Abstract
Background
Early detection for worsening renal function (WRF) is indispensable in patients with acute decompensated heart failure (HF). We tested the hypothesis that the difference in the circulating levels of each B‐type or brain natriuretic peptide (BNP) molecular form is associated with the occurrence of WRF.
Methods and Results
Circulating levels of proBNP, the NT‐proBNP (N‐terminal proBNP), and total BNP (proBNP+mature BNP) were prospectively measured in patients with acute decompensated HF using specific and sensitive enzyme immunochemiluminescent assays. An estimated mature BNP (emBNP) concentration was calculated by subtracting proBNP levels from total BNP levels. WRF was defined as a >20% decrease in the estimated glomerular filtration rate during the hospitalization. One‐way repeated‐measures ANOVA was used to compare the changes of variables between the patients with and without WRF. In patients with acute decompensated HF (New York Heart Association class III–IV; 96%) hospitalized for HF, NT‐proBNP levels did not differ during the hospitalization between patients with and without WRF (n=42 and 140, respectively). By contrast, emBNP levels were lower in patients with WRF than in those without WRF on day 3 after admission. NT‐proBNP/emBNP molar ratios were elevated on day 3 after admission in the patients with WRF, before estimated glomerular filtration rate declined, but were unchanged in patients without WRF. On day 3 after hospital admission, NT‐proBNP/emBNP ratios were strongly associated with percentage decreases in estimated glomerular filtration rate.
Conclusions
These findings suggest that elevation of NT‐proBNP/emBNP ratio precedes WRF in patients with acute HF and can be a potentially useful biomarker for risk stratification of cardiorenal syndrome.
Keywords: acute heart failure, BNP (B‐type or brain natriuretic peptide), cardiorenal syndrome, renal function
Subject Categories: Cardiorenal Syndrome, Heart Failure
Clinical Perspective
What Is New?
Little has been known about the changes in the circulating levels of each B‐type or brain natriuretic peptide (BNP) molecular form in patients with acute heart failure (HF) and worsening renal function (WRF).
This study found that in patients with acute decompensated HF, NT‐proBNP (N‐terminal proBNP) levels did not differ during the hospitalization between patients with and without WRF; by contrast, estimated mature BNP levels were lower in patients with WRF than in those without WRF on day 3 after admission.
NT‐proBNP/estimated mature BNP ratio, but not NT‐proBNP alone, was strongly associated with changes of renal function during hospitalization.
What Are the Clinical Implications?
Renal impairment is a strong prognostic indicator for acute HF, and early detection for WRF is indispensable.
WRF is often asymptomatic, and is detected only through increases in serum creatinine levels.
This study shows that elevation of NT‐proBNP/estimated mature BNP ratio precedes WRF in patients with acute HF; therefore, we propose that the NT‐proBNP/estimated mature BNP ratio could be a novel biomarker for early detection of WRF in patients during the acute phase after the onset of acute HF.
Introduction
Renal impairment is recognized to be an independent risk factor for morbidity and mortality in patients with heart failure (HF).1, 2 As a result of the diverse mechanisms underlying the pathophysiological features of HF, renal and cardiac functions have close and complementary interconnections; and worsening renal function (WRF) often complicates patients with HF. This is termed “cardiorenal syndrome.” Particularly in patients with acute decompensated HF (ADHF), risk factors for WRF, such as hemodynamic disorder and neurohormonal activation, underlie vulnerable phases after the acute onset of HF.3, 4, 5, 6 There is, thus, an unmet need for a biomarker for early detection of WRF, evaluation of HF severity, and prediction of clinical outcomes in patients with ADHF.
The B‐type or brain natriuretic peptide (BNP) gene encodes a 134–amino acid pre‐proBNP precursor, which is converted to the 108–amino acid proBNP (proBNP) through cleavage of a 26–amino acid signal peptide in endoplasmic reticulum.7 Further processing of proBNP leads to the production of 2 molecular forms: biologically active mature BNP and inactive NT‐proBNP (N‐terminal proBNP) in trans‐Golgi network.8 In addition, substantial amounts of proBNP are secreted into the blood without processing and can be detected in the plasma of both healthy subjects and patients with HF.9, 10 The conventional BNP assay kit using sandwich immunoassay usually cross‐reacts with proBNP. Consequently, the BNP level measured using a conventional BNP assay is the total BNP measured as the sum of the mature BNP and proBNP (Figure 1).
Figure 1.
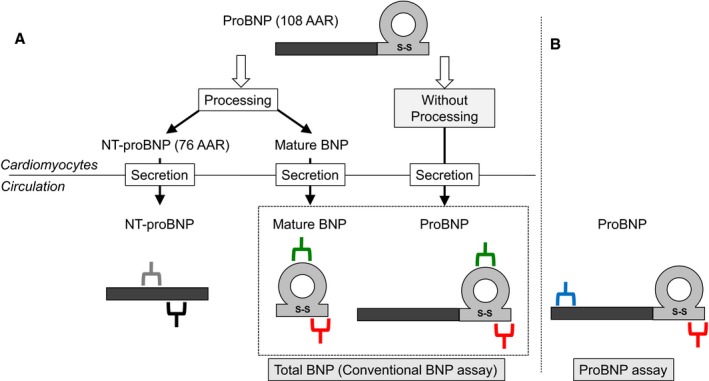
Schematic representation of NT‐proBNP (N‐terminal pro‐B‐type or brain natriuretic peptide) assay, (total) BNP assay, and proBNP‐specific assay. A, The schema of proBNP processing and secretion from cardiomyocytes into circulation. Gray/black and green/red bidentate bars indicate the antibodies specific for the corresponding region of NT‐proBNP and mature BNP/proBNP ratio, respectively. Total BNP indicates sum of mature BNP and proBNP. B, Blue/red bidentate bars indicate the antibodies specific for the corresponding region of proBNP. Thus, estimated mature BNP (emBNP) was calculated as follows: emBNP=total BNP−proBNP (pmol/L). AAR indicates amino acid residue.
We recently developed 2 chemiluminescent enzyme immunoassays (CLEIAs) for proBNP and total BNP; in the latter assay, proBNP and mature BNP are equally recognized on a molar basis. Using these assay systems, we can accurately measure the ratio of proBNP/total BNP and calculate the estimated mature BNP (emBNP) level by subtracting proBNP from total BNP.11 In addition, using this system, we recently reported that the ratio of proBNP/total BNP is increased in patients with severe HF.12 More important, definite differences exist in the clearance and excretion rates of mature BNP and NT‐proBNP: mature BNP is cleared via membrane‐bound natriuretic peptide receptors A and C (NPR‐A and NPR‐C, respectively), neprilysin, and an unknown protease, among others, whereas NT‐proBNP is mainly cleared through renal excretion.13, 14 The regulation of NPR‐A and NPR‐C expression in patients with HF compromised by WRF remains controversial. Some earlier clinical and experimental studies suggest NPRs are up‐regulated in patients with HF,15 hypertensive animal models,16 and a renal ischemic reperfusion animal model,17 suggesting that mature BNP plays a role in renal disease via NPR‐A/cGMP cascade.15 Furthermore, others and we have shown that mature BNP/total BNP ratio is increased in acute HF.18, 19 We hypothesized that the difference in the circulating levels of mature BNP and NT‐proBNP varies with the pathological condition such that their ratio is associated with the occurrence of WRF and future clinical outcome.
