Abstract
Background
Little attention has been paid to the importance of sex in the long‐term prognosis of patients undergoing cardiac surgery.
Methods and Results
We conducted a retrospective cohort study of Ontario residents, aged ≥40 years, who underwent coronary artery bypass grafting (CABG) and/or aortic, mitral, or tricuspid valve surgery between October 1, 2008, and December 31, 2016. The primary outcome was all‐cause mortality. The mortality rate in each surgical group was calculated using the Kaplan‐Meier method. The risk of death was assessed using multivariable Cox proportional hazard models. Sex‐specific mortality risk factors were identified using multiplicative interaction terms. A total of 72 824 patients were included in the study (25% women). The median follow‐up period was 5 (interquartile range, 3–7) years. The long‐term age‐standardized mortality rate was lowest in patients who underwent isolated CABG and highest among those who underwent combined CABG/multiple valve surgery. Women had significantly higher age‐standardized mortality rate than men after CABG and combined CABG/mitral valve surgery. Men had lower rates of long‐term mortality than women after isolated mitral valve repair, whereas women had lower rates of long‐term mortality than men after isolated mitral valve replacement. We observed a statistically significant association between female sex and long‐term mortality after adjustment for key risk factors.
Conclusions
Female sex was associated with long‐term mortality after cardiac surgery. Perioperative optimization and long‐term follow‐up should be tailored to younger women with a history of myocardial infarction and percutaneous coronary intervention and older men with a history of chronic obstructive pulmonary disease and depression.
Keywords: cardiac surgery, coronary revascularization, mortality, sex differences, valve repair, valve replacement
Subject Categories: Women, Cardiovascular Surgery, Mortality/Survival
Clinical Perspective
What Is New?
We systematically described the sex differences in long‐term mortality after major cardiac surgery in a large, real‐world cohort.
We observed a statistically significant association between female sex and long‐term mortality after adjustment for key risk factors.
Women experienced higher rates of mortality than men after coronary artery bypass grafting and combined coronary artery bypass grafting/mitral valve surgery, and mortality risk factors differed by sex.
What Are the Clinical Implications?
Perioperative optimization and long‐term follow‐up should be tailored to women with a history of recent myocardial infarction and diabetes mellitus and men with a history of chronic obstructive pulmonary disease and depression.
Given the substantial sex differences in patient presentation for coronary and valvular heart disease, further efforts need to be directed at the education of both physicians and patients in the early recognition of acute presentation of cardiac disease in women.
Given the poorer performance of standard risk scores in women, further research is needed to derive and validate sex‐specific risk prediction models in patients undergoing cardiac surgery.
Introduction
Globally, 2 million cardiac surgery procedures are being performed each year.1 Steady improvements in postoperative survival can be largely attributed to important advancements in surgical technique, perioperative risk stratification, and care.2, 3, 4 Despite the well‐established role of patient sex on disease presentation, comorbid conditions, and the development of postoperative complications,5, 6, 7 little attention has been paid to the importance of sex in the prognosis of patients undergoing cardiac surgery.6, 8 To date, most reports of sex differences in cardiac surgery outcomes have been focused on short‐term survival.9, 10, 11, 12, 13, 14 Existing sex‐stratified analyses of long‐term outcomes are dated, single center in nature, and/or limited to isolated coronary artery bypass grafting (CABG) or a single type of procedure.15, 16, 17, 18 A current and comprehensive understanding of sex‐specific long‐term prognosis is needed to better inform operative decisions and provide tailored follow‐up strategies in cardiac surgery patients.3, 19 We, therefore, examined the sex differences in long‐term survival of all patients who underwent CABG, valve, or combined CABG/valve procedures in Ontario, Canada, between 2008 and 2016.
Methods
A flow chart detailing the process used to select the study cohort is shown in Figure 1.
Figure 1.
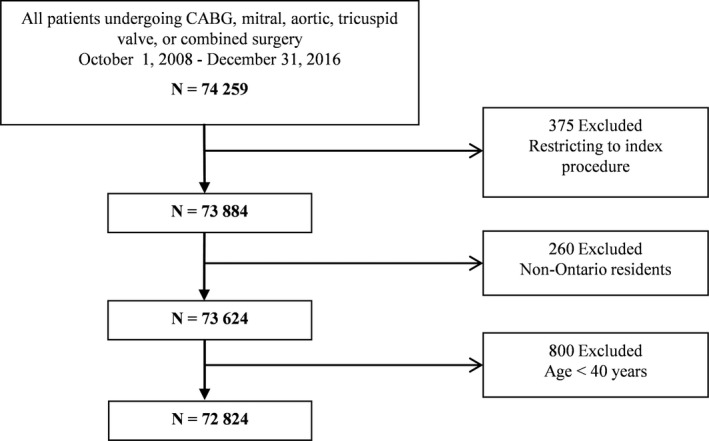
Cohort selection flow chart. CABG indicates coronary artery bypass grafting.
Design and Study Population
We conducted a population‐based, retrospective cohort study in Ontario, Canada. The Research Ethics Board of Sunnybrook Health Sciences Centre (Toronto, Canada) approved this study and waived the need for individual patient consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Included were adult Ontario residents, aged ≥40 years, who underwent CABG and/or aortic, mitral, or tricuspid valve surgery between October 1, 2008, and December 31, 2016. For those patients who underwent multiple cardiac procedures during the study period, the first procedure was considered the index procedure. Exclusion criteria were non‐Ontario residents, those with missing information on age and sex, and those who had concomitant arrhythmia, pulmonic valve, or thoracic aorta surgery. Ontario is Canada's most populous province, with a publicly funded, universal healthcare system that reimburses all covered services and providers.
Data Sources
We used the clinical registry data from CorHealth Ontario (https://www.corhealthontario.ca) and population‐level administrative healthcare databases with information on all Ontario residents, available at the Institute for Clinical Evaluative Sciences. Individuals who underwent specified cardiac procedures were identified from the CorHealth Ontario registry. CorHealth Ontario maintains a detailed prospective registry of all patients who undergo invasive cardiac procedures in Ontario. All 20 advanced cardiac hospitals in Ontario participate in the registry. It captures demographic, comorbidity, and procedural‐related information and has been validated through selected chart audits. In addition, CorHealth Ontario ejection fraction (EF) and angiographic data undergo core laboratory validation.20
Administrative databases were linked deterministically by using encrypted unique confidential codes that preserve patient confidentiality. We linked the CorHealth Ontario registry (date and type of cardiac procedures, physiologic and comorbidity data) with the Canadian Institute for Health Information's Discharge Abstract Database (comorbidities and hospital admissions) and Same Day Surgery database (comorbidities), Ontario Health Insurance Plan database (physician service claims), Registered Persons Database (ascertainment of vital statistics), and Canadian census. These administrative databases have been validated for many outcomes, exposures, and comorbidities, including heart failure (HF), chronic obstructive pulmonary disease (COPD), asthma, hypertension, myocardial infarction (MI), and diabetes mellitus.21, 22, 23, 24
Comorbidities
Comorbidities were identified from the CorHealth Ontario registry and supplemented with data from the Discharge Abstract Database, Same Day Surgery database, and Ontario Health Insurance Plan database using International Classification of Diseases, Tenth Revision (ICD‐10‐CA), codes25 within 5 years before the index procedure, according to validated algorithms.21, 23, 26, 27 We estimated socioeconomic status based on patients’ neighborhood median income in the Canadian census and determined their residence (rural versus urban) using the definitions from Statistics Canada.28 Procedural urgency was ascertained from the CorHealth Ontario registry. Height, weight, and body mass index were identified from the CorHealth Ontario registry; and they were used to define morbid obesity (weight >159 kg or body mass index ≥40 kg/m2).6, 7, 29 Frailty status was identified using the Johns Hopkins Adjusted Clinical Groups frailty‐defining diagnoses indicator, which is an instrument designed and validated for research of frailty‐related outcomes and resource use using administrative data.29, 30, 31, 32, 33, 34
Outcomes
The primary outcome was all‐cause mortality. We confirmed in‐hospital mortality using the Discharge Abstract Database and postdischarge mortality using the Registered Persons Database.
