Abstract
Background
Adults with a systemic right ventricle (sRV) have a high risk of cardiac complications. This study aimed to identify prognostic markers in adults with sRV based on clinical evaluation, echocardiography, and blood biomarkers.
Methods and Results
In this prospective cohort study, consecutive clinically stable adults with sRV caused by Mustard‐ or congenitally corrected transposition of the great arteries were included (2011–2013). Eighty‐six patients were included (age 37±9 years, 65% male, 83% New York Heart Association functional class I, 76% Mustard transposition of the great arteries, 24% congenitally corrected transposition of the great arteries). Venous blood sampling was performed including N‐terminal pro B‐type natriuretic peptide, high‐sensitive‐troponin‐T, high‐sensitivity C‐reactive protein, growth differentiation factor‐15, galectin‐3, red cell distribution width, estimated glomerular filtration rate, and hemoglobin. Besides conventional echocardiographic measurements, longitudinal, circumferential, and radial strain were assessed using strain analysis. During a median follow‐up of 5.9 (interquartile range 5.3–6.3) years, 19 (22%) patients died or had heart failure (primary end point) and 29 (34%) patients died or had arrhythmia (secondary end point). Univariable Cox regression analysis was performed using dichotomous or standardized continuous variables. New York Heart Association functional class >I, systolic blood pressure, and most blood biomarkers were associated with the primary and secondary end point (galectin‐3 not for primary, N‐terminal pro B‐type natriuretic peptide and high‐sensitivity C‐reactive protein not for secondary end point). Growth differentiation factor‐15 showed the strongest association with both end points (hazard ratios; 2.44 [95% CI 1.67–3.57, P<0.001], 2.00 [95% CI 1.46–2.73, P<0.001], respectively). End‐diastolic basal dimension of the subpulmonary ventricle was associated with both end points (hazard ratio: 1.95 [95% CI 1.34–2.85], P<0.001, 1.70 [95% CI 1.21–2.38, P=0.002], respectively). Concerning strain analysis, only sRV septal strain was associated with the secondary end point (hazard ratio 0.58 [95% CI 0.39–0.86], P=0.006).
Conclusions
Clinical, conventional echocardiographic, and blood measurements are important markers for risk stratification in adults with a sRV. The value of novel echocardiographic strain analysis seems limited.
Keywords: adult congenital heart disease, biomarker, risk stratification, speckle tracking echocardiography
Subject Categories: Congenital Heart Disease, Biomarkers, Echocardiography, Risk Factors
Clinical Perspective
What Is New?
In adults with a systemic right ventricle, blood biomarkers carry significant prognostic value, of which growth differentiation factor‐15 is the strongest prognosticator of all blood biomarkers, and not the conventional biomarker N‐terminal pro‐brain natriuretic peptide.
Echocardiographic strain analysis is of limited prognostic value in adults with a systemic right ventricle, while subpulmonary end‐diastolic dimension and tricuspid regurgitation are predictive of outcomes.
What Are the Clinical Implications?
Given the complexity of the failing systemic right ventricle, risk stratification should consist of clinical variables, blood‐ and echocardiographic markers, and implementation of blood biomarkers should be especially encouraged.
Introduction
Transposition of the great arteries (TGA) is a complex congenital heart defect, with an incidence of 0.31 per 1000 live births per year.1 Before the arterial switch operation was introduced, TGA patients were operated on according to the Mustard or Senning procedure (M‐TGA). During this procedure, a baffle is created at the levels of the atria (atrial switch operation) resulting in a right ventricle supporting the systemic circulation (sRV).2 Congenitally corrected TGA (ccTGA) is the name given to a morphologic situation where there is both atrioventricular and ventriculoarterial discordance, also resulting in a sRV.3
Since the right ventricle is designed for volume load and not pressure overload,4 the right ventricle will degenerate earlier, leading to an impaired ventricular function over time. Dysfunction of the sRV may subsequently lead to cardiac complications such as heart failure (HF), arrhythmias, and death.5, 6, 7, 8 Currently, it is not clear which patients are at higher risk of developing complications, nor is it clear how we can best monitor them over time. Identification of high‐risk patients is important to enable clinicians to intervene at the right time and also to establish a better expectation of management for patients. However, at the moment prospective longitudinal studies investigating prognostic factors in patients with a sRV are scarce. Studies have investigated prognosis in adult congenital heart disease,6 but rarely in a prospective manner pertaining exclusively to sRV patients.9
Therefore, this study aimed to identify the prognostic value of clinical variables, echocardiographic‐ and blood biomarkers in adult patients with a sRV.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Design
Patients with M‐TGA and ccTGA were extracted from a prospective cohort of consecutive, clinically stable adults with congenital heart disease who were included during a routine visit to the outpatient clinic of the Erasmus Medical Center, Rotterdam between April 2011 and April 2013. Exclusion criteria were the following: age <18 years, pregnancy, renal dysfunction (creatinine level >200 μmol/L), or patients not capable of understanding and signing informed consent. In our center, the Senning procedure in patients with TGA had not been performed; therefore, only M‐TGA patients were included in this study.
All patients underwent clinical examination by a cardiologist, 12‐lead electrocardiography, echocardiography, and venous blood sampling. The study protocol has been described in more detail previously.10 This study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committee. Written informed consent was obtained from all participants.
Echocardiography and Speckle‐Tracking Analysis
Two‐dimensional grayscale harmonic images were obtained with patients in the left lateral decubitus position using a commercially available iE33 or Epic C7 ultrasound system (Philips Medical Systems, Best, The Netherlands) equipped with a transthoracic broadband S5‐1 (1–5 MHz) or X5‐1 matrix transducer (composed of 3040 elements, with 1 to 5 MHz extended operating frequency range).
Echocardiographic assessment was performed according to the European Society of Cardiology (ESC) and American Society of Echocardiography (ASE) guidelines for echocardiographic chamber quantification.11, 12
Speckle tracking analysis was performed (RWG) using TomTec (TomTec 2D CPA using dedicated RV software; TomTec Imaging Systems, Unterschleissheim, Germany) following the current consensus document on strain analysis.13 Longitudinal strain in the apical 4‐ chamber view was assessed for all patients, and circumferential and radial strain were only assessed in the short‐axis view in M‐TGA patients as the appropriate view required for analysis is unobtainable in the ccTGA patients because of the orientation of the sRV in the thorax. Similarly, subpulmonary left ventricular diameters were not measured in the ccTGA group.
