Abstract
Background
Left ventricular (LV) involvement is common in arrhythmogenic right ventricular cardiomyopathy (ARVC). We aim to evaluate LV involvement in ARVC patients by cardiovascular magnetic resonance feature tracking.
Methods and Results
Sixty‐eight patients with ARVC and 30 controls were prospectively enrolled. ARVC patients were divided into 2 subgroups: the preserved LV ejection fraction (LVEF) group (LVEF ≥55%, n=27) and the reduced LVEF group (LVEF <55%, n=41). Cardiovascular magnetic resonance with late gadolinium enhancement (LGE) and cardiovascular magnetic resonance feature tracking were performed in all subjects. LV global and regional (basal, mid, apical) peak strain (PS) in radial, circumferential and longitudinal directions were assessed, respectively. Right ventricular global PS in three directions were also analyzed. Compared with the controls, LV global and regional PS were all significantly impaired in the reduced LVEF group (all P<0.05). However, only LV global longitudinal PS as well as mid and apical longitudinal PS were impaired in the preserved LVEF group (all P<0.05), and all these parameters were significantly associated with right ventricular global radial PS (r=−0.47, −0.47, and −0.49, respectively, all P<0.001). The reduced LVEF group showed significantly higher prevalence of LGE (95.10% versus 63.00%, P=0.002) than the preserved LVEF group. Moreover, LV radial PS was significantly reduced in LV segments with LGE (33.15±20.42%, n=46) than those without LGE (41.25±15.98%, n=386) in the preserved LVEF group (P=0.016).
Conclusions
In patients with ARVC, cardiovascular magnetic resonance feature tracking could detect early LV dysfunction, which was associated with LV myocardial LGE and right ventricular dysfunction.
Keywords: arrhythmogenic right ventricular dysplasia, magnetic resonance imaging, strain, ventricle
Subject Categories: Magnetic Resonance Imaging (MRI), Cardiomyopathy
Clinical Perspective
What Is New?
This is the first study to apply cardiovascular magnetic resonance feature tracking (CMR‐FT) to assess left ventricular (LV) dysfunction and its association with late gadolinium enhancement in arrhythmogenic right ventricular cardiomyopathy patients with preserved LV ejection fraction (LVEF).
Impaired LV global and regional peak strain could be detected by CMR‐FT in arrhythmogenic right ventricular cardiomyopathy patients with preserved LVEF.
LV dysfunction detected by CMR‐FT was associated with LV myocardial late gadolinium enhancement and right ventricular deformation mechanics.
What Are the Clinical Implications?
Findings of this study indicate that CMR‐FT provides a novel method for the evaluation of the global and regional myocardial contraction and could be more sensitive to detect early LV dysfunction than LVEF in arrhythmogenic right ventricular cardiomyopathy patients.
LV global and regional dysfunction detected by CMR‐FT was associated with late gadolinium enhancement and RV dysfunction. Since the presence of biventricular dysfunction was a stronger predictor of adverse outcomes than isolated right ventricular diseases, the early detection of LV involvement by CMR‐FT could be of clinical significance in risk stratification of arrhythmogenic right ventricular cardiomyopathy patients with preserved LVEF.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy characterized by progressive fibro‐fatty myocardial replacement, ventricular tachycardia, and ventricular dysfunction, preferentially affecting the right ventricle (RV).1 However, left ventricular (LV) involvement has been increasingly recognized across a broad spectrum of ARVC severity.2 Detection of LV involvement is of clinical importance because the presence of biventricular dysfunction was reported to be a stronger predictor of adverse outcomes including sudden cardiac death and heart failure than isolated RV disease.3
Cardiac magnetic resonance (CMR) plays a prominent role in the diagnosis of ARVC because of its unique capability in the detection of fibro‐fatty tissue by late gadolinium enhancement (LGE) and high reproducibility in the assessment of biventricular morphology and function.4 Left ventricular ejection fraction (LVEF) is a widely used parameter in clinical practice although it has been proven to be an insensitive marker of regional systolic dysfunction.5 Currently, LV strain analysis by CMR feature‐tracking (CMR‐FT) has been introduced for the quantitative evaluation of global and regional myocardial contraction.6 Recent studies suggest a potential role of stain analysis in early detection and objective quantification of contractile abnormalities in a variety of cardiovascular diseases.7, 8, 9, 10
Therefore, we aimed to investigate the diagnostic value of this novel technique for detection of LV involvement in ARVC patients with or without preserved LVEF, and to evaluate their relationships with LV myocardial LGE and RV dysfunction.
Methods
The data, analytic methods, and study materials will be made available to other researchers upon reasonable request.
Study Population
This study was approved by hospital institutional review board and informed consents were acquired in all subjects. From January 2016 to July 2017, 75 consecutive ARVC patients were prospectively enrolled in this study. All patients underwent systemic clinical evaluation and CMR examinations. According to the revised Task Force Criteria, the diagnosis of ARVC was made when 2 major, or 1 major plus 2 minor, or 4 minor criteria from different categories were present.11 Seven patients were excluded from further analyses because of inadequate image quality. Finally, a total of 68 patients with ARVC were included. Thirty age‐ and sex‐matched healthy subjects were recruited as controls.