Statistical Analysis
A.B.E. and L.Y.S. had full access to all the data in the study and take responsibility for their integrity and for the data analysis. All analyses were performed in the overall cohort and then stratified by sex. Continuous variables are expressed as mean (SD), and categorical variables are expressed as number (proportion). Outcomes were assessed through December 31, 2017. Patients were censored at the front end if they lost possession of a valid Ontario health insurance card for 2 consecutive eligibility quarters (ie, assumed to have left the province of Ontario). Event time was defined as the date of index surgery until the date of the event or the date of the last follow‐up, whichever occurred earlier. Mortality rates in each group were calculated by using the Kaplan‐Meier method, and differences in mortality between groups were assessed with the log‐rank test. The proportionality assumption was tested by using log‐log plots for sex and type of surgery. Sex‐specific mortality rates were standardized by age, using the 2012 Canadian population as the reference.
The risk of death was assessed using a multivariable Cox proportional hazard model with adjustment for all variables listed in Table 1. We explored the modifying effect of sex by using multiplicative interaction terms. For the covariates that had a significant interaction effect with sex, we reported sex‐stratified hazard ratios (HRs) for the covariate, as well as HRs for female sex, stratified by the presence or absence of that covariate.
Table 1.
Baseline Characteristics, Stratified by Sex
| Variable | Overall Population | No. (%) of Patientsa | P Value | |
|---|---|---|---|---|
| Women (N=17 874) | Men (N=54 950) | |||
| Surgery type | ||||
| Isolated CABG | 52 546 (72.2) | 10 743 (60.1) | 41 803 (76.1) | <0.001 |
| CABG+single valveb | 7936 (10.9) | 2098 (11.7) | 5838 (10.6) | |
| CABG+multiple valvec | 665 (0.9) | 202 (1.1) | 463 (0.8) | |
| Single valveb | 10 368 (14.2) | 4212 (23.6) | 6156 (11.2) | |
| Multiple valvec | 1309 (1.8) | 619 (3.5) | 690 (1.3) | |
| Age, mean±SD, y | 67.0±10.2 | 69.1±10.1 | 66.2±10.1 | <0.001 |
| 40–64 | 28 941 (39.7) | 5601 (31.3) | 23 340 (42.5) | <0.001 |
| 65–74 | 25 229 (34.6) | 6206 (34.7) | 19 023 (34.6) | |
| 75–84 | 16 806 (23.1) | 5363 (30.0) | 11 443 (20.8) | |
| ≥85 | 1848 (2.5) | 704 (3.9) | 1144 (2.1) | |
| Rural residence | 11 763 (16.2) | 2805 (15.7) | 8958 (16.3) | |
| Income quintile | ||||
| 1 (Lowest) | 13 448 (18.5) | 3831 (21.4) | 9617 (17.5) | <0.001 |
| 2 | 14 696 (20.2) | 3762 (21.0) | 10 934 (19.9) | |
| 3 | 14 759 (20.3) | 3559 (19.9) | 11 200 (20.4) | |
| 4 | 15 017 (20.6) | 3478 (19.5) | 11 539 (21.0) | |
| 5 (Highest) | 14 532 (20.0) | 3145 (17.6) | 11 387 (20.7) | |
| Missing | 372 (0.5) | 99 (0.6) | 273 (0.5) | |
| Hypertension | 62 852 (86.3) | 15 892 (88.9) | 46 960 (85.5) | <0.001 |
| Atrial fibrillation | 5165 (7.1) | 1621 (9.1) | 3544 (6.4) | <0.001 |
| Recent MI | 22 952 (31.5) | 5283 (29.6) | 17 669 (32.2) | <0.001 |
| Remote MI | 11 605 (15.9) | 2555 (14.3) | 9050 (16.5) | <0.001 |
| Previous PCI | 10 532 (14.5) | 2245 (12.6) | 8287 (15.1) | <0.001 |
| Heart failure | 19 847 (27.3) | 6378 (35.7) | 13 469 (24.5) | <0.001 |
| LVEF, % | ||||
| ≥50 | 48 185 (66.2) | 13 081 (73.2) | 35 104 (63.9) | <0.001 |
| 35–50 | 15 448 (21.2) | 3010 (16.8) | 12 438 (22.6) | |
| 20–35 | 6044 (8.3) | 1123 (6.3) | 4921 (9.0) | |
| <20 | 1113 (1.5) | 165 (0.9) | 948 (1.7) | |
| Missing | 2034 (2.8) | 495 (2.8) | 1539 (2.8) | |
| Cerebrovascular disease | 7420 (10.2) | 2012 (11.3) | 5408 (9.8) | <0.001 |
| Peripheral arterial disease | 9139 (12.5) | 2314 (12.9) | 6825 (12.4) | 0.065 |
| COPD or asthma | 21 419 (29.4) | 6232 (34.9) | 15 187 (27.6) | <0.001 |
| Diabetes mellitus | 32 812 (45.1) | 8458 (47.3) | 24 354 (44.3) | <0.001 |
| Morbid obesity | 28 391 (39.0) | 5449 (30.5) | 22 942 (41.8) | <0.001 |
| Hypothyroidism | 1419 (1.9) | 786 (4.4) | 633 (1.2) | <0.001 |
| Liver disease | 633 (0.9) | 161 (0.9) | 472 (0.9) | 0.60 |
| Anemia | 7347 (10.1) | 2564 (14.3) | 4783 (8.7) | <0.001 |
| Venous thromboembolism | 288 (0.4) | 73 (0.4) | 215 (0.4) | 0.99 |
| Dialysis | 1531 (2.1) | 390 (2.2) | 1141 (2.1) | 0.39 |
| Baseline creatinine, μmol/L | ||||
| 120–179 | 6635 (9.1) | 1219 (6.8) | 5416 (9.9) | <0.001 |
| <120 | 60 190 (82.7) | 15 258 (85.4) | 44 932 (81.8) | |
| ≥180 | 2435 (3.3) | 537 (3.0) | 1898 (3.5) | |
| Missing | 3564 (4.9) | 860 (4.8) | 2704 (4.9) | |
| Chronic renal disease | 3133 (4.3) | 771 (4.3) | 2362 (4.3) | 0.93 |
| Dementia | 176 (0.2) | 55 (0.3) | 121 (0.2) | 0.038 |
| Depression | 1089 (1.5) | 417 (2.3) | 672 (1.2) | <0.001 |
| Psychosis | 161 (0.2) | 58 (0.3) | 103 (0.2) | 0.001 |
| Primary tumor | 3770 (5.2) | 817 (4.6) | 2953 (5.4) | <0.001 |
| Metastatic cancer | 375 (0.5) | 114 (0.6) | 261 (0.5) | 0.008 |
| Charlson score, median (IQR) | 1 (0–3) | 2 (0–3) | 1 (0–3) | <0.001 |
| Frailtyc | 11 685 (16.0) | 3601 (20.1) | 8084 (14.7) | <0.001 |
CABG indicates coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Unless otherwise stated.
Mitral, aortic, or tricuspid surgery.
As women and men differed in baseline and operative characteristics, we conducted a sensitivity analysis by using inverse probability of treatment weighting using propensity scores to estimate the effect of sex. Specifically, we used logistic regression to estimate propensity scores of being women, using the same set of covariates as those used in the multivariable Cox proportional hazards model. We used a weighted Cox proportional hazards model to estimate the effect of sex on mortality in the sample weighted by inverse probability of treatment weighting. We then used a robust sandwich variance estimator to account for the within‐subject correlation in outcomes induced by weighting.
Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), with statistical significance defined by a 2‐sided P<0.05.
Results
A total of 72 824 patients were included in the study (24% women). The baseline patient characteristics are summarized in Table 1. Compared with men, women were older, were more frail, had lower socioeconomic status, and were more likely to have preserved left ventricular EF and comorbid conditions, such as valvular heart disease, atrial fibrillation, hypertension, cerebrovascular and peripheral arterial disease, COPD, diabetes mellitus, hypothyroidism, and anemia. In contrast, women were less likely than men to have had an MI, to have had a previous percutaneous coronary intervention (PCI), to undergo isolated CABG, to be morbidly obese, or to abuse alcohol.
