Abstract
Background
Metabolic syndrome (MetS) is a serious health problem over the world; thus, the aim of the present work was to develop a lifestyle intervention to decrease the dysbiosis of gut microbiota and reduce the biochemical abnormalities of MetS.
Methods and Results
The prevalence of MetS was evaluated in 1065 subjects of Mexico City, Mexico, and the gut microbiota in a subsample. Subjects with MetS were selected for a pragmatic study based on a lifestyle intervention with a low‐saturated‐fat diet, reduced‐energy intake, with functional foods and physical activity, and a second group was selected for a randomized control‐placebo study to assess the gut microbiota after the dietary intervention. Prevalence of MetS was 53%, and the higher the body mass index, the higher the gut microbiota dysbiosis. The higher the Homeostatic Model Assessment for Insulin Resistance, the lower the high‐density lipoprotein cholesterol concentration. The pragmatic study revealed that after 15 days on a low‐saturated‐fat diet, there was a 24% reduction in serum triglycerides; and after a 75‐day lifestyle intervention, MetS was reduced by 44.8%, with a reduction in low‐density lipoprotein cholesterol, small low‐density lipoprotein particles, glucose intolerance, lipopolysaccharide, and branched‐chain amino acid. The randomized control‐placebo study showed that after the lifestyle intervention, there was a decrease in the dysbiosis of the gut microbiota associated with a reduction in the Prevotella/ Bacteroides ratio and an increase in the abundance of Akkermansia muciniphila and Faecalibacterium prausnitzii.
Conclusions
A lifestyle intervention significantly decreased MetS components, small low‐density lipoprotein particle concentration, gut microbiota dysbiosis, and metabolic endotoxemia, reducing the risk of atherosclerosis.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03611140.
Keywords: functional foods, lipoprotein, metabolic endotoxemia, metabolic syndrome, microbiota
Subject Categories: Diet and Nutrition, Lifestyle, Obesity
Clinical Perspective
What Is New?
Of subjects with overweight or obesity, 53% had metabolic syndrome.
A lifestyle intervention decreased metabolic syndrome prevalence and dysbiosis of gut microbiota.
A lifestyle intervention decreased small low‐density lipoprotein and high‐density lipoprotein particles.
What Are the Clinical Implications?
The development of lifestyle interventions with the recommendations of the National Cholesterol Education Program and the addition of functional foods high in fiber, easily accessible, with bioactive compounds and with a low glycemic index can be useful in the control of the epidemic of obesity and metabolic syndrome.
Introduction
Metabolic syndrome (MetS) is estimated to be prevalent in at least a quarter of the adults in the world1 and is becoming a major public health issue in urban areas. In addition to genetic predisposition, there are various modern environmental factors, such as physical inactivity and dysbiosis of the gut microbiota associated with several hallmarks of MetS and with the onset of low‐grade inflammation,2 that can contribute to the development of MetS. Therefore, it is necessary to develop effective lifestyle interventions for real‐world clinical practice to control MetS and reduce the risk of developing diabetes mellitus and cardiovascular disease in the near future. The MetS includes a cluster of at least 3 of the following factors: central obesity (waist circumference ≥80 cm in women and ≥90 cm in men), fasting blood glucose ≥5.50 mmol/L, triglyceride concentration ≥1.65 mmol/L, low levels of high‐density lipoprotein (HDL) cholesterol (<1.00 mmol/L in men and <1.25 mmol/L in women), and systolic and/or diastolic blood pressure ≥130/85 mm Hg. These cardiovascular risk factors are now one of the major public health challenges worldwide. Substantial evidence shows that lifestyle intervention programs play a crucial role in the control and treatment of MetS.3 It has been suggested that a low‐saturated‐fat diet (LSFD), recommended by the Adult Treatment Program, is the first strategy to reduce cardiovascular disease.4 We developed a dietary portfolio with functional foods (soy protein, dehydrated nopal, chia seed, oat, and inulin) as a method to provide dietary support for more effective control of MetS. A dietary portfolio includes ≥2 functional foods with beneficial health properties for a specific condition on the basis of scientific evidence. The American Heart Association and the Adult Treatment Program recommend the use of dietary portfolio to control dietary lipids.5
Thus, the purpose of the present work was to study the effect of a lifestyle intervention into real‐world clinical practice in subjects with MetS and its effect on biochemical parameters, gut microbiota, and metabolic endotoxemia.
Subjects and Methods
Methods
The data will not be made available to other researchers for purposes of reproducing the results or replicating the procedures; however, the data that support the findings of this study are available from the corresponding author on request.
Study Design
The study was divided into 3 stages: (1) a cross‐sectional study to evaluate the prevalence of MetS in subjects of the urban area of Mexico City, Mexico, and its metabolic alterations in subjects with overweight and obesity and the gut microbiota in a subsample, to identify and recruit subjects for a lifestyle intervention; (2) in a sample of subjects with MetS, a pragmatic study was conducted to provide evidence for adoption of a lifestyle intervention into real‐world clinical practice6, 7; and (3) finally, a single‐center, randomized, placebo‐controlled, double‐blind, parallel‐arm study was performed to evaluate the effect of lifestyle intervention on the gut microbiota in a sample of healthy subjects and subjects with MetS and class III obesity (OCIII) (Figure S1).
Participants
This study was performed at the Department of Physiology of Nutrition of the Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán, Mexico City, from January 2013 to June 2018. The inclusion criteria for the cross‐sectional study to identify subjects with MetS were Mexican mestizos, aged 20 to 65 years, with body mass index (BMI) >18.5 kg/m2 without any chronic disease. Subjects were divided according to the presence of MetS criteria. For the pragmatic study, subjects who were aged 20 to 60 years, with BMI >25 and <40 kg/m2, and who satisfied 3 positive criteria for MetS were included. In the randomized control‐placebo study, the inclusion criteria for controls were healthy adults, BMI ≥20 to <25 kg/m2 with no criteria of positive MetS; and for cases with MetS, adults with a BMI ≥25 to ≤50 kg/m2 with 3 positive criteria of the MetS were selected.
The exclusion criteria included serum glucose ≥6.93 mmol/L, a history of cardiovascular events, weight loss >3 kg in the past 3 months, cancer, AIDS, kidney or liver disease, pregnancy, smoking, substance abuse, alcohol consumption, or having taken any medication. For the randomized control‐placebo study, the following exclusion criteria were added in addition to those previously mentioned: any drug or medication that activates intestinal motility, laxatives or antispasmodics 4 weeks before the study, treatment with antibiotics 2 months before the study, treatment with prosymbiotic/presymbiotic/symbiotic and high‐fiber foods (>15 g of fiber), patients with a digestive functional disorder (constipation, diarrhea, dyspepsia, or functional abdominal distension determined by questionnaire on the basis of the classification of Rome III), inflammatory bowel disease, irritable bowel syndrome, or other chronic gastrointestinal diseases, and patients with a major abdominal surgery.
Lifestyle Intervention
In the pragmatic study, the participants who met the selection criteria were invited to participate in the 2‐period lifestyle intervention. In the first period, the participants consumed a reduced‐energy diet tailored to provide a 2092 J/d deficit, as recommended by the National Institutes of Health,8 with respect to their habitual diet for 2 weeks and an LSFD, according to the Adult Treatment Program.9 During the second period of the study, participants consumed an LSFD and functional foods for 2 months. For each dietary period, participants in the study received 15 different eating plans (50%–60% carbohydrates, 15% protein, 25%‐35% fat, <7% saturated fat based on total energy, ≤200 mg cholesterol, and 20–30 g/d fiber). During the second period, the participants continued to consume the same reduced‐energy diet as in the first period minus the energy provided by the combination of functional foods (736 J/d).
The combination of functional foods consisted of a mixture of 14 g of dehydrated nopal, 4 g of chia seeds, 14 g of oats, 25 g of soybean protein, 4 g of inulin, 0.02 g of sweetener, and 0.15 g of flavoring. The functional foods were selected on the basis of their antihyperglycemic, antihyperinsulinemic, hypocholesterolemic, anti‐inflammatory, and antioxidant effects caused by the presence of omega‐3 fatty acids, β‐glycans, vegetable protein of good quality, soluble and insoluble fiber, polyphenols, and a low glycemic index. In addition, the physical activity of each participant was measured by a pedometer (YAMAX; Health & Sports Inc, Tokyo, Japan). Participants quantified the average steps for a week. Subsequently, the physical activity increased by 10% in the first 15 days and subsequently increased by 25% for 1 month and 50% in the second with respect to the basal physical activity.
In the randomized control‐placebo study, a subsample of healthy subjects and recruited subjects with MetS were instructed to follow the same lifestyle intervention as above for 2 weeks. After that, participants were randomly assigned to follow either lifestyle intervention similar to the pragmatic study or placebo for 2 months. The study was a randomized block‐design controlled trial. This random allocation was performed by an assistant not associated with any other aspect of the research. The placebo consisted of 30 g of calcium caseinate, 30 g of maltodextrin, 0.02 g of sweetener, and 1 g of flavoring. Each mix was placed into packages of 30 g that had similar energy, appearance, and taste to functional foods to ensure a double‐blind study. The product in each package was dissolved in 250 mL of water. Participants were instructed to consume a serving of the functional foods or placebo once in the morning and once at night for 2 months of treatment.
