Abstract
Background
Autonomic dysregulation represents a hallmark of coronary artery disease (CAD). Therefore, we investigated the effects of exercise‐based cardiac rehabilitation (CR) on autonomic function and neuro‐cardiovascular stress reactivity in CAD patients.
Methods and Results
Twenty‐two CAD patients (4 women; 62±8 years) were studied before and following 6 months of aerobic‐ and resistance‐training–based CR. Twenty‐two similarly aged, healthy individuals (CTRL; 7 women; 62±11 years) served as controls. We measured blood pressure, muscle sympathetic nerve activity, heart rate, heart rate variability (linear and nonlinear), and cardiovagal (sequence method) and sympathetic (linear relationship between burst incidence and diastolic blood pressure) baroreflex sensitivity during supine rest. Furthermore, neuro‐cardiovascular reactivity during short‐duration static handgrip (20s) at 40% maximal effort was evaluated. Six months of CR lowered resting blood pressure (P<0.05), as well as muscle sympathetic nerve activity burst frequency (48±8 to 39±11 bursts/min; P<0.001) and burst incidence (81±7 to 66±17 bursts/100 heartbeats; P<0.001), to levels that matched CTRL and improved sympathetic baroreflex sensitivity in CAD patients (P<0.01). Heart rate variability (all P>0.05) and cardiovagal baroreflex sensitivity (P=0.11) were unchanged following CR, yet values were not different pre‐CR from CTRL (all P>0.05). Furthermore, before CR, CAD patients displayed greater blood pressure and muscle sympathetic nerve activity reactivity to static handgrip versus CTRL (all P<0.05); yet, responses were reduced following CR (all P<0.05) to levels observed in CTRL.
Conclusions
Six months of exercise‐based CR was associated with marked improvement in baseline autonomic function and neuro‐cardiovascular stress reactivity in CAD patients, which may play a role in the reduced cardiac risk and improved survival observed in patients following exercise training.
Keywords: autonomic nervous system, coronary artery disease, exercise training, myocardial infarction, sympathetic nerve activity
Subject Categories: Autonomic Nervous System, Cardiovascular Disease, Chronic Ischemic Heart Disease, Myocardial Infarction, Exercise
Clinical Perspective
What Is New?
Six months of exercise‐training–based cardiac rehabilitation was associated with marked improvements in resting autonomic function and neural‐cardiovascular stress responses in coronary artery disease patients.
Following exercise‐based cardiac rehabilitation, indices of autonomic neural control at rest and in response to transient physical stress in coronary artery disease patients were normalized, such that values were no long different from that of similarly aged, healthy individuals without cardiovascular disease.
What Are the Clinical Implications?
Our finding of normalized autonomic nervous system regulation may play a role in the reduced cardiac risk and improved mortality rates observed in coronary artery disease patients following exercise training.
Our findings provide additional evidence that aerobic‐ and resistance‐exercise–based cardiac rehabilitation represents a feasible and efficacious, nonpharmacological intervention in the management of coronary artery disease.
Introduction
Autonomic nervous system dysregulation, manifested typically through sympathetic neural hyperactivity1 and persistent vagal (ie, parasympathetic) dysfunction,2, 3 represents a major feature of coronary artery disease (CAD). In turn, this chronic dysregulation has been linked to cardiovascular instability, cardiac and vascular damage and remodeling, increased risk for sudden death, and an overall elevated risk of mortality.4, 5 Therefore, clinical strategies aimed at reversing this dysfunction are of the utmost importance. Indeed, the value of exercise‐based cardiac rehabilitation (CR) for CAD patients has become increasingly recognized, most notably for its beneficial effects on overall quality of life and cardiovascular mortality.6 Yet, the effects of exercise‐based CR on autonomic function remains controversial. Specifically, although several studies in CAD patients have shown considerable benefits of exercise training on heart rate (HR) variability (HRV) and cardiovagal baroreflex sensitivity (BRS),7, 8, 9, 10, 11, 12 both indirect indices of cardiac autonomic regulation, others have not.13, 14, 15 Furthermore, only 2 groups to date have examined the effects of exercise training on direct measures of sympathetic outflow in CAD patients,16, 17 noting reductions in muscle sympathetic nerve activity (MSNA) of ≈20% to 50%. Therefore, additional data are required regarding the effects of exercise‐based CR on chronic cardiovagal and sympathetic outcomes from the same participants.
In addition to pathological consequences of chronic elevations in baseline autonomic output, exaggerated blood pressure (BP) and sympathetic responses to transient, short‐duration physical stress (eg, static handgrip exercise [SHG]) are also reported in several cardiac‐related disorders.18, 19 In turn, heightened stress reactivity has been linked to increased short‐20 and long‐term21 cardiac risk, whereas frequent episodic surges of BP and sympathetic outflow contribute to onset and progression of cardiovascular disease–related morbidity and mortality.22, 23 In this sense, attenuation of stress hyper‐reactivity has been hypothesized to be 1 mechanism by which exercise training improves cardiovascular outcomes,24 and recent evidence, albeit limited, suggests that exercise training may blunt neuro‐cardiovascular responses to SHG exercise in chronic heart failure patients.25 Whether exercise‐based CR improves neuro‐cardiovascular reactivity to physical stress in CAD patients remains unknown.
Therefore, we investigated the effects of 6 months of exercise‐based CR on autonomic function and neuro‐cardiovascular reactivity to physical stress in patients with CAD. Furthermore, we compared CAD patients with a reference group of similarly aged, healthy controls to investigate whether autonomic indices and neuro‐cardiovascular stress reactivity are normalized with CR to levels observed in healthy individuals.
Methods
The authors declare that all supporting data are available within the article.