Figure 2 demonstrates the relative frequencies of surgical procedures performed in women and men. Isolated CABG was the most common cardiac procedure, accounting for 72.2% of the provincial procedural volume. Although valvular procedures were performed in near equal frequencies in both sexes, most CABG and combined CABG/valve procedures were performed in men. Table 2 summarizes baseline patient characteristics according to surgery type. Isolated CABG was most likely to be performed in younger men with a history of reduced left ventricular EF and previous MI and PCI; but least likely to be performed in those with HF. Compared with those who underwent combined CABG/valve procedures, those who underwent isolated valve surgery were younger and were more likely to have preserved left ventricular EF and a lower burden of comorbidities, as evidenced by a lower Charlson comorbidity index. Those who underwent combined CABG/multiple valve surgery were among the frailest and burdened with the highest number of comorbidities. The proportionality assumption was satisfied for age and type of surgery.
Figure 2.
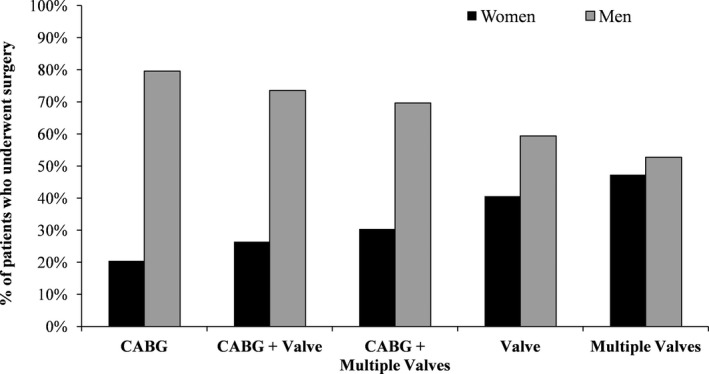
Percentage of men and women who underwent each of the 5 cardiac procedure categories. CABG indicates coronary artery bypass grafting.
Table 2.
Baseline Characteristics, Stratified by Type of Surgery
| Variable | Overall Population (N=72 824) | No. (%) of Patientsa | P Value | ||||
|---|---|---|---|---|---|---|---|
| Isolated CABG (N=52 546) | CABG+Single Valve (N=7936)b | CABG+Multiple Valves (N=665)c | Single Valve (N=10 368)b | Multiple Valves (N=1309)c | |||
| Age, mean±SD, y | 67.0±10.2 | 65.9±9.8 | 72.6±9.0 | 72.5±9.1 | 67.5±11.2 | 68.3±11.4 | <0.001 |
| 40–64 | 28 941 (39.7) | 22 925 (43.6) | 1460 (18.4) | 129 (19.4) | 3969 (38.3) | 458 (35.0) | <0.001 |
| 65–74 | 25 229 (34.6) | 18 626 (35.4) | 2795 (35.2) | 216 (32.5) | 3197 (30.8) | 395 (30.2) | |
| 75–84 | 16 806 (23.1) | 10 220 (19.4) | 3128 (39.4) | 269 (40.5) | 2801 (27.0) | 388 (29.6) | |
| ≥85 | 1848 (2.5) | 775 (1.5) | 553 (7.0) | 51 (7.7) | 401 (3.9) | 68 (5.2) | |
| Rural residence | 11 763 (16.2) | 8204 (15.6) | 1457 (18.4) | 143 (21.5) | 1756 (16.9) | 203 (15.5) | |
| Income quintile | |||||||
| 1 (Lowest) | 13 448 (18.5) | 10 003 (19.0) | 1370 (17.3) | 102 (15.3) | 1728 (16.7) | 245 (18.7) | <0.001 |
| 2 | 14 696 (20.2) | 10 724 (20.4) | 1608 (20.3) | 148 (22.3) | 1959 (18.9) | 257 (19.6) | |
| 3 | 14 759 (20.3) | 10 627 (20.2) | 1609 (20.3) | 157 (23.6) | 2117 (20.4) | 249 (19.0) | |
| 4 | 15 017 (20.6) | 10 714 (20.4) | 1705 (21.5) | 127 (19.1) | 2214 (21.4) | 257 (19.6) | |
| 5 (Highest) | 14 532 (20.0) | 10 184 (19.4) | 1617 (20.4) | 129 (19.4) | 2310 (22.3) | 292 (22.3) | |
| Missing | 372 (0.5) | 294 (0.6) | 27 (0.3) | ≤5 | 40 (0.4) | 9 (0.7) | |
| Hypertension | 62 852 (86.3) | 46 111 (87.8) | 7185 (90.5) | 595 (89.5) | 7954 (76.7) | 1007 (76.9) | <0.001 |
| Atrial fibrillation | 5165 (7.1) | 2373 (4.5) | 990 (12.5) | 155 (23.3) | 1343 (13.0) | 304 (23.2) | <0.001 |
| Recent MI | 22 952 (31.5) | 20 684 (39.4) | 1659 (20.9) | 132 (19.8) | 422 (4.1) | 55 (4.2) | <0.001 |
| Remote MI | 11 605 (15.9) | 9432 (17.9) | 1295 (16.3) | 108 (16.2) | 679 (6.5) | 91 (7.0) | <0.001 |
| Previous PCI | 10 532 (14.5) | 8773 (16.7) | 1004 (12.7) | 68 (10.2) | 617 (6.0) | 70 (5.3) | <0.001 |
| Heart failure | 19 847 (27.3) | 10 126 (19.3) | 3714 (46.8) | 430 (64.7) | 4729 (45.6) | 848 (64.8) | <0.001 |
| LVEF, % | |||||||
| ≥50 | 48 185 (66.2) | 32 237 (61.4) | 5630 (70.9) | 435 (65.4) | 8843 (85.3) | 1040 (79.4) | |
| 35–50 | 15 448 (21.2) | 12 762 (24.3) | 1398 (17.6) | 145 (21.8) | 967 (9.3) | 176 (13.4) | |
| 20–35 | 6044 (8.3) | 4911 (9.3) | 654 (8.2) | 66 (9.9) | 346 (3.3) | 67 (5.1) | |
| <20 | 1113 (1.5) | 905 (1.7) | 139 (1.8) | 13 (2.0) | 43 (0.4) | 13 (1.0) | |
| Missing | 2034 (2.8) | 1731 (3.3) | 115 (1.4) | 6 (0.9) | 169 (1.6) | 13 (1.0) | <0.001 |
| Cerebrovascular disease | 7420 (10.2) | 5132 (9.8) | 1073 (13.5) | 82 (12.3) | 970 (9.4) | 163 (12.5) | <0.001 |
| Peripheral arterial disease | 9139 (12.5) | 6424 (12.2) | 1389 (17.5) | 108 (16.2) | 1079 (10.4) | 139 (10.6) | <0.001 |
| COPD or asthma | 21 419 (29.4) | 14 702 (28.0) | 2726 (34.3) | 251 (37.7) | 3301 (31.8) | 439 (33.5) | <0.001 |
| Diabetes mellitus | 32 812 (45.1) | 25 267 (48.1) | 3639 (45.9) | 266 (40.0) | 3218 (31.0) | 422 (32.2) | <0.001 |
| Morbid obesity | 28 391 (39.0) | 20 490 (39.0) | 3106 (39.1) | 281 (42.3) | 3951 (38.1) | 563 (43.0) | 0.004 |
| Hypothyroidism | 1419 (1.9) | 969 (1.8) | 186 (2.3) | 18 (2.7) | 206 (2.0) | 40 (3.1) | <0.001 |
| Liver disease | 633 (0.9) | 351 (0.7) | 81 (1.0) | 11 (1.7) | 153 (1.5) | 37 (2.8) | <0.001 |
| Anemia | 7347 (10.1) | 4918 (9.4) | 1113 (14.0) | 136 (20.5) | 977 (9.4) | 203 (15.5) | <0.001 |
| Venous thromboembolism | 288 (0.4) | 173 (0.3) | 46 (0.6) | ≤5 | 57 (0.5) | 10 (0.8) | <0.001 |
| Dialysis | 1531 (2.1) | 1047 (2.0) | 233 (2.9) | 22 (3.3) | 195 (1.9) | 34 (2.6) | <0.001 |
| Baseline creatinine, μmol/L | |||||||
| 120–179 | 6635 (9.1) | 4487 (8.5) | 1037 (13.1) | 93 (14.0) | 855 (8.2) | 163 (12.5) | <0.001 |
| <120 | 60 190 (82.7) | 43 934 (83.6) | 6158 (77.6) | 522 (78.5) | 8538 (82.3) | 1038 (79.3) | |
| ≥180 | 2435 (3.3) | 1698 (3.2) | 381 (4.8) | 32 (4.8) | 261 (2.5) | 63 (4.8) | |
| Missing | 3564 (4.9) | 2427 (4.6) | 360 (4.5) | 18 (2.7) | 714 (6.9) | 45 (3.4) | |
| Chronic renal disease | 3133 (4.3) | 2109 (4.0) | 516 (6.5) | 43 (6.5) | 399 (3.8) | 66 (5.0) | <0.001 |
| Dementia | 176 (0.2) | 98 (0.2) | 41 (0.5) | 6 (0.9) | 27 (0.3) | ≤5 | <0.001 |
| Depression | 1089 (1.5) | 733 (1.4) | 149 (1.9) | 13 (2.0) | 157 (1.5) | 37 (2.8) | <0.001 |
| Psychosis | 161 (0.2) | 102 (0.2) | 17 (0.2) | ≤5 | 33 (0.3) | 6 (0.5) | 0.025 |
| Primary tumor | 3770 (5.2) | 2486 (4.7) | 539 (6.8) | 48 (7.2) | 601 (5.8) | 96 (7.3) | <0.001 |
| Metastatic cancer | 375 (0.5) | 248 (0.5) | 40 (0.5) | ≤5 | 72 (0.7) | 10 (0.8) | 0.03 |
| Charlson score, median (IQR) | 1 (0–3) | 2 (0–3) | 2 (0–3) | 2 (1–3) | 1 (0–2) | 1 (0–2) | <0.001 |
| Frailtyc | 11 685 (16.0) | 8623 (16.4) | 1491 (18.8) | 154 (23.2) | 1204 (11.6) | 213 (16.3) | <0.001 |
CABG indicates coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Unless otherwise stated.