Dietary assessment. Compliance to the diet and the placebo or combination of functional foods was evaluated using 3 methods: the 24‐hour diet recall, the 3‐day food record (food log), and measuring the number of empty packages returned. In addition, compliance with the combination of functional foods or placebo was monitored by weekly telephone calls by the nutritionists.
Procedures
In the cross‐sectional study, a medical history was obtained, and a physical activity questionnaire was completed. Anthropometric variables and serum biochemical parameters were measured in a blood sample. In a subsample of healthy and MetS subjects, stool and serum samples were taken to determine the gut microbiota and serum lipopolysaccharide. The pragmatic or randomized control‐placebo studies consisted of 4 visits during the monitoring period. In the first visit, a medical history, 2‐hour oral glucose tolerance test, and blood glucose and serum insulin levels were recorded for a period of 120 minutes after the administration of 75 g of oral glucose. In the randomized control‐placebo study, a stool sample for DNA isolation was collected. During each subsequent visit, a 24‐hour dietary recall was conducted, a physical activity questionnaire was completed, and anthropometric and serum biochemical variables were measured. During the last visit, the 2‐hour oral glucose tolerance test was repeated; and in the randomized control‐placebo study, a stool sample for DNA isolation was collected. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición (approval numbers 346 and 793). All participants were informed about the scope and procedures of the study, and before any study procedures, written informed consent was formally obtained.
Anthropometric Measurements
The anthropometric evaluation included measurements of the body weight, height, and waist circumference.10 The percentage of fat and lean mass was obtained using bioelectrical impedance analysis (Inbody 720; Inbody Co, LTD, South Korea) in the morning after 12 hours of fasting. The nutritional status of the subjects was evaluated by BMI classification, according to the World Health Organization.11
Biochemical Parameters
Blood samples were collected after a 12‐hour fasting period. Serum glucose, total cholesterol, triglycerides, HDL, low‐density lipoprotein (LDL), glycosylated hemoglobin, and CRP (C‐reactive protein) were determined using a COBAS c111. Total serum adiponectin, leptin, and insulin were measured using an ELISA kit (ALPCO, Salem, NH). Plasma branched‐chain amino acid (BCAA) levels were determined using a colorimetric assay kit following the manufacturer's instructions (Abcam, Cambridge, MA) with leucine as a standard, and serum lipopolysaccharide was determined by ELISA kit (Cloud‐Clone Corp).
Genotyping
DNA was extracted from leukocytes from a blood sample. Single‐nucleotide polymorphisms (SNPs) of ATP‐binding cassette transporter A1 (ABCA1) rs9282541, fat mass and obesity‐associated gene (FTO) rs9939609, glucose‐fructose oxidoreductase domain containing 2 (GFOD2) rs12449157, peroxisome proliferator activated receptor γ (PPARγ) rs1801282, transcription factor 7‐like 2 (TCF7L2) rs7903146, adiponectin (ADIPOQ) rs1501299, and apolipoprotein E (APOE) rs7412 were determined by allelic discrimination using a polymerase chain reaction end point TaqMan SNP Genotyping assay (ABI Prism 7900 HT Sequence Detection System; Applied Biosystems, Foster City, CA). These genotypes were distributed according to the Hardy‐Weinberg equilibrium.12
Lipoproteins
The lipoprotein analysis was performed by nuclear magnetic resonance, including lipoprotein subclass concentrations, mean sizes for very LDL (VLDL), LDL and HDL, and nuclear magnetic resonance–estimated total triglycerides, VLDL triglycerides, and HDL cholesterol by LipoScience, Inc (Raleigh, NC).13
DNA Isolation and 16S rRNA Gene Sequencing of Gut Microbiota
Fresh fecal samples were collected immediately, frozen, and stored at −70°C until use. Bacterial DNA content was extracted using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. The MiSeq platform was used for the sequencing of the samples, and then genomic libraries of the regions V3 and V4 of the 16S gene were generated using primers for those regions that also contained an overhang adapter specified by Illumina, as described before.14 The amplicons of the V3 and V4 regions were generated by polymerase chain reactions containing genomic DNA (5 ng/μL in 10 mmol/L Tris, pH 8.5), high‐fidelity DNA polymerase 2× KAPA HiFi HotStart ReadyMix, and primers (1 μmol/L). This mixture was placed into the thermal cycler and run through the following program: 3 minutes at 95°C, followed by 25 amplification cycles consisting of denaturation (30 seconds at 95°C), alignment (30 seconds at 55°C), and elongation (30 seconds at 72°C). The final elongation consisted of 5 minutes at 72°C. The amplicons were purified using AMPure XP beads, and their size was verified by capillary electrophoresis in a QIAxcel Advanced system (Qiagen, Germany), with an approximate size of 550 bp. After passing quality control, the samples were indexed using the Illumina Nextera XT Index Kit (v.2, set A). For this process, 5 μL of the first polymerase chain reaction product, High Fidelity DNA polymerase 2× KAPA HiFi HotStart ReadyMix, and primers (index) were mixed and returned to the thermocycler using the following program: 3 minutes at 95°C, followed by 8 amplification cycles consisting of denaturation (30 seconds at 95°C), alignment (30 seconds at 55°C), and extension (30 seconds at 72°C). The final extension consisted of 5 minutes at 72°C. This product was purified, and the integrity was analyzed. The amplicons had an approximate size of 610 bp. The concentration of double‐stranded DNA was determined by fluorometry (Qubit fluorometer 3.0, highly sensitive kit). The final library was mixed equimolarly and sequenced on the Illumina MiSeq platform (MiSeq Reagent Kit V.3, 600 cycles), following the supplier's instructions.
Sequence Analysis
For taxonomic composition analysis, Custom C# and Python scripts, as well as Python scripts in the Quantitative Insights Into Microbial Ecology 1.9 software pipeline, were used to process the sequencing files. The sequence outputs were filtered for low‐quality sequences (defined as any sequences that were <200 or >620 bp, sequences with any nucleotide mismatches to either the barcode or the primer, sequences with an average quality score of <30, and sequences with ambiguous bases >0). Sequences were chimera checked with Gold.fa, and chimeric sequences were filtered out. Analysis started by clustering sequences within a percentage sequence similarity into operational taxonomic units (OTUs); 93% of the sequences passed filtering, resulting in 57 342 sequences/sample with a 97% similarity threshold. OTU selection was performed using Quantitative Insights Into Microbial Ecology tools and the Usearch method. Representative sequences were aligned using PyNAST algorithms. OTUs were picked against the Greengenes 13.9 with a 97% similarity with the OTU reference database. After the resulting OTU, the result files were merged into one overall table, and taxonomy was assigned on the basis of the gg v13.9 reference taxonomy. Thus, 99.94%, 99.72%, 99.56%, 94.32%, 85.48%, and 44.41% of the reads were assigned to the phylum, class, order, family, genus, and species level, respectively. Species richness (observed, Chao1) and α diversity measurements (Shannon) were calculated, and we estimated the within‐sample diversity at a rarefaction depth of >10 189 reads per sample. Weighted and unweighted UniFrac distances were used to perform the principal coordinate analysis (PCoA).15
Microbial sequence data were pooled for OTU comparison and taxonomic abundance analysis but separated by batch in PCoA to obtain clear PCoA figures. For even sampling, a depth of 10 189 sequences/sample was used. PCoAs were produced using Emperor. Community diversity was determined by the number of OTUs and β diversity, measured by UniFrac unweighted and weighted distance matrices in Quantitative Insights Into Microbial Ecology. ANOSIM, a permutational multivariate ANOVA, was used to determine statistically significant clustering of groups on the basis of microbiota structure distances.
Statistical Analysis
Sample size for the pragmatic study was calculated on the basis of a previous study,16 with 80% power and α error=0.05 and considering a follow‐up loss of 20%. The sample size was estimated to be 146 participants for detecting 10% difference in all MetS biochemical variables for those who consumed the LSFD+functional foods.
The continuous variables were expressed as mean±SEM. The dichotomous variables were expressed as frequencies and percentages. All variables were log transformed before analysis to reduce the skewness and the variability of data, especially in data sets that include outlying observations. Anthropometric or biochemical analysis of each group, according to BMI (normal, overweight, obesity class I, obesity class II, and obesity class III), was compared using one‐way ANOVA. Anthropometric or biochemical analysis of each group, according to BMI and sex, was performed by 2‐way ANOVA. The comparison between anthropometric or biochemical parameters between groups (without MetS versus MetS or women versus men) was analyzed using an independent Student t test. The statistical analysis of the anthropometric and biochemical parameters in the pragmatic study was performed during the 4 visits at times 0, 15, 45, and 75 days after the intervention using repeated‐measures ANOVA. When the main effects were identified by the initial analysis, post hoc analysis using Bonferroni correction was conducted. To analyze only the differences between baseline and after lifestyle intervention, a paired t test was used. A dominant model was used for analysis of SNPs comparing the genotype of the common homozygote versus heterozygote genotype and no common homozygote genotype. Differences between groups were analyzed using an independent Student t test. The significance threshold was set at α=0.05. The data were analyzed using Statistical Package for Social Science, version 20 (SPSS Inc, Chicago, IL). In the randomized control‐placebo study, the differences between baseline and the final measurements of the gut microbiota between placebo and functional food groups were determined using linear discriminant analysis effect size to find biomarkers between 2 groups using relative abundances. The tool is hosted on a Galaxy web application.