Laboratory Results
Peripheral venous blood sampling was performed at the day of study inclusion and was for research purposes only. Blood samples were then transferred to the clinical chemistry laboratory of our center within 2 hours after withdrawal. N‐terminal pro B‐type natriuretic peptide (NT‐proBNP), red cell distribution width (RDW), and estimated glomerular filtration rate were directly determined in fresh blood samples, whereas the rest of the samples were aliquoted and stored at −80°C. Serum levels of growth differentiation factor‐15 (GDF‐15), high‐sensitive C‐reactive protein, galectin‐3, and high‐sensitive troponin T (hs‐TnT) were determined in batches. Samples were exposed to only 1 freeze–thaw cycle. The laboratory analyses of all biomarkers have been described in more detail previously.10, 14, 15, 16, 17
Definition of Events
Given the relatively low mortality rate in this population, we chose to take combined end points. The primary end point was composed of all‐cause mortality or HF. HF was defined as signs and symptoms of HF requiring hospital admission, or requiring initiation or change in HF medication. The secondary end point was a composite of all‐cause mortality or arrhythmia. We defined arrhythmia as any symptomatic and registered arrhythmia, or arrhythmias requiring treatment. Presence of premature ventricular complexes or premature atrial complexes were not regarded as arrhythmias. HF and arrhythmias were treated as separate end points because of potential event‐specific predictors because of a probable different mechanism of clinical worsening. Follow‐up was assured by yearly scheduled visits to the adult congenital heart disease outpatient clinic during the first 4 years of follow‐up, and end points were systematically tracked by 2 investigators (LG and JR) until January 1, 2018. Survival status was checked in the Municipal Population Register.
Statistical Analysis
Continuous variables with a normal distribution were presented as mean± SD, otherwise the median and interquartile range was presented. For blood biomarkers, the geometric mean was given in addition to the median. Patients were stratified according to diagnosis (M‐TGA or ccTGA). Continuous variables between these groups were compared using the unpaired t test or Mann–Whitney U test as appropriate. For frequencies, Fisher exact test was performed.
Missing data were addressed by multiple imputation before any further statistical analyses were performed. Multiple imputation was performed in SPSS using 5 imputations and based on all relevant patient characteristics, including the end points. End points were not imputed. Biomarker levels were log‐transformed to correct for a skewed distribution before further analysis.
Correlations between blood biomarkers and echocardiographic measurements were reflected by Spearman correlation coefficient. The Kaplan–Meier estimator was used to obtain survival curves stratified according to the diagnosis groups. Survival functions were compared using the log‐rank test.
Cox regression analysis was performed based on the entire study population. All continuous echocardiographic and blood biomarkers variables were transformed into z scores to obtain standardized hazard ratios for the associations with the end points. Tricuspid regurgitation was treated as continuous variable (0–3), but not standardized. Univariable Cox regression was performed to assess associations between variables and end points. Additionally, all univariable associations were alternately adjusted for New York Heart Association (NYHA) functional class (NYHA I versus NYHA II/III) in multivariable analyses. Comparisons of strength of associations were made on the basis of standardized hazard ratios. As post hoc analysis, a subgroup analysis was performed that was restricted to M‐TGA patients only.
A 2‐sided P value of <0.05 was considered statistically significant. Statistical tests were performed using SPSS Statistics, version 24.0.
Results
Baseline Characteristics
In this prospective cohort study, 86 patients were included: 65 patients with M‐TGA and 21 patients with ccTGA. The patient selection process is shown in Figure S1. Overall, the mean age was 37±9 years, 56 (65%) were men, and most patients were in NYHA class I (83%) (Table 1). Anticoagulants were more often prescribed in ccTGA patients than in M‐TGA patients. M‐TGA patients were more often in sinus rhythm than ccTGA patients and had an implantable cardioverter defibrillator less often. In patients with ccTGA, significantly higher levels of RDW and hs‐TnT were found, compared with M‐TGA patients. The sRV end‐diastolic annulus was 50.4±5.9 mm in the M‐TGA group, which was larger compared with the ccTGA group: 34.7±4.6 mm. Sixty patients (70%) had at least a moderately impaired sRV. Speckle tracking analysis showed that the mean longitudinal strain of the sRV free wall was −15.5±3.9% and the mean sRV circumferential strain was −12.0±3.8%.
Table 1.
Baseline Characteristics for All Patients With a sRV and Stratified According to Mustard‐TGA and ccTGA Patients
| Complete, n (%) | All Patients (n=86) | Mustard‐TGA (n=65) | ccTGA (n=21) | P Value | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, y | 86 (100) | 37±9 | 35±6 | 41±14 | 0.06 |
