Abstract
Background
Although it has been reported that renal function can improve after catheter ablation of atrial fibrillation (AF), long‐term changes in renal function and its relationship to rhythm outcomes have not yet been evaluated. We explored the 5‐year change in estimated glomerular filtration rate (eGFR) in AF patients depending on medical therapy and catheter ablation.
Methods and Results
Among 1963 patients who underwent AF catheter ablation and 14 056 with AF under medical therapy in the National Health Insurance Service database, we compared 571 with AF catheter ablation (59±10 years old, 72.3% male, and 66.5% paroxysmal AF) and 1713 with medical therapy after 1:3 propensity‐score matching. All participants had 5 years of serial eGFR data (Chronic Kidney Disease‐Epidemiology Collaboration [CKD‐EPI] method). Catheter ablation improved eGFR 5 yrs (P<0.001), but medical therapy did not. In 2284 matched patients, age (adjusted odds ratio [OR], 0.98 [0.97–0.99]; P<0.001) and AF catheter ablation (adjusted OR, 2.02 [1.67–2.46]; P<0.001) were independently associated with an improved eGFR 5 yrs. Among 571 patients who underwent AF ablation, freedom from AF/atrial tachycardia recurrence after the last AF ablation procedure was independently associated with an improved eGFR 5 yrs (adjusted OR, 1.44 [1.01–2.04]; P=0.043), especially in patients without diabetes mellitus (adjusted OR, 1.78 [1.21–2.63]; P=0.003, P for interaction=0.012). Although underlying renal dysfunction (<60 mL/min/1.73m2) was associated with atrial structural remodeling (adjusted OR, 1.05 [1.00–1.11]; P=0.046), it did not affect the AF ablation rhythm outcome.
Conclusions
AF catheter ablation significantly improved renal function over a 5‐year follow‐up, especially in patients maintaining sinus rhythm without preexisting diabetes mellitus.
Keywords: atrial fibrillation, catheter ablation, renal function
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator
Short abstract
See Editorial Wehner
Clinical Perspective
What Is New?
Atrial fibrillation (AF) catheter ablation improved renal function over the 5‐year follow‐up, whereas medical therapy did not.
No recurrence after the last AF ablation procedure was independently associated with an improved long‐term renal function, especially in patients without diabetes mellitus.
Impaired renal function was associated with an atrial structural remodeling in AF patients, but did not affect the rhythm outcome of AF catheter ablation.
What Are the Clinical Implications?
AF catheter ablation and reduction of AF burden can improve renal function over a long‐term period, especially in patients without diabetes mellitus.
Introduction
Atrial fibrillation (AF), a common arrhythmia that increases mortality by 2 to 3 times,1 is a progressive degenerative disease2 associated with strokes,1 dementia,1 and heart failure.1 Chronic kidney disease (CKD) has a well‐established association with cardiovascular disease, and all‐cause mortality and cardiovascular mortality progressively increase as renal function declines.3, 4 It has been shown that 7% to 18% of patients with a CKD stage of ≥3 (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) have concomitant AF, and 10% to 15% of AF patients have CKD.5, 6, 7 This relationship becomes more significant for those aged >70 years. Patients with both AF and CKD have an increased risk of bleeding and thromboembolisms.7 Washam et al8 reported that AF rhythm control by antiarrhythmic drugs did not affect all‐cause mortality and risk of a stroke in patients in the ORBIT‐AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) registry data. In contrast, AF catheter ablation has been known to be a more‐effective method of AF rhythm control than antiarrhythmic drugs,9 and it is known to lower the heart failure mortality10, 11 and risk of cerebral infarctions12 and improve the cognitive function.13, 14 However, studies on renal‐function changes after AF rhythm control by catheter ablation have been limited thus far. Takahashi et al reported that maintenance of sinus rhythm after a single AF catheter ablation resulted in a positive effect on renal function.15 After a successful AF ablation, left ventricular systolic function improves and mean heart rate increases because of autonomic nerve modulation.16, 17 As a result, cardiac output increases, and a positive effect on renal function is expected after a successful AF ablation. However, given that AF has a substantial long‐term recurrence rate after catheter ablation, there are no data on changes in renal function over a 5‐year long‐term period or after repeated procedures. Therefore, we hypothesized that an optimal AF rhythm control, including a repeat ablation, may improve long‐term renal function beyond 5 years. In this study, we compared the change in renal function in AF patients who underwent catheter ablation followed by a guideline‐based regular rhythm follow‐up (Yonsei AF ablation cohort) and those that underwent medical therapy alone (Korean National Health Insurance [NHIS] database) over a peirod of 5 years. We also explored the relationship of the change in long‐term renal function after AF catheter ablation to rhythm outcome or degree of atrial remodeling.
Methods
The data, analytic methods, and study materials that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
This study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Yonsei University Health System. The medical therapy group was selected from the NHIS database (NHIS‐2018‐2‐189), and the ablation therapy group was selected from the Yonsei AF Ablation cohort (registered at ClinicalTrials.gov Identifier: NCT02138695). Both groups were enrolled as nonvalvular AF patients with baseline and 5‐year follow‐up eGFR (estimated by Chronic Kidney Disease Epidemiology Collaboration [CKD‐EPI] equation) data. In the AF ablation group, all patients provided written informed consent for inclusion in the Yonsei AF Ablation study. The informed consent requirement in the medical therapy group from the NHIS database was waived. Among 1963 patients who underwent AF catheter ablation (AFCA) for symptomatic drug‐refractory AF, 571 patients (59±10 years, 72.3% male, and 66.5% paroxysmal AF) who had 5‐year serial eGFR data after AF ablation were enrolled in this study. All patients stopped their antiarrhythmic drugs for a period of time corresponding to at least 5 half‐lives before catheter ablation. During the 5‐year follow‐up period, 103 patients underwent a second ablation, and 3 underwent a third ablation for antiarrhythmic‐resistant recurrent AF (Figure 1). In the medical therapy group, among 14 056 patients with nonvalvular AF who were not treated with catheter ablation in the NHIS database, 1713 patients (59±11 years, 73.2% male) who had both baseline and 5‐year follow‐up renal function data were enrolled in this study for the control group after propensity‐score matching with the AF ablation group. Exclusion criteria were as follows: (1) permanent AF refractory to electrical cardioversion, (2) AF with rheumatic valvular disease, and (3) previous cardiac surgery with concomitant AF surgery or AF catheter ablation.
Figure 1.