Results
Cross‐Sectional Study
Prevalence of overweight and obesity in the studied population and its physiological/biochemical parameters according to BMI categories
In the cross‐sectional study, 1065 participants were recruited. As expected, women showed significantly lower values of anthropometric measurements than men. In our population, blood pressure was significantly higher in men than women. Also, triglyceride, HDL cholesterol, CRP, and leptin levels differed according to sex. We further classified the patients according to their BMI category to allow us a better characterization and comparison among groups. The percentage of subjects with normal weight (NW) was 3.3%; overweight, 29.4%; class I obesity (OCI), 38.8%; class II obesity (OCII), 19.8%; and OCIII, 8.7% (Table S1).
As expected, body fat increased significantly from NW to overweight and reached a rather stable percentage from OCI, whereas in the opposite direction, the percentage of lean mass showed a continuous and significant reduction (Figure 1A). Furthermore, blood glucose tended to increase as BMI increased; thus, patients with OCIII had a 21.8% and 13.7% increase compared with NW or overweight, respectively. Similarly, insulin levels augmented in a significant linear trend as the BMI increased among groups. Patients with OCII or OCIII had a 2‐ and 3‐fold increase, respectively, with respect to the NW group (Figure 1B). As a consequence, cumulative gaussian curves indicated that as BMI increased, the percentage of patients with Homeostatic Model Assessment for Insulin Resistance values ≥2.5 also increased (Figure 1C). In addition, obese patients had a significantly higher concentration of triglyceride and lower HDL values for both sexes than subjects with NW (Figure 1D and 1E). Finally, both leptin and CRP levels were higher in subjects with any class of obesity than NW subjects (Figure 1F and 1G); indeed, the increase in CRP in obese patients ranged from 3 to 7 times with regard to individuals having NW.
Figure 1.
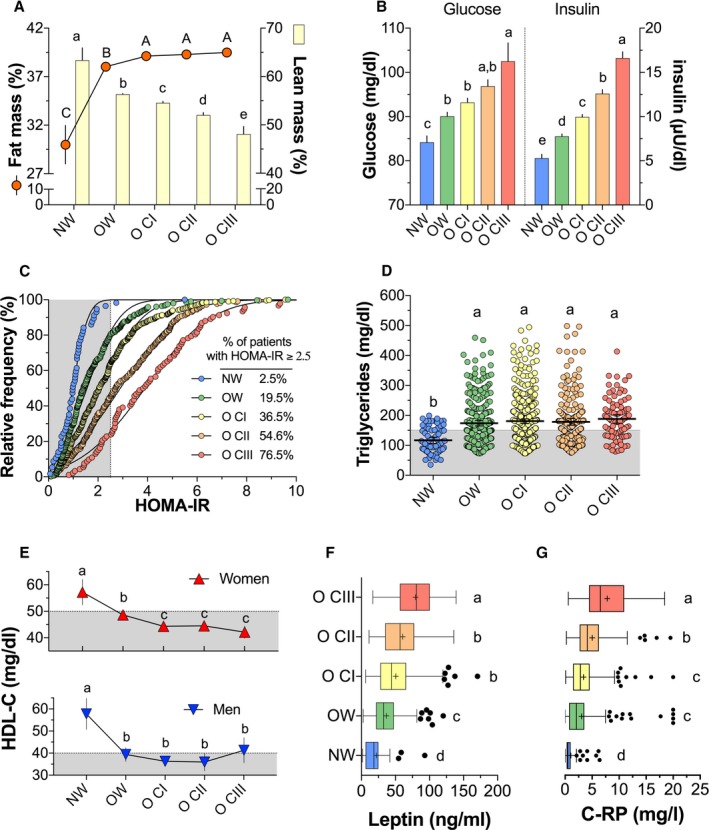
Physiological/biochemical parameters according to BMI categories in 1065 participants. A, Fat mass (%) and lean mass (%). B, Serum glucose and insulin. C, Relative frequency of subjects with Homeostatic Model Assessment for Insulin Resistance (HOMA‐IR) ≥2.5. D, Serum triglyceride concentration. E, Serum high‐density lipoprotein cholesterol (HDL‐C) concentration in women and men. F, Serum leptin. G, Serum CRP (C‐reactive protein). To compare the variables among body mass index categories, 1‐way ANOVA, followed by Tukey's post hoc test, was performed. Different letters indicate significant differences among groups: a>b>c>d (P<0.05). NW indicates normal weight; OW, overweight; OCI, class I obesity; OCII, class II obesity; OCIII, class III obesity.
Prevalence of MetS among groups of subjects with overweight and obesity
Of the 1032 participants with overweight and obesity, 542 (52.5%) presented with MetS. There was a significant trend toward an increased prevalence of MetS as the BMI increased: overweight, 41.8% (129/308); OCI, 55.0% (228/414); OCII, 57.3% (125/218); and OCIII, 65.2% (60/92) (Figure 2A). Interestingly, there were differences between sex. The prevalence of MetS in women was 40.6%, 51.7%, 57.2%, and 67.9% in overweight, OCI, OCII, and OCIII groups, respectively, whereas in men, the prevalence of MetS was 48.1%, 66.7%, 57.9%, and 50%, respectively (Figure 2B). As expected, subjects with overweight or OCI and OCII with MetS had higher values of systolic and diastolic blood pressure (Figure 2C). Likewise, the concentration of serum glucose, insulin, and, therefore, the Homeostatic Model Assessment for Insulin Resistance increased as the BMI increased (Figure 2D and 2E). In addition, serum triglyceride concentration was higher in subjects with MetS (Figure 2F). In contrast, according to sex, HDL cholesterol was significantly lower when MetS was present in each BMI category (Figure 2G). Approximately 72% of subjects with MetS showed a light physical activity, which indicates a sedentary lifestyle, and 22% of subjects studied (509 subjects) presented irritable bowel syndrome (Figure S2A and S2B), according to the criteria of functional gastrointestinal disorders, ROMA III.17
Figure 2.
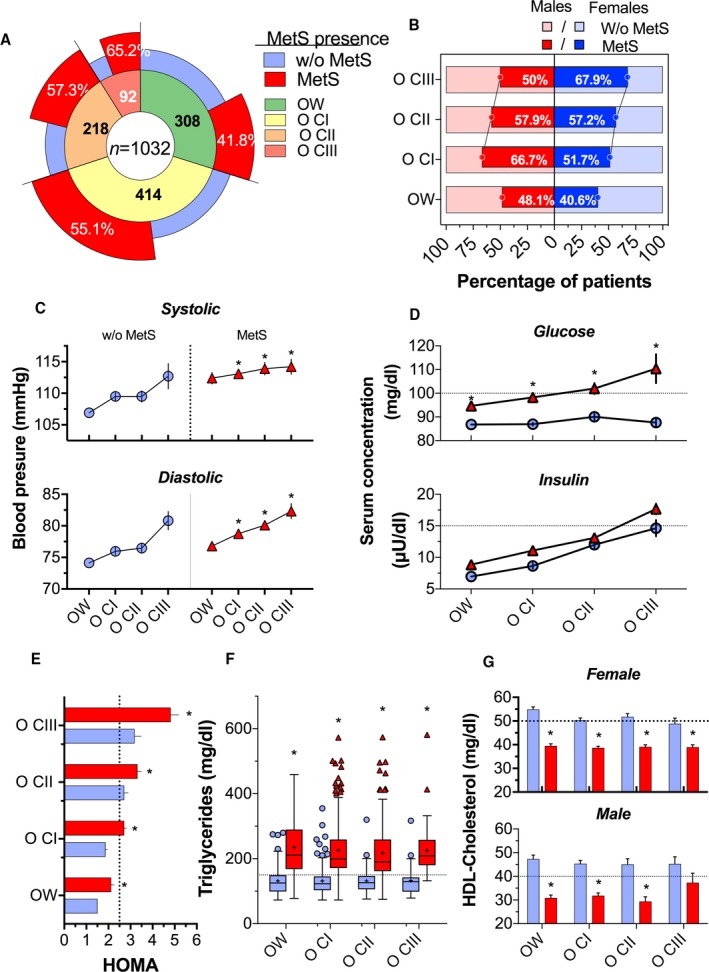
Biochemical parameters according to body mass index (BMI) categories and presence of metabolic syndrome (MetS) in 1032 participants. A, Percentage of participants with and without MetS according to the BMI category: overweight (OW), class I obesity (OCI), class II obesity (OCII), and class III obesity (OCIII). B, Percentage of participants with and without MetS, according to BMI categories and sex. C, Systolic and diastolic blood pressure, according to BMI categories. Circles in blue represent without MetS, and triangles in red represent with MetS. D, Serum glucose and insulin concentration, according to BMI categories. E, Homeostatic Model Assessment for Insulin Resistance (HOMA‐IR), according to BMI categories; the red bars represent with MetS, and the blue bars represent without MetS. F, Serum triglyceride concentration, according to BMI categories. G, High‐density lipoprotein (HDL) cholesterol concentration, according to BMI categories and sex. The differences in biochemical/physiological parameters between participants with and without MetS were determined using an unpaired Student t test.
The previous results suggested that several biochemical and clinical abnormalities (Table S2) in addition to physical inactivity observed in subjects with MetS could be associated with changes in the gut microbiota; therefore, fecal samples were collected to assess the gut microbiota.