Participants
Initially, we recruited 39 patients with CAD, of whom 1 was deemed ineligible to participate further (ie, because of uncontrolled hypertension) and 16 were lost to follow‐up (ie, because of noncompliance to exercise‐based CR and/or withdrawal for personal reasons). Therefore, 22 CAD patients (4 women; 62±8 years; range, 48–76) and 22 similarly aged, healthy individuals (CTRL; 7 women; 62±11 years; range, 45–79) participated in the current investigation. CAD patients with preserved left ventricular ejection fraction (ie, >50%) were eligible to participate if they were discharged from hospital following admission for acute coronary syndrome (ie, ST‐elevation or non‐ST elevation myocardial infarction or unstable angina), percutaneous coronary intervention, or coronary artery bypass graft surgery as documented by their attending physician. Exclusion criteria included those which might alter abnormally autonomic and cardiovascular function and may therefore confound study outcomes. Specifically, patients with unstable heart rhythm, congenital coronary abnormality, congestive heart failure, second‐ or third‐degree atrioventricular block, >2 previous myocardial infarctions, sick sinus syndrome, complicated arrhythmias, renal insufficiency, uncontrolled hypertension, and/or uncontrolled diabetes mellitus were ineligible to participate. Current smokers (ie, at the time of experimental testing) were also excluded.
Participant characteristics are provided in Table 1. In total, 19 CAD patients presented with acute ST‐elevation (n=9; 41%) or non‐ST elevation (n=10; 45%) myocardial infarction and 3 with unstable angina (14%), 15 patients underwent percutaneous coronary intervention (1.6±0.6 stents), and 5 patients underwent coronary artery bypass graft surgery (2.6±1.3 grafts). On average, pre‐CR testing occurred 68±31 days following hospital discharge. CAD patients were receiving, and remained on, conventional pharmacotherapy, including beta‐blockers (n=19; 86%), angiotensin‐converting enzyme inhibitors and/or angiotensin II receptor blockers (n=20; 91%), calcium channel blockers (n=5; 23%), diuretics (n=1; 5%), statins (n=21; 95%), and antiplatelet agents, including aspirin (n=22; 100%). Medication use was not altered throughout the study period, except in 1 patient who decreased their beta‐blocker dosage; however, this participant was not excluded, because removal of their data did not alter our results. CTRL participants were otherwise healthy with no history of cardiovascular, metabolic, inflammatory, or neurological disease (as assessed by a standardized health questionnaire) and were nonsmokers and nonmedicated. The current study was approved by the Health Sciences Research Ethics Board at Western University (Research Ethics Board no. 17810), and all participants provided informed written consent before study participation.
Table 1.
Participant Characteristics
| CAD Pre‐CR | CAD Post‐CR | CTRL | |
|---|---|---|---|
| Physical characteristics | |||
| n, males/females | 22, 18/4 | … | 22, 15/7 |
| Age, y | 62±8 | … | 62±11 |
| Height, cm | 172±9 | … | 170±8 |
| Weight, kg | 86±13* | 85±15† | 75±14 |
| BMI, kg/m | 29±4* | 29±4† | 26±4 |
| Resting hemodynamics | |||
| Heart rate, bpm | 59±7 | 58±8 | 58±8 |
| Mean arterial BP, mm Hg | 86±10 | 82±7† , ‡ | 88±8 |
| Systolic BP, mm Hg | 122±16 | 116±11‡ | 120±14 |
| Diastolic BP, mm Hg | 68±8 | 65±7† , ‡ | 71±8 |
| Echocardiographic characteristics | |||
| LVEF, % | 65±7 | 66±6 | 69±5 |
| LV mass index, g/m2 | 81±13* | 77±16 | 63±13 |
| Blood biochemistry | |||
| Glucose, mmol/L | 5.36±0.61 | 5.57±0.47† | 5.10±0.39 |
| Total CHO, mmol/L | 3.19±0.64* | 3.34±0.59 | 4.38±0.77 |
| HDL CHO, mmol/L | 0.97±0.17* | 1.07±0.30† , ‡ | 1.41±0.38 |
| LDL CHO, mmol/L | 1.65±0.48* | 1.71±0.39† | 2.53±0.70 |
| Triglycerides, mmol/L | 1.35±0.65* | 1.24±0.72 | 0.98±0.41 |
| HbA1C, % | 5.74±0.33 | 5.82±0.71 | 5.70±0.23 |
| CRP, mg/L | 1.85±1.86* | 1.97±2.41† | 0.64±0.45 |
| Exercise stress test | |||
| VO2peak, mL/kg/min | 26.5±6.9* | 28.7±8.9† , ‡ | 35.8±7.8 |
| Peak HR, bpm | 136±20* | 143±17† , ‡ | 161±19 |
| Peak systolic BP, mm Hg | 169±30 | 166±21 | 171±20 |
| Peak diastolic BP, mm Hg | 82±11 | 82±13 | 89±7 |
| RER | 1.16±0.13 | 1.14±0.11 | 1.21±0.10 |
Values are mean±SD. BMI indicates body mass index; BP, blood pressure; bpm, beats per minute; CAD, coronary artery disease; CHO, cholesterol; CR, cardiac rehabilitation; CRP, C‐reactive protein; CTRL, similarly aged, healthy controls; HbA1C, hemoglobin A1C; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; LV, left ventricular; LVEF, left ventricular ejection fraction; RER, respiratory exchange ratio; VO2peak, peak oxygen uptake.
*CAD pre‐CR vs CTRL, P<0.05; †CAD post‐CR vs CTRL, P<0.05; ‡CAD pre‐CR vs CAD post‐CR, P<0.05.
Experimental Protocol and Measures
CAD patients were studied before and following 6 months of exercise‐training–based CR. CTRL participants were studied at 1 time point only to serve as a comparison group. All studies were conducted in the morning following a 12‐hour fast and a 24‐hour abstinence from caffeine, alcohol, and vigorous exercise. All testing was completed in the supine position.