CMR Imaging Protocol
CMR imaging was performed on a 3‐T scanner (Discovery MR750, GE Healthcare, Milwaukee, USA) with a phased‐array cardiovascular coil, using electrocardiographic and respiratory gating. The protocol mainly consisted of: (1) cine imaging; (2) fat imaging; (3) first‐pass perfusion imaging; and (4) LGE imaging. The average acquisition time was ≈40 minutes. All images were acquired with breath holding. Cine images were acquired in 3 long‐axis views (LV 2‐chamber, 4‐chamber, and LV outflow tract) and series of short‐axis planes covering the entire LV using balanced steady state free precession sequence (b‐SSFP). Typical imaging parameters were: field of view=320×320 mm, matrix=192×224, repetition time (TR)=3.3 ms, echo time (TE)=1.7 ms, flip angle=50°, temporal resolution=46 to 60 ms, slice thickness=8 mm, slice gap=2 mm. Fat‐suppressed and non‐fat‐suppressed fast spin‐echo sequences were applied in the LV short‐axis views with double‐inversion recovery blood suppression pulses. Typical imaging parameters were: field of view=320×320 mm, matrix=192×224, TR=1 to 2 R‐R intervals, TE=10 ms, slice thickness=8 mm, slice gap=2 mm. The LGE images were obtained 10 to 15 minutes after intravenous administration of gadolinium‐DTPA (Magnevist, Bayer, Berlin, Germany) at a dose of 0.2 mmol/kg, using a segmented phase‐sensitive inversion recovery Turbo Fast Low Angle Shot sequence at the same position as cine images in end diastole. Typical imaging parameters were: field of view=380×320 mm, matrix=256×162, TR=8.6 ms, TE=3.36 ms, flip angle=25°, slice thickness=8 mm, slice gap=2 mm, nominal TI=300 to 350 ms.
CMR Analysis
All CMR images were analyzed using CVI42 (version 5.0, Circle Cardiovascular Imaging Inc., Calgary, Canada) by 2 radiologists with 8‐ and 10‐year experience of CMR imaging, respectively. The endocardial and epicardial contours of LV myocardium were manually traced at end‐diastole and end‐systole on short‐axis b‐SSFP cine images. Papillary muscles were excluded from volumes. Cardiac volumetric and functional parameters, including left/right ventricular end‐diastolic volume, left/right ventricular end‐systolic volume, and left/right ventricular ejection fraction (LVEF/RVEF), were automatically generated. All the volumetric parameters were indexed to body surface area. The presence of LGE in LV myocardium was visually analyzed using the American Heart Association (AHA) 16‐segment model by consensus reading of 2 independent observers. The regions of LGE were fine‐tuned by the operator to reduce false‐positive when necessary.
Myocardial Strain Analysis by CMR‐FT
The CMR‐FT analysis was performed on the acquired b‐SSFP cine images using CVI42 (Version 5.0). For right ventricle (RV), the endo‐and epicardial contours of RV free wall in 4‐chamber and short‐axis cine images were manually drawn at end‐diastole with subsequent automatic tracking throughout the cardiac cycle. The RV global peak strain (PS) in radial, circumferential and longitudinal directions were analyzed, respectively. The basis of LV strain algorithms has been previously described and their validity has been demonstrated.12, 13 Briefly, a set of cine images in short‐axis and three long‐axis views (2‐chamber, 4‐chamber, and LV outflow tract) were loaded into the feature tracking module. All endocardial and epicardial borders of LV at end‐diastole were manually delineated (Figure 1) with subsequent automatic tracking throughout the cardiac cycle. The quality of automatic tracking was checked and manually adjusted if needed. After defining the RV insertion points within the LV in short‐axis images, the LV global and regional (basal, mid and apical) PS in radial, circumferential and longitudinal mode were automatically derived by the software. The LV segmental strain parameters were also provided according to the American Heart Association 16‐segment model. The algorithm of LV regional PS was based on the average PS values of its corresponding segments. Lengthening or thickening of LV myocardium was defined as positive value (radial strain), while shortening or thinning was defined as negative value (circumferential and longitudinal strain).14
Figure 1.
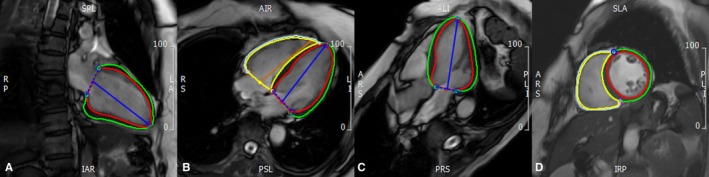
Measurement of biventricular strain by CMR‐FT. Biventricular endo‐ and epicardial contours were manually delineated at the phase of end‐diastole on the 2‐chamber (A), 4‐chamber (B), LV outflow tract (C), and short axis (D) cine images, respectively. CMR indicates cardiovascular magnetic resonance; FT, feature tracking.