Analysis of gut microbiota in healthy and MetS subjects
To study if there was a difference in gut microbiota among NW, MetS, and OCIII+MetS subjects, PCoA was used to display similarity among groups. PCoA revealed that the gut microbiota of subjects with MetS or OCIII+MetS was dissimilar from that of NW subjects, as observed in the PCoA analysis (ANOSIM r=0.6065; P=0.006) (Figure 3A). According to the Shannon index (Figure 3B), there was no difference in α diversity between NW subjects and subjects with MetS; however, there was a significant difference in the α diversity between NW and OCIII+MetS subjects. The relative abundance of the main phyla Bacteroidetes, Firmicutes, Proteobacteria, Tenericutes, Verrucomicrobia, and Cyanobacteria represented ≈98.62% of the sequences at the phylum level (Figure 3C). At the genus level, subjects with MetS and OCIII+MetS showed increases of ≈7.8% and 21.3%, respectively, in the relative abundance of Prevotella and significant decreases of 8.8% and 21.9%, respectively, in the relative abundance of Bacteroides with respect to the NW subjects (Figure 3D). Interestingly, there was a notable increase in serum lipopolysaccharide in subjects with MetS and OCIII+MetS with respect to the NW group, which is a major component of Gram‐negative bacterial cells (Figure 3E). In fact, subjects with MetS and OCIII showed a 33.7‐ and 32.1‐fold increase, respectively, in serum lipopolysaccharide compared with healthy subjects, indicative of chronic remarkable metabolic endotoxemia (Figure 3E). The linear discriminant analysis effect size indicated a clear difference in the gut microbiota between MetS or OCIII+MetS and healthy subjects, with increasing levels of mainly Prevotella copri in subjects with MetS and OCIII+MetS (Figure 3F). The ratio of Prevotella/Bacteroides was 0.085±0.04, 0.668±0.19, and 3.12±0.71 in NW, MetS, and OCIII+MetS subjects, with a significant difference between OCIII and NW or MetS (P<0.0001) (Figure 4A). In addition, there was a significant association (P<0.0001; r=0.604) between lipopolysaccharide and the Gram‐negative bacteria P copri, suggesting that the abundance of this bacteria is a causal agent of metabolic endotoxemia (Figure 4B). Interestingly, the higher the Prevotella/Bacteroides ratio, the higher the carbohydrate and the lower protein intake (Figure 4C).
Figure 3.
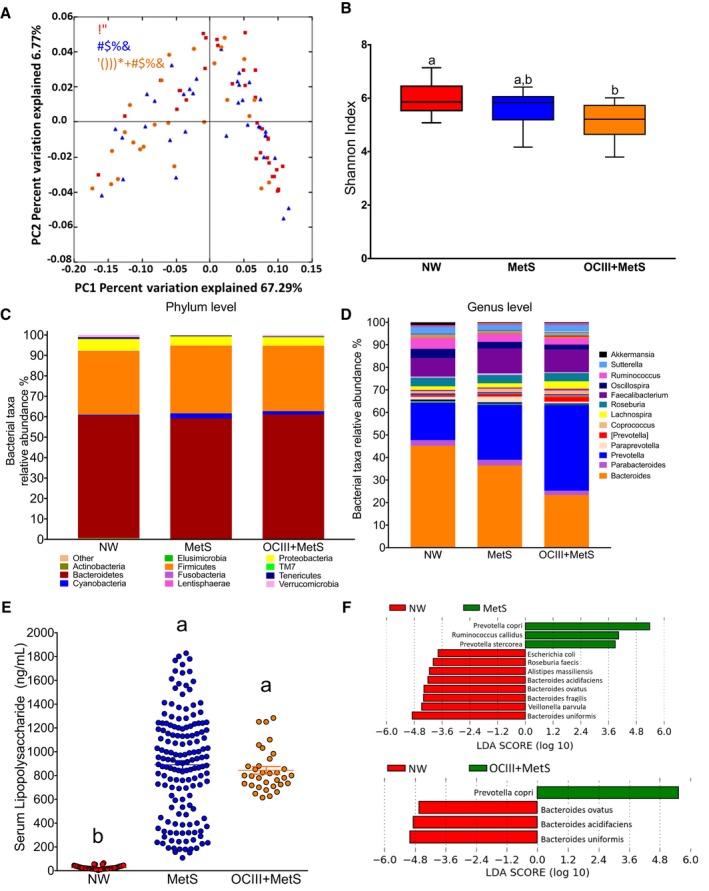
Metabolic syndrome (MetS) modifies the gut microbiota in Mexican adults. A, Principal coordinate analysis (PCoA) of normal weight (NW) subjects and subjects with MetS or class III obesity (OCIII)+MetS on the basis of the weighted UniFrac distances. The red squares represent samples of healthy subjects, blue triangles represent subjects with MetS, and orange circles represent subjects with OCIII+MetS. B, The α diversity by Shannon index in NW subjects indicates higher diversity in healthy subjects than subjects with OCIII+MetS. C and D, Taxonomic summary of the gut microbiota of NW subjects and subjects with MetS and OCIII+MetS at the phylum (C) and at the genus (D) levels. E, Serum lipopolysaccharide (LPS) in healthy subjects, subjects with MetS, and subjects with OCIII+MetS. F, Discriminative taxa at the species level in NW subjects and subjects with MetS and OCIII+MetS were determined using a linear discriminant analysis (LDA) effect size. The green bar chart represents the species that were more abundant in MetS and OCIII+MetS subjects, and the red bar chart represents the healthy subjects.
Figure 4.
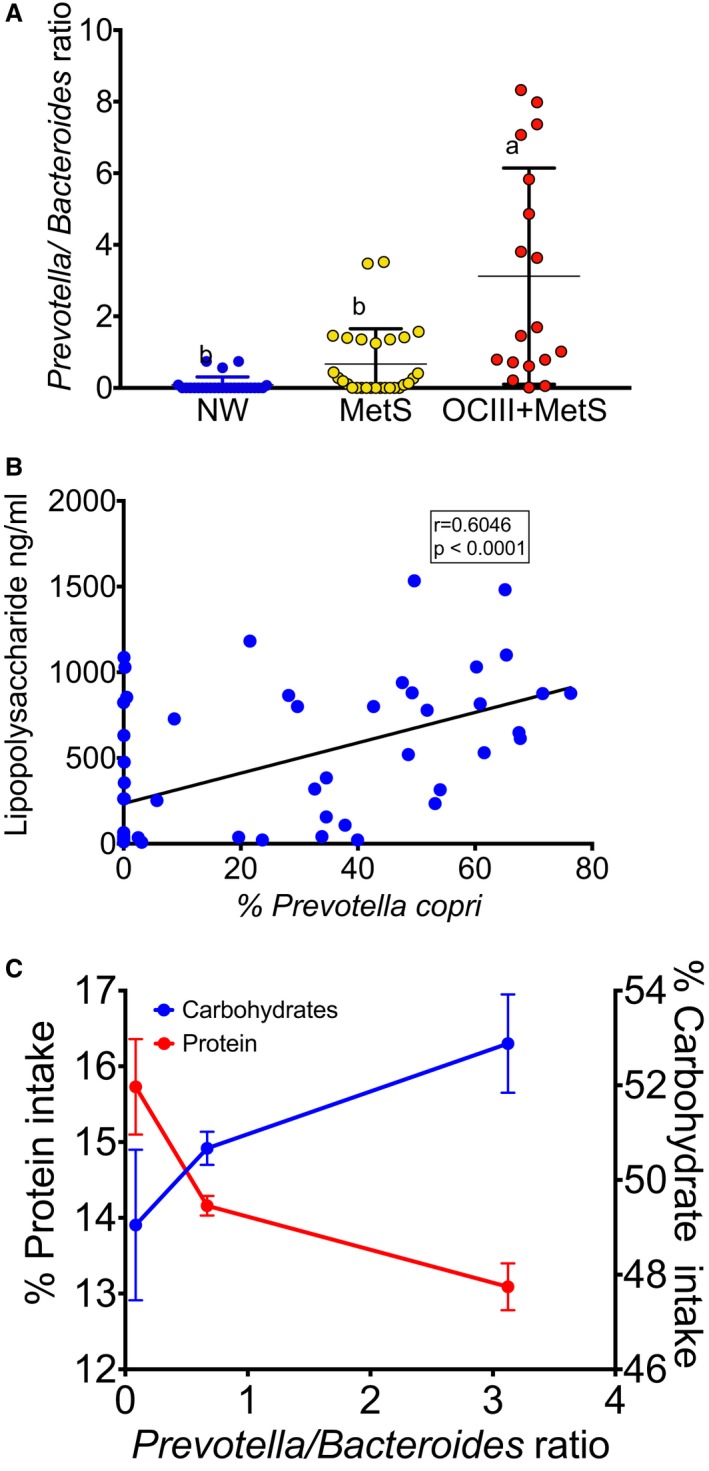
Relationship between specific genera and metabolic endotoxemia and body mass index categories and carbohydrate intake. A, Prevotella/Bacteroides ratio in normal weight (NW) subjects and subjects with metabolic syndrome (MetS) and class III obesity (OCIII)+MetS; statistical analysis was performed using 1‐way ANOVA, followed by Fisher's Least Significant Difference (LSD) post hoc test. Different letter indicates significant differences among groups: a>b>c (P<0.05). B, Correlation between serum lipopolysaccharide and Prevotella copri. The statistical analysis was performed using the correlation of Spearman. C, Association between Prevotella/Bacteroides ratio and percentage carbohydrates or percentage protein consumed in the diet.