Methods
Data, Materials, and Code Disclosure Statement
The data that support the findings of this study are available from the corresponding author (H.T.) on reasonable request.
Study Design
This was a prospective cross‐sectional study of changes in the plasma levels of several types of BNP in patients with ADHF admitted to the National Cerebral and Cardiovascular Center of Japan.
Study Population
Eligibility requirements were as follows: (1) age from 20 to 85 years, (2) hospitalization to our hospital because of ADHF, (3) enrollment within 48 hours after admission for ADHF, and (4) provided written informed consent. Patients on dialysis were excluded, as were patients with acute coronary syndrome. Ultimately, we analyzed 182 patients (aged 26–85 years) enrolled between June 2012 and October 2015. Blood samples for measurement of plasma NT‐proBNP and total BNP levels were collected within 48 hours of admission, 3 days after admission, 7 days after admission, and before discharge. The “before discharge” blood sampling was done a few days before the discharge date, when the patient was clinically stable.
Biomarker Testing
Blood samples were collected into polyethylene‐telephthalate tubes containing EDTA‐2Na (1.5 mg/mL) and aprotinin (500 kallikrein inhibitor units/mL) (Neotube NP‐EA0305; Nipro, Osaka, Japan). The collected blood samples were mixed well, stored at 4°C, and centrifuged within 6 hours. The resulting plasma samples were immediately frozen and stored at −80°C until measurements were made. The research group at Shionogi Corp, our collaborator in the development of the total BNP and proBNP assays, previously confirmed that use of polyethylene‐telephthalate tubes and addition of aprotinin at the time of blood collection greatly increase the stability of BNP immunoreactivity measured with an antibody pair that is identical to the pair used in the present total BNP assay. In these experiments, >90% of BNP immunoreactivity was always recovered under the blood collection and storage conditions used in the present study (H. Shimizu, PhD, K. Masuta, BS, Shionogi Corp, personal communications, 2012). Although several proteases, including neprilysin, dipeptidyl peptidase‐4, and insulin‐degrading enzyme, are known to quickly degrade mature BNP‐32, BNP immunoreactivity detected with the aforementioned antibody pair is believed to be quantitatively recovered and measured with this method and CLEIA system. Plasma total BNP and proBNP were measured using our recently developed CLEIAs, as previously described.19, 20 Briefly, in both CLEIAs, an antibody recognizing a common epitope in the C‐terminal region of proBNP and mature BNP was used as a capture antibody. An antibody recognizing the N‐terminal region of proBNP and one recognizing the ring region of BNP were used as detection antibodies in the proBNP and total BNP CLEIAs, respectively. More important, because the affinities of the 2 detection antibodies for their respective epitopes were nearly the same, and a common capture antibody was used, we were able to accurately measure the proBNP and total BNP and then estimate the mature BNP levels, as described previously.11, 19 The total BNP levels in this study correspond to those measured using the conventional BNP assays, which were measured as the sum of the mature BNP and proBNP and on an equimolar basis in this study. Thus, emBNP was calculated as follows: emBNP=total BNP (sum of proBNP and mature BNP)−proBNP, as shown in Figure 1. Glycosylated proBNP, with a relative molecular mass of 32 kDa (HyTest Ltd, Turku, Finland), served as the standard. Intra‐assay (8 replicates) and interassay (10 replicates) coefficients of variation of the standard peptide at 2.6, 8.9, and 76.7 pmol/L were in the ranges of 1.5% to 5.4% and 4.1% to 5.5%, respectively, in the proBNP assay. NT‐proBNP concentrations were measured using the Elecsys proBNP II assay (Roche Diagnostics, Basel, Switzerland). cGMP (cyclic guanosine monophosphate), a second messenger for BNP, was measured with a radioimmunoassay assay kit (Yamasa Shoyu, Tokyo, Japan). Estimated glomerular filtration rate (eGFR) was calculated using the equation for Japanese individuals: 194×serum creatinine−1.094×age−0.287 (×0.739 for women) (unit, mL/min per 1.73 m2).21
Echocardiography
Using medical records, we retrospectively reviewed the echocardiographic data recorded from the enrolled patients on admission. Left ventricular (LV) dimensions were measured according to American Society of Echocardiography guidelines. LV ejection fraction was measured using the Simpson biplane method or the semiquantitative 2‐dimensional visual estimate method, as previously described.22
Diagnosis of HF
ADHF was diagnosed using the Framingham criteria.23 The final diagnosis of cardiomyopathy was based on the definition from the World Health Organization/International Society and Federation of Cardiology Task Force.24 All patients with ischemic heart disease were diagnosed using coronary angiography or coronary computed tomography.
Definition of WRF
We compared the eGFRs measured at 2 time points, on admission and before discharge. In accordance with the previous studies,25, 26 WRF was defined as a >20% decrease in eGFR before discharge compared with levels on admission in this study.
Clinical Outcomes
After the admission date, we investigated all causes of death, implantation of a LV assist device, and rehospitalization for HF for the 2 years through medical chart review or a letter. Composite clinical events were defined as occurrences of the following events, at least as follows: all causes of death, implantation of an LV assist device, and rehospitalization for HF.
Ethics
Written informed consent was obtained from all subjects. This study was approved by our institutional ethics committee (M23‐090) and was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Data are expressed as medians and interquartile ranges. Categorical variables were expressed as patient number (N) and percentage. Fisher's exact test or χ2 statistic was used to compare categorical variables, as appropriate. For baseline patient characteristics, Wilcoxon's rank sum test was used for comparison of continuous variables between 2 groups (Figure 2, Tables 1, 2 through 3). One‐way repeated‐measures ANOVA was used to compare the changes of eGFR, each BNP level (Figure 3), NT‐proBNP/emBNP ratio (Figure 4A), and cGMP (Figure 4B) over time (1=admission, 2=3 days after admission, 3=7 days after admission, and 4=before discharge) between the patients with WRF and without WRF. Linear regression models were performed to test the difference in the relationship between dependent variables (percentage decreases in eGFR) and independent variables (NT‐proBNP/emBNP ratio) in Tables 4, 5 through 6. Linear regression was also used to assess the relationships between eGFR and each BNP level (Figure 5A through 5C). We also evaluated the group differences in the relationship (eGFR and NT‐proBNP/emBNP ratio) from below and above median NT‐proBNP level by the interaction term between NT‐proBNP (below or above median value) and NT‐proBNP/emBNP ratios (Figure 5D). Univariate survival analyses were performed with the Cox proportional hazards model (Table 7). Hazard ratios with 95% confidential intervals (CI)s and probability (P) values, determined using the likelihood ratio test, are presented. The area under receiver operating characteristic curve and C‐statistics were also calculated. Areas under receiver operating characteristic curves are compared using an algorithm developed by DeLong et al.27 The pairwise comparisons of area under multiple receiver operating characteristic curves were conducted by using roccomp command in STATA. Multivariable analysis/regression was used to test multiple covariates. All tests were 2 tailed, and P<0.05 was considered significant. All statistical analyses were performed using JMP 9 statistical analysis software (SAS Institute Japan, Inc, Tokyo, Japan) and Stata 15 (Stata Corporation LLC, College Station, TX).