Reproducibility Analysis
For the assessment of the inter‐observer and intra‐observer reproducibility, a randomly selected set of 20 ARVC patients were assessed by two experienced investigators. The same subjects were assessed by each investigator independently for inter‐observer analysis. To determine the intra‐observer variability, the measurement was repeated by one of the investigators 1 month later.
Statistical Analysis
Continuous variables were given as mean±SD or median values with interquartile range depending on normality of the variables. Categorical variables were presented as percentages. Comparisons for continuous data were performed using Student t‐test or one‐way ANOVA. Categorical variables were compared using the chi‐square test or Fisher exact test. Correlations were assessed by the Pearson or Spearman rank correlation coefficient. Receiver operating characteristics analysis was used to define the optimal cut‐off values. The intraclass correlation coefficient was used to assess the inter‐ and intra‐observer variability. All statistical analyses were conducted by using a statistical software package SPSS, Version 24.0 (IBM, SPSS Statistics). A 2‐tailed P<0.05 was considered statistically significant.
Results
Baseline Characteristics
There were 68 ARVC patients (mean age 39.28±13.88 years, 45 men) and 30 healthy controls (mean age 40.20±12.42 years, 17 men) in this study. The ARVC patients were further divided into 2 subgroups: the preserved LVEF group (LVEF ≥55%, n=27) and the reduced LVEF group (LVEF <55%, n=41). The baseline characteristics of the study population were presented in Table 1. No significant differences were observed in terms of baseline characteristics among 3 groups.
Table 1.
Baseline Characteristics and Conventional CMR Parameters
| Total ARVC (n=68) | ARVC (LVEF <55%) (n=41) | ARVC (LVEF ≥55%) (n=27) | Controls (n=30) | |
|---|---|---|---|---|
| Age, y | 39.28±13.88 | 37.24±14.37 | 42.50±12.66 | 40.20±12.42 |
| Male sex, n (%) | 45 (66.18) | 25 (61.00) | 20 (74.10) | 17 (56.67) |
| BSA, m2 | 1.74±0.21 | 1.70±0.20 | 1.79±0.21 | 1.77±0.24 |
| rTFC (major or minor), n (%) | ||||
| Structural abnormalities | 68 (100.00) | 41 (100.00) | 27 (100.00) | ··· |
| Repolarization abnormalities | 45 (66.18) | 28 (27.10) | 17 (17.90) | ··· |
| Depolarization abnormalities | 4 (5.88) | 3 (2.40) | 1 (1.60) | ··· |
| Ventricular arrhythmia | 42 (61.76) | 23 (25.30) | 19 (16.70) | ··· |
| Family history | 6 (8.82) | 3 (3.60) | 3 (2.40) | ··· |
| rTFC score, n (median) | 4 (4–5) | 5 (4–6) | 4 (4–5) | ··· |
| Conventional CMR parameters | ||||
| LVEF, % | 48.22±12.54a | 41.16±11.16b | 58.93±3.97c | 61.85±4.82 |
| LVESVi, mL/m2 | 40.20±22.05a | 49.68±23.73b | 25.82±5.47c | 28.34±7.42 |
| LVEDVi, mL/m2 | 73.74±22.44 | 80.89±24.87 | 62.88±11.94b, c | 73.61±13.80 |
| RVEF, % | 21.90±9.22a | 21.20±8.17b | 22.96±10.69b | 45.70±5.69 |
| RVESVi, mL/m2 | 99.67±46.54a | 101.61±47.26b | 96.74±46.16b, c | 37.74±8.99 |
| RVEDVi, mL/m2 | 125.07±49.56a | 126.36±50.97b | 123.13±48.22b | 58.84±11.60 |
| LGE presence in LV, n (%) | 46 (67.65) | 39 (95.10) | 7 (63.00)c | ··· |
| No. of LGE segments per patient | 4.44±2.53 | 4.78±2.79 | 3.57±1.45 | ··· |
Data were presented as percentages in parentheses, mean±SD, or median values with interquartile range in parentheses. ARVC indicates arrhythmogenic right ventricular cardiomyopathy; BSA, body surface area; CMR, cardiac magnetic resonance; EDVi, end‐diastolic volume index; EF, ejection fraction; ESVi, end‐systolic volume index; LGE, late gadolinium enhancement; LV, left ventricular; rTFC, revised Task Force Criteria; RV, right ventricular.
P<0.05 when compared with the controls between 2 groups (the total ARVC group and the controls).
P<0.05 when compared with the controls among 3 groups (the ARVC LVEF <55% group, the ARVC LVEF ≥55% group, and the controls).
P<0.05 when compared with the ARVC (LVEF <55%) group among 3 groups (the ARVC LVEF <55% group, the ARVC LVEF ≥55% group, and the controls).