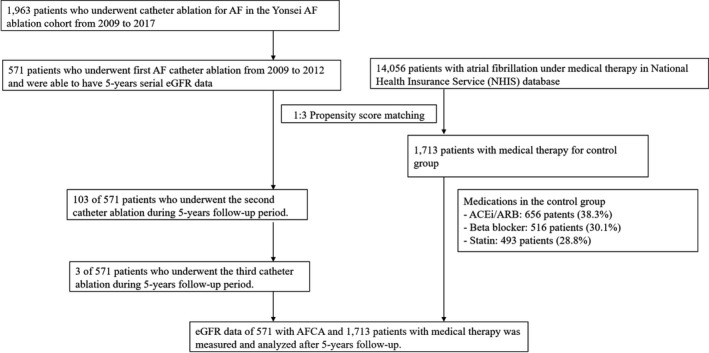
Study flow chart of the patient enrollment. ACEi indicates angiotensin‐converting‐enzyme inhibitor; AF, atrial fibrillation; AFCA, AF catheter ablation; ARB, angiotensin type II receptor blocker; eGFR, estimated glomerular filtration rate.
Electrophysiological Mapping and Radiofrequency Catheter Ablation
3D electroanatomical mapping (NavX; St. Jude Medical, Inc, Minnetonka, MN) was generated using a circumferential pulmonary vein (PV)‐mapping catheter (Lasso; Biosense‐Webster Inc, Diamond Bar, CA) through a long sheath (Schwartz left 1; St. Jude Medical, Inc). Multiview pulmonary venograms were obtained after the trans‐septal punctures. The 3D geometry of both the left atrium (LA) and PVs was generated using the NavX system and then merged with 3D spiral computed tomography images. Systemic anticoagulation with intravenous heparin was achieved to maintain an activated clotting time of 350 to 400 seconds during the procedure.
An open‐irrigated tip catheter (Celsius; Johnson & Johnson Inc, Diamond Bar, CA; NaviStar ThermoCool, Biosense Webster Inc; ThermoCool SF, Biosense Webster Inc; ThermoCool SmartTouch, Biosense Webster Inc; Coolflex, St. Jude Medical Inc; 30–35 W; 47°C; FlexAbility, St Jude Medical Inc; ThermoCool SmartTouch, Biosense Webster Inc, and TactiCath, St. Jude Medical Inc) was used for AFCA. All patients underwent a de novo procedure with a circumferential pulmonary vein isolation. The majority of the patients (94.2%) underwent a cavotricuspid isthmus block during the de novo procedure if there was no atrioventricular conduction disease. We conducted an additional linear ablation including a roof line, posterior inferior line (posterior box lesion), and anterior line, especially in patients with persistent AF. A left lateral isthmus ablation, right atrial ablation, and complex fractionated electrogram ablation were performed in a minority of the patients at the operator's discretion. The de novo procedure ended when there was no immediate recurrence of AF within 10 minutes after the cardioversion with an isoproterenol infusion (5–10 μg/min). In the case of mappable AF triggers or premature atrial beats, non‐PV foci were carefully mapped and ablated as much as possible.
The detailed technique and strategy for the repeat ablation procedures were presented in a previous study.18 If there were reconnections of PV potentials or a previous linear ablation, we completed the circumferential pulmonary vein isolation and accomplished bidirectional block of the circumferential pulmonary vein isolation, cavotricuspid isthmus, or linear ablation as much as possible. Then, we provoked extra‐PV foci with an isoproterenol infusion (5–10 μg/min) and carefully mapped and ablated mappable AF triggers or frequent atrial premature beats. If there were multiple extra‐PV triggers, we conducted an additional linear ablation or an electrogram‐guided ablation at the operators’ discretion.
Postablation Management and Follow‐up
Patients visited the outpatient clinic at 1, 3, 6, and 12 months and every 6 months thereafter or whenever symptoms developed after the AFCA. ECG was performed at every visit. Twenty‐four‐hour Holter monitoring was performed at 3, 6, and 12 months and then every 6 months after the AFCA according to the 2012 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society expert consensus statement guidelines.19 Patients underwent an annual laboratory examination (serum creatinine, blood urea nitrogen, urine hemoglobin, and dipstick urine protein). eGFR was estimated by the CKD‐EPI equation. Patients who suffered from symptoms of palpitations underwent Holter/event‐monitor examinations to investigate the possibility of an arrhythmia recurrence. We defined an AF recurrence as any episode of atrial tachycardia (AT) or AF lasting for 30 seconds or more. Any ECG documentation of an AF recurrence after a 3‐month blanking period was classified as a clinical recurrence.
Statistical Analysis
Continuous variables are expressed as mean±SD and were analyzed using a Student t test. Categorical variables are reported as numbers (percentages) and were analyzed using a chi‐square or Fisher's exact test. We used a paired t test to investigate changes in eGFR5 yr (ΔeGFR5 yr) in both the AFCA group and control group. Also, we compared ΔeGFR5 yr after AFCA according to freedom from AF/AT recurrence by using a paired t test. A multivariate logistic regression analysis was used to investigate whether AF catheter ablation improved renal function and identify predictors associated with an improvement in renal function in patients who underwent AF catheter ablation. The variables with a P value under 0.05 were included in the multivariate model. Furthermore, we did not use a step‐wise selection model in multivariate regression analysis. A Kaplan–Meier analysis was used to analyze probability of freedom from AF/AT recurrence after AFCA according to a concomitant impaired renal function. Propensity matching was performed without a replacement and with a caliper of 0.01 at a 1: 3 ratio of the AF ablation group and medical therapy group based on the following variables: age, sex, body mass index, congestive heart failure, hypertension, diabetes mellitus, previous history of a stroke or transient ischemic attack, previous history of vascular disease, CHA2DS2VASc score, baseline eGFR, and medications, including angiotensin‐converting enzyme inhibitors, angiotensin type II receptor blockers, β‐blockers, and statins. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 23.0; SPSS, Inc, Chicago, IL) software for Windows.