Figure 2.
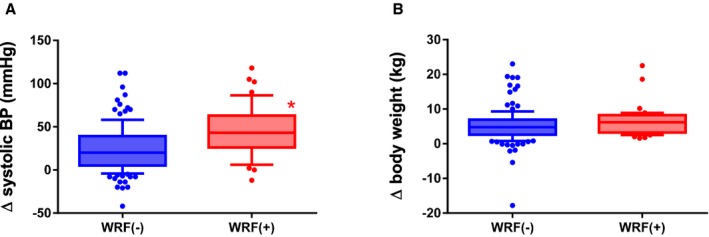
Differences in clinical backgrounds in patients with and without worsening renal function (WRF). A, Change (Δ) in systolic blood pressure in the patients with WRF and without WRF: Δsystolic blood pressure=systolic blood pressure on admission−systolic blood pressure before discharge. Larger decreases in systolic blood pressure were found in the patients with WRF (red) compared with those without WRF (blue); *P<0.05 vs the patients without WRF. B, ΔBody weight in the patients with WRF and without WRF: Δbody weight=body weight on admission−body weight before discharge. Body weight tended to be larger in the patients with WRF (red) compared with those without WRF (blue); P=0.07 vs the patients without WRF. BP, blood pressure.
Table 1.
Baseline Characteristics of Patients With and Without Occurrences of WRF
| Characteristics | WRF (−) | WRF (+) |
|---|---|---|
| Patients, N | 140 | 42 |
| Age, y | 73 (66–80) | 78 (72–81) |
| Female sex, N (%) | 43 (31) | 20 (48) |
| BMI, kg/m2 | 23.0 (20.5–23.3) | 23.5 (22.0–27.2) |
| NYHA class III or IV, N (%) | 133 (95) | 40 (95) |
| Cause, N (%) | ||
| Ischemic | 44 (31) | 13 (31) |
| Nonischemic | 32 (23) | 8 (19) |
| Valvular | 31 (22) | 10 (24) |
| Hypertensive | 26 (19) | 6 (14) |
| Others | 7 (5) | 5 (12) |
| History, N (%) | ||
| HF hospitalization | 60 (43) | 21 (50) |
| Hypertension | 95 (68) | 30 (73) |
| DM | 61 (44) | 15 (35) |
| Dyslipidemia | 72 (51) | 21 (50) |
| Vital signs on admission | ||
| Systolic blood pressure, mm Hg | 133 (112–154) | 147 (123–169)a |
| Heart rate, bpm | 85 (70–104) | 95 (78–115) |
| Echocardiography | ||
| LVDd, mm | 56 (48–64) | 52 (45–59) |
| LVDs, mm | 47 (35–56) | 39 (31–53) |
| LVEF, % | 32 (23–50) | 39 (29–48) |
| Laboratory data | ||
| eGFR, mL/min per 1.73 m2 | 42 (32–55) | 56 (36–75)a |
| BUN, mg/dL | 24 (19–33) | 18 (14–27) |
| Hemoglobin, g/dL | 12.5 (10.7–13.6) | 11.1 (9.9–12.9)a |
| CRP, mg/dL | 0.41 (0.14–1.31) | 0.48 (0.09–1.59) |
| Total BNP, pmol/L | 116.5 (59.6–192.4) | 92.6 (30.0–163.5) |
| emBNP, pmol/L | 43.1 (22.8–73.1) | 32.7 (13.0–61.8) |
| NT‐proBNP, pmol/L | 522.8 (263.4–1103.5) | 493.2 (148.6–982.0) |
| Medications, N (%) | ||
| ACEi or ARB | 86 (61) | 26 (62) |
| β Blockers | 99 (71) | 22 (52) |
| Loop diuretics | 53 (38) | 16 (38) |
Values are the median (interquartile range), unless otherwise specified. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B‐type or brain natriuretic peptide; bpm, beats per minute; BUN, blood urea nitrogen; CRP, C‐reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; emBNP, estimated mature BNP; HF, heart failure; LVDd, left ventricular end‐diastolic diameter; LVDs, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal proBNP; NYHA, New York Heart Association; WRF, worsening renal function.
P<0.05 vs WRF (−).
Table 2.
Total Dosage of Diuretic Agents in Patients With WRF and Without WRF for First 3 Days After Admission
| Diuretic Agents | WRF (−) | WRF (+) |
|---|---|---|
| Furosemide (intravenous), mga | 20 (1060) | 40 (200)b |
| Furosemide (oral), mg | 10 (240) | 30 (280) |
| Torsemide, mg | 0 (28) | 0 (16) |
| Azosemide, mg | 0 (180) | 0 (180) |
| Trichlormethiazide, mg | 0 (4) | 0 (4) |
| Spironolactone, mg | 0 (125) | 0 (125) |
| Tolvaptan, mg | 0 (45) | 0 (30) |
Values are expressed as median (maximum dose). Except furosemide (intravenous), all other agents were taken by oral administration. WRF indicates worsening renal function.
The dose is expressed as a total dosage of intravenous furosemide administration for the first 3 days after hospital admission.
P<0.05 vs WRF (−).
Table 3.