Conventional CMR Parameters
Compared with the controls, right ventricular end‐diastolic volume index and right ventricular end‐systolic volume index were significantly higher whereas RVEF was remarkably lower in ARVC patients (all P<0.05) (Table 1). The reduced LVEF group showed significantly lower LVEF and larger left ventricular end‐systolic volume index (all P<0.05) than the controls, while no significant differences were observed in the preserved LVEF group. In contrast, the preserved LVEF group had the lowest left ventricular end‐diastolic volume index compared with ARVC patients with LVEF <55% and the controls (P=0.002).
LGE was present in 46 (67%) of the 68 ARVC patients, and the reduced LVEF group showed higher prevalence of LGE (95.10% versus 63.00%, P=0.002) than the preserved LVEF group. The mean number of LGE‐positive segments per patient was 4.44±2.53, and there was no significant difference between these 2 subgroups (4.78±2.79 versus 3.57±1.45, P=0.053) (Table 1). Besides, LGE was most frequently detected in the LV mid anteroseptal segments (n=21; 31%) (Figure 2).
Figure 2.
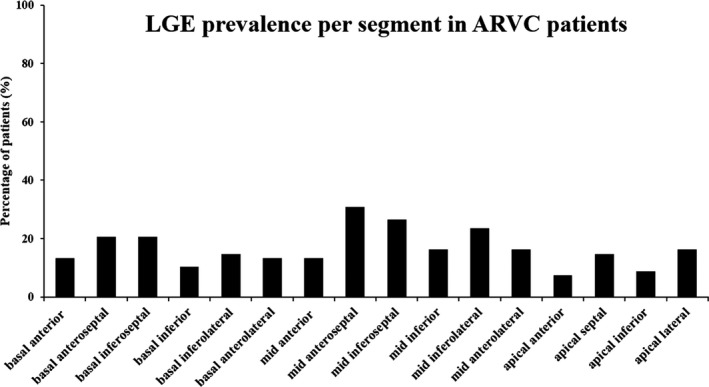
LGE prevalence per segment in ARVC patients according to AHA 16‐segment model. AHA indicates American Heart Association; ARVC, arrhythmogenic right ventricular cardiomyopathy; LGE, late gadolinium enhancement; LV, left ventricular.
Global and Regional Strain Analysis by CMR‐FT
ARVC patients showed significantly reduced RV global longitudinal, circumferential and radial PS than those of the controls (all P<0.05). Compared with the controls, the LV global and regional PS were all significantly impaired in the reduced LVEF group (all P<0.05). However, only LV global longitudinal PS as well as mid and apical longitudinal PS were impaired in the preserved LVEF group (all P<0.05) (Table 2). Among LV strain parameters, the receiver operating characteristic curve analysis demonstrated that LV global longitudinal PS (cut off value: −13.60%, sensitivity: 73.53%, specificity: 80%, area under curve: 0.822), longitudinal PS at apical (cut off value: −15.22%, sensitivity: 80.88%, specificity: 85%, area under curve: 0.836) and mid‐level (cut off value: −16.67%, sensitivity: 67.65%, specificity: 95%, area under curve: 0.820) were all good discriminators between ARVC patients and controls (Figure 3).
Table 2.
Biventricular Global and LV Regional Strain Analysis by CMR‐FT
| Total ARVC | ARVC (LVEF <55%) | ARVC (LVEF ≥55%) | Controls | |
|---|---|---|---|---|
| (n=68) | (n=41) | (n=27) | (n=30) | |
| RV longitudinal PS, % | ||||
| Global | −12.79±5.79a | −11.90±5.53b | −14.13±6.02b | −22.85±5.04 |
| RV circumferential PS, % | ||||
| Global | −4.58±4.34a | −4.26±4.11b | −5.06±4.71b | −7.86±5.39 |
| RV radial PS, % | ||||
| Global | 10.65±6.13a | 10.40±6.09b | 11.03±6.28b | 19.83±6.51 |
| LV longitudinal PS, % | ||||
| Global | −10.91±3.42a | −9.99±3.58b | −12.30±2.67b, c | −14.62±2.30 |
| Basal | −8.52±2.86a | −8.01±2.87b | −9.30±2.71 | −10.91±3.69 |
| Medial | −13.87±5.00a | −12.92±4.69b | −15.31±5.19b | −18.94±2.32 |
| Apical | −12.08±4.32a | −10.76±4.57b | −14.07±3.01b, c | −17.04±3.31 |
| LV circumferential PS, % | ||||
| Global | −13.85±4.80a | −11.50±4.33b | −17.41±2.96c | −16.82±2.22 |
| Basal | −12.16±4.06a | −10.18±3.43b | −15.17±2.95c | −15.28±1.94 |
| Medial | −15.70±5.20a | −13.25±5.08b | −19.42±2.45c | −18.73±3.41 |
| Apical | −14.18±5.81 | −11.53±4.88b | −18.21±4.73c | −16.35±5.54 |
| LV radial PS, % | ||||
| Global | 28.60±11.74a | 23.86±10.46b | 35.80±9.88c | 40.01±9.94 |
| Basal | 30.85±13.49a | 26.39±13.28b | 37.62±10.89b, c | 46.02±14.32 |
| Medial | 24.89±10.67a | 20.18±9.10b | 32.04±8.81c | 31.70±8.94 |
| Apical | 32.94±15.67a | 27.51±14.17b | 41.19±14.38c | 45.88±20.68 |
Data were presented as mean±SD. ARVC indicates arrhythmogenic right ventricular cardiomyopathy; CMR, cardiac magnetic resonance; FT, feature tracking; LV, left ventricular; LVEF, left ventricular ejection fraction; PS, peak strain; RV, right ventricular.