| Sex, man, n (%) | 86 (100) | 56 (65) | 43 (66) | 13 (62) | 0.79 |
| Age at initial repair, y | 64 (98) | ··· | 0.7 [0.4–2.2] | ··· | ··· |
| Concomitant heart defect, n (%) | 86 (100) | ||||
| Ventricular septal defect | 27 (31) | 21 (32) | 6 (29) | 1.00 | |
| Pulmonary outflow tract obstruction | 13 | 7 (11) | 5 (24) | 0.16 | |
| NHYA class ≥ II, n (%)a | 86 (100) | 15 (17) | 12 (19) | 3 (14) | 1.00 |
| Body mass index, kg/m2 | 84 (98) | 24.8±4.0 | 25.0±4.3 | 24.2±2.8 | 0.41 |
| Body surface area, m2 | 84 (98) | 1.91±0.20 | 1.92±0.19 | 1.88±0.21 | 0.47 |
| Systolic blood pressure, mm Hg | 83 (97) | 125±14 | 125±15 | 124±13 | 0.87 |
| Diastolic blood pressure, mm Hg | 83 (97) | 79±12 | 79±12 | 78±10 | 0.64 |
| Heart rate, beats/min | 84 (98) | 72±13 | 72±13 | 71±11 | 0.86 |
| Oxygen saturation >96%, n (%) | 78 (91) | 63 (81) | 44 (76) | 19 (95) | 0.10 |
| Cardiac medication use, n (%) | 86 (100) | ||||
| ACE‐inhibitor | 26 (30) | 17 (26) | 9 (43) | 0.18 | |
| ARBs | 5 (6) | 2 (10) | 3 (5) | 0.59 | |
| β‐blocker | 19 (22) | 11 (17) | 8 (38) | 0.07 | |
| Diuretics | 17 (20) | 11 (17) | 6 (29) | 0.34 | |
| Antiarrhythmic | 13 (15) | 10 (15) | 3 (14) | 0.90 | |
| Anticoagulants | 20 (23) | 11 (17) | 9 (43) | 0.034 | |
| Electrocardiography | |||||
| QRS duration, ms | 69 (80) | 114 [105–130] | 114 [105–127] | 113 [105–137] | 0.57 |
| Rhythm, n (%) | 86 (100) | 0.006b | |||
| Sinus rhythm | 59 (68) | 50 (77) | 9 (43) | ||
| Pacemaker rhythm | 17 (20) | 8 (12) | 9 (43) | ||
| Atrial fibrillation | 4 (5) | 2 (3) | 2 (9) | ||
| Other | 6 (7) | 5 (8) | 1 (5) | ||
| Device implantation, n (%) | 86 (100) | ||||
| Pacemaker | 17 (20) | 13 (20) | 4 (19) | 1.00 | |
| ICD | 9 (10) | 4 (6) | 5 (24) | 0.036 | |
| Echocardiography | |||||
| sRV dimensions | |||||
| End‐diastolic basal dimension, mm | 57 (66) | 59.6±8.4 | 58.9±8.2 | 61.1±9.0 | 0.35 |
| End‐diastolic annulus, mm | 67 (78) | 47.1±8.6 | 50.4±5.9 | 34.7±4.6 | <0.001 |
| End‐systolic area, cm² | 81 (95) | 30.7±7.9 | 30.6±7.5 | 30.8±9.5 | 0.93 |
| End‐diastolic area, cm² | 81 (95) | 41.0±9.2 | 40.4±8.7 | 42.9±10.7 | 0.30 |
| sRV systolic function | |||||
| ≥ Moderately impaired, n (%) | 86 (100) | 60 (70) | 47 (72) | 13 (62) | 0.42 |
| TAPSE, mm | 45 (52) | 13.0±3.0 | 12.9±3.1 | 13.6±2.3 | 0.64 |
| RV fractional area change, n (%) | 81 (95) | 25.4±8.2 | 24.3±7.5 | 28.9±9.5 | 0.03 |
| Tricuspid regurgitation, n (%) | 86 (100) | 0.038 | |||
| None | 12 (14) | 6 (9) | 6 (29) | ||
| Mild | 46 (53) | 40 (61) | 6 (29) | ||
| Moderate | 24 (28) | 16 (25) | 8 (38) | ||
| Severe | 4 (5) | 3 (5) | 1 (4) | ||
| Strain parameters | |||||
| LS of the RV free wall, % | 77 (90) | −15.5±3.9 | −15.0±3.2 | −16.7±5.5 | 0.21 |
| LS of the RV septal wall, % | 77 (90) | −12.4±3.3 | −12.4±3.3 | −12.3±3.4 | 0.95 |
| Global LS of sRV, % | 77 (90) | −13.8±3.4 | −13.6±3.1 | −14.2±4.1 | 0.50 |
| Global CS of sRV, % | 52 (80) | ··· | −12.0±3.8 | ··· | ··· |
| Global RS of sRV, % | 49 (75) | ··· | −18.9±13.5 | ··· | ··· |
| Subpulmonary LV dimensions | |||||
| LV end‐diastolic diameter, mm/m² | 51 (79) | ··· | 22±4 | ··· | ··· |
| LV end‐systolic diameter, mm/m² | 49 (75) | ··· | 15±4 | ··· | ··· |
| Subpulmonary LV function | |||||
| ≥ Moderately impaired, n (%) | 86 (100) | 2 (2) | 2 (3) | 0 | ··· |
| Laboratoryc | |||||
| Hemoglobin, mmol Fe/L | 81 (94) |
9.5 [9.2–10.0] 9.4 |
9.5 [9.2–10.0] 9.4 |
9.3 [9.0–10.0] 9.4 |
0.96 |
| RDW, % | 81 (94) |
13.1 [12.6–13.7] 13.2 |
13.0 [12.6–13.4] 13.1 |
13.5 [13.2–14.1] 13.5 |
0.027 |
| eGFR, mL/min per 1.73 m2 | 84 (98) |
90 [81–90] 85 |
90 [83–90] 85 |
89 [77–90] 83 |
0.16 |
| NT‐proBNP, pmol/L | 85 (99) |
30.9 [17.7–58.2] 34.5 |
27.4 [18.2–53.2] 32.7 |
44.3 [16.4–76.5] 41.1 |
0.36 |
| Hs‐troponin T, ng/L | 85 (99) |
6.0 [1.5–9.5] 2.4 |
5.0 [1.5–8.4] 4.6 |
8.9 [6.0–15.4] 9.1 |
0.004 |
| GDF‐15, ng/L | 84 (98) |
623 [501–886] 727 |
615 [498–862] 698 |
660 [519–1379] 830 |
0.29 |
| Hs‐CRP, mg/L | 85 (99) |
1.8 [0.8–3.5] 1.6 |
1.9 [0.7–3.6] 1.6 |
1.2 [0.8–2.7] 1.4 |
0.50 |
| Galectin‐3, ng/mL | 85 (99) |
12.7 [11.2–15.0] 12.7 |
12.7 [11.4–15.0] 12.7 |
12.8 [11.0–13.9] 13.0 |
0.99 |
ACE indicates angiotensin‐converting enzyme; ARBs, angiotensin II receptor blocker; CC‐TGA, congenitally corrected transposition of the great arteries; CRP, C‐reactive protein; CS, circumferential strain; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor‐15; Hs‐CRP, high‐sensitive C‐reactive protein; ICD, implantable cardioverter defibrillator; LS, longitudinal strain; LV, left ventricular; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; RDW, red cell distribution width; RS, radial strain; sRV, systemic right ventricle; TAPSE, transannular plane systolic excursion.
All patients were NYHA II except 1 patient who was NYHA class III.
Compares sinus rhythm vs all other rhythms.
For the laboratory measurements, the geometric mean is shown on the second line in addition to the median and interquartile range.
Correlations Between Echocardiographic Measurements and Blood Biomarkers
Both higher levels of NT‐proBNP and hs‐TnT were weakly correlated with a larger subpulmonary end diastolic dimension. Weak correlations were also found between higher levels of galectin‐3 and lower sRV global longitudinal strain and lower sRV longitudinal strain of the septal wall (Table 2).
Table 2.