Pragmatic Study
Effect of a lifestyle intervention on anthropometric and biochemical variables of the MetS
To study the effect of a lifestyle intervention in real‐world clinical practice, we studied 146 selected subjects who met the MetS criteria. Waist circumference, BMI, blood pressure, serum glucose, and HDL‐C were not modified in the first 15 days by the LSFD; however, serum triglycerides significantly decreased by 24.1% with respect to basal levels (Figure 5C). Interestingly, after 2 months of lifestyle intervention, waist circumference, BMI, and blood pressure (Figure 5A, 5B, and 5D) significantly decreased by 4.2%, 1.8%, and 5%, respectively, in women, and by 4.7%, 2.5%, and 5%, respectively, in men. There was no further decrease in serum triglycerides (Figure 5C), and serum glucose levels remained at normal levels (Figure 5E). Serum HDL‐C significantly increased by 8.6% only in women (Figure 5F).
Figure 5.
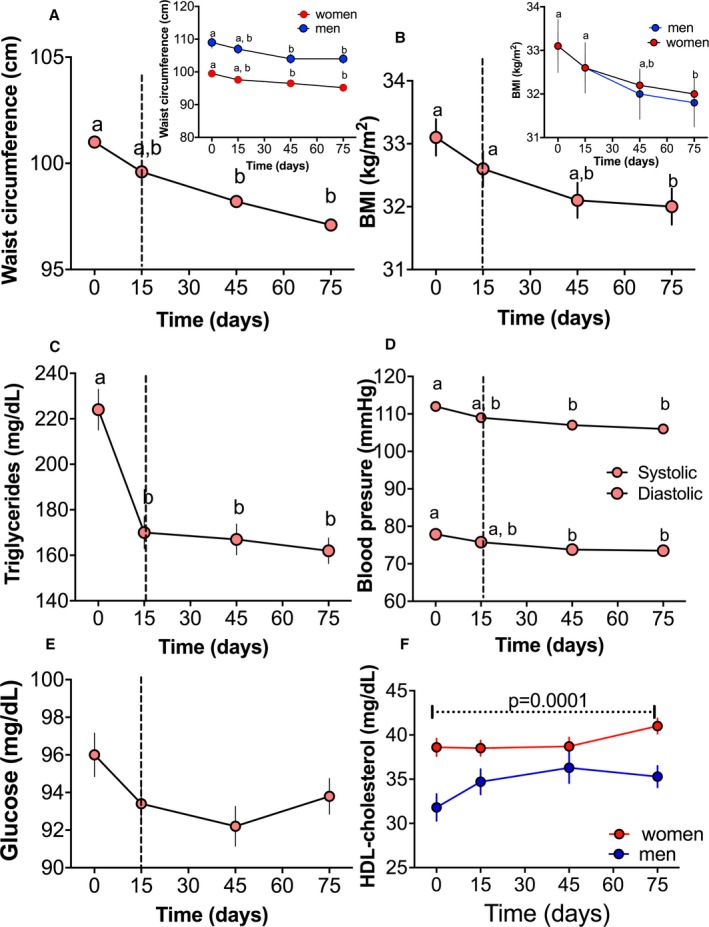
Effect of a lifestyle intervention with functional foods and energy reduction (−500 kcal) for 75 days on clinical and biochemical characteristics in 146 patients with metabolic syndrome. In the first stage, participants were instructed to consume a reduced‐energy diet for 15 days. This period is indicated by a vertical dotted line. During the second stage of the study, participants consumed the dietary intervention and functional foods in addition to the reduced‐energy diet for 60 days. A, Waist circumference in all patients and separated by sex. B, Body mass index (BMI) in all patients and separated by sex. C, Serum triglycerides. D, Systolic and diastolic blood pressure. E, Serum glucose. F, Serum high‐density lipoprotein (HDL) cholesterol separated by sex. Statistical analysis was performed using 1‐way ANOVA, followed by Bonferroni post hoc test. Logarithmic transformation was performed before the statistical analysis. Different letters indicate significant differences among groups: a>b>c>d (P<0.05).
In addition to the beneficial effect of the lifestyle intervention on all MetS criteria, there was a significant reduction of 20.4% and 31.5% in women and men, respectively, on serum leptin concentration in the different BMI categories (Figure 6A) and a significant reduction of 3.9% and 7.6% in body fat in women and men, respectively (Figure 6B). Homeostatic Model Assessment for Insulin Resistance and glycosylated hemoglobin were reduced by 12.3% and 5.4%, respectively (Figure 6C and 6D), which was more evident in subjects with OCII. The areas under the curve for glucose and insulin in all categories were significantly reduced by ≈8% and 12%, respectively (Figures 6E and 6F).
Figure 6.
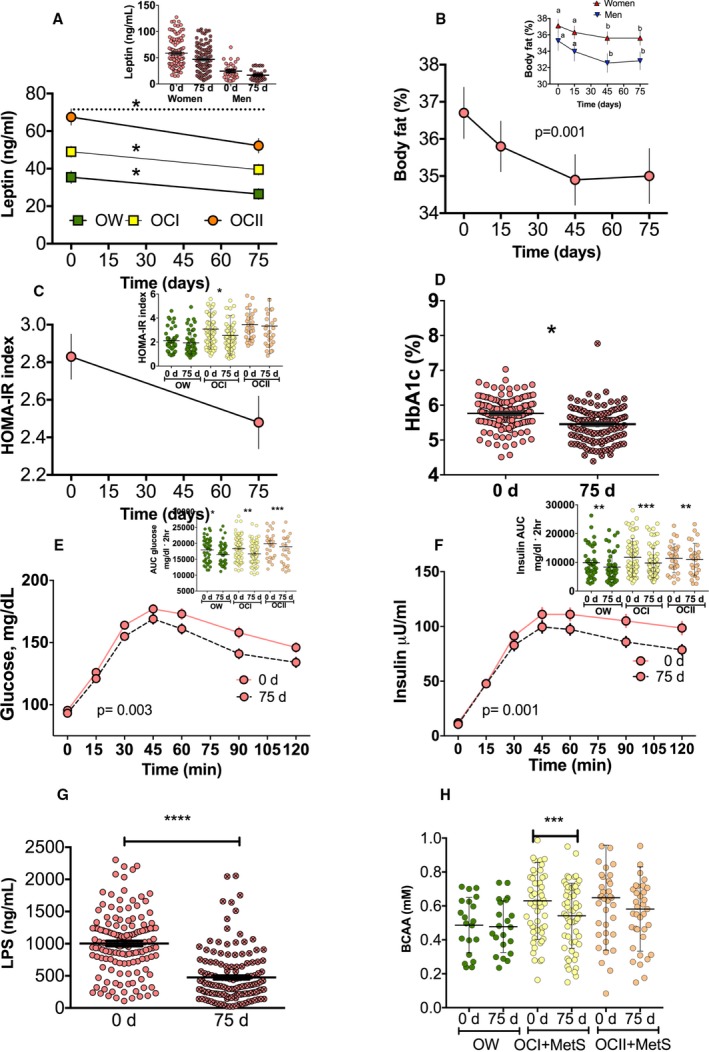
Effect of a lifestyle intervention with functional foods and energy reduction for 75 days on body composition and biochemical parameters in 146 patients with metabolic syndrome (MetS). In the first stage, participants were instructed to consume a reduced‐energy diet for 15 days. During the second stage of the study, participants consumed the functional foods in addition to the reduced‐energy diet for 60 days. A, Serum leptin concentration, according to body mass index (BMI) category (overweight [OW], class I obesity [OCI], and class II obesity [OCII]) and sex. B, Percentage of fat mass in all patients and separated by sex. C, Homeostatic Model Assessment for Insulin Resistance (HOMA‐IR) index. D, Percentage of glycosylated hemoglobin (HbA1c) in all patients and according to BMI category. E, Glucose serum concentration after an oral glucose tolerance test (OGTT) at 0 and 75 days after the dietary strategy in all patients and area under the curve (AUC), according to the BMI category. F, Insulin concentration after an OGTT at 0 and 75 days after the dietary strategy in all patients and AUC for insulin, according to BMI category. G, Serum lipopolysaccharide. H, Serum branched‐chain amino acid (BCAA), according to BMI category (n=111). Statistical analysis was performed using 1‐way ANOVA, followed by Bonferroni post hoc test. Logarithmic transformation was performed before the statistical analysis. Different letters indicate significant differences among groups: a>b>c>d (P<0.05). Comparisons between 2 groups were analyzed by a paired Student t test. Significant differences are shown. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Effect of a lifestyle intervention on serum LPS, BCAA and lipoproteins
As indicated above, increasing lipopolysaccharide levels are associated with dysbiosis of gut microbiota and with chronic low‐grade inflammation. Interestingly, the lifestyle intervention significantly decreased lipopolysaccharide in all groups, indicative of a reduction in the metabolic endotoxemia as a consequence of a reduction of dysbiosis of the gut microbiota (Figure 6G). A previous study in subjects with type 2 diabetes mellitus showed that an increase in P copri was associated with the synthesis of BCAA.18 In the present study, subjects with MetS increased serum BCAA by 22.7%; and after the lifestyle intervention, there was a decrease of ≈13.9% in circulating concentration of BCAA, indicative that the lifestyle intervention partially restored the gut microbiota (Figure 6H).