Baseline Characteristics of Patients With Below‐ or Above‐Median NT‐proBNP/emBNP Ratios
| Characteristics | Below‐Median NT‐proBNP/emBNP Ratio | Above‐Median NT‐proBNP/emBNP Ratio |
|---|---|---|
| Patients, N | 91 | 91 |
| Age, y | 73 (63–78) | 77 (70–81)a |
| Female sex, N (%) | 24 (26) | 37 (41)a |
| BMI, kg/m2 | 23.1 (20.4–26.3) | 23.1 (21.1–25.5) |
| NYHA class III or IV, N (%) | 87 (95) | 87 (96) |
| Cause, N (%) | ||
| Ischemic | 29 (32) | 28 (31) |
| Nonischemic cardiomyopathy | 20 (22) | 21 (23) |
| Valvular | 18 (20) | 23 (25) |
| Hypertensive | 17 (18) | 15 (16) |
| Others | 8 (9) | 4 (4) |
| History, N (%) | ||
| HF hospitalization | 32 (35) | 50 (55)a |
| Hypertension | 66 (73) | 59 (66) |
| DM | 33 (36) | 43 (47) |
| Dyslipidemia | 43 (48) | 51 (57) |
| Vital signs on admission | ||
| Systolic blood pressure, mm Hg | 133 (113–152) | 137 (110–161) |
| Heart rate, bpm | 88 (71–106) | 86 (70–106) |
| Echocardiography | ||
| LVDd, mm | 58 (49–64) | 53 (46–63) |
| LVDs, mm | 48 (37–58) | 41 (32–54) |
| LVEF, % | 33 (21–48) | 35 (24–51) |
| Laboratory data | ||
| eGFR, mL/min per 1.73 m2 | 55 (43–68) | 36 (24–48)a |
| BUN, mg/dL | 20 (16–26) | 29 (20–42)a |
| CRP, mg/dL | 0.30 (0.11–0.65) | 0.68 (0.18–2.58)a |
| Total plasma BNP, pmol/L | 102.9 (51.1–176.4) | 117.8 (60.1–190.5) |
| NT‐proBNP, pmol/L | 305.3 (140.2–658.5) | 914.3 (476.5–2073.9)a |
| cGMP, nmol/L | 14.0 (8.0–17.0) | 13.2 (8.1–19.3) |
| Medications, N (%) | ||
| ACEi or ARB | 57 (63) | 56 (62) |
| β Blockers | 66 (66) | 62 (69) |
| Loop diuretics | 58 (64) | 61 (69) |
Values are the median (interquartile range), unless otherwise specified. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B‐type or brain natriuretic peptide; bpm, beats per minute; BUN, blood urea nitrogen; CRP, C‐reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; emBNP, estimated mature BNP; HF, heart failure; LVDd, left ventricular end‐diastolic diameter; LVDs, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal proBNP; NYHA, New York Heart Association.
P<0.05 vs the patients with below‐median NT‐proBNP/emBNP ratio.
Figure 3.
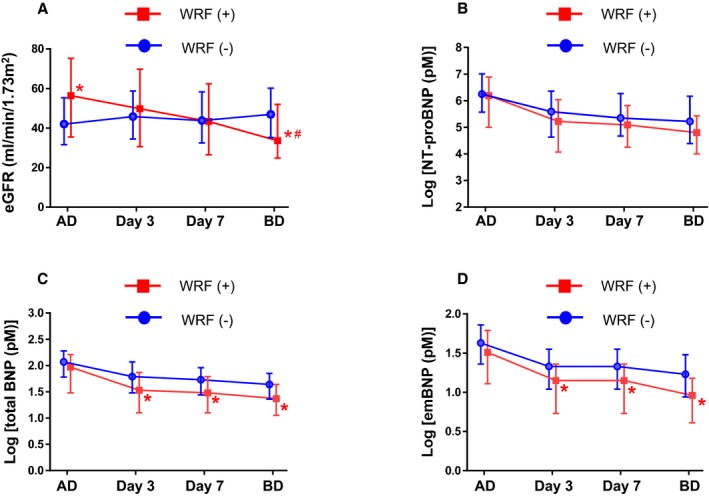
Time course changes in estimated glomerular filtration rate (eGFR) and each B‐type or brain natriuretic peptide (BNP) molecular form during hospitalization of patients with acute decompensated heart failure (ADHF). Time course showing eGFR (A), log NT‐proBNP (N‐terminal pro‐BNP; B), log total BNP (C), and log estimated mature BNP (D) values on hospital admission, 3 days (day 3) and 7 days (day 7) after admission, and before discharge (BD) in patients with ADHF and with worsening renal function (WRF) (red) and without WRF (blue). *P<0.05 vs the patients without WRF at the corresponding times, # P<0.05 vs the overall time course in those without WRF (1‐way repeated ANOVA). AD; admission.
Figure 4.
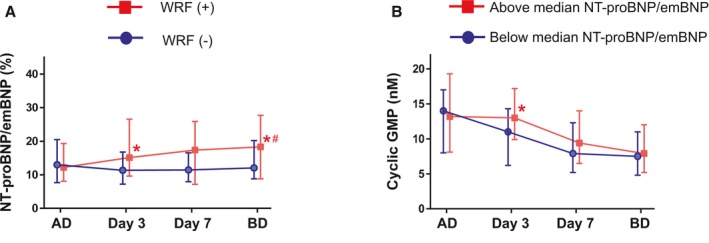
Time course changes in NT‐proBNP (N‐terminal pro‐B‐type or brain natriuretic peptide)/estimated mature BNP (emBNP) ratio and cGMP during hospitalization of patients with acute decompensated heart failure (ADHF). A, Time course of NT‐proBNP/emBNP ratios in patients with ADHF and with (red) and without (blue) worsening renal function (WRF). *P<0.05 vs the patients without WRF at the corresponding times, # P<0.05 vs the overall time course in those without WRF (1‐way repeated ANOVA). B, Time course changes in plasma cGMP in patients with above‐median NT‐proBNP/emBNP ratio (red) and with below‐median NT‐proBNP/emBNP ratio (blue). One‐way repeated ANOVA through protocol: no statistical significance. BD indicates before discharge. *P<0.05 vs the patients without WRF at each time point. AD; admission.
Table 4.
Association of emBNP and NT‐proBNP Levels and NT‐proBNP/emBNP Molar Ratio on Day 3 With Percentage Decreases in eGFR (Unadjusted)
| Variable | Parameter Estimate | 95% CI | P Value |
|---|---|---|---|
| emBNP level | −0.087 | −0.523 to 0.350 | 0.696 |
| NT‐proBNP level | −0.027 | −0.048 to −0.006 | 0.011 |
| NT‐proBNP/emBNP ratio | −1.437 | −2.162 to −0.712 | <0.001 |
eGFR indicates estimated glomerular filtration rate; emBNP, estimated mature B‐type natriuretic peptide; NT‐proBNP, N‐terminal B‐type or brain natriuretic peptide.
Table 5.
Association of the NT‐proBNP Levels on Day 3 With Percentage Decreases in eGFR
| Model | Parameter Estimate | 95% CI | P Value |
|---|---|---|---|
| 1 | −0.029 | −0.050 to −0.008 | 0.007 |
| 2 | −0.024 | −0.046 to −0.002 | 0.033 |
| 3 | −0.022 | −0.043 to −0.002 | 0.034 |
| 4 | −0.031 | −0.052 to −0.009 | 0.005 |
| 5 | −0.017 | −0.033 to −0.002 | 0.032 |
Model 1, adjusted for age and sex. Model 2, adjusted for age, sex, and eGFR (day 3). Model 3, adjusted for age, sex, and total dosage of intravenous furosemide for first 3 days. Model 4, adjusted for age, sex, and systolic blood pressure (BP) on admission. Model 5, adjusted for age, sex, eGFR, total dosage of intravenous furosemide administration for first 3 days, and systolic BP on admission. eGFR indicates estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type or brain natriuretic peptide.