Results
AF Catheter Ablation Versus Medical Therapy
Baseline characteristics in the AF catheter ablation group and propensity‐score–matched control group are presented in Table 1. There were no significant differences in patient characteristics, medication use, and baseline eGFR between the AFCA group and medical therapy group. Nonetheless, eGFR measured at the 5‐year follow‐up in patients who underwent AFCA was significantly higher than that in patients with medical therapy (84.6±19.4 versus 82.4±18.1 mL/min/1.73 m2; P=0.014; Figure 2A). ΔeGFR5 yr was also significantly higher in patients with AFCA as compared with patients with medical therapy (3.2±13.6 versus 0.7±16.8 mL/min/1.73 m2; P<0.001; Figure 2B). Among the overall 2284 patients, a young age (adjusted odds ratio [OR], 0.98 [0.97–0.99]; P<0.001; Table 2) and AF catheter ablation (adjusted OR, 2.02 [1.67–2.46]; P<0.001; Table 2) were independently associated with an improved renal function (ΔeGFR5 yr, >0 mL/min/1.73 m2) after the 5‐year follow‐up after when adjusting for preexisting hypertension and angiotensin‐converting enzyme inhibitor/angiotensin type II receptor blocker use. We tested the different thresholds of ΔeGFR5 yr based on previous studies,15, 20, 21, 22 and found that AFCA was a consistent variable of long‐term improvement in renal function (Tables S1 through S3).
Table 1.
Comparison of the Baseline Characteristics and eGFR in AF Patients With AFCA or Medical Therapy
| Overall | AFCA | Medical therapy | P Value | ASMD | |
|---|---|---|---|---|---|
| (n=2284) | (n=571) | (n=1713) | |||
| Age, y | 59±10 | 59±10 | 59±11.0 | 0.895 | 0.006 |
| Male | 1667 (73%) | 413 (72.3%) | 1254 (73.2%) | 0.683 | 0.020 |
| Body mass index | 24.8±3.0 | 24.9±2.8 | 24.8±3.0 | 0.312 | 0.046 |
| CHA2DS2VASc score | 1.7±1.5 | 1.8±1.5 | 1.7±1.6 | 0.546 | 0.029 |
| CHF | 110 (4.8%) | 30 (5.3%) | 80 (4.7%) | 0.573 | 0.027 |
| Hypertension | 1163 (50.9%) | 290 (50.8%) | 873 (51%) | 0.942 | 0.004 |
| Diabetes mellitus | 394 (17.3%) | 99 (17.3%) | 295 (17.2%) | 0.949 | 0.003 |
| Stroke/TIA | 263 (11.5%) | 67 (11.7%) | 196 (11.4%) | 0.850 | 0.009 |
| Vascular disease | 336 (14.7%) | 92 (16.1%) | 244 (14.2%) | 0.275 | 0.052 |
| ACEi/ARB use | 862 (37.8%) | 206 (36.1%) | 656 (38.3%) | 0.358 | 0.046 |
| Beta‐blocker use | 676 (29.6%) | 160 (28.1%) | 516 (30.1%) | 0.353 | 0.046 |
| Statin use | 653 (28.6%) | 160 (28.1%) | 493 (28.8%) | 0.745 | 0.017 |
| Baseline eGFR | 81.7±16.9 | 81.4±18.5 | 81.8±16.3 | 0.685 | 0.02 |
The patients under medical therapy were included in this study after propensity‐score matching for the age, sex, body mass index, CHF, hypertension, diabetes mellitus, stroke/TIA, vascular disease, CHA2DS2VASc score, baseline eGFR, ACEi/ARB use, beta‐blocker use, and statin use. ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AFCA, AF catheter ablation; ARB, angiotensin type II receptor blocker; ASMD, absolute standardized mean differences; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack.
Figure 2.
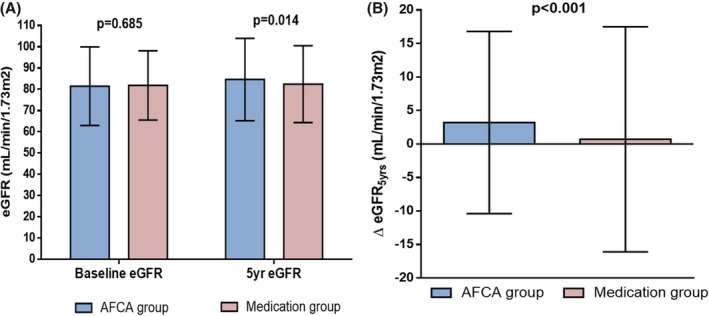
Mean estimated glomerular filtration rate (eGFR) measured at baseline and 5 years of follow‐up between the AFCA and medication groups (A). Comparison of the increase in the eGFR from baseline to 5 years (▵eGFR 5 yrs) between the AFCA and medication groups (B). AFCA indicates atrial fibrillation catheter ablation.
Table 2.
Logistic Regression Analysis for Variables Predicting an Improved Renal Function (ΔeGFR5 yr >0) After 5 Years of Follow‐up (n=2284)
| Univariate | Multivariate Model | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 0.980 (0.972–0.987) | <0.001a | 0.980 (0.972–0.989) | <0.001a |
| Male | 0.915 (0.761–1.101) | 0.347 | ||
| Body mass index | 1.015 (0.988–1.044) | 0.279 | ||
| CHA2DS2VASc score | 0.946 (0.897–0.999) | 0.044a | ||
| CHF | 0.746 (0.505–1.102) | 0.141 | ||
| Hypertension | 0.840 (0.712–0.990) | 0.037a | 1.005 (0.826–1.222) | 0.961 |
| Diabetes mellitus | 0.888 (0.714–1.105) | 0.288 | ||
| Stroke/TIA | 0.914 (0.706–1.183) | 0.494 | ||
| Vascular disease | 1.037 (0.822–1.308) | 0.759 | ||
| ACEi/ARB use | 0.801 (0.675–0.949) | 0.010a | 0.871 (0.715–1.060) | 0.169 |
| BB use | 0.855 (0.714–1.024) | 0.089 | ||
| Statin use | 0.985 (0.821–1.181) | 0.870 | ||
| AFCA | 2.021 (1.666–2.450) | <0.001a | 2.023 (1.666–2.457) | <0.001a |
The CHA2DS2VASC score was not included in multivariate model in Table 2 because the age and hypertension variables had already been considered to calculate the CHA2DS2VASc score. ACEi indicates angiotensin‐converting enzyme inhibitor; AFCA, atrial fibrillation catheter ablation; ARB, angiotensin type II receptor blocker; BB, beta blocker; CHF, congestive heart failure; OR, odds radio; TIA, transient ischemic attack.
Statistical significance.
Patient Characteristics With a Renal Function Improvement After AFCA
Table 3 summarizes the comparison of baseline characteristics, including echocardiographic parameters and clinical rhythm outcome of 571 patients according to improvement in renal function (ΔeGFR5 yr, >0 mL/min/1.73 m2). Patients whose renal function improved during the 5‐year follow‐up were younger (P=0.008), had a lower CHA2DS2VASc score (P=0.015) and lower hypertension (P=0.003), took less angiotensin‐converting enzyme inhibitors/angiotensin type II receptor blockers after the de novo ablation procedure (P=0.046), and were more likely to maintain sinus rhythm after the last ablation procedure (P=0.036) than those without renal function improvement.