On the other hand, it has been demonstrated that an increase in soluble fiber is associated with a reduction in LDL cholesterol concentration.19 Because of the soluble fiber content in the different functional foods used in this study, we evaluated the effect of the lifestyle intervention on the different lipoprotein particles. As shown in Figure 7A, serum cholesterol levels were in the normal range because this biochemical parameter is not associated with MetS. However, the total LDL particle concentration decreased after the lifestyle intervention, and this reduction was caused by a decrease in intermediate‐density lipoprotein and small LDL particle concentrations (Figure 7B). In addition, there was a modest but significant decrease in HDL‐C. Interestingly, the total HDL particle concentration decreased because of a reduction in the small HDL particle concentration, indicating a potential decrease in the risk of atherosclerosis (Figure 7C). Measurement of serum triglycerides by nuclear magnetic resonance showed a significant reduction after lifestyle intervention. In particular, we observed a similar decrease in VLDL and chylomicron triglycerides (Figure 7D). The reduction in these lipoproteins was associated with a decrease in large and medium VLDL and chylomicrons (Figure 7E). The analysis of the mean particle size revealed that the lifestyle intervention reduced the VLDL particle size by 8.33% (Figure 7F).
Figure 7.
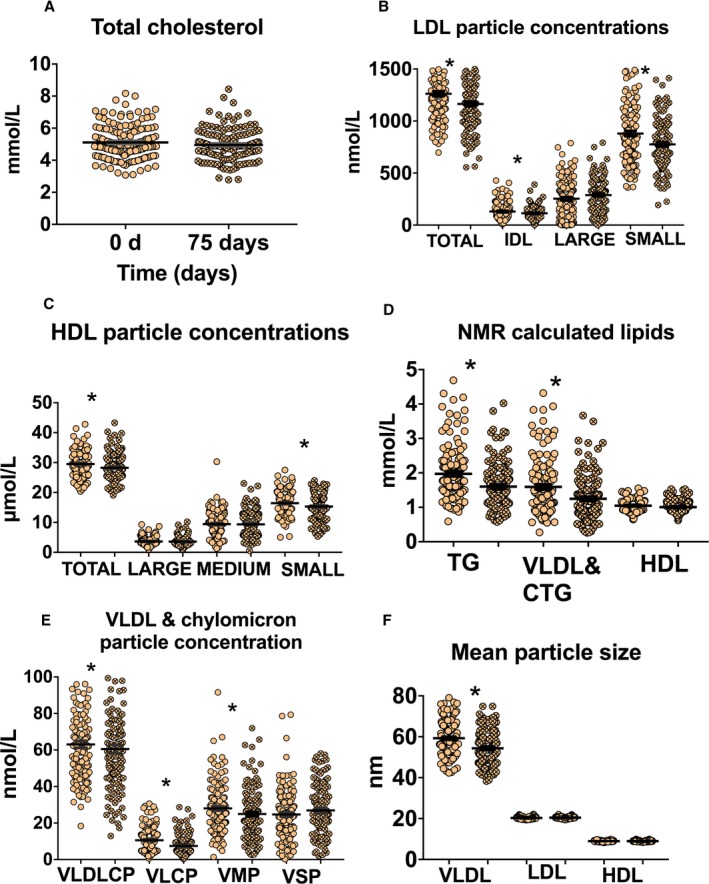
Effect of lifestyle intervention with functional foods and energy reduction for 75 days on lipoprotein profile in 146 patients with metabolic syndrome. A, Serum total cholesterol concentration. B, Nuclear magnetic resonance–calculated lipids: plasma triglycerides (TG), very‐low‐density lipoprotein (VLDL), chylomicron triglycerides (CTGs), and high‐density lipoprotein (HDL) cholesterol. C, Plasma VLDL and chylomicron particle concentration: VLDL and chylomicron particles (VLDLCPs), large VLDL and chylomicron particles (VLCPs), medium VLDL particles (VMPs) and small VLDL particles (VSPs). D, Plasma low‐density lipoprotein concentration (LDL): total LDL particles, intermediate‐density lipoprotein (IDL) particles, large LDL particles (LARGE), and small LDL particles (SMALL). E, Plasma HDL particle concentration: total HDL particle (TOTAL), large HDL particles (LARGE), medium HDL particles (MEDIUM), small HDL particles (SMALL). F, Mean particle size of VLDL, LDL, and HDL. Statistical analysis was performed by a paired Student t test. Significant differences are shown. *P<0.05.
Association of some polymorphisms with serum lipids, BMI, fat mass and glucose tolerance after the lifestyle intervention
Several studies have demonstrated that some metabolic alterations observed in MetS are associated with the presence of specific SNPs.20 Some of these SNPs may contribute to the diverse response to specific dietary treatments on the levels of circulating lipids and glucose.21 Therefore, we studied 7 different SNPs related to lipid and glucose metabolism in subjects with MetS in the pragmatic study. The presence of GT+TT genotypes in the ADIPO Q gene showed an increase in HDL‐C and a decrease in triglycerides, VLDL, and chylomicron triglycerides after the lifestyle intervention. The presence of the AG+GG genotypes in the GFOD2 gene was associated with a decrease in body weight, percentage fat mass, and BMI. Subjects with the ABCA1 (R230C+C230C) or ApoE isoform ε3/4 showed an increase in LDL concentration and LDL particles, respectively. In addition, the presence of the T allele in the TCF7L2 polymorphism was associated with a decrease in the area under the curve for glucose after the oral glucose tolerance test (Figure 8).
Figure 8.
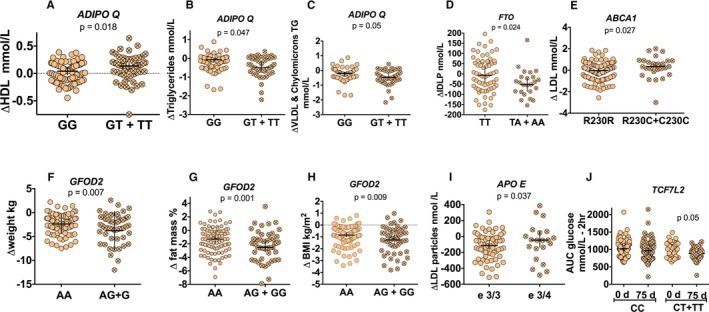
Effect of a lifestyle intervention on changes in anthropometric and biochemical parameters, according to the presence of specific polymorphisms in patients with metabolic syndrome. A, High‐density lipoprotein (HDL) cholesterol. B, Triglycerides. C, Very‐low‐density lipoprotein (VLDL) and chylomicron triglyceride concentration after dietary strategy, according to the presence of ADIPOQ genotype. D, Serum intermediate‐density lipoprotein (IDLP) after dietary strategy, according to the presence of FTO genotype. E, Serum LDL cholesterol after dietary strategy, according to the presence of ABCA1 genotypes. F, Weight. G, Fat mass. H, Body mass index (BMI) after dietary strategy, according to the presence of GFOD2 genotypes. I, Serum LDL particles after dietary strategy, according to presence of APOE isoforms. J, Serum area under the curve (AUC) of glucose after dietary strategy, according to presence of TCF7L2 genotypes.
Randomized Control‐Placebo Study
Lifestyle intervention modifies gut microbiota
Because we observed important changes in the biochemical parameters in subjects with MetS after the lifestyle intervention, we and other researchers have shown that this may be caused by changes in the intestinal microbiota.18, 22 Thus, gut microbiota analysis was performed in a subsample of NW subjects and subjects with MetS and OCIII+MetS. The α diversity, estimated by the Shannon index, was higher in the NW subjects, followed by the MetS and OCIII+MetS groups (Figure 9A). Interestingly, the lifestyle intervention significantly increased the diversity in both groups with MetS (P<0.05) and OCIII+MetS (P<0.005). These results indicated that the lifestyle intervention increased the species richness and diversity compared with the placebo groups (Figure 9A). Bacteroidetes, Firmicutes, and Proteobacteria represented ≈97% of the sequences at the phylum level. At the genus level, the subjects with NW and lifestyle intervention showed a significant increase in Prevotella, Faecalibacterium, Ruminococcus, and Roseburia by 10.8%, 2.94%, 12.14%, and 1.89%, respectively, and a decrease in Bacteroides by 15.2% with respect to the placebo group (Figure 9B). In contrast, subjects with MetS that followed the lifestyle intervention increased Bacteroides and Faecalibacterium by 1.2% and 3.1%, respectively, and decreased Prevotella and Roseburia by 0.97% and 1.4%, respectively, compared with the placebo group. On the other hand, Bacteroides, Faecalibacterium, and Oscillospira showed significant increases of 5.94%, 0.9%, and 0.8%, respectively, and a decrease in Prevotella by 9.21% in subjects with OCIII+MetS with respect to the placebo group (Figure 9B). The Prevotella/Bacteroides ratio increased in the groups that received placebo; however, this ratio significantly decreased in subjects with MetS (P<0.05) and OCIII+MetS (P<0.0001) who followed the lifestyle intervention, indicative of a decrease in dysbiosis of the gut microbiota (Figure 9C).