Correlations Between Blood Biomarker and Echocardiographic (Strain) Measurements and Mutual Biomarker Correlations
| HB | RDW | eGFR | NT‐proBNP | Hs‐TnT | GDF‐15 | Hs‐CRP | Galectin‐3 | |
|---|---|---|---|---|---|---|---|---|
| r | r | r | r | r | r | r | r | |
| Echocardiography | ||||||||
| Tricuspid regurgitation | 0.08 | −0.01 | 0.09 | −0.12 | 0.18 | −0.06 | −0.08 | −0.01 |
| sRV end‐diastolic annulus | 0.07 | −0.11 | 0.13 | −0.04 | −0.06 | −0.05 | 0.06 | 0.21 |
| sRV global LS§ | −0.18 | −0.09 | 0.13 | −0.21 | −0.20 | −0.07 | −0.02 | −0.25* |
| sRV GCS§ | 0.05 | −0.07 | −0.07 | −0.24 | −0.10 | −0.10 | 0.01 | 0.01 |
| sRV LS freewall§ | −0.16 | −0.03 | 0.21 | −0.19 | −0.17 | −0.03 | −0.03 | −.022 |
| sRV LS septal wall§ | −0.17 | −0.21 | −0.01 | −0.19 | −0.17 | −0.11 | −0.03 | −0.27* |
| LV EDD∥ | −0.19 | −0.08 | −0.07 | 0.29* | 0.35* | 0.09 | 0.02 | 0.05 |
| Blood biomarkers | ||||||||
| RDW | −0.09 | |||||||
| eGFR | 0.07 | −0.30† | ||||||
| NT‐proBNP | −0.19 | 0.34† | −0.43‡ | |||||
| Hs‐TnT | 0.09 | 0.44‡ | −0.33† | 0.48‡ | ||||
| GDF‐15 | −0.19 | 0.40† | −0.43‡ | 0.43‡ | 0.32† | |||
| Hs‐CRP | −0.14 | 0.27* | −0.22* | 0.39‡ | 0.21‡ | 0.39‡ | ||
| Galectin‐3 | −0.04 | 0.31† | −0.28† | 0.20 | 0.26* | 0.37† | 0.31† | |
BSA indicates body surface area; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; GCS, global circumferential strain; GDF‐15, growth differentiation factor‐15; HB, hemoglobin; Hs, high sensitive; LS, longitudinal strain; LV EDD, left ventricular end‐diastolic dimension; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; RDW, red cell distribution width; sRV, systemic right ventricle; TnT, troponin T.
Level of significance is indicated by the following symbols: *P<0.05, † P<0.01, ‡ P<0.001.
§Variables were transformed to positive numbers for easier interpretation.
∥Indexed for BSA.
Mutual blood biomarker correlations were present, though no strong correlations were found. The strongest correlation was found between NT‐proBNP and hs‐TnT (r=0.48, P<0.001) (Table 2).
Follow‐Up
Follow‐up was complete in 99% of the patients. After a median follow‐up period of 5.9 (interquartile range 5.3–6.3) years, 19 patients (22%) reached the primary end point and 29 patients (34%) reached the secondary end point. In 1 patient, an event of HF overlapped with the presence of arrhythmia and therefore reached both end points at the same time. Considering all components of the end points separately (ie, patients were not censored at the time of another end point than the end point of interest), the occurrence of events were 5 deaths, 18 HF events, and 26 arrhythmias. Causes of death were end‐stage HF (n=2), cardiac arrest (n=1), sudden death, presumed cardiac (n=1), and hemorrhagic shock (n=1). In 9 cases, HF required hospitalization; in the other cases HF medication was initiated or changed. The nature of the arrhythmias were 5 ventricular tachycardias and 21 supraventricular tachycardias. Supraventricular tachycardias included atrial flutter (n=6), atrial fibrillation (n=4), AV (nodal) re‐entry tachycardia (n=2), and other supraventricular tachycardias (n=8). One patient had an AV‐block.
Associations With End Points
Kaplan–Meier curves showed no significant differences in the risk of both end points among the M‐TGA and ccTGA patients. Cumulative HF‐free survival at 6 years was 81% in ccTGA patients versus 76% in M‐TGA patients. Cumulative arrhythmia‐free survival was 67% versus 64%, respectively (Figure 1).
Figure 1.
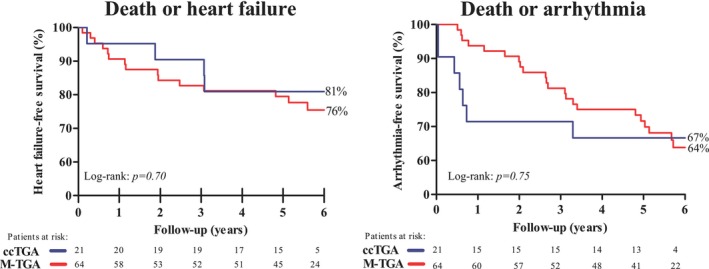
Heart failure–free survival (primary end point) and arrhythmia‐free survival (secondary end point) stratified according to ccTGA and M‐TGA patients. Red line indicates the event‐free survival according to M‐TGA patients. Blue line indicates the event‐free survival according to ccTGA patients. ccTGA indicates congenitally corrected transposition of the great arteries; M‐TGA, transposition of the great arteries corrected by the Mustard operation.
A lower systolic blood pressure, NYHA class >1, and loss of sinus rhythm were significantly associated with an increased risk of the primary end point. All blood biomarkers showed a significant association with the primary end point, except galectin‐3. GDF‐15 showed the strongest association with the primary end point, and a relatively strong association was found between high‐sensitive C‐reactive protein and RDW and the primary end point (Figure 2). (Standard deviations of all variables are presented in Table S1).
Figure 2.
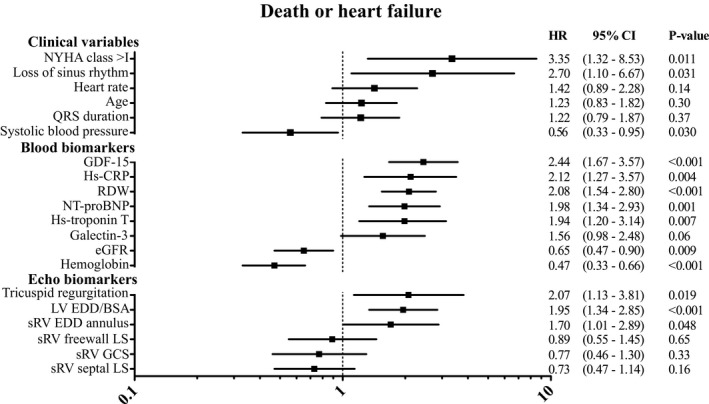
Associations between clinical variables, blood‐, and echocardiographic biomarkers, and the primary end point (death or heart failure) in patients with a systemic right ventricle. Hazard ratios are expressed per increase in standard deviation, except for categorical variables (NYHA class, loss of sinus rhythm, and tricuspid regurgitation). All blood biomarker levels were log transformed before standardization. sRV GCS and LV EDD/BSA were only measured in M‐TGA patients. Inversed hazard ratios (for comparison purposes); hemoglobin=2.13, eGFR=1.54. BSA indicates body surface area; CRP, C‐reactive protein; EDD, end‐diastolic dimension; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor‐15; GCS, global circumferential strain; GLS, global longitudinal strain; LS, longitudinal strain; M‐TGA, Mustard transposition of the great arteries; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association functional class; RDW, red cell distribution width; sRV, systemic right ventricle.
With regard to the secondary end point, systolic blood pressure and NYHA functional class were again significantly associated with the end point. Notably, loss of sinus rhythm was not associated with the risk of death or arrhythmias. GDF‐15, RDW, and galectin‐3 showed the strongest association of all biomarkers with the secondary end point. NT‐proBNP and high‐sensitive C‐reactive protein were the only blood biomarkers not associated with the secondary end point (Figure 3).
