within 3 to 12 months. Patients with metabolic disturbance are encouraged to modify lifestyle behaviors such as regular exercise, smoking cessation, or alcohol abstinence with or without the start of medications. According to this policy, early detection and medical consultation could be used to reduce metabolic abnormalities and obesity and ultimately prevent CVD risk.
Our study has some limitations. First, it is necessary to use a universally accepted, international definition of metabolic syndrome such as National Cholesterol Education Program's Adult Treatment Panel III, International Diabetes Federation, or World Health Organization criteria to compare our results directly with those of other studies. However, in establishing metabolic health, only total cholesterol level was available in the NHIS‐HEALS database, and we thus used this as a substitute for triglyceride and high‐density lipoprotein cholesterol levels to evaluate dyslipidemia. In addition, we used BMI to define obesity. While waist circumference might more accurately depict an individual's visceral obesity,36 waist circumference values were not available from the database. Thus, future studies are needed to adopt other measures reflecting delicate metabolic health status using triglyceride or high‐density lipoprotein cholesterol and waist circumference or waist‐hip ratio. Second, we were unable to examine the exact reason for the weight change. There is a possibility that those who decided to reduce their weight had already experienced worsening health conditions or secondary change after suffering malignancy or gastrointestinal surgery, which could affect the weight. To overcome this limitation, we did additional subgroup analysis in groups with an initial Charlson comorbidity index score of 0 or >1 (Table S5). A similar association was observed in both groups. Moreover, liver and kidney diseases or cancers are known to cause unintentional weight loss, but also lead to higher risk for CVD risk.37 Therefore, we conducted sensitivity analysis among participants after excluding those with comorbidities that can lead to weight loss (Table S6), the results of which were consistent with the main results. Third, we adopted the final study population after excluding those who did not participate in both the first and second health screenings, had missing covariates values, or had been diagnosed or died before the index date. To overcome the short‐term exposure duration, we conducted an additional analysis, taking into account the 6‐year exposure period, which showed a consistent association with the main results. However, our study design could result in selection bias, and future studies are needed to further validate our findings. Fourth, the study participants from NHIS‐HEALS were limited to those aged ≥40 years. Even though NHIS is single insurer with an enrollment rate of 97%, the participation rate of national health screenings during our exposure period was 43.9%, 48%, 51.3%, and 51.6% in 2002, 2003, 2004, and 2005, respectively.38 Moreover, it was reported that up to 80% of participants were employed or householders. Data from unemployed or nonhouseholder participants were less included, which could have resulted in a high proportion of healthy participants. Finally, the number of improvements from obesity to nonobesity or metabolically unhealthy to metabolically healthy was small, which might in part account for the lack of significance. However, the protective tendency was observed in groups with improvements from MUNO to MHNO, MUO to MHNO, or MUO to MHO. Nonetheless, future studies are needed to explore improvements with a sufficient sample size for those who had improved metabolic health.
Despite these limitations, this study also has a number of strengths. To our knowledge, this is the first study to consider the effect of concurrent changes in metabolic health and BMI on CVD among a large Asian population. Moreover, while most of the previous studies regarding MHO were investigated among Western populations,10 we conducted a prospective cohort study in an Asian population reflecting the criteria of obesity for Asians. BMI in the NHIS database was measured by trained health professionals, which could be more accurate records than a self‐reported questionnaire survey to evaluate weight change. Furthermore, all records of medical diagnosis and death registry were available for each individual from the database, making the outcomes highly reliable. In addition, we included men and adjusted for various risk factors including sociodemographics, comorbidities, and health behavior such as smoking, alcohol consumption, and physical activity, thus enhancing the generalizability of our results.
In conclusion, changes toward metabolically unhealthy or obese status were significantly associated with increased risk of CVD. Improvements in metabolic health or weight were associated with reduced CVD risk. Metabolically unhealthy or obese patients who improve metabolic health or lose weight may benefit from reduced CVD risk.
Author Contributions
Dr Park had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs Bae, Choi, and Park; acquisition of data: Drs Bae and Choi; analysis and interpretation of data: Drs Bae, Choi, Park, Chang, K. Kim, and S. M. Kim; drafting of the manuscript: Drs Bae, Choi, and Park; critical revision of the manuscript: Drs Bae, Choi, Park, K. Lee, Son, H. Lee, M. H. Cho, Koo, and I. Y. Cho; statistical analysis: Dr Choi; administrative, technical, or material support: Dr Bae.