Mitral, aortic, or tricuspid valve surgery.
Survival at 30 Days
Overall, women were more likely to die than men during the first 30 days after surgery (2.7% versus 1.6%; P<0.001). Thirty‐day mortality rates after isolated CABG, CABG/single‐valve, CABG/multiple‐valve, single‐valve, and multiple‐valve surgery were 1.4%, 4.0%, 7.5%, 1.7%, and 5.2%, respectively (P<0.001). The sex‐ and procedure‐stratified 30‐day crude and age‐standardized mortality rates (AMRs) are summarized in Table 3.
Table 3.
CMRs and AMRs Within 30 Days and in Long‐Term Follow‐Up After Surgery
| Surgery | Sex | No. of Deaths | Person‐Years | CMR per 1000 Person‐Years (95% CI) | AMR per 1000 Person‐Years (95% CI) | P Value |
|---|---|---|---|---|---|---|
| 30‐d Mortality | ||||||
| CABG | Women | 235 | 317 222 | 0.7 (0.7–0.8) | 0.6 (0.5–0.7) | <0.001 |
| Men | 506 | 1 244 223 | 0.4 (0.4–0.4) | 0.4 (0.3–0.4) | ||
| CABG+single valvea | Women | 112 | 60 505 | 1.9 (1.5–2.2) | 1.6 (1.1–2.4) | 0.008 |
| Men | 207 | 171 205 | 1.2 (1.1–1.4) | 0.9 (0.7–1.2) | ||
| CABG+multiple valvea | Women | 22 | 5588 | 3.9 (2.5–6.0) | 2.0 (0.9–3.9) | 0.95 |
| Men | 28 | 13 298 | 2.1 (1.4–3.0) | 2.0 (0.9–3.7) | ||
| Single valvea | Women | 87 | 124 712 | 0.7 (0.6–0.9) | 0.6 (0.4–0.7) | 0.11 |
| Men | 90 | 183 088 | 0.5 (0.4–0.6) | 0.4 (0.3–0.5) | ||
| Multiple valvea | Women | 30 | 17 971 | 1.7 (1.13–2.38) | 1.2 (0.7–2.0) | 0.17 |
| Men | 38 | 19 951 | 1.9 (1.35–2.61) | 1.9 (1.2–2.7) | ||
| Long‐term mortality | ||||||
| CABG | Women | 1749 | 49 723 | 35.2 (33.6–36.9) | 28.4 (26.7–30.1) | <0.001 |
| Men | 5219 | 196 646 | 26.5 (25.8–27.3) | 23.6 (22.8–24.5) | ||
| CABG+single valvea | Women | 647 | 8869 | 73.0 (67.4–78.8) | 58.2 (49.7–67.6) | 0.01 |
| Men | 1567 | 24 420 | 64.2 (61.0–67.4) | 46.5 (42.9–50.3) | ||
| CABG+multiple valvea | Women | 79 | 687 | 115.0 (91.1–143.4) | 75.2 (51.4–106.2) | 0.59 |
| Men | 149 | 1758 | 84.8 (71.7–99.5) | 66.6 (49.5–87.7) | ||
| Single valve | Women | 802 | 18 803 | 42.7 (39.8–45.7) | 29.7 (27.0–32.6) | 0.64 |
| Men | 1055 | 27 352 | 38.6 (36.3–41.0) | 30.5 (28.4–32.7) | ||
| Multiple valve | Women | 126 | 2253 | 55.9 (46.6–66.6) | 41.7 (32.0–53.3) | 0.11 |
| Men | 165 | 2685 | 61.5 (52.4–71.6) | 53.7 (44.3–64.4) | ||
AMR indicates age‐standardized mortality rate; CABG, coronary artery bypass grafting; CMR, crude mortality rate.
Mitral, aortic, or tricuspid valve surgery.
Sex Differences in Long‐Term Survival
The median follow‐up period was 5 years (interquartile range, 3–7 years), and the maximum follow‐up period was 9 years. Women were more likely than men to die within 1 year after a cardiac procedure (6.2% versus 4.1%; P<0.001) and during long‐term follow‐up (19.0% versus 14.8%; P<0.001). The multivariable adjusted long‐term survival curves for women and men are illustrated in Figure 3.
Figure 3.

Adjusted Kaplan‐Meier curves of long‐term survival in women and men.
Sex modified the correlation between mortality and type of surgery, age, recent and remote MI, previous PCI, COPD/asthma, and depression. The HRs for sex, stratified by the presence or absence of these characteristics, are presented in Table 4; the sex‐stratified HRs for the same characteristics are presented in Table 5. Taken together, women who underwent surgery involving CABG had a higher risk of death than men (Table 4); and this observation was especially evident in women who underwent CABG/multiple valve reconstruction (Table 5). Although female sex accentuated the risk of long‐term mortality in patients aged <75 years (Table 4), the association between sex and long‐term mortality was more accentuated in men aged >75 years compared with women of the same age group (Table 5). In addition, although remote MI, recent MI, and previous PCI were associated with higher risks of death in women (Table 4), previous PCI was associated with a lower risk of death in men and not in women (Table 5). Both COPD/asthma and depression were associated with a slightly higher risk of death in men compared with women (Table 5).
Table 4.
Impact of Female Sex, Stratified by the Presence and Absence of Characteristics That Have a Significant Interaction Effect With Sex
| Characteristics | Adjusted HR for Women (95% CI) | Interaction P Value | |
|---|---|---|---|
| Presence of Characteristic | Absence of Characteristic | ||
| Surgery | |||
| CABG | 1.15 (1.08–1.21) | … | 0.03 |
| CABG+single valvea | 1.11 (1.01–1.22) | … | |
| CABG+multiple valvesa | 1.34 (1.02–1.77) | … | |
| Single valvea | 1.06 (0.96–1.16) | … | |
| Multiple valvesa | 0.82 (0.65–1.04) | … | |
| Age group, y | |||
| 40–64 | 1.22 (1.11–1.34) | … | 0.001 |
| 65–74 | 1.20 (1.11–1.29) | … | |
| 75–84 | 1.04 (0.97–1.10) | … | |
| ≥85 | 0.97 (0.84–1.13) | … | |
| Recent MI | 1.27 (1.19–1.36) | 1.03 (0.98–1.09) | <0.001 |
| Remote MI | 1.21 (1.11–1.32) | 1.09 (1.04–1.14) | 0.03 |
| Previous PCI | 1.24 (1.11–1.39) | 1.10 (1.05–1.15) | 0.04 |
| COPD/asthma | 1.06 (1.00–1.13) | 1.16 (1.09–1.22) | 0.05 |
| Depression | 0.88 (0.71–1.11) | 1.12 (1.07–1.17) | 0.04 |
CABG indicates coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Mitral, aortic, or tricuspid valve surgery.