Figure 3.
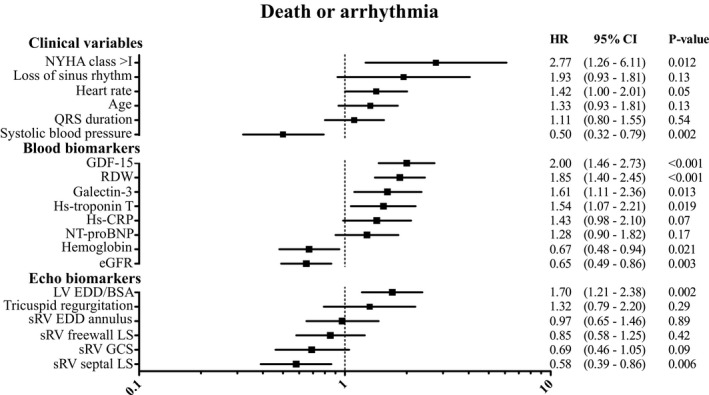
Associations between clinical variables, blood and echocardiographic biomarkers, and the secondary end point (death or arrhythmia). HRs are expressed per increase in standard deviation, except for categorical variables (NYHA class, loss of sinus rhythm, and tricuspid regurgitation). All blood biomarker levels were log transformed before standardization. sRV GCS and LV EDD/BSA were only measured in M‐TGA patients. Inversed HRs (for comparison purposes): hemoglobin=1.49, eGFR=1.54. BSA indicates body surface area; CRP, C‐reactive protein; EDD, end‐diastolic dimension; eGFR, estimated glomerular filtration rate; GCS, global circumferential strain; GDF‐15, growth differentiation factor‐15; GLS, global longitudinal strain; HRs, hazard ratios; Hs‐troponin‐T, high‐sensitive troponin T; LS, longitudinal strain; LV, left ventricular; M‐TGA, Mustard transposition of the great arteries; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association functional class; RDW, red cell distribution width; sRV, systemic right ventricle.
Regarding the echocardiographic variables (Figures 2 and 3), end‐diastolic diameters of the sRV as well as subpulmonary left ventricle were significantly associated with both end points. Tricuspid regurgitation was associated with the primary but not the secondary end point. Of all strain variables, only septal longitudinal strain showed a significant association with the secondary end point.
When adjusting associations between blood biomarkers and the primary end point for NYHA class, only estimated glomerular filtration rate was no longer statistically significant. All clinical variables and sRV end diastolic dimension did not remain associated with the primary end point, while tricuspid regurgitation did show an independent association with the primary end point. GDF‐15, RDW, estimated glomerular filtration rate, systolic blood pressure, and heart rate were independently associated with the secondary end point as well as 2 echo markers: longitudinal strain of the sRV septal wall and sRV global circumferential strain (Table S2).
Subgroup analysis restricted to M‐TGA patients only showed significant associations with both end points for age, systolic blood pressure, NYHA class, and loss of sinus rhythm. Of all blood biomarkers, NT‐proBNP yielded the strongest association with the primary end point and GDF‐15 was most strongly associated with the secondary end point. Longitudinal strain of the free wall was significantly associated with the secondary end point (Table S3).
Discussion
This prospective longitudinal cohort study consisting of 86 adults with a sRV showed that these patients are at high risk of HF, arrhythmias, and death. This emphasizes the importance of adequate risk stratification. Higher NYHA class, lower systolic blood pressure, and loss of sinus rhythm were significantly associated with an increased risk of adverse cardiac outcomes. Concerning echocardiographic measurements, both subpulmonary ventricular end‐diastolic dimension and sRV septal strain measurements were found to be associated with end points. This study also confirmed tricuspid regurgitation as a risk factor for HF. Most blood biomarkers were associated with adverse cardiac events, of which GDF‐15 yielded the strongest association, even stronger than NT‐proBNP. Risk stratification in adults with sRV should therefore focus on clinical variables, echocardiographic analysis, and blood biomarkers; however, strain analysis may be less feasible compared with the use of clinical and blood biomarkers.
Previous Studies
NT‐proBNP is the most widely used and studied blood biomarker for risk stratification in the adult congenital heart disease population18 and is one of the few blood biomarkers that has previously been investigated in sRV patients. Westhoff‐Bleck et al investigated NT‐proBNP in a prospective cohort study of 116 patients with TGA corrected by the atrial switch procedure and found that NT‐proBNP was an independent prognostic factor for adverse cardiac outcomes. Hemoglobin was also assessed, but showed no significant association.19 Popelova et al also found a strong independent prognostic value of NT‐proBNP in TGA patients after the atrial switch operation.20 One cross‐sectional study showed that hs‐TnT was better in detecting patients with sRV dysfunction than NT‐proBNP.21 Surprisingly, our results showed that NT‐proBNP was not the strongest prognostic factor. To the best of our knowledge, GDF‐15, galectin‐3, and RDW have never been described before in this specific ACHD population, although according to our study, these biomarkers may be better predictors for adverse cardiac events than NT‐proBNP. In a large prospective cohort study including all types of ACHD patients, higher levels of RDW have been investigated and were shown to be predictive of adverse cardiac outcomes.22 This same group also demonstrated a significant association between high‐sensitive C‐reactive protein and outcomes in ACHD patients.23
Although some cross‐sectional studies have been published, only a few studies investigated the prognostic value of echocardiographic measures. Conventional measures such as sRV ejection fraction based on magnetic resonance imaging or sRV fractional area change have been linked to clinical outcome,24, 25 with longitudinal strain on magnetic resonance imaging being the most promising. Tricuspid regurgitation is also a known risk factor for adverse outcomes in these patients.26, 27 Diller et al showed that in patients with a sRV, there is an adverse ventricular–ventricular interaction, similar to tetralogy of Fallot patients.24 This adverse interaction impedes the subpulmonary left ventricular function in a number of ways; shared myocardial fibers, dyssynchrony, and septal shift may all play a role. Another mechanism is because of backward failure of the sRV, leading to increased stress on the subpulmonary left ventricle. This was also present in our study, demonstrated by the association between left ventricular diameters and clinical events. Current literature is ambiguous regarding the prognostic value of strain analysis in sRV patients.24, 28, 29 In our study, the prognostic value of strain analysis was limited, though we did find an association between septal longitudinal strain and death or arrhythmia.
Death and HF
The increased risk of HF in patients with a sRV may arise from different mechanisms: myocardial ischemia caused by the single coronary artery that has to supply the right ventricle, and perioperative damage to the myocardium and the right ventricle which has to deal with a pressure overload. In M‐TGA patients, baffle stenosis or leakages can occur and are associated with a worse outcome.7, 30, 31
Regarding the clinical variables, NYHA class >1 was associated with death or HF, as well as a lower systolic blood pressure. Lower systolic blood pressure could be a sign of subclinical systemic ventricular failure, though mean systolic blood pressure still was 125±14 mm Hg.