Table 6.
Association of the NT‐proBNP/emBNP Molar Ratio on Day 3 With Percentage Decreases in eGFR
| Model | Parameter Estimate | 95% CI | P Value |
|---|---|---|---|
| 1 | −1.504 | −2.239 to −0.769 | <0.001 |
| 2 | −1.364 | −2.156 to −0.573 | <0.001 |
| 3 | −1.226 | −1.974 to −0.477 | 0.002 |
| 4 | −1.504 | −2.242 to −0.767 | <0.001 |
| 5 | −0.942 | −1.800 to −0.085 | 0.032 |
Model 1, adjusted for age and sex. Model 2, adjusted for age, sex, and eGFR (day 3). Model 3, adjusted for age, sex, and total dosage of intravenous furosemide for first 3 days. Model 4, adjusted for age, sex, and systolic blood pressure (BP) on admission. Model 5, adjusted for age, sex, eGFR, total dosage of intravenous furosemide administration for first 3 days, and systolic BP on admission. eGFR indicates estimated glomerular filtration rate; emBNP, estimated mature B‐type or brain natriuretic peptide; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 5.
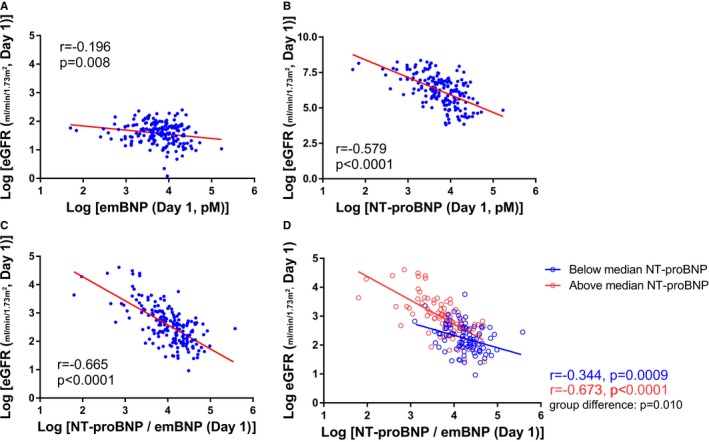
Association between NT‐proBNP (N‐terminal pro‐B‐type or brain natriuretic peptide)/estimated mature BNP (emBNP) ratios and estimated glomerular filtration rate (eGFR) in patients with acute decompensated heart failure. A, Association between the log emBNP levels and log eGFR. B, Association between the log NT‐proBNP levels and log eGFR. C, Association between the log NT‐proBNP/emBNP ratio and log eGFR. D, Association between the log NT‐proBNP/emBNP ratio and log eGFR in patients with above‐median NT‐proBNP (≥525.4 pmol/L) (red plot) or below‐median NT‐proBNP (<525.4 pmol/L) (blue plot). The interaction term between NT‐proBNP and NT‐proBNP/emBNP ratio was significant (P=0.010).
Table 7.
Predictive Values for Composite Clinical Events in Patients With ADHF
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Admission | |||
| Univariate analysis | |||
| WRF (−) | |||
| Log NT‐proBNP | 1.44 | 1.09–1.94 | 0.011 |
| Log NT‐proBNP/emBNP ratio | 1.05 | 0.67–1.67 | 0.815 |
| WRF (+) | |||
| Log NT‐proBNP | 1.05 | 0.67–1.67 | 0.815 |
| Log NT‐proBNP/emBNP ratio | 2.24 | 1.14–4.31 | 0.020 |
| Discharge | |||
| Univariate analysis | |||
| WRF (−) | |||
| Log NT‐proBNP | 1.65 | 1.27–2.16 | <0.001 |
| Log NT‐proBNP/emBNP ratio | 1.28 | 0.86–1.83 | 0.217 |
| WRF (+) | |||
| Log NT‐proBNP | 1.42 | 0.92–2.15 | 0.110 |
| Log NT‐proBNP/emBNP ratio | 1.78 | 0.98–3.34 | 0.060 |
ADHF indicates acute decompensated heart failure; emBNP indicates estimated mature B‐type or brain natriuretic peptide; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; WRF, worsening renal function.
Results
Characteristics of Patients With WRF Occurrences During Hospitalization
Among the 182 enrolled patients, WRF occurred in 42 during their hospitalization (median length of hospital stay, 22 days; interquartile range, 14–35 days), and the follow‐up term (from the baseline blood collection date to the blood collection before discharge) was a median of 18 days (interquartile range, 13–29 days). Table 1 summarizes the characteristics of patients with and without occurrences of WRF during hospitalization for ADHF. The patients with WRF tended to be older and were more likely to be women. There were no statistical differences in the cause of HF, comorbidities, or echocardiographic findings between those who experienced WRF and those who did not. Systolic blood pressure and eGFR were higher and hemoglobin and total BNP tended to be lower in the patients with WRF than in those without it. As shown in Figure 2, in the patients who experienced WRF during hospitalization, the change in systolic blood pressure between admission to the hospital and discharge (Δ systolic blood pressure) was larger in patients with WRF than in those without WRF. As shown in Table 2, total dosages of furosemide by intravenous administration for the first 3 days were higher in the patients with WRF than in those without WRF (P<0.05). Dosages of other diuretic agents did not differ between the 2 groups. A frequency of carperitide administration did not differ between the 2 groups. Decreases in body weight during hospitalization also tended to be larger in the patients with WRF than in those without it (P=0.053).
Time Course Changes in NT‐proBNP, Total BNP, and emBNP in Patients With and Without WRF
Figure 3 presented time course changes in eGFR (Figure 3A), NT‐proBNP (Figure 3B), total BNP (Figure 3C), and emBNP (Figure 3D) in the patients with and without WRF. Before day 3, only 5 WRF events (11% of overall patients with WRD) had occurred. Patients received blood collection for natriuretic peptides at day 3 (n=164), at day 7 (n=159), and before discharge (n=144); and we exclude from the analysis for time course changes those who did not received all 3 blood collections. Significant differences between the patients with and without WRF were found at each time point after day 3 in total BNP and mature BNP, but not in NT‐proBNP. For time course changes, NT‐proBNP levels did not differ in the patients with WRF and those without WRF (Figure 3B). In contrast, both total BNP and emBNP were lower in the patients with WRF compared with those without WRF after day 3 (Figure 3C and 3D, respectively).