Sources of Funding
Dr Choi received a grant from the BK21‐plus education program from the National Research Foundation of Korea. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (grant number 2017R1D1A1B03033721) and the Seoul National University Hospital research fund (grant number 04‐2018‐0370). The Ministry of Education of Korea had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting information
Table S1. Descriptive Characteristics of the Study Population According to Development of Cardiovascular Disease
Table S2. Hazard Ratios for Cardiovascular Disease According to the Change in Metabolic Health and Body Mass Index, With Obesity Defined as Body Mass Index of ≥30.0 kg/m2
Table S3. Hazard Ratios for Cardiovascular Disease According to the Change in Metabolic Health and Body Mass Index, With Metabolic Health Defined Using Clinical Parameters and Medication Use
Table S4. Hazard Ratios for Cardiovascular Disease According to the Change in Metabolic Health and Body Mass Index With Secondary Metabolic Health and Obesity Measured During 2008–2009
Table S5. Stratified Analysis on the Association of Changes in Metabolic Health and Body Mass Index on Cardiovascular Disease According to Subgroups of Comorbidities
Table S6. Sensitivity Analysis on the Association of Changes in Metabolic Health and Body Mass Index on Cardiovascular Disease After Excluding Participants With Liver Disease, Kidney Disease, or Cancer
Table S7. Hazard Ratios for Cardiovascular Disease According to Change in Body Mass Index Among Persistently Metabolically Healthy and Unhealthy Participants
Figure S1. Survival curves for cardiovascular disease for secondary metabolic health and obesity groups according to initial metabolic health and obesity status.
(J Am Heart Assoc. 2019;8:e011825 DOI: 10.1161/JAHA.118.011825.)
References
- 1. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body‐mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie‐Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168:1617–1624. [DOI] [PubMed] [Google Scholar]
- 3. Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring H‐U. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. [DOI] [PubMed] [Google Scholar]
- 4. Blüher M. The distinction of metabolically “healthy” from “unhealthy” obese individuals. Curr Opin Lipidol. 2010;21:38–43. [DOI] [PubMed] [Google Scholar]
- 5. Plourde G, Karelis AD. Current issues in the identification and treatment of metabolically healthy but obese individuals. Nutr Metab Cardiovasc Dis. 2014;24:455–459. [DOI] [PubMed] [Google Scholar]
- 6. Rey‐López JP, de Rezende LF, de Sá TH, Stamatakis E. Is the metabolically healthy obesity phenotype an irrelevant artifact for public health? Am J Epidemiol. 2015;182:737–741. [DOI] [PubMed] [Google Scholar]
- 7. Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219–227. [DOI] [PubMed] [Google Scholar]
- 8. Eckel N, Meidtner K, Kalle‐Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2016;23:956–966. [DOI] [PubMed] [Google Scholar]
- 9. Ärnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle‐aged men. Circulation. 2010;121:230–236. [DOI] [PubMed] [Google Scholar]
- 10. Zheng R, Zhou D, Zhu Y. The long‐term prognosis of cardiovascular disease and all‐cause mortality for metabolically healthy obesity: a systematic review and meta‐analysis. J Epidemiol Community Health. 2016;70:1024–1031. DOI: 10.1136/jech-2015-206948. [DOI] [PubMed] [Google Scholar]
- 11. Fan J, Song Y, Chen Y, Hui R, Zhang W. Combined effect of obesity and cardio‐metabolic abnormality on the risk of cardiovascular disease: a meta‐analysis of prospective cohort studies. Int J Cardiol. 2013;168:4761–4768. [DOI] [PubMed] [Google Scholar]
- 12. Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der A DL, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado‐Yohar SM, Engstrom G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina‐Portillo E, Moreno‐Iribas C, Norberg M, Overvad K, Panico S, Quiros JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjonneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan‐European case‐cohort analysis. Eur Heart J. 2018;39:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lissner L, Odell PM, D'Agostino RB, Stokes J III, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–1844. [DOI] [PubMed] [Google Scholar]
- 14. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70:1429–1437. [DOI] [PubMed] [Google Scholar]
- 15. De Ycaza AE, Donegan D, Jensen MD. Long‐term metabolic risk for the metabolically healthy overweight/obese phenotype. Int J Obes (Lond). 2018;42:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei M, Gibbons LW, Mitchell TL, Kampert JB, Stern MP, Blair SN. Low fasting plasma glucose level as a predictor of cardiovascular disease and all‐cause mortality. Circulation. 2000;101:2047–2052. [DOI] [PubMed] [Google Scholar]
- 17. Barr E, Boyko E, Zimmet P, Wolfe R, Tonkin A, Shaw J. Continuous relationships between non‐diabetic hyperglycaemia and both cardiovascular disease and all‐cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia. 2009;52:415–424. [DOI] [PubMed] [Google Scholar]