Following 30 minutes of supine rest, a venous blood sample was obtained from the antecubital vein for the analysis of blood glucose, blood lipids, and glycated hemoglobin. Next, supine resting BP was determined from the brachial artery using manual sphygmomanometry, and the average of 3 values was used for final analysis, as well as to calibrate the BP values obtained using finger photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). As such, beat‐to‐beat BP was acquired continuously throughout the experimental protocol. Heart rate (HR) was determined from a standard 3‐lead ECG (HP 78354A; Hewlett Packard, Andover, MA). Finally, direct sympathetic neural recordings were obtained by microneurography at the right peroneal nerve (662C‐3; Bioengineering of University of Iowa, Iowa City, IA), using standard procedures described previously in detail.26 All data were collected online using the LabChart7 and PowerLab data acquisition system (ADInstruments, Colorado Springs, CO).
Baseline data were collected for 5 minutes for determination of resting hemodynamics, MSNA, HRV, and cardiovagal and sympathetic BRS. Next, for the ensuing SHG protocol, participants completed 2 maximal voluntary contractions with their left hand (nondominant hand in 100% and 95% of CAD patients and CTRL, respectively), and the largest of the 2 was used to calculate relative exercise intensity. All SHG exercise was completed at 40% maximal voluntary contraction. Participants performed 4, 20‐second contractions, separated by 1‐minute rest periods. Throughout the SHG protocol, visual feedback of exercise intensity was provided to aid in the maintenance of target force, and participants were instructed to breathe spontaneously throughout the exercise period. Upon completion, participants rated their level of perceived exertion using the 20‐point Borg Rating of Perceived Exertion Scale.27
Data Analysis
All data were analyzed by a trained investigator who was blinded to participant group (ie, CAD or CTRL) and time point (ie, pre‐CR or post‐CR). Sympathetic bursts were identified visually from the integrated MSNA neurogram and quantified as burst frequency (the number of sympathetic bursts per minute [bursts/min]) and burst incidence (the number of sympathetic bursts per 100 heart beats [bursts/100 hb]).
HRV was analyzed using linear (ie, time and frequency domain) and nonlinear methodology (Kubios HRV Analysis Software 2.1; Biosignal Analysis and Medical Imaging Group, University of Eastern Finland, Kuopio, Finland). Briefly, in the time domain, the standard deviation of normal RR intervals, root mean square of successive RR interval differences, and the percentage of consecutive RR intervals that differ by >50 ms are reported. In the frequency domain, RR intervals were analyzed using fast Fourier transformation to obtain low‐frequency (0.04–0.15 Hz) and high‐frequency (0.15–0.40 Hz) power spectral components. Finally, nonlinear measures assessed included the short‐term fractal scaling exponent (α1) and sample entropy.
Cardiovagal BRS was assessed using the sequence method.28, 29 Briefly, spontaneous sequences of ≥3 consecutive cardiac cycles, in which systolic BP and RR interval either increased or decreased in the same direction, were identified. Data were analyzed using either a lag 0 (86% of data sets), lag 1 (11% of data sets), or lag 2 (3% of data sets) criterion to ensure the greatest number of sequences. On average, in CAD patients, analysis resulted in 44±18 baroreflex sequences for analysis pre‐CR and 41±18 baroreflex sequences for analysis post‐CR. In CTRL, analysis resulted in 47±18 baroreflex sequences for analysis. Linear regressions were performed, and the mean slope of the identified sequences was taken to represent cardiovagal BRS. All regressions had an r 2>0.85. Sympathetic BRS was determined as the relationship between MSNA burst incidence and diastolic BP.30 Briefly, diastolic BP values were averaged into 2 mm Hg bins, and the probability of a sympathetic burst occurring within each bin (from 0% to 100%) represented sympathetic burst incidence. The slope of this spontaneous relationship was taken to represent sympathetic BRS.
For the SHG protocol, HR, BP, and MSNA were calculated over the 30 seconds preceding each SHG bout (ie, baseline rest) and for the last 10 seconds of each SHG exercise period. Because no differences were observed in the baseline values or the response to each of the 4 handgrips, the average of the 4 SHG bouts was determined for each individual and used in the final analysis.
Cardiorespiratory Fitness
All participants completed a physician‐supervised graded treadmill exercise stress test to exhaustion. Specifically, breath‐by‐breath measurements of oxygen uptake and carbon dioxide production were acquired continuously (over 3‐second intervals) throughout the exercise test until volitional exhaustion (Quark b2; COSMED srl, Rome, Italy). Each participant's VO2peak was estimated from the graded exercise test as per standard clinical guidelines.31
Cardiac Rehabilitation Program
Exercise‐based CR consisted of both aerobic and resistance exercise training and was completed in accord with current guidelines at the time of study execution.32 Specifically, in Ontario, Canada, 6 months of CR represents standard clinical care for patients with CAD. Sessions were supervised as part of an existing community‐based CR program and supplemented with home‐based workouts. Specifically, aerobic exercise training was performed 3 to 7 days/week, consisting of 20 to 60 minutes of exercise at 40% to 70% of HR reserve. Aerobic exercise consisted of walking, running, and/or cycling exercise. Exercise intensity and duration were gradually increased on an individual patient basis throughout the 6‐month period. Resistance exercise training was completed 3 to 4 days/week. Resistance exercise entailed a full‐body circuit, consisting of 8 to 12 exercises, completed for 1 to 3 sets at 10 to 15 repetitions. Exercise intensity started at 30% to 40% 1‐repetition maximum and 50% to 60% 1‐repetition maximum for upper and lower body exercises, respectively, and resistance was increased gradually (ie, ~5%) once the upper limit of repetitions (ie, 12–15) could be completed comfortably.
Statistical Analysis
The effect of exercise‐based CR in CAD patients (ie, pre‐ to post‐CR) was tested using 2‐tailed paired t tests. Non‐normally distributed variables were analyzed using Wilcoxon signed‐rank tests. CAD patients were also compared to the reference CTRL group at pre‐CR and post‐CR using 2‐tailed unpaired t tests, with Bonferroni correction for multiple comparisons. Non‐normally distributed variables were analyzed using Mann–Whitney rank‐sum tests. Statistical significance was set at P<0.05 and all data are means±SD, unless otherwise indicated. All statistical analyses were performed using SigmaPlot software (version 12.0; Systat Software, San Jose, CA).