Figure 9.
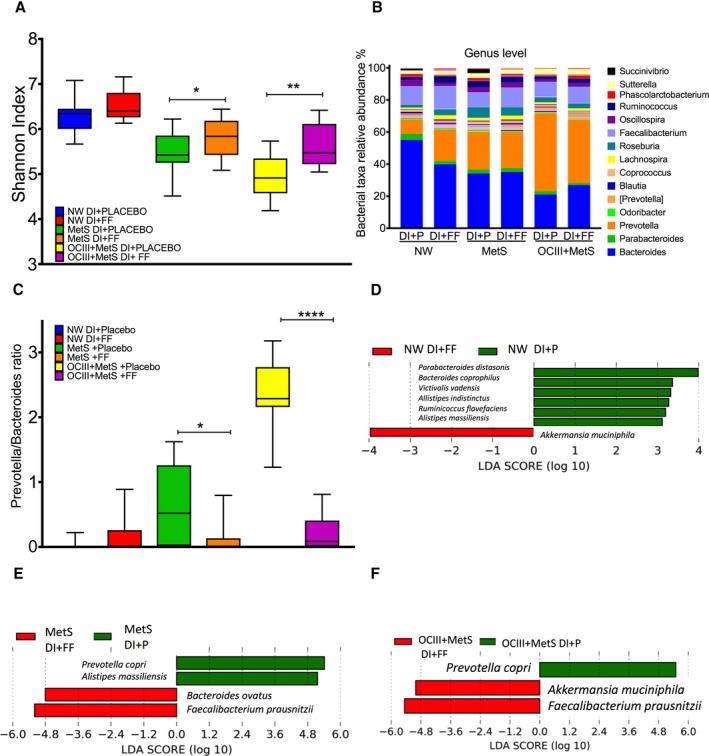
Effect of a lifestyle intervention with functional foods (FFs) or placebo (P) on gut microbiota in patients with metabolic syndrome (MetS). A, The α diversity by Shannon index, according to the body mass index category after dietary intervention (DI) with placebo or FFs. Normal weight (placebo=9, FFs=11), MetS (placebo=17, FFs=18), class III obesity (OCIII)–MetS (placebo=10, FFs=13). B, Taxonomic summary of gut microbiota at the genus level. C, Prevotella/Bacteroides ratio after the dietary strategy with placebo or FFs. Discriminative taxa at the species level in normal weight (NW; D), MetS (E), and OCIII+MetS (F) subjects using a linear discriminant analysis (LDA) effect size. The green bar chart represents the species that were more abundant in subjects consuming the DI+placebo, and the red bar chart represents the subjects consuming the DI+FFs. Comparisons between 2 groups were analyzed by an unpaired Student t test. Significant differences are shown. *P<0.05, **P<0.01, ****P<0.0001.
Lifestyle intervention increases Akkermansia muciniphila and Faecalibacterium prausnitzii
The linear discriminant analysis effect size analysis indicated a well‐defined difference in gut microbiota between subjects who followed the lifestyle intervention among groups, with increasing levels of Akkermansia muciniphila in the NW group (Figure 9D), Bacteroides ovatus and Faecalibacterium prausnitzii in the MetS group (Figure 9E), and A muciniphila and F prausnitzii in subjects with OCIII+MetS (Figure 9F). Clinical and biochemical characteristics of these subjects are shown in Tables S3 through S5. Although improvement was seen in some parameters, the same effect as in pragmatic study was not observed because of the sample size. There were no adverse effects reported by the participants with the dietary intervention.
Discussion
In recent years, obesity and MetS have been suggested as immediate precursors of type 2 diabetes mellitus and cardiovascular disease.23, 24 The results from a meta‐analysis indicated that MetS doubles the risk of cardiovascular disease outcomes, increases all‐cause mortality by 1.5 times, and was associated with a 2‐fold increase in the risk of myocardial infarction.25 This burden of obesity and MetS in developing countries has created an urgent need to develop different types of strategies to manage this epidemic of obesity.26 Lifestyle modification is the mainstay of prevention and treatment for MetS and type 2 diabetes mellitus.27 This work showed the situation and frequency of MetS in subjects of the urban area of Mexico City and how a change in lifestyle might affect each of the 5 components of the MetS and gut microbiota. Alarmingly, 1 in 2 subjects in this study presented MetS (Table S2), which may be in part caused by the susceptibility of specific ethnic groups, including Hispanics.23
Notably, overweight subjects initiated significant abnormalities in different biochemical parameters related to MetS, including an increase in 8% fat mass, 49% in serum triglycerides, 17% in insulin resistance, 49.6% in serum leptin, and 2.7‐fold in CRP, whereas there was a 31.8% and 15% decrease in serum HDL concentration in women and men, respectively. Compared with a healthy weight person, an overweight individual is 3 times more likely to develop diabetes mellitus within 10 years. This risk increases dramatically to 23 times at the higher BMI levels (BMI ≥35 kg/m2).28 In women, the higher the BMI, the higher the presence of MetS, whereas in men, the highest presence of MetS occurred in OCI. Likewise, subjects with MetS showed higher blood pressure, glucose, Homeostatic Model Assessment for Insulin Resistance, triglycerides, total cholesterol, LDL cholesterol, and CRP and lower HDL‐C concentration and adiponectin than subjects without MetS.
Interestingly, the metabolic abnormalities in subjects with MetS and OCIII+MetS were accompanied by lower α diversity in gut microbiota and with a higher Prevotella/Bacteroides ratio than the control group. These results were associated with a higher consumption of carbohydrates than the control group (52.8±1.04 versus 49.0±1.5; P=0.04). In addition, there was a significant association between lipopolysaccharide and the Gram‐negative bacteria P copri, indicating the presence of metabolic endotoxemia mediated by lipopolysaccharide. A recent study in subjects with type 2 diabetes mellitus demonstrated the association between P copri and BCAA,18 and high levels of these amino acids are associated with presence of insulin resistance.29 In the present study, we found an increase of 29.4% in serum BCAA in subjects with MetS who also had insulin resistance.
Although there is no single drug therapy for MetS and the associated comorbidities, there is growing interest in the use of naturally occurring compounds in lowering the risk and progression of MetS.30 Strategies should be cost‐effective, culturally sensitive, and adapted to local practices. It has been reported that, despite the thousands of publications related to all aspects of MetS, therapies developed for MetS are based on therapeutic lifestyle changes.31 There is good evidence that consumption of high fiber in an LSFD accompanied with daily exercise can reduce the incidence of diabetes mellitus by almost 60%.32 Interestingly, the lifestyle intervention in this study significantly decreased the biochemical and anthropometric abnormalities of MetS as well as serum leptin concentration, percentage body fat, insulin resistance, glycosylated hemoglobin, glucose intolerance, and metabolic endotoxemia. The goal of weight reduction is a loss of 7% to 10% over a period of 6 to 12 months.30 In our study, there was a reduction in body weight by 3.4% over a period of 2.5 months. This loss of body weight was associated with a significant decrease between 3.5% and 5.0% in BMI, waist circumference, percentage body fat, blood pressure, insulin, HOMA, and glycosylated hemoglobin, as well as a decrease of 14.3% in BCAA and an increase of 9.9% in HDL‐C concentration only in women. Interestingly, there was a significant reduction of triglyceride levels in the first 15 days with the LSFD, as recommended by the Adult Treatment Program.23
On the other hand, the consumption of total fiber in subjects with MetS was ≈19.2±3.9 g, and the dietary intervention with functional foods provided an additional 10.5 g of total fiber. This amount of fiber could, in part, explain the decrease in the VLDL, chylomicron, LDL, and small HDL particle concentrations. There was no further decrease in serum triglycerides with the addition of functional foods because it has been demonstrated that dietary fiber does not affect serum triglyceride concentrations.33 Overall, at the end of the study, 44.8% of the subjects studied were without MetS, and 15.5% decreased glucose intolerance.
Interestingly, we found that the α diversity significantly increased in OCIII+MetS subjects who followed the lifestyle intervention with respect to the placebo group. The Prevotella/Bacteroides ratio significantly decreased in subjects with MetS and OCIII+MetS who received the lifestyle intervention, indicative of a decrease in dysbiosis of the gut microbiota and metabolic endotoxemia. Interestingly, the increases of A muciniphila, F prausnitzii, and B ovatus were the main modifications by the lifestyle intervention. An increase in A muciniphila has been associated with an increase in insulin sensitivity,34 and F prausnitzii has been considered a marker of human health.35
In our cross‐sectional study, the average intake of calcium was 603±7.3 mg/d, whereas the daily recommend intake of calcium in adults is ≈1000 to 1200 mg/d.36 It has been reported that there is a positive association of calcium intake with insulin sensitivity.37 These results partially explain why half of the population studied showed insulin resistance; however, more studies are needed to demonstrate the health significance.