Table 5.
Sex‐Specific Risk Factors of Long‐Term Mortality
| Characteristics | Adjusted HR (95% CI) | Interaction P Value | |
|---|---|---|---|
| Women | Men | ||
| Surgery | |||
| CABG | Reference | Reference | 0.03 |
| CABG+single valvea | 1.39 (1.27–1.53) | 1.43 (1.35–1.53) | |
| CABG+multiple valvesa | 1.92 (1.53–2.42) | 1.64 (1.39–1.94) | |
| Single valvea | 1.23 (1.12–1.35) | 1.34 (1.24–1.44) | |
| Multiple valvesa | 1.24 (1.03–1.49) | 1.73 (1.47–2.03) | |
| Age group, y | |||
| 40–64 | Reference | Reference | 0.001 |
| 65–74 | 1.71 (1.54–1.89) | 1.74 (1.64–1.85) | |
| 75–84 | 2.78 (2.52–3.07) | 3.28 (3.09–3.49) | |
| ≥85 | 4.24 (3.66–4.91) | 5.31 (4.79–5.9) | |
| Recent MI | 1.30 (1.21–1.40) | 1.05 (1.00–1.11) | <0.001 |
| Remote MI | 1.27 (1.17–1.38) | 1.14 (1.08–1.20) | 0.03 |
| Previous PCI | 1.06 (0.96–1.17) | 0.93 (0.87–0.99) | 0.04 |
| COPD/asthma | 1.26 (1.18–1.35) | 1.37 (1.31–1.43) | 0.05 |
| Depression | 1.20 (1.00–1.44) | 1.52 (1.32–1.75) | 0.04 |
CABG indicates coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Mitral, aortic, or tricuspid valve surgery.
In the sensitivity analysis in which we used inverse probability of treatment weighting of the propensity score, we found female sex to be associated with a higher risk of death (HR, 1.10; 95% CI, 1.05–1.16).
Long‐Term Survival by Surgery Type
The sex‐ and procedure‐specific mortality rates are shown in Table 5. Overall, long‐term AMRs were lowest in patients who underwent isolated CABG, followed by single‐valve, multiple‐valve, CABG/single‐valve, and CABG/multiple‐valve surgeries. AMRs after isolated CABG and combined CABG/single‐valve procedures were significantly higher in women than men. After CABG/multiple‐valve surgeries, women had numerically higher AMR than men, whereas after multiple‐valve surgery, men had numerically higher AMR than women. There were no sex‐based differences in long‐term survival after single‐valve surgery. The adjusted Kaplan‐Meier survival curves (Figure 4) also support these findings.
Figure 4.
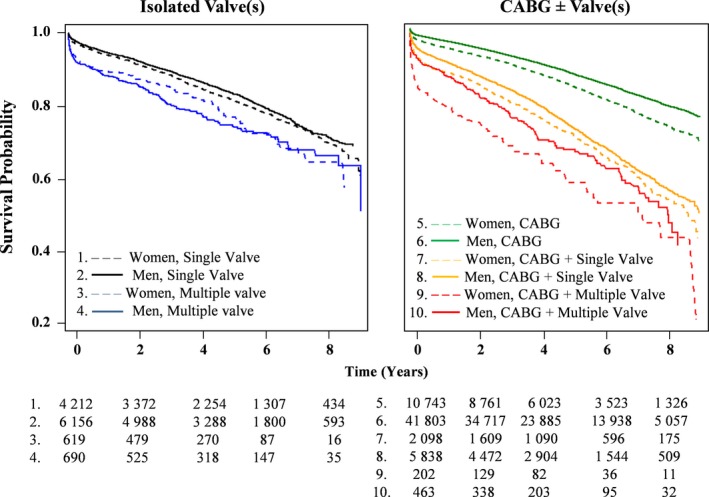
Adjusted survival curves in patients who underwent isolated valvular procedures (left) and those who underwent procedures involving coronary revascularization (right). CABG indicates coronary artery bypass grafting.
As long‐term survival differed by surgery type, we also determined the valve‐specific mortality rates and HRs for the most commonly performed surgical procedures (ie, aortic valve, mitral valve, CABG/aortic valve, CABG/mitral valve, and combined mitral/aortic valve) (Figure S1). We found that women had higher AMRs compared with men after isolated CABG (P<0.001) and combined CABG/mitral valve procedures (P=0.005). There were no statistically significant differences in long‐term survival between sexes after procedures other than isolated CABG and combined CABG/mitral valve surgery.
We then examined those who had isolated mitral or aortic valve procedures for any sex‐specific differences in outcomes. Specifically, we compared the long‐term mortality of women versus men, who underwent the following: (1) mitral valve repair versus replacement (Figure 5) and (2) simple (ie, isolated) aortic valve replacement (AVR) versus complex AVR (ie, involving root reconstruction and/or coronary reimplantation) (Figure 6). In patients who underwent isolated mitral valve surgery, those who underwent repair had higher rates of long‐term survival compared with those who had replacement surgery. In addition, in patients who had mitral valve repair, men had higher rates of long‐term survival than women. Conversely, in those who had mitral valve replacement (MVR), women had higher rates of long‐term survival than men. In patients who underwent isolated AVR, there were no statistically significant differences in long‐term survival when comparing simple with complex procedure and women with men.
Figure 5.
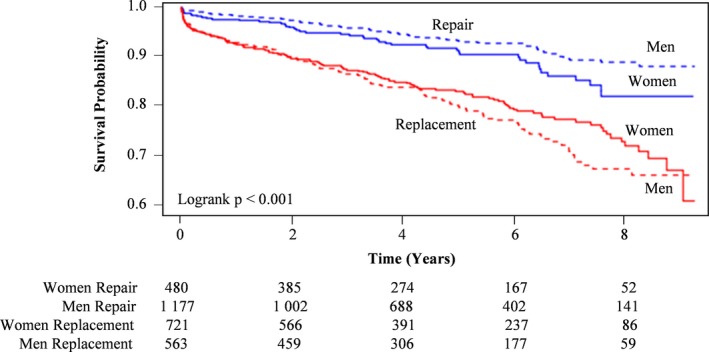
Kaplan‐Meier survival curves in women and men who underwent mitral valve repair vs replacement.
Figure 6.
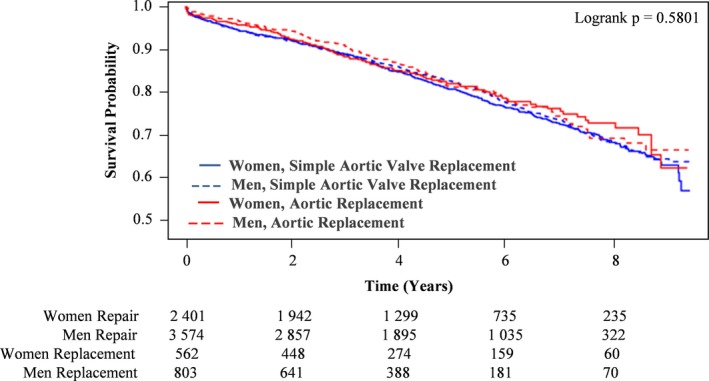
Kaplan‐Meier survival curves of women and men who underwent simple vs complex aortic valve replacement.
We conducted a post hoc analysis to determine whether there were sex differences in the type of surgery performed during an acute, nonelective cardiac admission (Table S1). We found that women were more likely than men to have isolated CABG during an acute cardiac admission. There were no sex differences in other urgent procedures performed.