The fact that many blood biomarkers were significantly associated with the primary end point may indicate that the pathophysiology of sRV deterioration is complex and involves multiple processes and cellular pathways. GDF‐15 has been suggested as a cardiac biomarker by Wollert et al and is identified as an independent predictor of mortality in many different populations, such as acute and chronic HF, atrial fibrillation, coronary artery disease, and in the community‐dwelling elderly.32 It is therefore thought that GDF‐15 is secreted in response to multiple processes, including hypoxia, inflammation, and oxidative stress. In contrast, NT‐proBNP is primarily induced by myocardial stretch, and this might be only a partial reflection of deterioration of sRV function. This could explain why GDF‐15 seems to be even better as predictor for adverse outcomes than NT‐proBNP according to our data. As proposed by the study of Opotowsky et al, inflammation might play a role in the pathophysiology of ACHD.23 Our study supports this by the associations found between hs‐CPR and both end points in this study. Mechanisms contributing to hs‐TnT secretion in sRV patients might be a combination of loss of myocardiocytes caused by increased wall stress and ischemia caused by failing oxygen supply of the right ventricle by the single coronary artery. However, as mechanisms of hs‐TnT secretion in the case of chronic HF are still not fully elucidated, secretion of hs‐TnT in sRV is even more speculative.33
Pettersen et al described that the sRV undergoes certain changes in order to be able to support a high‐pressure circulation: Longitudinal shortening diminishes, while circumferential shortening significantly enhances.34 In the present study, we found no association between strain measurements and the primary end point. Two recent studies reported absent associations between sRV systolic function on cardiac magnetic resonance imaging and exercise capacity and therefore concluded that the value of sRV imaging at rest seems to be limited.28, 29 We can corroborate these findings with this prospective study, though the difficulty in analyzing sRV should also be taken into account.
This study showed that ventricular dilatation measured by the left ventricular end‐diastolic dimension was associated with death or HF, which can be explained by backward failure of the sRV resulting in subpulmonary ventricular dilatation. A similar process is seen in HF patients with anatomically normal hearts; the deterioration of the contralateral ventricle is an ominous sign of end‐stage HF. Enlarged left ventricular end‐diastolic dimension could also be the result of a residual shunt or baffle leak. However, patients with severe residual shunt of baffle would have undergone an intervention, so therefore it is less likely the enlarged dimensions are the results of residual shunts or baffle leaks. Nevertheless, former shunts and leaks may still have contributed to the enlarged left ventricular dimension.
Another prevalent problem in sRV patients is tricuspid regurgitation. Although it is still debatable whether tricuspid regurgitation leads to sRV dysfunction or whether sRV dysfunction causes tricuspid regurgitation, tricuspid regurgitation has been associated with the risk of HF.26 Our data support the presence of tricuspid regurgitation as a risk factor for HF. Notably, tricuspid regurgitation was not associated with the occurrence of arrhythmias.
Arrhythmias
Similar to the primary end point, NYHA class >1 and lower systolic blood pressure were significantly associated with an increased risk of the secondary end point. Notably, loss of sinus rhythm did not have these increased risks. An explanation for this might be that patients who at baseline already have a pacemaker rhythm or atrial fibrillation are excluded from the occurrence of some atrial arrhythmias, while patients who are still in sinus rhythm at baseline can lose their sinus rhythm and may therefore be more likely to experience an event during follow‐up.
Studies about the prognostic value of biomarkers in relation to arrhythmias are limited in these patients, even though they are characterized by a high burden of arrhythmias.6 This is confirmed by our study. There are several probable causes for this, the first of which is the congenital defect itself. ccTGA patients have an abnormal conduction pathway in which the conduction system takes a longer course to the ventricles. Secondly, perioperative damage may result in myocardial fibrosis, which may lead to arrhythmias. Thirdly, myocardial stretch caused by HF may give rise to arrhythmias.
NT‐proBNP was not associated with arrhythmias in our study, whereas GDF‐15 and RDW did show a strong association with the occurrence of arrhythmias. An explanation may be that arrhythmias do not originate solely from myocardial stretch or hypertrophy but may be caused by a combination of myocardial fibrosis, inflammation, hypoxia, and myocardial stress.30 GDF‐15 has been associated with cardiac fibrosis in nonischemic cardiomyopathy35 and RDW has been suggested as being “an overall cardiovascular barometer” because of its probable reflection of diverse pathophysiological processes.36 This might explain the strong prognostic value found for both biomarkers for arrhythmias.
Aggregating the data from our study, and taking into consideration the lack of strong associations between echocardiography and blood biomarkers and the predictive ability of blood biomarkers in particular, it is likely that blood biomarkers are secreted in an earlier onset of subclinical deterioration in sRV patients, while echocardiographic changes may be revealed in a later disease stage and are therefore less predictive of cardiac events. The fact that most blood biomarkers were associated with the end points even after adjustment for NYHA class strengthens the idea that blood biomarkers reflect subclinical sRV failure.
Clinical Implications
A wide range of blood biomarkers yielded prognostic value in this study. Besides NT‐proBNP, other biomarkers play a pivotal role, and GDF‐15 specifically can be a potential biomarker for risk stratification. Some disadvantages of blood biomarkers are the high laboratory costs and the sometimes restricted availability. GDF‐15 is a relative expensive biomarker and often not available in standard laboratories. On the other hand, RDW also showed strong prognostic value and furthermore this biomarker is widely available, low cost, and incorporated in a standard complete blood count.
For daily clinical practice, findings such as an abnormal NYHA class and lower blood pressures can indicate high‐risk patients, and these patients should probably be followed up more closely.
While echocardiography plays a key role in the follow‐up of patients with complex ACHD, high‐quality image acquisition requires skilled sonographers, is hampered by complex anatomy and orientation in the thorax, and is highly user‐dependent, which may limit its prognostic value. Newer echocardiography techniques such as strain analysis seem to have little prognostic value in sRV patients and their role in clinical practice is currently unclear. Nevertheless, more easily obtainable conventional echocardiographic measurements such as subpulmonary end‐diastolic dimension can still be valuable measures for risk stratification in daily clinical practice.
Limitations
This study is limited by the relatively small sample size, and conclusions drawn from this study should therefore be treated with caution. We did not observe differences in event‐free survival in ccTGA and M‐TGA patients; however, underlying mechanisms of HF and arrhythmias may be different in these patients, and therefore prognostic biomarkers and echocardiographic measurements could behave differently in each diagnosis subgroup. Unfortunately, the low sample size allowed us to only perform subgroup analysis in M‐TGA patients. Also, this study lacked statistical power to adjust for multiple potential confounders and biomarkers in multivariable analysis. Because we used composite end points, it should be taken into account that associations could be driven more by 1 of the specific components.