Results
On average, as part of a supervised, community‐based CR program, CAD patients engaged in aerobic and resistance exercise 2.1±0.6 days per week, which was supplemented with home‐based workouts.
Before CR, CAD patients had lower cardiorespiratory fitness in comparison to CTRL (Table 1; P<0.001). Following exercise‐based CR, CAD patients exhibited a significant improvement in cardiorespiratory fitness (P=0.02); yet, levels in CAD patients post‐CR remained lower than CTRL (P=0.01).
As displayed in Table 1, before CR, resting BP did not differ between CAD patients and CTRL (all P>0.05). In CAD patients, resting systolic BP, diastolic BP, and mean arterial BP were reduced following CR (Table 1). Post‐CR, CAD patients had lower diastolic BP (P=0.01) and mean arterial BP (P=0.02) than CTRL, whereas systolic BP did not differ (P=0.28). Resting HR was not different between CAD and CTRL pre‐ (P=0.84) or post‐CR (P=0.92), nor was it altered following exercise‐based CR in CAD patients (P=0.55).
Table 2 displays all indices of HRV in CAD patients before and following CR and in CTRL participants. Before CR, no differences between CAD and CTRL were observed for any index of HRV (all P>0.05). Furthermore, no changes in HRV were observed in CAD patients after exercise‐based CR (all P>0.05), such that no differences in HRV existed between CAD post‐CR and CTRL (all P>0.05).
Table 2.
Heart Rate Variability Indices Before and Following Cardiac Rehabilitation in CAD Patients and in Healthy Controls
| CAD Pre‐CR | CAD Post‐CR | CTRL | |
|---|---|---|---|
| Time domain | |||
| Mean RRI, ms | 1039±121 | 1058±159 | 1057±148 |
| SDNN | 37±16 | 37±18 | 38±12 |
| RMSSD, ms | 25±11 | 27±20 | 26±16 |
| pNN50, % | 6.5±11 | 8.7±17 | 6.5±11 |
| Frequency domain | |||
| LF power, ms2 | 296±301 | 442±711 | 390±317 |
| HF power, ms2 | 231±243 | 353±667 | 274±385 |
| LF power, nu | 55±22 | 54±22 | 64±16 |
| HF power, nu | 45±22 | 46±22 | 36±16 |
| Nonlinear | |||
| Sample entropy | 1.49±0.30 | 1.58±0.31 | 1.42±0.26 |
| α1 | 1.03±0.36 | 1.06±0.27 | 1.17±0.26 |
Values are mean±SD. CAD indicates coronary artery disease; CR, cardiac rehabilitation; CTRL, similarly aged, healthy controls; HF, high frequency; LF, low frequency; nu, normalized units; pNN50, percentage of consecutive RR intervals that differ by more than 50 ms; RMSSD, root mean square of successive interval differences; RRI, RR interval, SDNN, standard deviation of normal RR intervals; α1, short‐term fractal scaling exponent.
Figure 1 displays levels of resting MSNA in CAD patients before and following CR and in CTRL participants. Before CR, both burst frequency (48±8 versus 36±8 bursts/min; P<0.001) and burst incidence (81±7 versus 62±10 bursts/100 hb; P<0.001) were elevated in CAD patients versus CTRL. However, both burst frequency (48±8 to 39±11 bursts/min; P<0.001) and burst incidence (81±7 to 66±17 bursts/100 hb; P<0.001) were lowered significantly in CAD patients post‐CR, such that neither variable was different post‐CR from CTRL (both P>0.05).
Figure 1.
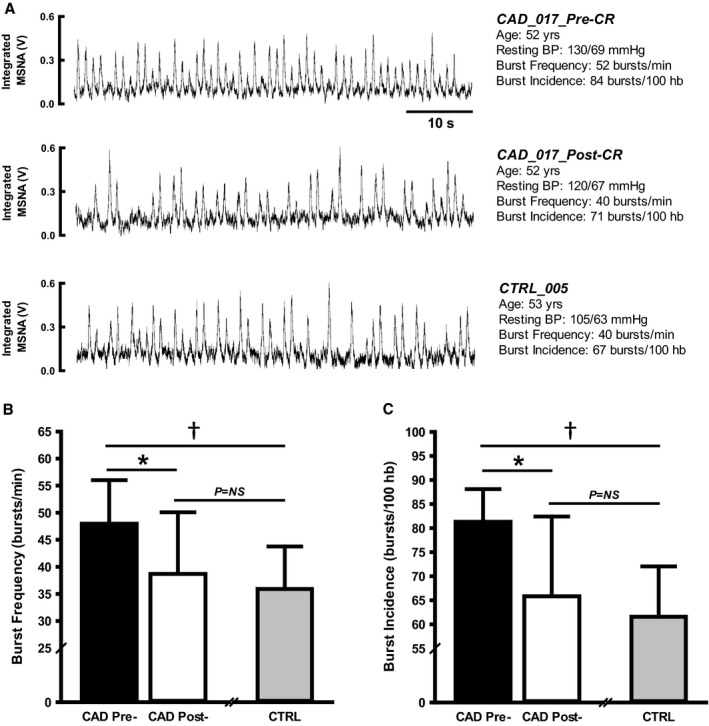
Resting muscle sympathetic nerve activity (MSNA) in coronary artery disease (CAD) patients before and following exercise‐based cardiac rehabilitation (CR) and in similarly aged, healthy control (CTRL) participants. A, Representative tracings from the integrated MSNA neurogram at rest from 1 CAD patient pre‐ and post‐CR and in 1 CTRL participant. B, MSNA burst frequency and (C) burst incidence in CAD patients pre‐ and post‐CR and in CTRL. *CAD pre‐CR vs CAD post‐CR, P<0.01; † CAD pre‐CR vs CTRL, P<0.05. BP indicates blood pressure; hb, heart beats; NS, not significant; V, volts.