Table 3.
Comparison of the Baseline Characteristics, Catheter Ablation, and Clinical Rhythm Outcomes According to an Improved Renal Function (ΔeGFR5 yr >0) Among the Overall Patients With AFCA (n=571)
| Overall Patients With AFCA | Improved Renal Function | Nonimproved Renal Function | P Value | |
|---|---|---|---|---|
| (n=571) | (n=342) | (n=229) | ||
| Age, y | 59±10 | 58±10 | 60±10 | 0.008a |
| Male | 413 (72.3%) | 245 (71.6%) | 168 (73.4%) | 0.652 |
| PAF at procedure | 380 (66.5%) | 231 (67.5%) | 149 (65.1%) | 0.538 |
| Body mass index | 24.9±2.8 | 24.9±2.9 | 24.9±2.8 | 0.850 |
| Body surface area | 1.80±0.17 | 1.81±0.18 | 1.80±0.17 | 0.445 |
| CHA2DS2VASc score | 1.8±1.5 | 1.7±1.5 | 2.0±1.5 | 0.015a |
| Congestive heart failure | 30 (5.3%) | 13 (3.8%) | 17 (7.4%) | 0.057 |
| Hypertension | 290 (50.8%) | 156 (45.6%) | 134 (58.5%) | 0.003a |
| Diabetes mellitus | 99 (17.3%) | 56 (16.4%) | 43 (18.8%) | 0.457 |
| Stroke/TIA | 67 (11.7%) | 38 (11.1%) | 29 (12.7%) | 0.572 |
| Vascular disease | 92 (16.1%) | 55 (16.1%) | 37 (16.2%) | 0.981 |
| LA dimension, mm | 41.9±6.1 | 41.9±6.1 | 41.9±6.1 | 0.911 |
| LVEF, % | 63.4±8.0 | 63.3±7.6 | 63.5±8.7 | 0.785 |
| E/Em | 10.6±5.0 | 10.6±5.3 | 10.6±4.5 | 0.999 |
| Postablation ACEi/ARB use | 206 (36.1%) | 112 (32.8%) | 94 (41.0%) | 0.046a |
| Postablation BB use | 160 (28.1%) | 89 (26.1%) | 71 (31%) | 0.201 |
| Postablation Statin use | 160 (28.1%) | 96 (28.2%) | 64 (27.9%) | 0.957 |
| Postablation AAD use | 128 (22.4%) | 68 (19.9%) | 60 (26.2%) | 0.076 |
| Advanced CKD at baseline | 67 (11.7%) | 33 (9.6%) | 34 (14.8%) | 0.059 |
| Repeat ablations | 106 (18.6%) | 70 (20.5%) | 36 (15.7%) | 0.153 |
| Freedom from AF/AT recurrence after last AFCA | 366 (64.1%) | 231 (67.5%) | 135 (59%) | 0.036a |
ΔeGFR5 yr indicates the increase in the eGFR from baseline to 5 years after the AF ablation; AAD, antiarrhythmic drugs; ACEi, angiotensin‐converting‐enzyme inhibitor; AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; ARB, angiotensin type II receptor blocker; AT, atrial tachycardia; BB, beta blocker; CKD, chronic kidney disease; E/Em, the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); eGFR, estimated glomerular filtration rate; LA, left atrium; LVEF, left ventricular ejection fraction; PAF, paroxysmal atrial fibrillation; TIA, transient ischemic attack.
Statistical significance.
Factors Associated With Improvement in Renal Function in Patients That Underwent AFCA
Among the overall 571 patients, 465 underwent a single ablation procedure and the others underwent multiple procedures during the follow‐up period. In the patients with a single procedure alone, 64.1% (298 of 465) were free from an AF/AT recurrence, and in the overall patients with single or multiple procedures, 64.1% (366/571) were free from an AF/AT recurrence after the last ablation procedure during the 5‐year follow‐up period. Mean follow‐up duration after the last ablation session in the 571 overall patients was 42.4±21.3 months, and in the 106 patients who underwent multiple procedures was 27.8±18.0 months. The 5‐year follow‐up eGFR was significantly higher than baseline eGFR in the patients without an AF/AT recurrence in both the overall ablation group (82.1±17.8–86.2±18.8 mL/min/1.73 m2; P<0.001; Figure 3A) and single‐procedure group (81.4±17.9–85.2±19.4 mL/min/1.73 m2; P<0.001; Figure 3D). However, there was no significant improvement in 5‐year follow‐up eGFR in patients with an AF/AT recurrence (Figure 3B and 3E). Degree of eGFR increase (ΔeGFR5 yrs) was significantly greater in patients who remained in sinus rhythm than in those with an AF/AT recurrence after the last ablation procedure (4.1±13.6 versus 1.5±13.4 mL/min/1.73 m2; P=0.029; Figure 3C). After adjusting for the CHA2DS2VASc score and postablation angiotensin‐converting enzyme inhibitor/angiotensin type II receptor blocker use, a multivariate logistic regression analysis demonstrated that freedom from an AF/AT recurrence after the last ablation procedure was independently associated with an improvement in renal function (adjusted OR, 1.44 [1.01–2.04]; P=0.043; Table 4).
Figure 3.
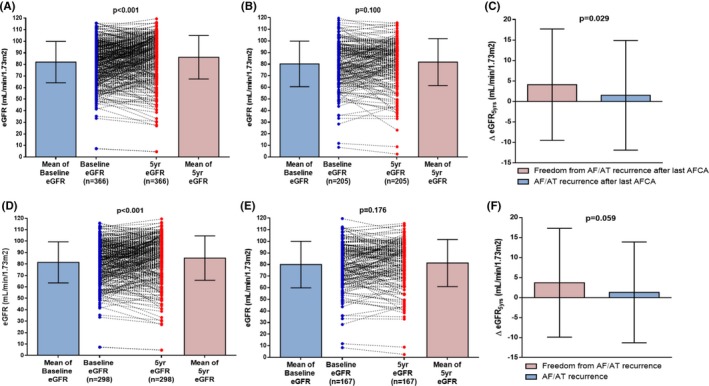
Scatter plot and mean changes in the estimated glomerular filtration rate (eGFR) from baseline to 5 years after AF ablation in the overall patients without an AF/AT recurrence (A) and those with an AF/AT recurrence (B), and in the single procedure alone group without an AF/AT recurrence (D) and those with an AF/AT recurrence (E). Comparison of the increase in the eGFR from baseline to 5 years (▵eGFR 5 yrs) according to AF/AT recurrence after the AF ablation among the patients with repeat procedures (C) and a single procedure alone (F). ACEi indicates angiotensin‐converting‐enzyme inhibitor; AF, atrial fibrillation; AFCA, AF catheter ablation; AT, atrial tachycardia.