Discussion
In this population‐based study, we found patient sex to be an important determinant of long‐term survival after cardiac surgery. Three main findings were derived from this study. (1) Long‐term AMR was lowest in patients who underwent isolated CABG and highest among those who underwent combined CABG/multiple valve surgery. (2) Women had significantly higher AMRs than men after CABG and combined CABG/mitral valve surgery. Overall, AMR was numerically higher in women than men after any procedure involving coronary revascularization. (3) Men had higher rates of long‐term survival than women after isolated mitral valve repair, whereas women had higher rates of long‐term survival than men after isolated MVR.
Sex Differences in Long‐Term Survival After Cardiac Surgery
It is well documented that compared with men, women who present for CABG are older, have a smaller body surface area, are more likely to require an urgent/emergent operation, and have a greater burden of comorbid conditions, such as diabetes mellitus, hypertension, HF, cerebrovascular disease, and anemia.6, 11, 12, 35, 36, 37 Similar sex differences in comorbidities have been noted in patients presenting for isolated mitral,17, 38, 39 aortic valve,15 and concomitant CABG and valve surgeries.40 Our results corroborate these findings in that women presenting for cardiac surgery were older, more frail, and more medically complex than men. In addition, women with cardiovascular diseases often experience a delay in diagnosis and treatment when compared with men, which may, in part, explain the more advanced coronary or valvular pathological conditions observed in women at the time of surgery.6, 41, 42 These established sex differences explain our overall observation of lower estimated long‐term survival in women after cardiac surgery, and of female sex being an independent risk factor for long‐term mortality.
The Impact of Procedure Type on Survival
We found that valvular procedures were performed in near equal frequencies in both sexes, whereas procedures involving coronary revascularization were performed much more frequently in men. These findings are consistent with the literature.6, 43, 44 Female sex has been reported as an independent risk factor for perioperative mortality as well as mortality within 5 years of CABG.6, 7, 35, 45, 46 However, sex does not consistently influence survival after 5 years.9, 14, 40, 47, 48 Nicolini et al,48 in a study of 1332 women and 5976 men who underwent isolated CABG in Italy between 2003 and 2013, found no sex difference in survival at mean follow‐up of 8 years. In a study of 607 women and 3326 men who underwent isolated CABG in Stockholm, Sweden, between 1980 and 1989, significant sex differences in survival were observed at 30 days but not in long‐term (5‐year) follow‐up.49 This study compared 5‐year mortality in women and men who survived the index surgical admission, thus removing the contribution of early female mortality to the calculation of long‐term risk. In addition, this study has a small sample size, especially in the female group; and temporal changes in surgical outcomes, as well as geographic differences in surgical practice and health‐seeking behavior, may in part explain these differences in results. A recent meta‐analysis45 showed that female sex was an independent risk factor for mortality at 5 years after isolated CABG (random‐effects odds ratio, 1.14; 95% CI (confidence interval), 1.08–1.20; I2=0%). This observation is in keeping with the current study, in which we found the HR associated with female sex to be 1.10 (95% CI, 1.05–1.16) after inverse probability of treatment weighting using the propensity score. In addition, poorer survival after CABG in women may be attributed to smaller coronary size,50 which may contribute to technical challenges,12, 51 the risk of graft‐coronary mismatch, and incomplete revascularization.52, 53 The diagnosis of coronary artery disease may be delayed in women compared with men, possibly because of the likelihood for an atypical presentation to coronary artery disease for women.54 Women are also older, have a higher burden of comorbidities, are more likely to have been hospitalized for HF within 90 days and 1 year before CABG,6 and have higher rates of postoperative complications, such as new‐onset dialysis and late cardiac readmission.6, 48, 55, 56 In addition, our post hoc analysis found that women were more likely than men to have isolated CABG during an acute cardiac admission, which may, in part, explain their poorer prognosis.
Limited knowledge has been generated to date on the long‐term outcomes after combined CABG/valve surgery. We found that among those who underwent valvular surgery with concomitant CABG, women had numerically, albeit nonstatistically significantly, higher AMRs when compared with men. Sex was not a risk factor for long‐term survival after combined CABG/valve surgery. Our findings corroborate a small case series of 1567 patients who had combined CABG and aortic, mitral, or multiple‐valve surgeries in Toronto, Canada, between 1990 and 2000, in which women and men had similar rates of survival at 5.3 years of follow‐up.40 In a more recent cohort of 5867 patients undergoing combined CABG and aortic valve surgery (33% women), the risk of in‐hospital mortality was higher in women compared with men (odds ratio, 2.00; 95% CI, 1.44–2.79). Long‐term outcomes were not examined.44 These authors concluded that substantial sex differences existed in patient presentation and mortality risk factors, and that standard risk scores, such as the EuroSCORE, did not perform as well in women as in men.
The sex differences in long‐term outcomes after combined CABG/mitral valve surgery have not been systematically reported. In the current study, we found a significantly higher AMR in women undergoing CABG/mitral valve surgery. This could be attributed to the poorer prognosis after CABG in women with HF,6, 7 as those needing combined CABG/mitral valve surgery are more likely to have HF with reduced EF. The presence of HF compounds the sex‐specific challenges in patients with coronary artery disease and further places women at risk.
We found no sex differences in long‐term survival after single‐valve surgery. This finding was likely driven by a higher overall number of AVRs performed compared with mitral valve surgery. In a nationwide study of all patients undergoing MVR in the Netherlands between 2007 and 2011 (N=3411; 42% women), no sex differences were reported for mortality in the hospital.43 Long‐term outcomes were not evaluated in this study. In a study of 743 patients (28% women) who underwent mitral repair for degenerative mitral regurgitation between 2001 and 2014 in Ottawa, Canada,57 sex differences in survival were not observed at 5 years. However, the authors found that women were more likely to present with more advanced disease than men at the time of surgery. A study of 183 792 Medicare beneficiaries, between 2000 and 2009, found no difference in long‐term survival by sex after mitral valve surgery. However, the authors found that women were more likely to receive mitral replacement instead of repair, and that repair restored life expectancy for men but not for women.18 In a cohort of 3118 (40.4% women) who had MVR or AVR in Ottawa, Canada, between 1976 and 2006, no statistically significant sex differences were observed in survival after MVR.58 The same authors also found women to have a lower risk of mortality 5.6 years after bioprosthetic AVR (adjusted HR, 0.5; 95% CI, 0.3–0.6), but no difference in survival between sexes after mechanical AVR. We also found that life expectancy was higher in men than women after mitral valve repair; however, women had higher life expectancy after MVR than men. In our subgroup of patients who underwent isolated AVR, we found no sex differences in long‐term survival irrespective of the complexity of surgery. The findings of the Ottawa study58 pertaining to survival after bioprosthetic AVR differ from the current province‐wide study and may reflect center‐based practice and experience with selection of mechanical versus bioprosthetic valves.
Limitations
This study has several limitations. First, the sex differences in outcomes are representative of surgical practice in Ontario. Similar research needs to be conducted in other settings to confirm the generalizability of our findings. Second, our data sources lacked details about the specific valve implant type (ie, bioprosthetic versus mechanical, and brand name) or repair technique. This precluded us from being able to examine sex‐specific differences in outcomes in detail. Third, we lacked some details about patient characteristics, such as race and ethnicity, which some studies have shown may be independent predictors of mortality after cardiac surgery.59 The inability to measure, and thereby adjust for, differences in such characteristics could have explained, in part, the differences in mortality observed in this study. Third, cohort studies are by nature subject to residual confounding.
Conclusions
We described the sex differences in long‐term outcomes after a variety of cardiac procedures in a large, real‐world cohort. We found a statistically significant association between female sex and long‐term mortality after cardiac surgery. Specifically, women experienced higher rates of mortality than men after CABG and combined CABG/mitral valve surgery, and mortality risk factors also differed by sex. Perioperative optimization and long‐term follow‐up should be tailored to younger women with a history of MI and previous PCI and older men with a history of COPD and depression. Given the substantial sex differences in patient presentation for coronary and valvular heart disease, further efforts need to be directed at the education of both physicians and patients in the early recognition of acute presentation of cardiac disease in women. In addition, given the poorer performance of standard risk scores in women, further research is needed to derive and validate sex‐specific risk prediction models in patients undergoing cardiac surgery.