Cardiovagal BRS was not different between CAD patients and CTRL before CR (14±10 versus 17±11 ms/mm Hg; P=0.28). Following CR, cardiovagal BRS was not changed in CAD patients (14±10 to 18±14 ms/mm Hg; P=0.11; Figure 2A), such that no differences existed between CAD post‐CR and CTRL (P=0.92). In contrast, sympathetic BRS was lower in CAD patients pre‐CR versus CTRL (−3.5±1.9 versus −6.0±3.3 bursts/100 hb/mm Hg; P=0.02), yet improved in CAD patients following exercise‐based CR (−3.5±1.9 to −5.9±3.0 bursts/100 hb/mm Hg; P<0.01; Figure 2B). As such, differences in sympathetic BRS were no longer observed between CAD patients post‐CR and CTRL (P=0.92).
Figure 2.
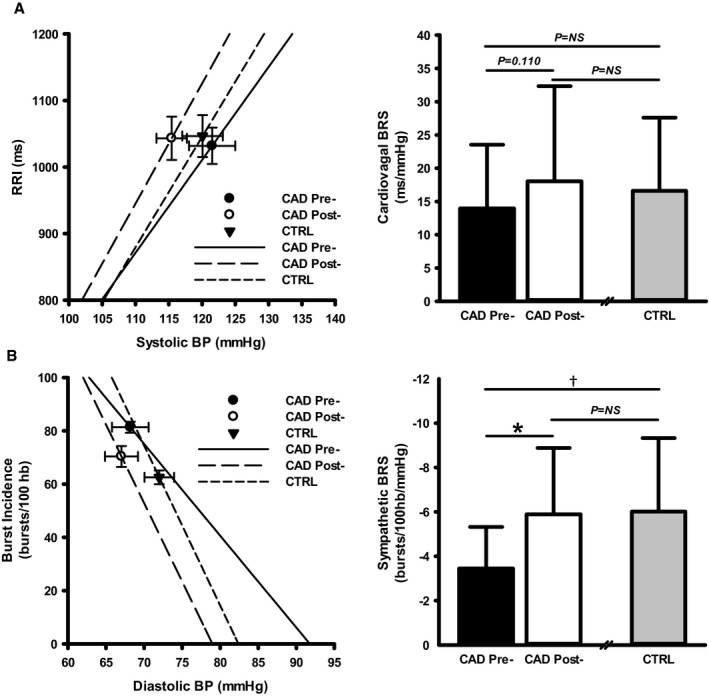
Cardiovagal and sympathetic baroreflex sensitivity (BRS) in coronary artery disease (CAD) patients before and following exercise‐based cardiac rehabilitation (CR) and in similarly aged, healthy control (CTRL) participants. A, Cardiovagal BRS in CAD patients pre‐ and post‐CR and in CTRL. B, Sympathetic BRS in CAD patients pre‐ and post‐CR and in CTRL. Left panels are mean±SEM. *CAD pre‐CR vs CAD post‐CR, P<0.01; † CAD pre‐CR vs CTRL, P<0.05. BP indicates blood pressure; hb, heart beats; NS, not significant; RRI, RR interval.
Neuro‐Cardiovascular Reactivity to SHG
Figure 3 displays the hemodynamic and MSNA responses to SHG exercise in CAD patients before and following CR and in CTRL participants. Absolute maximal voluntary contractions voltage and Borg rating of perceived exertion scores did not differ between CAD and CTRL before or following CR, nor were they altered with CR in CAD patients (all P>0.05). Before CR, all indices of BP increased in response to SHG exercise in CAD patients and CTRL (all P<0.001); however, magnitude of increases in BP was greater in CAD patients (all P<0.05). Before CR, HR increased in both groups (both P<0.001), with no differences in magnitude of response (P=0.10). Finally, before CR, both MSNA burst frequency and burst incidence increased in response to SHG in CAD patients (both P<0.001), but were unchanged in CTRL (both P>0.05), such that MSNA response was greater in CAD patients compared with CTRL (both P<0.001). In response to exercise‐based CR, all BP and MSNA responses to SHG exercise were lowered significantly in CAD patients (all P<0.05), whereas HR showed no change (P=0.23). As such, BP and MSNA reactivity in CAD patients post‐CR was no longer different from CTRL (all P>0.05).
Figure 3.