Table 4.
Logistic Regression Analysis of the Variables Predicting an Improved Renal Function (ΔeGFR5 yr >0) After 5 Years of Follow‐up Among the Overall Patients With AFCA (n=571)
| Univariate | Multivariate Model 1 | Multivariate Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 0.977 (0.961–0.994) | 0.008a | 0.984 (0.966–1.001) | 0.066 | ||
| Male | 0.917 (0.630–1.335) | 0.652 | ||||
| PAF at procedure | 1.117 (0.785–1.591) | 0.539 | ||||
| Body mass index | 1.006 (0.948–1.067) | 0.850 | ||||
| Body surface area | 1.462 (0.553–3.862) | 0.444 | ||||
| CHA2DS2VASc score | 0.873 (0.781–0.975) | 0.016a | 0.898 (0.796–1.015) | 0.084 | ||
| Congestive heart failure | 0.493 (0.235–1.035) | 0.062 | ||||
| Hypertension | 0.595 (0.424–0.834) | 0.003a | 0.665 (0.421–1.050) | 0.080 | ||
| Diabetes mellitus | 0.847 (0.546–1.313) | 0.458 | ||||
| Stroke/TIA | 0.862 (0.515–1.443) | 0.572 | ||||
| Vascular disease | 0.994 (0.631–1.567) | 0.981 | ||||
| LA dimension | 0.998 (0.971–1.026) | 0.910 | ||||
| LVEF | 0.997 (0.976–1.018) | 0.784 | ||||
| E/Em | 1.000 (0.966–1.035) | 0.999 | ||||
| Postablation ACEi/ARB use | 0.702 (0.496–0.994) | 0.046a | 0.991 (0.626–1.569) | 0.970 | 0.817 (0.558–1.196) | 0.298 |
| Postablation BB use | 0.786 (0.543–1.138) | 0.202 | ||||
| Postablation stain use | 1.010 (0.696–1.467) | 0.957 | ||||
| Postablation AAD use | 0.699 (0.470–1.039) | 0.077 | ||||
| Advanced CKD at baseline | 0.613 (0.367–1.021) | 0.060 | ||||
| Repeat ablations | 1.380 (0.887–2.147) | 0.154 | ||||
| Freedom from AF/AT recurrence after last AFCA | 1.449 (1.024–2.051) | 0.036a | 1.408 (0.990–2.002) | 0.057 | 1.436 (1.012–2.037) | 0.043a |
ΔeGFR5 yr indicates the increase in the eGFR from baseline to 5 years after AF ablation; AAD, antiarrhythmic drugs; ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; ARB, angiotensin type II receptor blocker; AT, atrial tachycardia; BB, beta blocker; CKD, chronic kidney disease; E/Em, the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); LA, left atrium; LVEF, left ventricular ejection fraction; OR, odds radio; PAF, paroxysmal atrial fibrillation; TIA, transient ischemic attack.
Statistical significance.
Which Patients Exhibit a Significant Improvement in Renal Function After AF Ablation?
We performed subgroup analyses to assess whether maintaining sinus rhythm after the last ablation procedure in some groups of patients helped improve 5‐year follow‐up eGFR. The analyses showed that the beneficial effect of maintaining sinus rhythm proved to be more prominent in patients with a CHA2DS2VASc score <2, left atrial diameter <45 mm, or left ventricular ejection fraction ≥50% and without preexisting comorbidities, including hypertension, diabetes mellitus, vascular disease, or advanced CKD (eGFR <60 mL/min/1.73 m2; Figure 4). In particular, the positive effect of rhythm control on renal function improvement was more pronounced in patients without diabetes mellitus than in those with diabetes mellitus (P for interaction=0.012; Figure 4).
Figure 4.
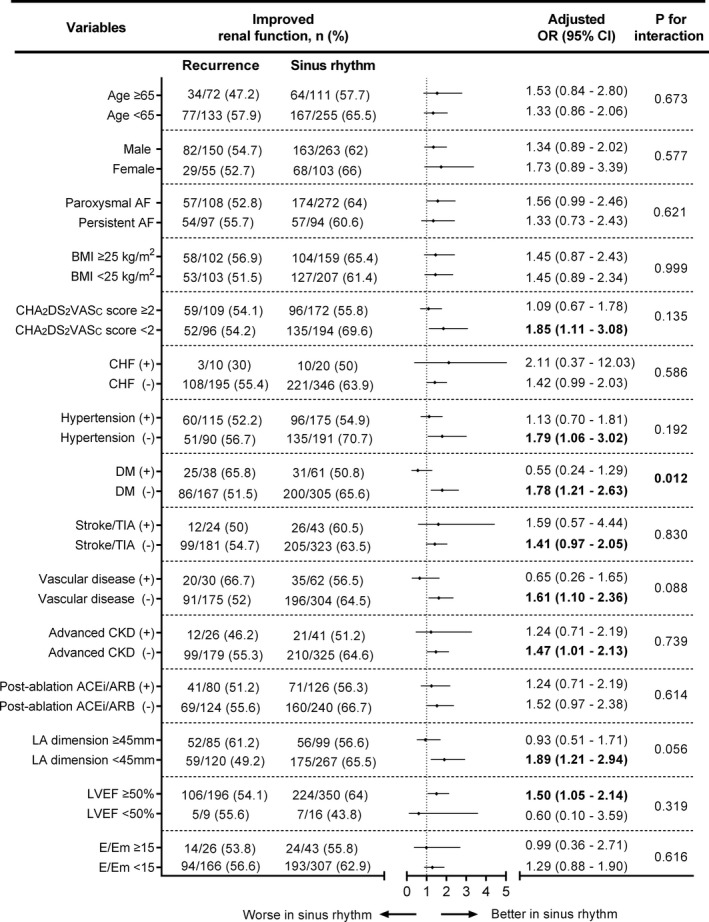
Efficacy of the freedom form AF/AT recurrence after the last AFCA in subgroup analyses. AF indicates atrial fibrillation; AFCA, AF catheter ablation; ARB, angiotensin type II receptor blocker; AT, atrial tachyarrhythmia; BMI, body mass index; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; E/Em, the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); LA, left atrium, LVEF, left ventricular ejection fraction; OR, odds ratio; TIA,transient ischemic attack.