Figure 4A shows the time courses of the changes in NT‐proBNP/emBNP ratios during hospitalization in the patients with and without WRF. NT‐proBNP/emBNP ratios were elevated on day 3 after admission in the WRF group but were unchanged throughout the hospitalization in those without WRF (Figure 4A). Figure 4B shows the time course of changes in plasma cGMP in patients with above‐ and below‐median NT‐proBNP/emBNP ratios. Although 1‐way repeated ANOVA revealed no overall differences throughout the protocol, on day 3 after admission, plasma cGMP levels were higher in patients with above‐median NT‐proBNP/emBNP ratios than in those with below‐median NT‐proBNP/emBNP ratios (P<0.05). As the basic characteristics, patients with above‐median NT‐proBNP/emBNP ratios were significantly older and more likely to be women than those with below‐median NT‐proBNP/emBNP ratios (Table 3). Higher frequency of HF hospitalization history was observed in patients with above‐median NT‐proBNP/emBNP ratios than those with below‐median NT‐proBNP/emBNP ratios. Lower eGFR (P<0.05), higher blood urea nitrogen (P<0.05), and smaller LV size (P=0.086) were observed in those with above‐median NT‐proBNP/emBNP ratios compared with those with below‐median NT‐proBNP/emBNP ratios. LV ejection fraction values were comparable between the 2 groups. Plasma total BNP levels also did not differ between them. As the diuretics, furosemide dosages (intravenous administration) did not differ between below‐ and above‐median NT‐proBNP/emBNP ratio groups (median value for both, 20 mg; P=0.138). In addition, there were no statistical differences in use of nitrate (intravenous administration) or phosphodiesterase type 5 inhibitor between the patients with below‐ and above‐median NT‐proBNP/emBNP ratio. NT‐proBNP levels, but not emBNP, were well associated with eGFR (Figure 5A and 5B). Log [NT‐proBNP/emBNP] was well correlated with log eGFR on hospital admission (Figure 5C). Moreover, in patients with above‐median NT‐proBNP levels on admission, the correlation was stronger than in those with below‐median NT‐proBNP levels (Figure 5D; group difference, P=0.010).
The data summarized in Tables 4, 5 through 6 show the association of emBNP, NT‐proBNP levels, and NT‐proBNP/emBNP ratios on day 3 after hospital admission with percentage decreases in eGFR during hospitalization. The emBNP levels on day 3 themselves were not associated with percentage decreases in eGFR (P=0.696; Table 4). The NT‐proBNP levels on day 3 were moderately associated with percentage decreases in eGFR (Table 5). The NT‐proBNP/emBNP ratio was strongly associated with percentage decreases in eGFR, even after adjustments for age, sex, and other confounding factors (Table 6, models 2–5). Moreover, the ratio persisted even after adjustments for serum CRP (C‐reactive protein) values (parameter estimate, −1.58; P<0.0001). In addition, we analyzed predictability of these parameters for WRF occurrences. The association of both eGFR and NT‐proBNP/emBNP ratio (day 3) with the clinical events (WRF) was not statistically significant (both receiver operating characteristic curves and C‐statistics, P>0.05).
Predictive Value of NT‐proBNP/emBNP Ratios for Future Adverse Clinical Outcomes in Patients With ADHF
During the follow‐up period (median, 472 days; interquartile range, 200–712 days), composite clinical events occurred in 70 patients (16 events in patients with WRF, 54 events in patients without WRF). Table 7 shows the predictive values of BNPs and the NT‐proBNP/emBNP ratio in the enrolled patients with and without WRF. The upper rows of Table 7 show the predictive value of NT‐proBNP alone and NT‐proBNP/emBNP ratios measured on admission. In the patients without WRF, NT‐proBNP was associated with clinical outcomes, but NT‐proBNP alone was not associated with clinical outcomes in patients with WRF. Conversely, NT‐proBNP/emBNP ratios were associated with clinical outcomes in patients with WRF, but not in those without WRF. The lower rows of Table 7 show the predictive value of NT‐proBNP alone and NT‐proBNP/emBNP ratios measured before discharge. At that time, NT‐proBNP levels were strongly associated with clinical outcomes in the patients without WRF but were not associated with clinical outcome in those with WRF. NT‐proBNP/emBNP ratios at discharge had a borderline association with clinical outcomes in those with WRF.
Discussion
The present study revealed significant differences in the time course of changes in total BNP and emBNP, but not NT‐proBNP, between patients with and without WRF (Figure 3). As shown in Table 4, the association of emBNP itself with percentage decreases in eGFR was weak. In contrast, the ratio of NT‐proBNP/emBNP was strongly associated with percentage decreases in eGFR. Thus, we propose that combination of NT‐proBNP/emBNP is useful for prediction of WRF, to account for baseline NT‐proBNP levels, which is influenced by renal clearance. Indeed, the NT‐proBNP/emBNP ratio is strongly associated with renal function in patients with ADHF (Figure 5). In particular, the ratio was elevated on day 3 after hospital admission in patients with WRF, although eGFR was not lower in patients with WRF than in those without WRF during the first 7 days after admission (Figures 3A and 4A). These findings suggest that elevation of NT‐proBNP/emBNP ratio precedes WRF in patients with acute HF.
Early Detection for WRF in Patients With Cardiorenal Syndrome
WRF is often asymptomatic and is detected only through increases in serum creatinine levels. There is, thus, an unmet need for early detection of WRF during the short‐term phase of decompensated HF. Novel biomarkers for detecting WRF linked to HF severity are desired, but several confounding factors (eg, volume state) influence biomarker levels. Indeed, tubulospecific biomarkers, such as kidney injury molecule 1, neutrophil gelatinase‐associated lipocalin, and N‐acetyl‐β‐d‐glucosaminidase, are useful for early detection of renal tubular dysfunction in HF; however, their levels are significantly affected by diuretic therapy.28 Therefore, biomarkers for detection of both HF severity and renal function are clinically required for prediction of cardiorenal syndrome in patients with HF. Generally, elevation of serum creatinine levels takes several days after acute renal impairment, which means a significant time lag for detection of WRF. By contrast, expression of BNP mRNA is rapidly induced; and peptide levels of BNP are elevated within 1 hour after myocardial stretch.29 We, therefore, propose that the NT‐proBNP/emBNP ratio could be a novel tool for early detection of WRF in patients during the short‐term phase after the onset of acute HF. Consistent with this idea, we demonstrated herein that NT‐proBNP/emBNP ratios were elevated on day 3 after hospital admission in the patients with WRF (Figure 4A), before declines in eGFR. These findings support our hypothesis that NT‐proBNP/emBNP ratio is potentially useful for early detection of WRF in patients with acute HF much more sensitively than changes in eGFR. The patients with WRF were mainly characterized by hypertensive HF with fluid accumulation (Figure 2), and with higher doses of furosemide intravenous administration (Table 2). Although our data also suggest that diuretics therapy enhances serum creatinine levels, statistically strong differences in diuretics dose were not found between below‐ and above‐median NT‐proBNP/emBNP groups (P=0.138, furosemide intravenous administration dose for first 3 days after admission). These findings also support the idea that this ratio can be a useful biomarker for risk stratification for patients with WRF, with less association with diuretics dosages.