We also did not take into account the concomitant congenital heart defects in both TGA groups, which can influence the prognosis. We acknowledge the considerable limitation concerning the limited sample size of this study, yet to the best of our knowledge this is the largest study that provided prospective longitudinal data including a wide range of biomarkers and echocardiographic strain analysis in patients with a sRV.
Conclusions
High event rates in adults with a sRV demand adequate risk stratification. Assessing clinical characteristics and blood biomarkers as well as conventional echocardiographic measurements seems most expedient for adequate risk assessment in these patients. However, the contribution of novel echocardiographic strain measurements seems to be limited in this perspective. This study suggests that the underlying pathophysiology of ventricular deterioration in patients with a sRV is complex, requiring a comprehensive clinical approach.
Sources of Funding
This study was supported by a grant from the Dutch Heart Foundation, The Hague, The Netherlands (grant number 2015T029) to Baggen and by a grant from the Erasmus MC Thorax Foundation, Rotterdam, The Netherlands to van Grootel.
Disclosures
None.
Supporting information
Table S1. Standard Deviations of Variables Included in Univariable Cox Regression, for Interpretative Purposes of Standardized Hazard Ratios
Table S2. Associations Between Clinical Variables, Blood‐ and Echocardiographic Markers, and the Primary‐ (Death or Heart Failure) and Secondary End Point (Death or Arrhythmia), With Adjustment for New York Heart Association Functional Class in Adults With a Systemic Right Ventricle
Table S3. Associations Between Clinical Variables, Blood‐ and Echocardiographic Markers, and the Primary and Secondary End Point, Restricted to Adult Patients With Transposition of the Great Arteries Operated by the Mustard Procedure
Figure S1. Flowchart of the patient selection process of the prospective observational cohort study consisting of adults with a systemic right ventricle.
(J Am Heart Assoc. 2019;8:e013745 DOI: 10.1161/JAHA.119.013745.)
Ms Geenen and Mr van Grootel contributed equally to this work.
References
- 1. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos‐Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:2241–2247. [DOI] [PubMed] [Google Scholar]
- 2. Konstantinov IE, Alexi‐Meskishvili VV, Williams WG, Freedom RM, Van Praagh R. Atrial switch operation: past, present, and future. Ann Thorac Surg. 2004;77:2250–2258. [DOI] [PubMed] [Google Scholar]
- 3. Connelly MS, Liu PP, Williams WG, Webb GD, Robertson P, McLaughlin PR. Congenitally corrected transposition of the great arteries in the adult: functional status and complications. J Am Coll Cardiol. 1996;27:1238–1243. [DOI] [PubMed] [Google Scholar]
- 4. Iriart X, Roubertie F, Jalal Z, Thambo JB. Quantification of systemic right ventricle by echocardiography. Arch Cardiovasc Dis. 2016;109:120–127. [DOI] [PubMed] [Google Scholar]
- 5. Dimas AP, Moodie DS, Sterba R, Gill CC. Long‐term function of the morphologic right ventricle in adult patients with corrected transposition of the great arteries. Am Heart J. 1989;118:526–530. [DOI] [PubMed] [Google Scholar]
- 6. Cuypers JA, Eindhoven JA, Slager MA, Opic P, Utens EM, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, Rizopoulos D, Meijboom FJ, Bogers AJ, Roos‐Hesselink JW. The natural and unnatural history of the mustard procedure: long‐term outcome up to 40 years. Eur Heart J. 2014;35:1666–1674. [DOI] [PubMed] [Google Scholar]
- 7. Roos‐Hesselink JW, Meijboom FJ, Spitaels SE, van Domburg R, van Rijen EH, Utens EM, McGhie J, Bos E, Bogers AJ, Simoons ML. Decline in ventricular function and clinical condition after mustard repair for transposition of the great arteries (a prospective study of 22–29 years). Eur Heart J. 2004;25:1264–1270. [DOI] [PubMed] [Google Scholar]
- 8. Oechslin E, Jenni R. 40 years after the first atrial switch procedure in patients with transposition of the great arteries: long‐term results in Toronto and Zurich. Thorac Cardiovasc Surg. 2000;48:233–237. [DOI] [PubMed] [Google Scholar]
- 9. Couperus LE, Vliegen HW, Zandstra TE, Kies P, Jongbloed MRM, Holman ER, Zeppenfeld K, Hazekamp MG, Schalij MJ, Scherptong RWC. Long‐term outcome after atrial correction for transposition of the great arteries. Heart. 2019;105:790–796. [DOI] [PubMed] [Google Scholar]
- 10. Eindhoven JA, van den Bosch AE, Ruys TP, Opic P, Cuypers JA, McGhie JS, Witsenburg M, Boersma E, Roos‐Hesselink JW. N‐terminal pro‐B‐type natriuretic peptide and its relationship with cardiac function in adults with congenital heart disease. J Am Coll Cardiol. 2013;62:1203–1212. [DOI] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 12. Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio‐Thoracic S , Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 13. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. [DOI] [PubMed] [Google Scholar]
- 14. Baggen VJM, van den Bosch AE, Eindhoven JA, Menting ME, Witsenburg M, Cuypers J, Boersma E, Roos‐Hesselink JW. Prognostic value of galectin‐3 in adults with congenital heart disease. Heart. 2018;104:394–400. [DOI] [PubMed] [Google Scholar]
- 15. Eindhoven JA, Roos‐Hesselink JW, van den Bosch AE, Kardys I, Cheng JM, Veenis JF, Cuypers JA, Witsenburg M, van Schaik RH, Boersma E. High‐sensitive troponin‐T in adult congenital heart disease. Int J Cardiol. 2015;184:405–411. [DOI] [PubMed] [Google Scholar]
- 16. Eindhoven JA, van den Bosch AE, Oemrawsingh RM, Baggen VJ, Kardys I, Cuypers JA, Witsenburg M, van Schaik RH, Roos‐Hesselink JW, Boersma E. Release of growth‐differentiation factor 15 and associations with cardiac function in adult patients with congenital heart disease. Int J Cardiol. 2016;202:246–251. [DOI] [PubMed] [Google Scholar]
- 17. Baggen VJ, van den Bosch AE, Eindhoven JA, Schut AW, Cuypers JA, Witsenburg M, de Waart M, van Schaik RH, Zijlstra F, Boersma E, Roos‐Hesselink JW. Prognostic value of N‐terminal pro‐B‐type natriuretic peptide, troponin‐T, and growth‐differentiation factor 15 in adult congenital heart disease. Circulation. 2017;135:264–279. [DOI] [PubMed] [Google Scholar]