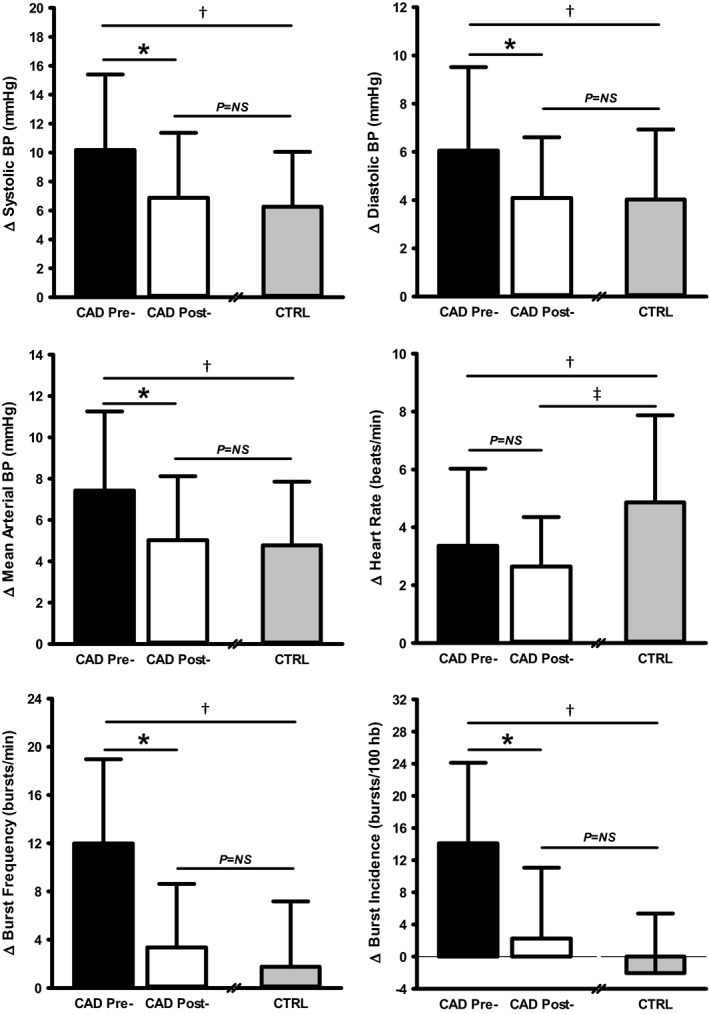
Neuro‐cardiovascular reactivity to static handgrip exercise in coronary artery disease (CAD) patients before and following exercise‐based cardiac rehabilitation (CR) and in similarly aged, healthy control (CTRL) participants. *CAD pre‐CR vs CAD post‐CR, P<0.01; † CAD pre‐CR vs CTRL, P<0.05; ‡ CAD post‐CR vs CTRL, P<0.05. BP indicates blood pressure; hb, heart beats; NS, not significant.
Discussion
The current study provides important and novel insight into the effects of exercise‐based CR on autonomic function and neuro‐cardiovascular stress reactivity in patients with CAD. First, we show that sympathetic nerve outflow was reduced and sympathetic BRS improved in CAD patients following exercise‐based CR, such that levels were no longer different than that of similarly aged, healthy individuals without disease. Conversely, cardiac autonomic regulation remained unchanged in patients following exercise training. Second, we report that CAD patients exhibit greater pressor and sympathetic nerve responses to short‐duration SHG, responses that were reduced to levels observed in healthy controls following exercise‐based CR. Taken together, our findings demonstrate that 6 months of exercise‐based CR was associated with marked improvements in sympathetic autonomic function and neuro‐cardiovascular stress responses in CAD patients.
Exercise‐based CR represents a cornerstone feature in the nonpharmacological treatment and management of patients with CAD, given its significant benefit on patient mortality and rehospitalization rates.6 However, the effect of exercise‐based CR on autonomic dysfunction, a hallmark of the disease, remains somewhat equivocal. Certainly, some of the debate pertains to the branch of the autonomic nervous system in question. For example, CAD is characterized by excessive sympathetic nerve activation,1 which, in turn, is linked to cardiovascular instability and adverse outcomes.33 Furthermore, chronic elevations in MSNA may be independently associated with end‐organ damage34 and increased mortality in cardiovascular disease.4 Here, we demonstrate that exercise‐based CR was associated with markedly lowered muscle sympathetic nerve outflow, to levels that matched healthy controls, and improved baroreflex control of sympathetic nerve activity in patients with CAD. These findings are consistent with previous reports in CAD patients,16, 17 as well as other cardiac‐related pathologies,35 and highlight the robust benefit of exercise training on sympathetic neural control in CAD. In contrast, concurrent improvements in indices reflecting cardiovagal control were not observed in this group of CAD patients, a finding similar to some studies,13, 14, 15 but not all.7, 8, 9, 10, 11, 12 Differences in training paradigms, medication statuses, and severity of CAD make comparisons between studies difficult. Also, a training threshold for improvement may exist in cardiac patients.36 Perhaps the modest (albeit significant) increase in cardiorespiratory fitness observed in our patients (~8% or 0.63 metabolic equivalents) reflected a suboptimal training load to elicit beneficial changes in cardiac vagal control, although other studies reflect similar rates of improvement in fitness.15, 17 Moreover, accurate tests of cardiorespiratory fitness are difficult to achieve in patients receiving beta‐blockade treatment. Importantly, however, even minor improvements in cardiorespiratory fitness elicit marked reductions in risk of all‐cause mortality.37, 38 Specifically, Myers et al38 demonstrated that each 1 metabolic equivalent increase in exercise capacity confers an ≈12% improvement in survival, highlighting the robust benefit of seemingly modest improvements. Furthermore, magnitude of benefit likely depends on how “impaired” CAD patients are to begin with. In this sense, indices of HRV and cardiovagal BRS were not different between CAD patients and CTRL before exercise‐based CR in the present study. However, it is worth noting that a modest trend for enhanced cardiovagal BRS (ie, ∆4±11 ms/mm Hg; P=0.11) was observed in our patients following CR, which may have great clinical relevance. This conjecture receives some support from the results of La Rovere et al,39 who demonstrated, in a 10‐year follow‐up study, that post–myocardial infarction patients with an exercise‐induced improvement in cardiovagal BRS of >3 ms/mm Hg had dramatically lower cardiac mortality than those patients without such an increase. Altogether, these data highlight the considerable protective and clinical benefit of seemingly small changes in autonomic cardiovascular control produced by exercise‐based CR in CAD patients.
A major novel finding of the current study is that CAD patients display greater pressor and sympathetic nerve responses to acute physical stress in comparison with similarly aged, healthy counterparts. In fact, sympathetic firing did not increase in response to short‐duration SHG in CTRL participants, consistent with the idea that SHG does not elicit major increases in MSNA until after ≈1 minute in healthy individuals.40 However, the current data support previous findings of rapid‐onset and aberrant neuro‐circulatory responses to physical stress in cardiovascular disease.19 Of importance, however, was the observation that 6 months of aerobic‐ and resistance‐based exercise training was associated with significant attenuation of arterial BP and sympathetic nerve response to acute physical stress in our patients. Moreover, neuro‐cardiovascular responses were no longer different than that of healthy controls following CR.