BNP Clearance in Patients With Acute HF
In contrast to the multistep clearance of mature BNP via NPR‐A or NPR‐C,30 NT‐proBNP is cleared mainly through urinary excretion. As a result, its levels are strongly associated with renal function. The association between NT‐proBNP/emBNP ratios and eGFR can be seen in Figure 5C. Consistent with earlier studies,31, 32 NT‐proBNP levels tended to be higher in older patients, women, and those with higher CRP levels. We, therefore, performed the multivariable analysis of these associations summarized in Table 5.
Interestingly, NPR‐C is reportedly upregulated in patients with HF15; and NPR‐A is also reportedly upregulated in experimental models of hypertension.16 It is also reported that this upregulated expression is transiently decreased after ischemic reperfusion in kidney, but subsequently recovers.17 The study suggests NPRs are upregulated within several days after renal impairment. Another study reported there is no difference between fractional extraction of BNP and NT‐proBNP, suggesting that there are no differences in the clearance behavior of the 2 peptides after glomerular filtration in patients with hypertension33; nonetheless, in this study, it is probable that enhanced expression of receptors mediating biological activity or clearance could potentially contribute to elevating NT‐proBNP/emBNP ratios in the first several days after admission. Consistent with this idea, we observed that cGMP levels, a second messenger for mature BNP, were higher in patients with above‐median NT‐proBNP/emBNP ratio than in those with below‐median NT‐proBNP/emBNP ratio only on day 3 after hospital admission (Figure 4B); however, no differences were found in use of carperitide between the 2 groups. In addition, although it is well known that plasma cGMP levels are influenced by nitric oxide (NO). donor therapies, there were no significant differences in use of these therapies, such as nitrate and phosphodiesterase type 5 inhibitor, between the patients with below‐ and above‐median NT‐proBNP/emBNP ratios. These findings raise the possibility that NPR‐A is upregulated at this time, and NPR‐A–mediated consumption of mature BNP is associated with elevation of cGMP levels. Furthermore, emBNP levels were lower in the patients with WRF than in those without WRF on day 3 after admission and thereafter (Figure 3D), but there was no significant difference in NT‐proBNP levels (Figure 3B). These findings are consisted with our hypothesis that NPRs mediate consumption of mature BNP in patients with WRF, leading to elevation of NT‐proBNP/emBNP ratios in patients with WRF.
In addition, it is widely known that the NT‐proBNP has several times longer half‐life than mature BNP (120 versus 20 minutes). As shown in Figure 5, association of eGFR with NT‐proBNP/emBNP ratio on admission was better than that of eGFR with each BNP form (NT‐proBNP and emBNP). Interestingly, NT‐proBNP was also well associated with eGFR compared with emBNP. On the basis of the above hypothesis of rapid consumption of emBNP via NPR‐A, using combination of NT‐proBNP, NT‐proBNP/emBNP ratio may be a reasonable predictor for WRF in patients with ADHF because NT‐proBNP was well correlated with eGFR at each time point.
Risk Stratification in Patients With Cardiorenal Syndrome
Our data demonstrated that the NT‐proBNP/emBNP ratio on admission was strongly associated with clinical outcomes in patients with acute HF and WRF (Table 7). By contrast, previous studies have suggested that BNP levels are higher in patients with renal impairment than in those with similar LV end‐diastolic pressure and function, but without renal impairment,34 and that BNP (total BNP in this study) alone is less predictive of future adverse clinical events in patients with advanced stages of chronic kidney disease.35 Indeed, in patients with WRF, association of NT‐proBNP levels, measured on hospital admission, with future adverse clinical events was weak. These findings of measurement timing of BNP are consistent with earlier studies showing that the predictiveness of BNP on admission was weaker than at the time of discharge.36 Interestingly, a stronger association between NT‐proBNP/emBNP ratios and eGFR was found in patients with above‐median NT‐proBNP levels (indicating more severe acute HF) than in patients with below‐median NT‐proBNP levels (indicating milder acute HF) (Figure 5D). This suggests HF severity, which was indicated by NT‐proBNP levels, is also associated with a steeper correlation between eGFR and NT‐proBNP/emBNP ratios. These findings suggest that this ratio might be influenced by pathophysiological characteristics in both the heart and kidney.
Taken together, the present study raises a possibility that the NT‐proBNP/emBNP ratio is a novel predictor candidate of clinical outcome and is useful for risk stratification of patients with acute HF complicated by WRF. Circulating BNP levels are mainly regulated by cardiac production and provide information about cardiac status, whereas combined assessments of NT‐proBNP and emBNP add useful information about renal status and can predict occurrences of WRF.
Limitations
The present study has several limitations. First, this was a single‐center investigation with a limited number of patients. However, we were able to confirm changes in the ratio of BNP molecular forms during the short‐term phase of HF. Second, there was no significant association between the NT‐proBNP/emBNP ratio or eGFR on hospitalization day 3 and occurrences of WRF (receiver operating characteristic curves and C‐statistics). Given the number of enrolled patients, this suggests the study lacked sufficient power to determine whether those parameters are predictive of WRF occurrence in the patients with acute HF. We, therefore, think that larger‐scale investigations are needed to confirm our findings. Nonetheless, we demonstrated that there are changes in the natriuretic peptide profile during hospitalization and that changes in the NT‐proBNP/emBNP ratio precede the changes in renal function. These findings may provide important evidence that could help in the design of future clinical studies.
Third, some of the patients did not receive a blood test after day 3 (n=18; 10% of the overall patient group), after day 7 (n=23; 13% of the overall patient group), or before discharge (n=38; 21% of the overall patient group). We excluded patients for whom we did not have all 3 blood samples (n=38; 21% of overall patients) from the analysis of the time‐dependent changes in the natriuretic peptide profile. Consequently, that analysis was performed with 79% of the enrolled patients. Because this study aimed to elucidate the association between natriuretic peptide levels at admission and future clinical outcomes during hospitalization, for all other analyses, we did not exclude the data from those patients.
Fourth, it is difficult to elucidate the relation between the interaction of the respective BNP molecular forms with NPRs and levels of circulating mature BNP in clinical settings. Fifth, we did not measure levels of other biomarkers, such as tubulospecific markers, that had not been planned in this study. Future investigations will be necessary to determine their relation to the NT‐proBNP/emBNP ratio and to clarify the relation between this ratio and tubular function in the patients with and without WRF.