Baseline Renal Function and Long‐Term Rhythm Outcome After AF Ablation
We compared advanced CKD (CKD stage ≥3; eGFR <60 mL/min/1.73 m2) and early CKD (CKD stages 1 and 2; Table 5). Age, proportion with persistent AF at the de novo procedure, CHA2DS2VASc score, and proportion with a comorbid disease, including hypertension, diabetes mellitus, and vascular disease, and LA dimension, were significantly greater in AF patients with advanced CKD than in those with early CKD (Table 5). After adjusting for AF type during the de novo procedure, congestive heart failure, hypertension, diabetes mellitus, and a previous history of vascular disease, old age (adjusted OR, 1.11 [1.07–1.15]; P<0.001) and baseline LA dimension (adjusted OR, 1.05 [1.00–1.11]; P=0.046) were independently associated with baseline advanced CKD (Table S4). However, baseline CKD stage did not affect rhythm outcome of the AF catheter ablation after a single procedure (log rank, P=0.904) or after multiple procedures (log rank, P=0.381; Figure S1).
Table 5.
Comparison of the Baseline Characteristics Among the Overall Patients With or Without Concomitant Advanced CKD
| Advanced CKD | Early CKD | P Value | |
|---|---|---|---|
| (n=67) | (n=504) | ||
| Age, y | 66.6±7.6 | 58.1±10.0 | <0.001a |
| Male | 50 (74.6%) | 363 (72.0%) | 0.655 |
| PAF at procedure | 37 (55.2%) | 343 (68.1%) | 0.036a |
| Body mass index | 25.2±2.7 | 24.9±2.9 | 0.318 |
| Body surface area | 1.81±0.15 | 1.80±0.18 | 0.790 |
| CHA2DS2VASc score | 2.8±1.6 | 1.7±1.5 | <0.001a |
| Congestive heart failure | 7 (10.4%) | 23 (4.6%) | 0.071 |
| Hypertension | 50 (74.6%) | 240 (47.6%) | <0.001a |
| Diabetes mellitus | 22 (32.8%) | 77 (15.3%) | <0.001a |
| Stroke/TIA | 10 (14.9%) | 57 (11.3%) | 0.388 |
| Vascular disease | 17 (25.4%) | 75 (14.9%) | 0.028a |
| LA dimension, mm | 44.1±5.6 | 41.6±6.1 | 0.001a |
| LVEF, % | 62.4±11.0 | 63.6±7.6 | 0.407 |
| E/Em | 11.6±4.4 | 10.5±5.1 | 0.099 |
CKD indicates chronic kidney disease; E/Em, the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); LA, left atrium; LVEF, left ventricular ejection fraction; PAF, paroxysmal atrial fibrillation; TIA, transient ischemic attack.
Statistical significance.
Discussion
Major Findings
In this study, we sought to determine how AF ablation contributed to long‐term renal function as compared with medical therapy alone by comparing an AF ablation cohort database and an NHIS database after retrospective propensity‐score matching. AF catheter ablation, but not medical therapy, significantly improved 5‐year follow‐up eGFR, and this improvement in long‐term renal function was more significant in patients who remained in sinus rhythm after the last AF ablation session and in those without preexisting diabetes mellitus. We also found that preexisting CKD was associated with degree of atrial structural remodeling before the catheter ablation, but did not affect the rhythm outcome of the AF catheter ablation.
AF and Renal Function
AF and CKD are both progressive degenerative diseases, and their prevalence increases with age. AF has been reported in ≈7% to 18% of CKD patients, and 12% to 25% of elderly individuals over 70 years of age with CKD have AF, which is 2 to 3 times that of the general population.5, 23, 24 In contrast, CKD is also associated with 10% to 15% of AF patients.25 Deterioration of renal function serves as an independent risk factor that increases AF prevalence, even in the early stages of CKD.26 It may be because of the mechanistic association between an impaired renal function and AF. Both AF and CKD have several common pathophysiologies, including neurohormonal activation of the renin‐angiotensin‐aldosterone system,27, 28, 29, 30 inflammatory factors,31, 32 and oxidative stress.33, 34, 35 Therefore, AF and renal dysfunction might adversely affect each other over time. In addition, CKD is important and clinically challenging in patients requiring anticoagulation, given that it simultaneously raises the thromboembolic and bleeding risks.36 However, given that CKD has a very heterogeneous nature37 and most major clinical trials related to AF management exclude advanced renal disease directly or indirectly,38 it is hard to define a comprehensive treatment policy for AF patients with CKD.
Renal Function and AF Catheter Ablation
The irregular rhythm of AF reduces myocardial function39 and microvascular function,40 and a loss of atrial kick causes negative hemodynamic effects. Therefore, restoring sinus rhythm may improve renal function by changing the hemodynamics,41, 42, 43, 44 improving ventricular function,16 raising mean heart rate,17 and reducing inflammatory/oxidative reactions,20, 33, 35, 45 especially after catheter ablation. Although there have been multiple clinical studies that evaluated renal function after AF catheter ablation within 1 to 2 years of follow‐up,15, 20, 21, 22, 46 renal function is hard to determine because eGFR declines by 0.5 to 1 mL/min/1.73 m2 per year in the general population and 1 to 2.5 mL/min/1.73 m2 per year in patients with CKD.47 The present study included a longer‐term follow‐up of over 5 years in a higher number of patients, so we were able to more accurately evaluate the small differences in eGFR changes than in previous studies. We also conducted a consistent rhythm‐monitoring and follow‐up protocol based on the guidelines over 5 years.19 We found that aggressive rhythm control and a reduction in AF burden through repeat ablation sessions were associated with an improved renal function during the long‐term follow‐up period. However, such beneficial effects of strict rhythm control by AF ablation were not shown in patients with preexisting diabetes mellitus. It might be because diabetes mellitus is associated with LA fibrosis and electroanatomical LA remodeling, which result in impairment of the LA reservoir and pump function.48, 49 Diabetes mellitus is also a well‐known risk factor of CKD and progressive deterioration of renal function.50 These adverse effects on the heart and kidneys of diabetes mellitus appear to have little effect on long‐term renal function improvement, despite active AF rhythm control by AFCA.