- 18. Whittle J. Blood pressure variability and cardiovascular risk. BMJ. 2016;354:i4190. [DOI] [PubMed] [Google Scholar]
- 19. Dowse GK, Gareeboo H, Alberti KGM, Zimmet P, Tuomilehto J, Purran A, Fareed D, Chitsono P, Collins VR, Hemraj F. Changes in population cholesterol concentrations and other cardiovascular risk factor levels after five years of the non‐communicable disease intervention programme in Mauritius. BMJ. 1995;311:1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mongraw‐Chaffin M, Foster MC, Anderson CA, Burke GL, Haq N, Kalyani RR, Ouyang P, Sibley CT, Tracy R, Woodward M. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71:1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow‐up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714–724. [DOI] [PubMed] [Google Scholar]
- 22. Park B, Sung J, Park K, Seo S, Kim S. Report of the Evaluation for Validity of Discharged Diagnoses in Korean Health Insurance Database. Seoul: Seoul National University; 2003:19–52. [Google Scholar]
- 23. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, Lee EJ, Ae Shin S. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, Kang HJ, Do CH, Song JS, Lee EJ, Ha S, Shin SA, Jeong SL. Cohort profile: the National Health Insurance Service‐National Health Screening Cohort (NHIS‐HEALS) in Korea. BMJ Open. 2017;7:e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee G, Choi S, Kim K, Yun JM, Son JS, Jeong SM, Kim SM, Park SM. Association of hemoglobin concentration and its change with cardiovascular and all‐cause mortality. J Am Heart Assoc. 2018;7:e007723 DOI: 10.1161/JAHA.117.007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Who EC. Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157. [DOI] [PubMed] [Google Scholar]
- 27. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J‐P, Fullerton HJ. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. DOI: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 28. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 29. Lee G, Kim SM, Choi S, Kim K, Jeong S‐M, Son JS, Yun J‐M, Park SM. The effect of change in fasting glucose on the risk of myocardial infarction, stroke, and all‐cause mortality: a nationwide cohort study. Cardiovasc Diabetol. 2018;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeong S‐M, Choi S, Kim K, Kim S‐M, Lee G, Son JS, Yun J‐M, Park SM. Association of change in total cholesterol level with mortality: a population‐based study. PLoS One. 2018;13:e0196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol. 2000;85:251–255. [DOI] [PubMed] [Google Scholar]
- 32. Choi S, Kim K, Kim SM, Lee G, Jeong SM, Park SY, Kim YY, Son JS, Yun JM, Park SM. Association of obesity or weight change with coronary heart disease among young adults in South Korea. JAMA Intern Med. 2018;178:1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson W, Bell JA, Robson E, Norris T, Kivimaki M, Hamer M. Do worse baseline risk factors explain the association of healthy obesity with increased mortality risk? Whitehall II Study. Int J Obes (Lond). 2019;43:1578‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee SE, Han K, Kang YM, Kim S‐O, Cho YK, Ko KS, Park J‐Y, Lee K‐U; Koh EHJPo . Trends in the prevalence of metabolic syndrome and its components in South Korea: findings from the Korean National Health Insurance Service Database (2009–2013). PLoS One. 2018;13:e0194490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJJJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. [DOI] [PubMed] [Google Scholar]
- 36. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 37. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Service NHI . Statistical yearbooks of health examinations. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Descriptive Characteristics of the Study Population According to Development of Cardiovascular Disease
Table S2. Hazard Ratios for Cardiovascular Disease According to the Change in Metabolic Health and Body Mass Index, With Obesity Defined as Body Mass Index of ≥30.0 kg/m2
Table S3. Hazard Ratios for Cardiovascular Disease According to the Change in Metabolic Health and Body Mass Index, With Metabolic Health Defined Using Clinical Parameters and Medication Use
Table S4. Hazard Ratios for Cardiovascular Disease According to the Change in Metabolic Health and Body Mass Index With Secondary Metabolic Health and Obesity Measured During 2008–2009
Table S5. Stratified Analysis on the Association of Changes in Metabolic Health and Body Mass Index on Cardiovascular Disease According to Subgroups of Comorbidities
Table S6. Sensitivity Analysis on the Association of Changes in Metabolic Health and Body Mass Index on Cardiovascular Disease After Excluding Participants With Liver Disease, Kidney Disease, or Cancer
Table S7. Hazard Ratios for Cardiovascular Disease According to Change in Body Mass Index Among Persistently Metabolically Healthy and Unhealthy Participants
Figure S1. Survival curves for cardiovascular disease for secondary metabolic health and obesity groups according to initial metabolic health and obesity status.