Overall, the current data suggest that exercise‐based CR reverses the chronic elevations in baseline arterial BP and sympathetic nerve activity observed in CAD, as well as reactivity in these variables to exercise stress. These observations align with the growing understanding that sympatho‐excitation to various stressors contributes to the development and progression of cardiovascular disease,23 and that attenuation of stress hyper‐reactivity may contribute to exercise‐training–induced improvements in cardiovascular outcomes.24
Limitations
First, we recognize that a control group of “usual care” CAD patients (ie, no exercise‐based CR) would have strengthened our conclusions. Unfortunately, in our hands, those patients who decline their referral to exercise‐based CR are also unlikely to volunteer in research studies of this magnitude. Furthermore, withholding exercise‐based CR in post–cardiac event patients is not acceptable given its well‐documented positive effects on quality of life and cardiovascular mortality,6 as well as the fact that CR represents the guideline‐recommended standard of care.32 Second, CAD patients remained medicated throughout, and the effects of continued pharmacotherapy (in conjunction with exercise‐based CR) on autonomic regulation remain largely unknown. However, the impact of cardiac blockade on cardiovagal indices appears to be minimal and therefore is not believed to have impacted the current data.41 Medications in CAD patients were not withdrawn before testing to avoid abrupt, rebound cardiovascular effects.42, 43 Third, given that exercise training was completed as part of an existing, community‐based CR program, we do not have data with respect to average exercise volume in our patients and therefore cannot quantify the relationship between individual exercise dose and magnitude of autonomic benefit. Fourth, the mechanisms underlying improvements in sympathetic neural control following exercise‐based CR are beyond the scope of the current study design, but may include centrally mediated mechanisms44, 45 and/or improved endothelial function. Finally, the long‐term maintenance of these benefits remains to be determined. Future studies are required to address these issues.
Perspectives
CAD continues to be among the most prevalent forms of cardiovascular disease and represents a leading cause of morbidity and mortality worldwide. Specifically, recent estimates suggest that at the age of 40 years, 1 in 2 men and 1 in 3 women will develop CAD.46 Therefore, the investigation into potential feasible and effective therapeutic interventions, as well as their mechanisms, remains a crucial effort toward improving patient outcomes and reducing the economic burden of disease. Here, we support earlier observations that CAD is associated with chronic elevations in baseline MSNA and that 6 months of exercise‐based CR reverses these changes. The current data show further that CR may attenuate exaggerated autonomic and cardiovascular stress reactivity in patients with CAD. Moreover, following exercise training, indices of neural control in CAD patients were indistinguishable from that of similarly aged, healthy individuals without cardiovascular disease. Given the deleterious impact of hyperadrenergic states on cardiac and vascular tissue,34 this normalization of autonomic nervous system regulation may play a role in reduced cardiac risk and improved mortality rates observed in patients following exercise training. Finally, these findings provide additional evidence that aerobic‐ and resistance‐exercise–based CR represents a feasible and efficacious, nonpharmacological intervention in the management of CAD.
Sources of Funding
This study was funded by the Canadian Institutes of Health Research Team Grant in Physical Activity, Mobility, and Neural Health (Grant No. 217532; JKS) and a Canadian Institutes of Health Research Doctoral Research Award (MBB). JKS is a Tier 1 Canada Research Chair.
Disclosures
None.
Acknowledgment
We thank the study volunteers for their participation.
(J Am Heart Assoc. 2019;8:e012257 DOI: 10.1161/JAHA.119.012257.)
References
- 1. Graham LN, Smith PA, Stoker JB, Mackintosh AF, Mary DA. Time course of sympathetic neural hyperactivity after uncomplicated acute myocardial infarction. Circulation. 2002;106:793–797. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz PJ, Zaza A, Pala M, Locati E, Beria G, Zanchetti A. Baroreflex sensitivity and its evolution during the first year after myocardial infarction. J Am Coll Cardiol. 1988;12:629–636. [DOI] [PubMed] [Google Scholar]
- 3. Lombardi F, Sandrone G, Pernpruner S, Sala R, Garimoldi M, Cerutti S, Baselli G, Pagani M, Malliani A. Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction. Am J Cardiol. 1987;60:1239–1245. [DOI] [PubMed] [Google Scholar]
- 4. Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135:302–307. [DOI] [PubMed] [Google Scholar]
- 5. La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 6. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016;67:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Stâhle A, Nordlander R, Bergfeldt L. Aerobic group training improves exercise capacity and heart rate variability in elderly patients with a recent coronary event: a randomized controlled study. Eur Heart J. 1999;20:1638–1646. [DOI] [PubMed] [Google Scholar]
- 8. Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: a randomized, controlled study. Circulation. 2000;102:2588–2592. [DOI] [PubMed] [Google Scholar]
- 9. Fujimoto S, Uemura S, Tomoda Y, Yamamoto H, Matsukura Y, Horii M, Iwamoto E, Hashimoto T, Dohi K. Effects of exercise training on the heart rate variability and QT dispersion of patients with acute myocardial infarction. Jpn Circ J. 1999;63:577–582. [DOI] [PubMed] [Google Scholar]
- 10. Malfatto G, Facchini M, Sala L, Branzi G, Bragato R, Leonetti G. Effects of cardiac rehabilitation and beta‐blocker therapy on heart rate variability after first acute myocardial infarction. Am J Cardiol. 1998;81:834–840. [DOI] [PubMed] [Google Scholar]