Limitations
This study had several limitations. It was an observational cohort study from a single center, so highly selected patients referred for AF were included in this study. Because we considered that there was an improvement in renal function in patients whose ΔeGFR5 yrs was above 0 mL/min/1.73 m2, this study might not be able to represent an improvement in CKD stage. Because many of the patients who underwent AFCA did not have advanced CKD, this study might not be able to represent a broad spectrum of patients with an impaired renal function. We could not investigate the mechanism of the association between renal function improvement and maintenance of sinus rhythm by comparing the change in hemodynamics and inflammatory markers; however, it was not the main subject of this study.
Conclusions
AF catheter ablation significantly improved renal function during the 5‐year follow‐up. Reduction in AF burden, even with repeat ablation sessions, was independently associated with an improvement in renal function in patients without preexisting diabetes mellitus as well as in the overall patients. However, patients with preexisting diabetes mellitus had no beneficial improvement in renal function; however, sinus rhythm was maintained after repeat ablation sessions.
Sources of Funding
This work was supported by a grant (HI18C0070) from the Korea Health 21 R&D Project, Ministry of Health and Welfare and a grant (NRF‐2017R1A2B4003983) from the Basic Science Research Program run by the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT, and Future Planning (MSIP). This study used the NHIS‐NSC data (NHIS‐2018‐2‐189) made by the National Health Insurance Service (NHIS).
Disclosures
None.
Supporting information
Table S1. Logistic Regression Analysis for Variables Predicting an Improved Renal Function (ΔeGFR5 yr ≥1) After 5 Years of Follow‐up (n=2284)
Table S2. Logistic Regression Analysis for Variables Predicting an Improved Renal Function (ΔeGFR5 yr >5) After 5 Years of Follow‐up (n=2284)
Table S3. Linear Regression Analysis for Variables Predicting a Change in eGFR (ΔeGFR5 yr) After 5 Years of Follow‐up (n=2284)
Table S4. Logistic Regression Analysis of the Baseline Risk Factors Predicting Advanced CKD Among the Overall Patients With AFCA (n=571)
Figure S1. Kaplan–Meier analysis of an AF/AT recurrence after a single AF ablation (A) and the last AF ablation (B) according to baseline advanced CKD.
Acknowledgment
We thank Mr John Martin for his linguistic assistance.
(J Am Heart Assoc. 2019;8:e013204 DOI: 10.1161/JAHA.119.013204.)
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 2. Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey P, Le Heuzey JY, Merioua I, Pedrazzini L, Prystowsky EN, Schwartz PJ, Torp‐Pedersen C, Weintraub W. Real‐life observations of clinical outcomes with rhythm‐ and rate‐control therapies for atrial fibrillation RECORDAF (registry on cardiac rhythm disorders assessing the control of atrial fibrillation). J Am Coll Cardiol. 2011;58:493–501. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 4. Ninomiya T, Kiyohara Y, Tokuda Y, Doi Y, Arima H, Harada A, Ohashi Y, Ueshima H. Impact of kidney disease and blood pressure on the development of cardiovascular disease: an overview from the Japan Arteriosclerosis Longitudinal Study. Circulation. 2008;118:2694‐2701. [DOI] [PubMed] [Google Scholar]
- 5. Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, Dries DL, Bazzano L, Mohler ER, Wright JT, Feldman HI. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010;159:1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, Lerma EV. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nimmo C, Wright M, Goldsmith D. Management of atrial fibrillation in chronic kidney disease: double trouble. Am Heart J. 2013;166:230–239. [DOI] [PubMed] [Google Scholar]
- 8. Washam JB, Holmes DN, Thomas LE, Pokorney SD, Hylek EM, Fonarow GC, Mahaffey KW, Gersh BJ, Kowey PR, Ansell JE, Go AS, Reiffel JA, Freeman JV, Singer DE, Naccarelli G, Blanco R, Peterson ED, Piccini JP. Pharmacotherapy for atrial fibrillation in patients with chronic kidney disease: insights from ORBIT‐AF. J Am Heart Assoc. 2018;7:e008928 DOI: 10.1161/JAHA.118.008928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli R, Raviele A, Themistoclakis S, Rossillo A, Bonso A, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. [DOI] [PubMed] [Google Scholar]
- 10. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bansch D. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 11. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck KH, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al‐Khalidi HR, Lee KL. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansour M, Heist EK, Agarwal R, Bunch TJ, Karst E, Ruskin JN, Mahapatra S. Stroke and cardiovascular events after ablation or antiarrhythmic drugs for treatment of patients with atrial fibrillation. Am J Cardiol. 2018;121:1192–1199. [DOI] [PubMed] [Google Scholar]
- 13. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Patients treated with catheter ablation for atrial fibrillation have long‐term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:839–845. [DOI] [PubMed] [Google Scholar]
- 14. Jin MN, Kim TH, Kang KW, Yu HT, Uhm JS, Joung B, Lee MH, Kim E, Pak HN. Atrial fibrillation catheter ablation improves 1‐year follow‐up cognitive function, especially in patients with impaired cognitive function. Circ Arrhythm Electrophysiol. 2019;12:e007197. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi Y, Takahashi A, Kuwahara T, Okubo K, Fujino T, Takagi K, Nakashima E, Kamiishi T, Hikita H, Hirao K, Isobe M. Renal function after catheter ablation of atrial fibrillation. Circulation. 2011;124:2380–2387. [DOI] [PubMed] [Google Scholar]
- 16. Kim IS, Kim TH, Shim CY, Mun HS, Uhm JS, Joung B, Hong GR, Lee MH, Pak HN. The ratio of early transmitral flow velocity (E) to early mitral annular velocity (Em) predicts improvement in left ventricular systolic and diastolic function 1 year after catheter ablation for atrial fibrillation. Europace. 2015;17:1051–1058. [DOI] [PubMed] [Google Scholar]
- 17. Kang KW, Kim TH, Park J, Uhm JS, Joung B, Hwang C, Lee MH, Pak HN. Long‐term changes in heart rate variability after radiofrequency catheter ablation for atrial fibrillation: 1‐year follow‐up study with irrigation tip catheter. J Cardiovasc Electrophysiol. 2014;25:693–700. [DOI] [PubMed] [Google Scholar]
- 18. Kim TH, Park J, Uhm JS, Joung B, Lee MH, Pak HN. Pulmonary vein reconnection predicts good clinical outcome after second catheter ablation for atrial fibrillation. Europace. 2017;19:961–967. [DOI] [PubMed] [Google Scholar]