Sources of Funding
This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. We also acknowledge support from an operating grant from the Ontario Ministry of Health and Long‐Term Care (Grant No. 4708). Dr Lee is supported by the Ted Rogers Chair in Heart Function Outcomes and a Mid‐Career Investigator Award from the Heart and Stroke Foundation. Dr Sun is supported by the Ottawa Heart Institute Research Corporation.
Disclosures
None.
Supporting information
Table S1. Proportion of Patients With Surgeries Performed During an Acute, Non‐Elective Hospital Admission, Stratified by Sex and Procedure Type
Figure S1. Crude and age‐standardized mortality rates and adjusted hazard ratios, stratified by sex and procedure type.
Acknowledgments
The authors acknowledge that the clinical registry data used in this analysis are from participating hospitals through CorHealth Ontario, which serves as an advisory body to the Ontario Ministry of Health and Long‐Term Care, is funded by the Ontario Ministry of Health and Long‐Term Care, and is dedicated to improving the quality, efficiency, access, and equity in the delivery of the continuum of adult cardiac and stroke services in Ontario, Canada. The authors also acknowledge the use of data compiled and provided by the Canadian Institute for Health Information. These data sets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences. The analyses, conclusions, opinions, and statements expressed in the article are those of the authors, and do not necessarily reflect those of the above agencies.
(J Am Heart Assoc. 2019;8:e013260 DOI: 10.1161/JAHA.119.013260.)
References
- 1. Kang H, Chung M. Images in clinical medicine: peripheral artery disease. N Engl J Med. 2007;357:e19. [DOI] [PubMed] [Google Scholar]
- 2. Favaloro R. Critical analysis of coronary artery bypass graft surgery: a 30‐year journey. J Am Coll Cardiol. 1998;31:1B–63B. [DOI] [PubMed] [Google Scholar]
- 3. Karim M, Reid C, Huq M, Brilleman S, Cochrane A, Tran L, Billah B. Predicting long‐term survival after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2018;26:257–263. [DOI] [PubMed] [Google Scholar]
- 4. Baillot R, Joanisse D, Stevens L, Doyle D, Dionne B, Lellouche F. Recent evolution in demographic and clinical characteristics and in‐hospital morbidity in patients undergoing coronary surgery. Can J Surg. 2009;52:394–400. [PMC free article] [PubMed] [Google Scholar]
- 5. Blasberg J, Schwartz G, Balaram S. The role of gender in coronary surgery. Eur J Cardiothorac Surg. 2011;40:715–721. [DOI] [PubMed] [Google Scholar]
- 6. Sun L, Tu J, Bader Eddeen A, Liu P. Prevalence and long‐term survival after coronary artery bypass grafting in men and women with heart failure and preserved versus reduced ejection fraction. J Am Heart Assoc. 2018;7:e008902 DOI: 10.1161/JAHA.118.008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun L, Tu J, Lee D, Beanlands R, Ruel M, Austin P, Bader Eddeen A, Liu P. Disability‐free survival after coronary artery bypass grafting in women and men with heart failure. Open Heart. 2018;5:e000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun L, Tu J, Coutinho T, Turek M, Rubens F, McDonnell L, Tulloch H, Mielniczuk L. Sex differences in heart failure outcomes in an ambulatory, population‐based cohort. CMAJ. 2018;190:E848–E854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arif R, Farag M, Gertner V, Szabó G, Weymann A, Veres G, Ruhparwar A, Bekeredjian R, Bruckner T, Karck M, Kallenbach K, Beller C. Female gender and differences in outcome after isolated coronary artery bypass graft surgery: does age play a role? PLoS One. 2016;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ennker I, Albert A, Pietrowski D, Bauer K, Ennker J, Florath I. Impact of gender on outcome after coronary artery bypass surgery. Asian Cardiovasc Thorac Ann. 2009;17:253–258. [DOI] [PubMed] [Google Scholar]
- 11. Faerber G, Zacher M, Reents W, Boergermann J, Kappert U, Boening A, Diegeler A, Doenst T. Female sex is not a risk factor for post procedural mortality in coronary bypass surgery in the elderly: a secondary analysis of the GOPCABE trial. PLoS One. 2017;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fisher L, Kennedy J, Davis K, Maynard C, Fritz J, Kaiser G, Myers W. Association of sex, physical size, and operative mortality after coronary artery bypass in the Coronary Artery Surgery Study (CASS). J Thorac Cardiovasc Surg. 1982;84:334–341. [PubMed] [Google Scholar]
- 13. King K, Clark P, Hicks G. Patterns of referral and recovery in women and men undergoing coronary artery bypass grafting. Am J Cardiol. 1992;69:179–182. [DOI] [PubMed] [Google Scholar]
- 14. Saxena A, Dinh D, Smith J, Shardey G, Reid C, Newcomb A. Sex differences in outcomes following isolated coronary artery bypass graft surgery in Australian patients: analysis of the Australasian Society of Cardiac and Thoracic Surgeons cardiac surgery database. Eur J Cardiothorac Surg. 2012;41:755–762. [DOI] [PubMed] [Google Scholar]
- 15. Hamed O, Persson P, Engel A, McDonough S, Smith J. Gender differences in outcomes following aortic valve replacement surgery. IJS. 2009;7:214–217. [DOI] [PubMed] [Google Scholar]
- 16. Pfannmueller B, Eifert S, Seeburger J, Misfeld M, Borger M, Mende M, Garbade J, Mohr F. Gender‐dependent differences in patients undergoing tricuspid valve surgery. Thorac Cardiovasc Surg. 2013;61:37–41. [DOI] [PubMed] [Google Scholar]
- 17. Seeburger J, Eifert S, Pfannmüller B, Garbade J, Vollroth M, Misfeld M, Borger M, Mohr F. Gender differences in mitral valve surgery. Thorac Cardiovasc Surg. 2012;61:042–046. [DOI] [PubMed] [Google Scholar]
- 18. Vassileva C, McNeely C, Mishkel G, Boley T, Markwell S, Hazelrigg S. Gender differences in long‐term survival of Medicare beneficiaries undergoing mitral valve operations. Ann Thorac Surg. 2013;96:1367–1373. [DOI] [PubMed] [Google Scholar]
- 19. Gao D, Grunwald G, Rumsfeld J, Schooley L, MacKenzie T, Shroyer A. Time‐varying risk factors for long‐term mortality after coronary artery bypass graft surgery. Ann Thorac Surg. 2006;81:793–799. [DOI] [PubMed] [Google Scholar]
- 20. Tu J, Ko D, Guo H, Richards J, Walton N, Natarajan M, Wijeysundera H, So D, Latter D, Feindel C, Kingsbury K, Cohen E; Cardiac Care Network of Ontario's Variations in Revascularization Practice in Ontario Working Group . Determinants of variations in coronary revascularization practices. CMAJ. 2012;184:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tu K, Campbell N, Chen Z, Cauch‐Dudek K, McAlister F. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 22. Juurlink D, Preya C, Croxford R, Chong A, Austin P, Tu J, Laupacis A. Canadian Institute for Health Information discharge abstract database: a validation study. Institute for Clinical Evaluative Sciences. 2006. https://www.ices.on.ca/Publications/Atlases-and-Reports/2006/Canadian-Institute-for-Health-Information. Accessed August 4, 2019.
- 23. Hux J, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516. [DOI] [PubMed] [Google Scholar]
- 24. Austin P, Daly P, Tu J. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–296. [DOI] [PubMed] [Google Scholar]
- 25. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J, Saunders L, Beck C, Feasby T, Ghali W. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130‐1139. [DOI] [PubMed] [Google Scholar]
- 26. Gershon A, Wang C, Guan J, Vasilevska‐Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD. 2009;6:388–394. [DOI] [PubMed] [Google Scholar]
- 27. Schultz S, Rothwell D, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33:160–166. [PubMed] [Google Scholar]
- 28. du Plessis V, Beshiri R, Bollman R, Clemeson H. Definitions of “Rural”: Agriculture and Rural Working Paper Series, No. 61. Ottawa, ON, Canada: Statistics Canada; 2002. [Google Scholar]
- 29. Tran D, Tu J, Dupuis J, Bader Eddeen A, Sun L. Association of frailty and long‐term survival in patients undergoing coronary artery bypass grafting. J Am Heart Assoc. 2018;7:e009882 DOI: 10.1161/JAHA.118.009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abrams C, Lieberman R, Weiner J. Development and evaluation of the Johns Hopkins University risk adjustment models for Medicare+choice plan payment. 2003. https://www.hopkinsacg.org/document/development-and-evaluation-of-the-johns-hopkins-university-risk-adjustment-models-for-medicarechoice-plan-payment/. Accessed August 4, 2019.