On the other hand, it is important to consider that there are genetic variants that make a person have a higher risk of presenting MetS. Interestingly, we observed that subjects with MetS and the presence of ADIPOQ T allele, TCF7L2 T allele, and GFOD2 G allele, which play an important role in the pathogenesis of type 2 diabetes mellitus and lipid metabolism,38, 39, 40 are better responders to lifestyle intervention and showed an improvement in HDL‐C, triglyceride, and VLDL cholesterol concentrations and glucose sensitivity. However, the presence of the isoform ε3/4 increased the LDL particles, indicative of an increased risk of atherosclerosis.41
The results of this study suggest that the beneficial effects of lifestyle intervention depend on multiple factors, including dysbiosis in specific genera in the gut microbiota, particularly Bacteroides and Prevotella, the presence of specific genetic variants, patient compliance with dietary intervention, and the type of functional foods. One of the limitations in this work was the length with the lifestyle intervention. Finally, this strategy is safe, beneficial, and cost‐effective for a population with a high prevalence of MetS in a country.
Author Contributions
Dr Torres conceived and designed the study. Dr Guevara‐Cruz, M. Aguilar‐López, Dr Medina‐Vera, M. Sánchez‐Tapia, and A. Flores‐López performed the experiments. Dr Torres, Dr Guevara‐Cruz, and Daniel Diaz analyzed the data. Drs Torres, Guevara‐Cruz, and Tovar performed analysis and/or interpretation. The manuscript was written by Drs Torres, Guevara‐Cruz, and Tovar. All authors have seen and approved the final version of the manuscript.
Sources of Funding
This work was supported by Medix (Dr Torres) and Consejo Nacional de Ciencia y Tecnología (Mexico) grants 181685 (Dr Guevara‐Cruz) and 261079 (Dr Torres).
Disclosures
None.
Supporting information
Table S1. Anthropometric and biochemical parameters in 1065 subjects according obesity class and sex.
Table S2. Anthropometric and biochemical parameters in subjects with overweight
and obesity with and without metabolic syndrome (MetS).
Table S3. Clinical and biochemical characteristics of normal weight group before and after the lifestyle intervention.
Table S4. Clinical and biochemical characteristics of metabolic syndrome group before and after the lifestyle intervention.
Table S5. Clinical and biochemical characteristics of metabolic syndrome+obesity class III group before and after the lifestyle intervention.
Figure S1. Flow chart of the randomized control‐placebo study.
Figure S2. Physical activity and presence of intestinal bowel syndrome prevalence in the population. A, Physical activity in 1032 participants with and without metabolic syndrome and (B) presence of intestinal bowel syndrome in 509 participants.
Acknowledgments
Soy protein isolate was donated by DuPont. Agave Inulin was donated by I+D+I Bustar Alimentos (Mexico).
(J Am Heart Assoc. 2019;8:e012401 DOI: 10.1161/JAHA.119.012401.)
References
- 1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83. [DOI] [PubMed] [Google Scholar]
- 3. American Heart Association Nutrition C , Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris‐Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie‐Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 4. National Cholesterol Education Program Expert Panel on Detection, Evalution, and Treatment of High Blood Cholesterol in Adults . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 5. Jenkins DJ, Josse AR, Wong JM, Nguyen TH, Kendall CW. The portfolio diet for cardiovascular risk reduction. Curr Atheroscler Rep. 2007;9:501–507. [DOI] [PubMed] [Google Scholar]
- 6. Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454–463. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20:637–648. [DOI] [PubMed] [Google Scholar]
- 8. Expert Panel on the Identification, evaluation, and treatment of overweight in adults . Clinical guidelines on the identification evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 9. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association, National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 10. Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books: Champaign, IL: ; 1988. [Google Scholar]
- 11. WHO Consultation on Obesity . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000. [PubMed]
- 12. Emigh TH. A comparison of tests for Hardy‐Weinberg equilibrium. Biometrics. 1980;36:627–642. [PubMed] [Google Scholar]
- 13. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. [DOI] [PubMed] [Google Scholar]
- 14. Sanchez‐Tapia M, Aguilar‐Lopez M, Perez‐Cruz C, Pichardo‐Ontiveros E, Wang M, Donovan SM, Tovar AR, Torres N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci Rep. 2017;7:4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamady M, Knight R. Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Res. 2009;19:1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guevara‐Cruz M, Tovar AR, Aguilar‐Salinas CA, Medina‐Vera I, Gil‐Zenteno L, Hernandez‐Viveros I, Lopez‐Romero P, Ordaz‐Nava G, Canizales‐Quinteros S, Guillen Pineda LE, Torres N. A dietary pattern including nopal, chia seed, soy protein, and oat reduces serum triglycerides and glucose intolerance in patients with metabolic syndrome. J Nutr. 2012;142:64–69. [DOI] [PubMed] [Google Scholar]
- 17. Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 18. Medina‐Vera I, Sanchez‐Tapia M, Noriega‐Lopez L, Granados‐Portillo O, Guevara‐Cruz M, Flores‐Lopez A, Avila‐Nava A, Fernandez ML, Tovar AR, Torres N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019;45:122–131. [DOI] [PubMed] [Google Scholar]
- 19. Bazzano L. Effect of soluble fiber on low density lipoprotein cholesterol and coronary heart disease risk. Curr Atheroscler Rep. 2008;10:473–477. [DOI] [PubMed] [Google Scholar]
- 20. Abou Ziki MD, Mani A. Metabolic syndrome: genetic insights into disease pathogenesis. Curr Opin Lipidol. 2016;27:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guevara‐Cruz M, Medina‐Vera I, Flores‐Lopez A, Aguilar‐Lopez M, Smith CE, Parnell LD, Lee YC, Lai CQ, Tovar AR, Ordovas JM, Torres N. Development of a genetic score to predict an increase in HDL cholesterol concentration after a dietary intervention in adults with metabolic syndrome. J Nutr. 2019;149:1116–1121. [DOI] [PubMed] [Google Scholar]
- 22. Zhang LS, Davies SS. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grundy SM, Hansen B, Smith SC Jr, Cleeman JI, Kahn RA; American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association . Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol. 2004;24:e19–e24. [DOI] [PubMed] [Google Scholar]
- 24. Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. [DOI] [PubMed] [Google Scholar]
- 25. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 26. Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–S30. [DOI] [PubMed] [Google Scholar]
- 27. The Expert Panel. National Cholesterol Education Program . Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA. 1993;269:3015–3023. [PubMed] [Google Scholar]
- 28. Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10‐year period. Arch Intern Med. 2001;161:1581–1586. [DOI] [PubMed] [Google Scholar]
- 29. Serralde‐Zuniga AE, Guevara‐Cruz M, Tovar AR, Herrera‐Hernandez MF, Noriega LG, Granados O, Torres N. Omental adipose tissue gene expression, gene variants, branched‐chain amino acids, and their relationship with metabolic syndrome and insulin resistance in humans. Genes Nutr. 2014;9:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matfin G. Therapeutic advances in endocrinology and metabolism: new crossroads in innovation, research, and clinical practice. Ther Adv Endocrinol Metab. 2010;1:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wagh A, Stone NJ. Treatment of metabolic syndrome. Expert Rev Cardiovasc Ther. 2004;2:213–228. [DOI] [PubMed] [Google Scholar]
- 33. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol‐lowering effects of dietary fiber: a meta‐analysis. Am J Clin Nutr. 1999;69:30–42. [DOI] [PubMed] [Google Scholar]
- 34. Naito Y, Uchiyama K, Takagi T. A next‐generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr. 2018;63:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier‐Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe‐Masselot C, Langella P, Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo‐Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 dietary reference intakes for calcium and vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111:524–527. [DOI] [PubMed] [Google Scholar]
- 37. Ma B, Lawson AB, Liese AD, Bell RA, Mayer‐Davis EJ. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: approaches to modeling a dose‐dependent association. Am J Epidemiol. 2006;164:449–458. [DOI] [PubMed] [Google Scholar]
- 38. Chu H, Wang M, Zhong D, Shi D, Ma L, Tong N, Zhang Z. AdipoQ polymorphisms are associated with type 2 diabetes mellitus: a meta‐analysis study. Diabetes Metab Res Rev. 2013;29:532–545. [DOI] [PubMed] [Google Scholar]
- 39. Ding W, Xu L, Zhang L, Han Z, Jiang Q, Wang Z, Jin S. Meta‐analysis of association between TCF7L2 polymorphism rs7903146 and type 2 diabetes mellitus. BMC Med Genet. 2018;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guevara‐Cruz M, Lai CQ, Richardson K, Parnell LD, Lee YC, Tovar AR, Ordovas JM, Torres N. Effect of a GFOD2 variant on responses in total and LDL cholesterol in Mexican subjects with hypercholesterolemia after soy protein and soluble fiber supplementation. Gene. 2013;532:211–215. [DOI] [PubMed] [Google Scholar]
- 41. Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res. 2009;50 (suppl):S183–S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Anthropometric and biochemical parameters in 1065 subjects according obesity class and sex.
Table S2. Anthropometric and biochemical parameters in subjects with overweight
and obesity with and without metabolic syndrome (MetS).
Table S3. Clinical and biochemical characteristics of normal weight group before and after the lifestyle intervention.
Table S4. Clinical and biochemical characteristics of metabolic syndrome group before and after the lifestyle intervention.
Table S5. Clinical and biochemical characteristics of metabolic syndrome+obesity class III group before and after the lifestyle intervention.
Figure S1. Flow chart of the randomized control‐placebo study.
Figure S2. Physical activity and presence of intestinal bowel syndrome prevalence in the population. A, Physical activity in 1032 participants with and without metabolic syndrome and (B) presence of intestinal bowel syndrome in 509 participants.