- 19. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 20. Koike H, Morita T, Tatebe J, Watanabe I, Koike M, Yao S, Shinohara M, Yuzawa H, Suzuki T, Fujino T, Ikeda T. The relationship between serum indoxyl sulfate and the renal function after catheter ablation of atrial fibrillation in patients with mild renal dysfunction. Heart Vessels. 2019;34:641–649. [DOI] [PubMed] [Google Scholar]
- 21. Navaravong L, Barakat M, Burgon N, Mahnkopf C, Koopmann M, Ranjan R, Kholmovski E, Marrouche N, Akoum N. Improvement in estimated glomerular filtration rate in patients with chronic kidney disease undergoing catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26:21–27. [DOI] [PubMed] [Google Scholar]
- 22. Kornej J, Hindricks G, Banerjee A, Arya A, Sommer P, Rolf S, Husser D, Lip GY, Bollmann A. Changes in renal function after catheter ablation of atrial fibrillation are associated with CHADS2 and CHA2DS2‐VASc scores and arrhythmia recurrences. Heart. 2015;101:126–131. [DOI] [PubMed] [Google Scholar]
- 23. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 24. Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, Hou Q, Shireman TI. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid‐eligible dialysis patients. Kidney Int. 2012;81:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 26. Iguchi Y, Kimura K, Kobayashi K, Aoki J, Terasawa Y, Sakai K, Uemura J, Shibazaki K. Relation of atrial fibrillation to glomerular filtration rate. Am J Cardiol. 2008;102:1056–1059. [DOI] [PubMed] [Google Scholar]
- 27. Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. [DOI] [PubMed] [Google Scholar]
- 28. Iravanian S, Dudley SC Jr. The renin‐angiotensin‐aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm. 2008;5(6 Suppl):S12–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goette A, Staack T, Rocken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal‐regulated kinase and angiotensin‐converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–1677. [DOI] [PubMed] [Google Scholar]
- 30. Ruster C, Wolf G. Renin‐angiotensin‐aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. [DOI] [PubMed] [Google Scholar]
- 31. Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 32. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nam JH, Park KH, Lee JH, Lee CH, Son JW, Kim U, Park JS, Shin DG. Discordant relationships between systemic inflammatory markers and burden of oxidative stress in patients with atrial fibrillation. Korean Circ J. 2017;47:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl. 2009;S3–S11. [DOI] [PubMed] [Google Scholar]
- 36. Reinecke H, Brand E, Mesters R, Schabitz WR, Fisher M, Pavenstadt H, Breithardt G. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol. 2009;20:705–711. [DOI] [PubMed] [Google Scholar]
- 37. Miller LM, Sood MM, Sood AR, Reslerova M, Komenda P, Rigatto C, Bueti J. Cardiovascular disease in end‐stage renal disease: the challenge of assessing and managing cardiac disease in dialysis patients. Int Urol Nephrol. 2010;42:1007–1014. [DOI] [PubMed] [Google Scholar]
- 38. Reddan DN. Therapy for cardiovascular disease in patients with chronic kidney disease: appropriate caution or the absence of data. Am Heart J. 2002;144:206–207. [DOI] [PubMed] [Google Scholar]
- 39. Ling LH, Khammy O, Byrne M, Amirahmadi F, Foster A, Li G, Zhang L, dos Remedios C, Chen C, Kaye DM. Irregular rhythm adversely influences calcium handling in ventricular myocardium: implications for the interaction between heart failure and atrial fibrillation. Circ Heart Fail. 2012;5:786–793. [DOI] [PubMed] [Google Scholar]
- 40. Lim HE, Choi CU, Na JO, Choi JI, Kim SH, Kim JW, Kim EJ, Han SW, Park SW, Rha SW, Park CG, Seo HS, Oh DJ, Hwang C, Kim YH. Effects of iatrogenic myocardial injury on coronary microvascular function in patients undergoing radiofrequency catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:318–326. [DOI] [PubMed] [Google Scholar]
- 41. Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie JL, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. [DOI] [PubMed] [Google Scholar]
- 42. Machino‐Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:1857–1865. [DOI] [PubMed] [Google Scholar]
- 43. Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. [DOI] [PubMed] [Google Scholar]
- 44. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 45. Tarnowski D, Plichta L, Forkmann M, Quick S, Ulbrich S, Heidrich FM, Wiedemann S, Christoph M, Poitz DM, Wunderlich C, Ibrahim K, Strasser RH, Pfluecke C. Reduction of atrial fibrillation burden by pulmonary vein isolation leads to a decrease of CD11b expression on inflammatory cells. Europace. 2018;20:459–465. [DOI] [PubMed] [Google Scholar]
- 46. Wang Q, Zhang XD, Liu X, Yang YQ. Renal function after repeat catheter ablation for long‐standing persistent atrial fibrillation: low CHA2DS2‐VASc score and sinus rhythm predict improved renal function. Herz. 2016;41:331–341. [DOI] [PubMed] [Google Scholar]
- 47. Chapter 2: definition, identification, and prediction of CKD progression. Kidney Int Suppl (2011). 2013;3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Q, Liu T, Ng CY, Li G. Diabetes mellitus and atrial remodeling: mechanisms and potential upstream therapies. Cardiovasc Ther. 2014;32:233–241. [DOI] [PubMed] [Google Scholar]
- 49. Tadic M, Ilic S, Cuspidi C, Ivanovic B, Bukarica L, Kostic N, Marjanovic T, Kocijancic V, Celic V. Left and right atrial phasic function and deformation in untreated patients with prediabetes and type 2 diabetes mellitus. Int J Cardiovasc Imaging. 2015;31:65–76. [DOI] [PubMed] [Google Scholar]
- 50. Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, Southern DA, McLaughlin K, Mortis G, Culleton BF. Progression of kidney dysfunction in the community‐dwelling elderly. Kidney Int. 2006;69:2155–2161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Logistic Regression Analysis for Variables Predicting an Improved Renal Function (ΔeGFR5 yr ≥1) After 5 Years of Follow‐up (n=2284)
Table S2. Logistic Regression Analysis for Variables Predicting an Improved Renal Function (ΔeGFR5 yr >5) After 5 Years of Follow‐up (n=2284)
Table S3. Linear Regression Analysis for Variables Predicting a Change in eGFR (ΔeGFR5 yr) After 5 Years of Follow‐up (n=2284)
Table S4. Logistic Regression Analysis of the Baseline Risk Factors Predicting Advanced CKD Among the Overall Patients With AFCA (n=571)
Figure S1. Kaplan–Meier analysis of an AF/AT recurrence after a single AF ablation (A) and the last AF ablation (B) according to baseline advanced CKD.