- 18. Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos‐Hesselink JW. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol. 2012;60:2140–2149. [DOI] [PubMed] [Google Scholar]
- 19. Westhoff‐Bleck M, Podewski E, Tutarel O, Wenzel D, Cappello C, Bertram H, Bauersachs J, Widder J. Prognostic value of NT‐proBNP in patients with systemic morphological right ventricles: a single‐centre experience. Int J Cardiol. 2013;169:433–438. [DOI] [PubMed] [Google Scholar]
- 20. Popelova JR, Tomkova M, Tomek J. NT‐proBNP predicts mortality in adults with transposition of the great arteries late after Mustard or Senning correction. Congenit Heart Dis. 2017;12:448–457. [DOI] [PubMed] [Google Scholar]
- 21. Kowalik E, Klisiewicz A, Rybicka J, Biernacka EK, Hoffman P. High sensitivity cardiac troponin T and systemic right ventricular function in adults with congenitally corrected transposition of the great arteries. Int J Cardiol. 2017;241:168–172. [DOI] [PubMed] [Google Scholar]
- 22. Alshawabkeh L, Rajpal S, Landzberg MJ, Emani S, Ephrem G, Gray C, Singh MN, Wu F, Opotowsky AR. Relationship of red cell distribution width to adverse outcomes in adults with congenital heart disease (from the Boston Adult Congenital Heart Biobank). Am J Cardiol. 2018;122:1557–1564. [DOI] [PubMed] [Google Scholar]
- 23. Opotowsky AR, Valente AM, Alshawabkeh L, Cheng S, Bradley A, Rimm EB, Landzberg MJ. Prospective cohort study of C‐reactive protein as a predictor of clinical events in adults with congenital heart disease: results of the Boston Adult Congenital Heart Disease Biobank. Eur Heart J. 2018;39:3253–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diller GP, Radojevic J, Kempny A, Alonso‐Gonzalez R, Emmanouil L, Orwat S, Swan L, Uebing A, Li W, Dimopoulos K, Gatzoulis MA, Baumgartner H. Systemic right ventricular longitudinal strain is reduced in adults with transposition of the great arteries, relates to subpulmonary ventricular function, and predicts adverse clinical outcome. Am Heart J. 2012;163:859–866. [DOI] [PubMed] [Google Scholar]
- 25. Kalogeropoulos AP, Deka A, Border W, Pernetz MA, Georgiopoulou VV, Kiani J, McConnell M, Lerakis S, Butler J, Martin RP, Book WM. Right ventricular function with standard and speckle‐tracking echocardiography and clinical events in adults with D‐transposition of the great arteries post atrial switch. J Am Soc Echocardiogr. 2012;25:304–312. [DOI] [PubMed] [Google Scholar]
- 26. Graham TP Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, Gersony WM, Gessner IH, Hurwitz RA, Kaemmerer H, Kugler JD, Murphy DJ, Noonan JA, Morris C, Perloff JK, Sanders SP, Sutherland JL. Long‐term outcome in congenitally corrected transposition of the great arteries: a multi‐institutional study. J Am Coll Cardiol. 2000;36:255–261. [DOI] [PubMed] [Google Scholar]
- 27. Prieto LR, Hordof AJ, Secic M, Rosenbaum MS, Gersony WM. Progressive tricuspid valve disease in patients with congenitally corrected transposition of the great arteries. Circulation. 1998;98:997–1005. [DOI] [PubMed] [Google Scholar]
- 28. Tutarel O, Orwat S, Radke RM, Westhoff‐Bleck M, Vossler C, Schulke C, Baumgartner H, Bauersachs J, Rontgen P, Diller GP. Assessment of myocardial function using MRI‐based feature tracking in adults after atrial repair of transposition of the great arteries: reference values and clinical utility. Int J Cardiol. 2016;220:246–250. [DOI] [PubMed] [Google Scholar]
- 29. Helsen F, De Meester P, Van De Bruaene A, Gabriels C, Santens B, Claeys M, Claessen G, Goetschalckx K, Buys R, Gewillig M, Troost E, Voigt JU, Claus P, Bogaert J, Budts W. Right ventricular systolic dysfunction at rest is not related to decreased exercise capacity in patients with a systemic right ventricle. Int J Cardiol. 2018;260:66–71. [DOI] [PubMed] [Google Scholar]
- 30. Rydman R, Gatzoulis MA, Ho SY, Ernst S, Swan L, Li W, Wong T, Sheppard M, McCarthy KP, Roughton M, Kilner PJ, Pennell DJ, Babu‐Narayan SV. Systemic right ventricular fibrosis detected by cardiovascular magnetic resonance is associated with clinical outcome, mainly new‐onset atrial arrhythmia, in patients after atrial redirection surgery for transposition of the great arteries. Circ Cardiovasc Imaging. 2015;8:e002628. [DOI] [PubMed] [Google Scholar]
- 31. Singh TP, Humes RA, Muzik O, Kottamasu S, Karpawich PP, Di Carli MF. Myocardial flow reserve in patients with a systemic right ventricle after atrial switch repair. J Am Coll Cardiol. 2001;37:2120–2125. [DOI] [PubMed] [Google Scholar]
- 32. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–151. [DOI] [PubMed] [Google Scholar]
- 33. Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J. 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 34. Pettersen E, Helle‐Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, Smiseth OA, Andersen K. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol. 2007;49:2450–2456. [DOI] [PubMed] [Google Scholar]
- 35. Lok SI, Winkens B, Goldschmeding R, van Geffen AJ, Nous FM, van Kuik J, van der Weide P, Klopping C, Kirkels JH, Lahpor JR, Doevendans PA, de Jonge N, de Weger RA. Circulating growth differentiation factor‐15 correlates with myocardial fibrosis in patients with non‐ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur J Heart Fail. 2012;14:1249–1256. [DOI] [PubMed] [Google Scholar]
- 36. Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, Lenihan DJ, Oren RM, Wagoner LE, Schwartz TA, Adams KF Jr. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Standard Deviations of Variables Included in Univariable Cox Regression, for Interpretative Purposes of Standardized Hazard Ratios
Table S2. Associations Between Clinical Variables, Blood‐ and Echocardiographic Markers, and the Primary‐ (Death or Heart Failure) and Secondary End Point (Death or Arrhythmia), With Adjustment for New York Heart Association Functional Class in Adults With a Systemic Right Ventricle
Table S3. Associations Between Clinical Variables, Blood‐ and Echocardiographic Markers, and the Primary and Secondary End Point, Restricted to Adult Patients With Transposition of the Great Arteries Operated by the Mustard Procedure
Figure S1. Flowchart of the patient selection process of the prospective observational cohort study consisting of adults with a systemic right ventricle.