- 31. Sternberg S, Bentur N, Abrams C, Spalter T, Karpati T, Lemberger J, Heymann A. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18:e392–e397. [PubMed] [Google Scholar]
- 32. Weiner J, Abrams C. The Johns Hopkins Adjusted Clinical Groups Technical Reference Guide, Version 9.0. Baltimore, MD: Johns Hopkins University Press; 2009. [Google Scholar]
- 33. Bronskill S, Carter M, Costa A, Esensoy A, Gill S, Gruneir A, Henry D, Hirdes J, Jaakkimainen R, Poss J, Wodchis W. Aging in Ontario: an ICES chartbook of health service use by older adults. Institute for Clinical Evaluative Sciences. 2010. https://www.ices.on.ca/Publications/Atlases-and-Reports/2010/Aging-in-Ontario. Accessed August 4, 2019.
- 34. Bronskill SCX, Gruneir A, Ho M. Health System Use by Frail Ontario Seniors: An In‐Depth Examination of Four Vulnerable Cohorts. Ontario, Canada: ICES; 2011. [Google Scholar]
- 35. Blankstein R, Ward R, Arnsdorf M, Jones B, Lou Y, Pine M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: contemporary analysis of 31 midwestern hospitals. Circulation. 2005;112:I323–I327. [DOI] [PubMed] [Google Scholar]
- 36. Khan S, Nessim S, Gray R, Czer L, Chaux A, Matloff J. Increased mortality of women in coronary artery bypass surgery: evidence for referral bias. Ann Intern Med. 1990;112:561–567. [DOI] [PubMed] [Google Scholar]
- 37. Weintraub W, Wenger N, Jones E, Craver J, Guyton R. Changing clinical characteristics of coronary surgery patients: differences between men and women. Circulation. 1993;88:II79‐II86. [PubMed] [Google Scholar]
- 38. McNeely C, Vassileva C. Mitral valve surgery in women: another target for eradicating sex inequality. Circ Cardiovasc Qual Outcomes. 2016;9:S94–S96. [DOI] [PubMed] [Google Scholar]
- 39. Song H, Grab J, O'Brien S, Welke K, Edwards F, Ungerleider R. Gender differences in mortality after mitral valve operation: evidence for higher mortality in perimenopausal women. Ann Thorac Surg. 2008;85:2040–2045. [DOI] [PubMed] [Google Scholar]
- 40. Doenst T, Ivanov J, Borger M, David T, Brister S. Sex‐specific long‐term outcomes after combined valve and coronary artery surgery. Ann Thorac Surg. 2006;81:1632–1636. [DOI] [PubMed] [Google Scholar]
- 41. Maas A, Appelman Y. Gender differences in coronary heart disease. Neth Heart J. 2010;18:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J. Lisheng L; Interheart Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 43. Mokhles M, Siregar S, Versteegh M, Noyez L, van Putte B, Vonk A, Roos‐Hesselink J, Bogers A, Takkenberg J; Data Registry Committee of the Netherlands Association for Cardio‐Thoracic Surgery . Male‐female differences and survival in patients undergoing isolated mitral valve surgery: a nationwide cohort study in the Netherlands. Eur J Cardiothorac Surg. 2016;50:482–487. [DOI] [PubMed] [Google Scholar]
- 44. Mokhles M, Soloukey Tbalvandany S, Siregar S, Versteegh M, Noyez L, van Putte B, Vonk A, Roos‐Hesselink J, Bogers A, Takkenberg J. Male‐female differences in aortic valve and combined aortic valve/coronary surgery: a national cohort study in the Netherlands. Open Heart. 2018;5:e000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alam M, Bandeali S, Kayani W, Ahmad W, Shahzad S, Jneid H, Birnbaum Y, Kleiman N, Coselli J, Ballantyne C, Lakkis N, Virani S. Comparison by meta‐analysis of mortality after isolated coronary artery bypass grafting in women versus men. Am J Cardiol. 2013;112:309–317. [DOI] [PubMed] [Google Scholar]
- 46. Filardo G, Hamman B, Pollock B, da Graca B, Sass D, Phan T, Edgerton J, Prince S, Ring W. Excess short‐term mortality in women after isolated coronary artery bypass graft surgery. Open Heart. 2016;3:e000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guru V, Fremes S, Tu J. Time‐related mortality for women after coronary artery bypass graft surgery: a population‐based study. J Thorac Cardiovasc Surg. 2004;127:1158–1165. [DOI] [PubMed] [Google Scholar]
- 48. Nicolini F, Vezzani A, Fortuna D, Contini G, Pacini D, Gabbieri D, Zussa C, De Palma R, Gherli T. Gender differences in outcomes following isolated coronary artery bypass grafting: long‐term results. J Cardiothorac Surg. 2016;11:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saxena A, Poh C, Dinh D, Reid C, Smith J, Shardey G, Newcomb A. Does patient gender affect outcomes after concomitant coronary artery bypass graft and aortic valve replacement? An Australian Society of Cardiac and Thoracic Surgeons database study. Cardiology. 2011;119:116–123. [DOI] [PubMed] [Google Scholar]
- 50. Dodge J, Brown B, Bolson E, Dodge H. Lumen diameter of normal human coronary arteries: influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86:232–246. [DOI] [PubMed] [Google Scholar]
- 51. O'Connor G, Morton J, Diehl M, Olmstead E, Coffin L, Levy D, Maloney C, Plume S, Nugent W, Malenka D, Hernandez F, Clough R, Birkmeyer J, Marrin C, Leavitt B; The Northern New England Cardiovascular Disease Study Group. Differences between men and women in hospital mortality associated with coronary artery bypass graft surgery. Circulation. 1993;88:2104–2110. [DOI] [PubMed] [Google Scholar]
- 52. Crosby I, Wellons H, Taylor G, Maffeo C, Beller G, Muller W. Critical analysis of the preoperative and operative predictors of aortocoronary bypass patency. Ann Surg. 1981;193:743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bjork V, Ekestrom S, Henze A, Ivert T, Landou C. Early and late patency of aortocoronary vein grafts. Scand J Thorac Cardiovasc Surg. 1981;15:11–21. [DOI] [PubMed] [Google Scholar]
- 54. Jarvie J, Foody J. Recognizing and improving health care disparities in the prevention of cardiovascular disease in women. Curr Cardiol Rep. 2010;12:488–496. [DOI] [PubMed] [Google Scholar]
- 55. den Ruijter H, Haitjema S, van der Meer M, van der Harst P, Rouleau J, Asselbergs F, van Gilst W; IMAGINE Investigators . Long‐term outcome in men and women after CABG: results from the IMAGINE trial. Atherosclerosis. 2015;241:284–288. [DOI] [PubMed] [Google Scholar]
- 56. Guru V, Fremes S, Austin P, Blackstone E, Tu J. Gender differences in outcomes after hospital discharge from coronary artery bypass grafting. Circulation. 2006;113:507–516. [DOI] [PubMed] [Google Scholar]
- 57. Chan V, Chen L, Elmistekawy E, Ruel M, Mesana T. Determinants of late outcomes in women undergoing mitral repair of myxomatous degeneration. Interact Cardiovasc Thorac Surg. 2016;23:779–783. [DOI] [PubMed] [Google Scholar]
- 58. Kulik A, Lam B, Rubens F, Hendry P, Masters R, Goldstein W, Bédard P, Mesana T, Ruel M. Gender differences in the long‐term outcomes after valve replacement surgery. Heart. 2009;95:318–326. [DOI] [PubMed] [Google Scholar]
- 59. Bridges C. Cardiac surgery in African Americans. Ann Thorac Surg. 2003;76:S1356–S1362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Proportion of Patients With Surgeries Performed During an Acute, Non‐Elective Hospital Admission, Stratified by Sex and Procedure Type
Figure S1. Crude and age‐standardized mortality rates and adjusted hazard ratios, stratified by sex and procedure type.