- 11. Leitch JW, Newling RP, Basta M, Inder K, Dear K, Fletcher PJ. Randomized trial of a hospital‐based exercise training program after acute myocardial infarction: cardiac autonomic effects. J Am Coll Cardiol. 1997;29:1263–1268. [DOI] [PubMed] [Google Scholar]
- 12. Malfatto G, Facchini M, Bragato R, Branzi G, Sala L, Leonetti G. Short and long term effects of exercise training on the tonic autonomic modulation of heart rate variability after myocardial infarction. Eur Heart J. 1996;17:532–538. [DOI] [PubMed] [Google Scholar]
- 13. La Rovere MT, Mortara A, Sandrone G, Lombardi F. Autonomic nervous system adaptations to short‐term exercise training. Chest. 1992;101:299S–303S. [DOI] [PubMed] [Google Scholar]
- 14. Currie KD, Rosen LM, Millar PJ, McKelvie RS, MacDonald MJ. Heart rate recovery and heart rate variability are unchanged in patients with coronary artery disease following 12 weeks of high‐intensity interval and moderate‐intensity endurance exercise training. Appl Physiol Nutr Metab. 2013;38:644–650. [DOI] [PubMed] [Google Scholar]
- 15. Oliveira NL, Ribeiro F, Teixeira M, Campos L, Alves AJ, Silva G, Oliveira J. Effect of 8‐week exercise‐based cardiac rehabilitation on cardiac autonomic function: a randomized controlled trial in myocardial infarction patients. Am Heart J. 2014;167:753–761. [DOI] [PubMed] [Google Scholar]
- 16. Martinez DG, Nicolau JC, Lage RL, Toschi‐Dias E, de Matos LD, Alves MJ, Trombetta IC, Dias da Silva VJ, Middlekauff HR, Negrão CE, Rondon MU. Effects of long‐term exercise training on autonomic control in myocardial infarction patients. Hypertension. 2011;58:1049–1056. [DOI] [PubMed] [Google Scholar]
- 17. Mimura J, Yuasa F, Yuyama R, Kawamura A, Iwasaki M, Sugiura T, Iwasaka T. The effect of residential exercise training on baroreflex control of heart rate and sympathetic nerve activity in patients with acute myocardial infarction. Chest. 2005;127:1108–1115. [DOI] [PubMed] [Google Scholar]
- 18. Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299:H1318–H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greaney JL, Edwards DG, Fadel PJ, Farquhar WB. Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J Hum Hypertens. 2015;29:402–408. [DOI] [PubMed] [Google Scholar]
- 20. Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion: protection against triggering by regular exertion. N Eng J Med. 1993;329:1677–1683. [DOI] [PubMed] [Google Scholar]
- 21. Filipovský J, Ducimetière P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle‐aged men. Hypertension. 1992;20:333–339. [DOI] [PubMed] [Google Scholar]
- 22. Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep. 2012;14:421–431. [DOI] [PubMed] [Google Scholar]
- 23. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. [DOI] [PubMed] [Google Scholar]
- 24. Matthews KA, Weiss TM, Detre T, Dembroski TM, Falkner B, Manuck SB, Williams RB, eds. Handbook of Stress, Reactivity, and Cardiovascular Disease. New York, NY: John Wiley & Sons; 1986. [Google Scholar]
- 25. Soares‐Miranda L, Franco FG, Roveda F, Martinez DG, Rondon MU, Mota J, Brum PC, Antunes‐Correa LM, Nobre TS, Barretto AC, Middlekauff HR, Negrão CE. Effects of exercise training on neurovascular responses during handgrip exercise in heart failure patients. Int J Cardiol. 2011;146:122–125. [DOI] [PubMed] [Google Scholar]
- 26. Badrov MB, Usselman CW, Shoemaker JK. Sympathetic neural recruitment strategies: responses to severe chemoreflex and baroreflex stress. Am J Physiol Regul Integr Comp Physiol. 2015;309:R160–R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 28. Blaber AP, Yamamoto Y, Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol Heart Circ Physiol. 1995;268:H1682–H1687. [DOI] [PubMed] [Google Scholar]
- 29. Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans: comparison with drug‐induced responses. Hypertension. 1995;25:1058–1068. [DOI] [PubMed] [Google Scholar]
- 30. Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest: relationship to blood pressure and age. J Physiol. 1978;274:621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 32. Stone JA, Arthur HM, Suskin N, eds. Canadian Guidelines for Cardiac Rehabilitation and Cardiovascular Disease Prevention: Translating Knowledge into Action. 3rd ed Winnipeg, Canada: Canadian Association for Cardiac Rehabilitation; 2009. [Google Scholar]
- 33. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation. 2011;124:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. [DOI] [PubMed] [Google Scholar]
- 35. Carter JR, Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci. 2015;188:36–43. [DOI] [PubMed] [Google Scholar]
- 36. Pardo Y, Merz CN, Velasquez I, Paul‐Labrador M, Agarwala A, Peter CT. Exercise conditioning and heart rate variability: evidence of a threshold effect. Clin Cardiol. 2000;23:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 38. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 39. La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise‐induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. [DOI] [PubMed] [Google Scholar]
- 40. Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. [DOI] [PubMed] [Google Scholar]
- 41. Airaksinen KEJ, Niemelä MJ, Huikuri HV. Effect of beta‐blockade on baroreflex sensitivity and cardiovascular autonomic function tests in patients with coronary artery disease. Eur Heart J. 1994;15:1482–1485. [DOI] [PubMed] [Google Scholar]
- 42. Miller RR, Olson HG, Amsterdam EA, Mason DT. Propranolol‐withdrawal rebound phenomenon. N Engl J Med. 1975;293:416–418. [DOI] [PubMed] [Google Scholar]
- 43. Tygesen H, Andersson B, Di Lenarda A, Rundqvist B, Sinagra G, Hjalmarson Å, Waagstein F, Wennerblom B. Potential risk of β‐blockade withdrawal in congestive heart failure due to abrupt autonomic changes. Int J Cardiol. 1999;68:171–177. [DOI] [PubMed] [Google Scholar]
- 44. Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. [DOI] [PubMed] [Google Scholar]
- 45. Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev. 2004;32:107–111. [DOI] [PubMed] [Google Scholar]
- 46. Lloyd‐Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. [DOI] [PubMed] [Google Scholar]