Significant difference compared with the controls between 2 groups (the total ARVC group and the controls).
Significant difference compared with the controls among 3 groups (the ARVC LVEF <55% group, the ARVC LVEF ≥55% group, and the controls).
Significant difference compared with the ARVC (LVEF <55%) group among 3 groups (the ARVC LVEF <55% group, the ARVC LVEF ≥55% group, and the controls).
Figure 3.

Receiver operating characteristic analysis of left ventricular strain parameters for differentiation of patients with arrhythmogenic right ventricular cardiomyopathy with the controls. ARVC indicates arrhythmogenic right ventricular cardiomyopathy; AUC, area under curve; PS, peak strain.
Association Between LV Strain and LGE
Representative cases of LV segmental strain and LGE according to American Heart Association 16‐segment model among 3 groups were shown in Figure 4. LV segments with LGE (n=185) showed impaired LV radial, circumferential, and longitudinal PS in ARVC patients than those without LGE (n=903) (all P<0.001). In the preserved LVEF group, LV segments with LGE had significantly reduced LV radial PS (33.15±20.42%, n=46) compared with segments without LGE (41.25±15.98%, n=386) (P=0.016) (Table 3).
Figure 4.
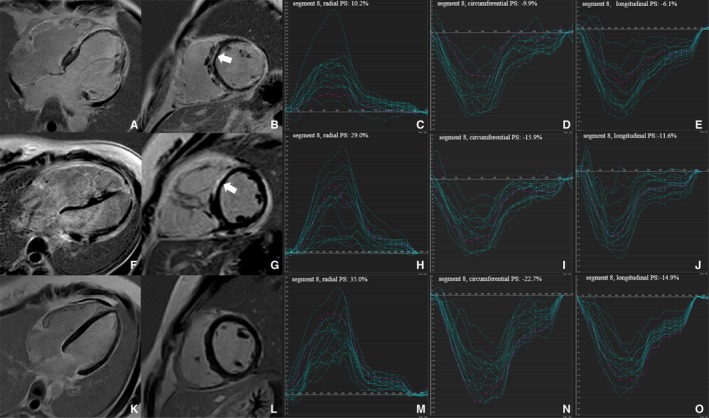
Representative cases of left ventricular (LV) segmental peak strain and late gadolinium enhancement according to AHA 16‐segment model from the reduced left ventricular ejection fraction group (A through E), the preserved left ventricular ejection fraction group (F through J) and the controls (K through O), respectively. Late gadolinium enhancement images were demonstrated in the 4‐chamber (A, F, and K) and mid‐short‐axis (B, G, and L) view, respectively. The white arrow (B and G) indicates late gadolinium enhancement in the segment 8 (LV mid‐anteroseptal wall). LV 16‐segment PS curves were shown in the radial (C, H, and M), circumferential (D, I, and N), and longitudinal (E, J, and O) directions, respectively. The red‐color curves indicate the PS curves of segment 8 (LV mid‐anteroseptal wall) in 3 groups, respectively. AHA indicates American Heart Association; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; PS, peak strain.
Table 3.
Comparisons of LV Segmental Strain Parameters With and Without LGE in ARVC
| LGE (−) | LGE (+) | P Value | |
|---|---|---|---|
| Total ARVC (n=68) | 903 | 185 | |
| LV longitudinal PS, % | −12.07±7.05 | −8.60±7.68 | <0.001 |
| LV circumferential PS, % | −15.50±5.72 | −9.80±6.99 | <0.001 |
| LV radial PS, % | 34.81±21.48 | 18.83±17.80 | <0.001 |
| Preserved LVEF group (n=27) | 386 | 46 | |
| LV longitudinal PS, % | −12.56±8.09 | −12.29±5.96 | 0.828 |
| LV circumferential PS, % | −18.12±5.32 | −17.03±4.09 | 0.178 |
| LV radial PS, % | 41.25±15.98 | 33.15±20.42 | 0.016 |
Data are presented as mean±SD. ARVC indicates arrhythmogenic right ventricular cardiomyopathy; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; PS, peak strain.
Association of Biventricular Strain Parameters
In this study, no significant correlations were observed between RVEF and LV strain parameters. However, RV global radial PS moderately correlated with LV global longitudinal PS (r=−0.47, P<0.001), longitudinal PS at mid (r=−0.47, P<0.001) and apical (r=−0.49, P<0.001) level, respectively.
Intra‐Observer and Inter‐Observer Variability
The intraclass correlation coefficients of the global strain parameters for both ventricles and LV regional PS were summarized in Table 4. All the strain parameters showed good to excellent inter‐observer (0.851–0.915) and intra‐observer (0.876–0.947) agreement.
Table 4.
Inter‐ and Intra‐Observer Variability of CMR‐FT‐Derived Global and Regional Strain Parameters
| Intra‐Observer | Inter‐Observer | |||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| RV longitudinal PS, % | ||||
| Global | 0.907 | 0.780 to 0.962 | 0.886 | 0.735 to 0.953 |
| RV circumferential PS, % | ||||
| Global | 0.897 | 0.759 to 0.958 | 0.856 | 0.673 to 0.940 |
| RV radial PS, % | ||||
| Global | 0.881 | 0.723 to 0.951 | 0.851 | 0.662 to 0.938 |
| LV longitudinal PS, % | ||||
| Global | 0.936 | 0.821 to 0.978 | 0.915 | 0.768 to 0.971 |
| Basal | 0.881 | 0.682 to 0.958 | 0.855 | 0.671 to 0.940 |
| Mid | 0.908 | 0.783 to 0.963 | 0.878 | 0.677 to 0.957 |
| Apical | 0.876 | 0.672 to 0.951 | 0.860 | 0.680 to 0.942 |
| LV circumferential PS, % | ||||
| Global | 0.930 | 0.804 to 0.976 | 0.907 | 0.745 to 0.968 |
| Basal | 0.926 | 0.795 to 0.975 | 0.867 | 0.651 to 0.953 |
| Mid | 0.913 | 0.762 to 0.970 | 0.860 | 0.634 to 0.951 |
| Apical | 0.896 | 0.720 to 0.964 | 0.884 | 0.690 to 0.960 |
| LV radial PS, % | ||||
| Global | 0.947 | 0.851 to 0.982 | 0.895 | 0.716 to 0.963 |
| Basal | 0.884 | 0.690 to 0.960 | 0.863 | 0.640 to 0.952 |
| Mid | 0.881 | 0.684 to 0.959 | 0.851 | 0.614 to 0.947 |
| Apical | 0.904 | 0.739 to 0.967 | 0.865 | 0.646 to 0.953 |
CMR indicates cardiac magnetic resonance; FT, feature tracking; ICC, intraclass correlation coefficient; LV, left ventricular; PS, peak strain; RV, right ventricular.
Discussion
The main findings of this study were as follows: (1) CMR‐FT could detect impaired LV global and regional longitudinal PS in ARVC patients with preserved LVEF; (2) LV radial PS was reduced in segments with LGE compared with those without LGE in ARVC patients with preserved LVEF; (3) Impaired LV strain was associated with RV deformation mechanics in ARVC patients.
Myocardial Strain Derived by CMR‐FT
Myocardial deformation is a sensitive marker of myocardial dysfunction in a broad of cardiovascular diseases.9, 10, 15, 16 CMR‐FT is a rapidly emerging approach for quantification of global and regional myocardial deformation. Several studies have validated its accuracy against CMR tagging or echocardiographic speckle tracking.17, 18 The major advantages of CMR‐FT are that it can be applied to routine cine CMR images and the post‐processing analysis is relatively easy. Preliminary studies have confirmed the feasibility of this technique for evaluation of LV myocardial strain.19, 20 All the strain parameters showed good to excellent inter‐observer and intra‐observer reproducibility, which was consistent with previous studies.21, 22
LV Strain of ARVC Patients With Preserved LVEF
Genotype/phenotype studies have demonstrated that ARVC, which was initially described as an isolated or predominant RV disease, may exhibit frequent LV involvement.2, 23 Regional dysfunction because of patchy fibro‐fatty displacement may occur before the onset of global changes. Recently, Mast et al found that only 16% of ARVC patients had reduced LVEF, whereas 55% had reduced strain derived by echocardiographic speckle tracking.24 Similarly, in our study, abnormal global and regional LV longitudinal strain were found to be significantly impaired in ARVC patients with preserved LVEF, implying that minor LV systolic dysfunction could be identified by CMR‐FT before the presence of LVEF reduction. LV apical longitudinal strain outperformed LVEF to be the best marker for early detection of LV involvement. These findings suggested that regional strain analysis was potentially more sensitive than global strain for detection of minor LV involvement. This was theoretically supported by previous reports, that ARVC begins as a regional rather than a global disease.2, 25 In short, LV strain is more sensitive than LVEF in detecting early LV involvement and may complement the conventional parameters for comprehensive evaluation of ARVC.
Association Between LV Strain and LGE
In accordance with previous studies,4, 23 LGE in LV myocardium was frequently detected in ARVC patients, even in those with preserved LVEF. Unlike other cardiomyopathies (eg hypertrophic cardiomyopathy) in which LGE mainly represents myocardial fibrosis,26 LGE positive area in ARVC could also be the consequence of fibro‐fatty change. In addition, we found that the LV segments with LGE showed impaired radial, circumferential and longitudinal strain in ARVC patients compared with those without LGE. However, only radial strain was significantly reduced in LGE‐positive segments in the preserved LVEF group. This was conceivable because radial strain was appreciated to be more representative of the outer fibers of LV myocardium.5 Besides, LGE was frequently observed without visual wall motion abnormality assessed by conventional echocardiographic or CMR.27 The possible explanation was, in contrast to objective quantification by regional strain analysis, the diagnosis of visual wall motion abnormality was more subjective and dependent on personal expertise with significant inter‐observer variability.15 Thus, mild regional abnormalities were likely to be overlooked.
Association of Biventricular Strain Parameters
RV‐LV coupling has already been recognized in selected populations.28, 29 In this study, RV global radial strain parameters were significantly associated with LV global and regional longitudinal PS, indicating that in addition to LGE, the impaired RV mechanics may also contribute to LV dysfunction in patients with ARVC.
There were several limitations in this study. Firstly, the sample size of the current study cohort was relatively small; further validation of our results in studies containing large numbers of patients might be warranted. Secondly, a direct comparison between CMR‐FT and CMR tagging, which has been considered as the reference standard of myocardial strain analysis, was not feasible because the tagging sequence was not included in the present study protocol. Thirdly, the lack of long‐term follow‐up data prevented definite conclusion of prognostic value of LV strain analysis in ARVC patients.
Conclusions
CMR‐FT could detect impaired LV global and regional deformation in ARVC patients with preserved LVEF. The LV dysfunction could be associated with LV fibro‐fatty replacement and RV dysfunction.
Sources of Funding
This study is supported by National Natural Science Foundation of China (grant no. 81701659 and grant no. 81620108015) and Capital Characteristic and Clinical Application Research Fund from the Beijing Municipal Commission of Science and Technology (Z161100000516110).
Disclosures
None.
Acknowledgments
Dr Xiuyu Chen made contributions to conception and design of study, critical revision of the manuscript; Dr Lu Li drafted the manuscript, and was responsible for statistical analysis of the data; Dr Yanyan Song collected conventional CMR data; Dr Keshan Ji, Lin Chen and Gang Yin were in charge of post‐processing of CMR‐FT analysis. Dr Huaibin Cheng collected and analyzed clinical data. Prof Minjie Lu made critical revisions of the manuscript; Prof Shihua Zhao did substantial contribution to conception and design and critical revision of the manuscript. All authors read and approved the final manuscript.
(J Am Heart Assoc. 2019;8:e012989 DOI: 10.1161/JAHA.119.012989.)
References
- 1. Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:784–804. [DOI] [PubMed] [Google Scholar]
- 2. Te Riele AS, James CA, Philips B, Rastegar N, Bhonsale A, Groeneweg JA, Murray B, Tichnell C, Judge DP, Van Der Heijden JF, Cramer MJ, Velthuis BK, Bluemke DA, Zimmerman SL, Kamel IR, Hauer RN, Calkins H, Tandri H. Mutation‐positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol. 2013;24:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinamonti B, Dragos AM, Pyxaras SA, Merlo M, Pivetta A, Barbati G, Di Lenarda A, Morgera T, Mestroni L, Sinagra G. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10‐year registry. Eur Heart J. 2011;32:1105–1113. [DOI] [PubMed] [Google Scholar]
- 4. Etoom Y, Govindapillai S, Hamilton R, Manlhiot C, Yoo SJ, Farhan M, Sarikouch S, Peters B, McCrindle BW, Grosse‐Wortmann L. Importance of CMR within the Task Force Criteria for the diagnosis of ARVC in children and adolescents. J Am Coll Cardiol. 2015;65:987–995. [DOI] [PubMed] [Google Scholar]
- 5. Chungsomprasong P, Hamilton R, Luining W, Fatah M, Yoo SJ, Grosse‐Wortmann L. Left ventricular function in children and adolescents with arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2017;119:778–784. [DOI] [PubMed] [Google Scholar]
- 6. Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016;9:e004077. [DOI] [PubMed] [Google Scholar]
- 7. Bertini M, Ng AC, Antoni ML, Nucifora G, Ewe SH, Auger D, Marsan NA, Schalij MJ, Bax JJ, Delgado V. Global longitudinal strain predicts long‐term survival in patients with chronic ischemic cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:383–391. [DOI] [PubMed] [Google Scholar]
- 8. Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, Andre F, Ehlermann P, Franke J, Taeger T, Frankenstein L, Steen H, Meder B, Giannitsis E, Katus HA, Korosoglou G. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:307–315. [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Yang ZG, Xu HY, Shi K, Guo YK. Assessment of left ventricular myocardial deformation by cardiac MRI strain imaging reveals myocardial dysfunction in patients with primary cardiac tumors. Int J Cardiol. 2018;253:176–182. [DOI] [PubMed] [Google Scholar]
- 10. Liu X, Zhang Q, Yang ZG, Shi K, Xu HY, Xie LJ, Jiang L, Diao KY, Guo YK. Assessment of left ventricular deformation in patients with Ebstein's anomaly by cardiac magnetic resonance tissue tracking. Eur J Radiol. 2017;89:20–26. [DOI] [PubMed] [Google Scholar]
- 11. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Circulation. 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bistoquet A, Oshinski J, Skrinjar O. Left ventricular deformation recovery from cine MRI using an incompressible model. IEEE Trans Med Imaging. 2007;26:1136–1153. [DOI] [PubMed] [Google Scholar]
- 13. Bistoquet A, Oshinski J, Skrinjar O. Myocardial deformation recovery from cine MRI using a nearly incompressible biventricular model. Med Image Anal. 2008;12:69–85. [DOI] [PubMed] [Google Scholar]
- 14. Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bourfiss M, Vigneault DM, Aliyari Ghasebeh M, Murray B, James CA, Tichnell C, Mohamed Hoesein FA, Zimmerman SL, Kamel IR, Calkins H, Tandri H, Velthuis BK, Bluemke DA, te Riele ASJM. Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy: a multisoftware feasibility and clinical implementation study. J Cardiovasc Magn Reson. 2017;19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong DT, Leong DP, Weightman MJ, Richardson JD, Dundon BK, Psaltis PJ, Leung MC, Meredith IT, Worthley MI, Worthley SG. Magnetic resonance‐derived circumferential strain provides a superior and incremental assessment of improvement in contractile function in patients early after ST‐segment elevation myocardial infarction. Eur Radiol. 2014;24:1219–1228. [DOI] [PubMed] [Google Scholar]
- 17. Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al‐Khalidi HR, Chung ES, Benson DW, Mazur W. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3:144–151. [DOI] [PubMed] [Google Scholar]
- 18. Obokata M, Nagata Y, Wu VC, Kado Y, Kurabayashi M, Otsuji Y, Takeuchi M. Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur Heart J Cardiovasc Imaging. 2016;17:525–532. [DOI] [PubMed] [Google Scholar]
- 19. Satriano A, Heydari B, Narous M, Exner DV, Mikami Y, Attwood MM, Tyberg JV, Lydell CP, Howarth AG, Fine NM, White JA. Clinical feasibility and validation of 3D principal strain analysis from cine MRI: comparison to 2D strain by MRI and 3D speckle tracking echocardiography. Int J Cardiovasc Imaging. 2017;33:1979–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu B, Dardeer AM, Moody WE, Hayer MK, Baig S, Price AM, Leyva F, Edwards NC, Steeds RP. Reference ranges for three‐dimensional feature tracking cardiac magnetic resonance: comparison with two‐dimensional methodology and relevance of age and gender. Int J Cardiovasc Imaging. 2018;34:761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B, Townend JN, Hor KN, Steeds RP, Mazur W, Leyva F. Myocardial strain measurement with feature‐tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16:871–881. [DOI] [PubMed] [Google Scholar]
- 22. Xu HY, Chen J, Yang ZG, Li R, Shi K, Zhang Q, Liu X, Xie LJ, Jiang L, Guo YK. Early marker of regional left ventricular deformation in patients with hypertrophic cardiomyopathy evaluated by MRI tissue tracking: the effects of myocardial hypertrophy and fibrosis. J Magn Reson Imaging. 2017;46:1368–1376. [DOI] [PubMed] [Google Scholar]
- 23. Dalal D, Tandri H, Judge DP, Amat N, Macedo R, Jain R, Tichnell C, Daly A, James C, Russell SD, Abraham T, Bluemke DA, Calkins H. Morphologic variants of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy a genetics‐magnetic resonance imaging correlation study. J Am Coll Cardiol. 2009;53:1289–1299. [DOI] [PubMed] [Google Scholar]
- 24. Mast TP, Teske AJ, vd Heijden JF, Groeneweg JA, Te Riele AS, Velthuis BK, Hauer RN, Doevendans PA, Cramer MJ. Left ventricular involvement in arrhythmogenic right ventricular dysplasia/cardiomyopathy assessed by echocardiography predicts adverse clinical outcome. J Am Soc Echocardiogr. 2015;28:1103–1113.e9. [DOI] [PubMed] [Google Scholar]
- 25. Te Riele A, Tandri H, Sanborn DM, Bluemke DA. Noninvasive multimodality imaging in ARVD/C. JACC Cardiovasc Imaging. 2015;8:597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli‐Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. [DOI] [PubMed] [Google Scholar]
- 27. Sen‐Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–1720. [DOI] [PubMed] [Google Scholar]
- 28. Nielsen EA, Okumura K, Sun M, Hjortdal VE, Redington AN, Friedberg MK. Regional septal hinge‐point injury contributes to adverse biventricular interactions in pulmonary hypertension. Physiol Rep. 2017;5:e13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jing L, Pulenthiran A, Nevius CD, Mejia‐Spiegeler A, Suever JD, Wehner GJ, Kirchner HL, Haggerty CM, Fornwalt BK. Impaired right ventricular contractile function in childhood obesity and its association with right and left ventricular changes: a cine DENSE cardiac magnetic resonance study. J Cardiovasc Magn Reson. 2017;19:49. [DOI] [PMC free article] [PubMed] [Google Scholar]