Conclusion
We demonstrated the differences in changes in NT‐proBNP and emBNP between patients with acute HF with and without WRF. NT‐proBNP/emBNP molar ratios are strongly associated with renal function during the short‐term phase of acute HF and with adverse clinical events. This ratio provides information about patients with cardiorenal syndrome that cannot be derived from measurements of total BNP or eGFR individually and also provides valuable information about pathophysiological characteristics in both the heart and kidney.
Sources of Funding
This work was partly supported by the Intramural Research Fund of the National Cerebral and Cardiovascular Center of Japan (grants 22‐1‐4 and 27‐1‐5 to Dr Minamino) and Grant in Aid for Scientific Research, Japan Society for the Promotion of Science (grant 18K08057 to Dr Takahama).
Disclosures
This study was performed as collaborative research with Shionogi & Co Ltd, and the measurement kits for pro‐B‐type natriuretic peptide (BNP) and total BNP were donated by this company. The authors have no other disclosures to report.
Acknowledgments
The authors thank to Tomomi Takahashi and Mitsuko Nakatani of Department of Molecular Pharmacology for assistance with management of this study and with the sample managements and measurements of B‐type natriuretic peptides.
(J Am Heart Assoc. 2019;8:e011468 DOI: 10.1161/JAHA.118.011468.)
References
- 1. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. [DOI] [PubMed] [Google Scholar]
- 2. Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta‐analysis. J Am Coll Cardiol. 2006;47:1987–1996. [DOI] [PubMed] [Google Scholar]
- 3. Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. [DOI] [PubMed] [Google Scholar]
- 4. Di Lullo L, Bellasi A, Russo D, Cozzolino M, Ronco C. Cardiorenal acute kidney injury: epidemiology, presentation, causes, pathophysiology and treatment. Int J Cardiol. 2017;227:143–150. [DOI] [PubMed] [Google Scholar]
- 5. Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G, Teerlink JR, Greenberg BH, Filippatos G, Teichman SL, Metra M. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre‐RELAX‐AHF. Eur J Heart Fail. 2011;13:961–967. [DOI] [PubMed] [Google Scholar]
- 6. Takahama H, Kitakaze M. Pathophysiology of cardiorenal syndrome in patients with heart failure: potential therapeutic targets. Am J Physiol Heart Circ Physiol. 2017;313:H715–H721. [DOI] [PubMed] [Google Scholar]
- 7. Sudoh T, Maekawa K, Kojima M, Minamino N, Kangawa K, Matsuo H. Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem Biophys Res Commun. 1989;159:1427–1434. [DOI] [PubMed] [Google Scholar]
- 8. Tonne JM, Campbell JM, Cataliotti A, Ohmine S, Thatava T, Sakuma T, Macheret F, Huntley BK, Burnett JC Jr, Ikeda Y. Secretion of glycosylated pro‐B‐type natriuretic peptide from normal cardiomyocytes. Clin Chem. 2011;57:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seferian KR, Tamm NN, Semenov AG, Mukharyamova KS, Tolstaya AA, Koshkina EV, Kara AN, Krasnoselsky MI, Apple FS, Esakova TV, Filatov VL, Katrukha AG. The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clin Chem. 2007;53:866–873. [DOI] [PubMed] [Google Scholar]
- 10. Dries DL, Ky B, Wu AH, Rame JE, Putt ME, Cappola TP. Simultaneous assessment of unprocessed proBNP1‐108 in addition to processed BNP32 improves identification of high‐risk ambulatory patients with heart failure. Circ Heart Fail. 2010;3:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nishikimi T, Okamoto H, Nakamura M, Ogawa N, Horii K, Nagata K, Nakagawa Y, Kinoshita H, Yamada C, Nakao K, Minami T, Kuwabara Y, Kuwahara K, Masuda I, Kangawa K, Minamino N. Direct immunochemiluminescent assay for proBNP and total BNP in human plasma proBNP and total BNP levels in normal and heart failure. PLoS One. 2013;8:e53233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakagawa Y, Nishikimi T, Kuwahara K, Fujishima A, Oka S, Tsutamoto T, Kinoshita H, Nakao K, Cho K, Inazumi H, Okamoto H, Nishida M, Kato T, Fukushima H, Yamashita JK, Wijnen WJ, Creemers EE, Kangawa K, Minamino N, Kimura T. MiR30‐GALNT1/2 axis‐mediated glycosylation contributes to the increased secretion of inactive human prohormone for brain natriuretic peptide (proBNP) from failing hearts. J Am Heart Assoc. 2017;6:e003601 DOI: 10.1161/JAHA.116.003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weber M, Hamm C. Role of B‐type natriuretic peptide (BNP) and NT‐proBNP in clinical routine. Heart. 2006;92:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishikimi T, Ikeda M, Takeda Y, Ishimitsu T, Shibasaki I, Fukuda H, Kinoshita H, Nakagawa Y, Kuwahara K, Nakao K. The effect of glycosylation on plasma N‐terminal proBNP‐76 levels in patients with heart or renal failure. Heart. 2012;98:152–161. [DOI] [PubMed] [Google Scholar]
- 15. Andreassi MG, Del Ry S, Palmieri C, Clerico A, Biagini A, Giannessi D. Up‐regulation of “clearance” receptors in patients with chronic heart failure: a possible explanation for the resistance to biological effects of cardiac natriuretic hormones. Eur J Heart Fail. 2001;3:407–414. [DOI] [PubMed] [Google Scholar]
- 16. Nuglozeh E, Gauquelin G, Garcia R, Tremblay J, Schiffrin EL. Atrial natriuretic peptide receptors in renal papilla of DOCA‐salt hypertensive rats. Am J Physiol. 1990;259:F130–F137. [DOI] [PubMed] [Google Scholar]
- 17. Cha SA, Park BM, Jung YJ, Kim SM, Kang KP, Kim W, Kim SH. Regional heterogeneity of expression of renal NPRs, TonEBP, and AQP‐2 mRNAs in rats with acute kidney injury. Peptides. 2015;69:33–39. [DOI] [PubMed] [Google Scholar]
- 18. Vodovar N, Seronde MF, Laribi S, Gayat E, Lassus J, Boukef R, Nouira S, Manivet P, Samuel JL, Logeart D, Ishihara S, Cohen Solal A, Januzzi JL Jr, Richards AM, Launay JM, Mebazaa A. Post‐translational modifications enhance NT‐proBNP and BNP production in acute decompensated heart failure. Eur Heart J. 2014;35:3434–3441. [DOI] [PubMed] [Google Scholar]
- 19. Takahama H, Takashio S, Nishikimi T, Hayashi T, Nagai‐Okatani C, Nakagawa Y, Amaki M, Ohara T, Hasegawa T, Sugano Y, Kanzaki H, Yasuda S, Kangawa K, Minamino N, Anzai T. Ratio of pro‐B‐type natriuretic peptide (BNP) to total BNP is decreased in mild, but not severe, acute decompensated heart failure patients: a novel compensatory mechanism for acute heart failure. Int J Cardiol. 2018;258C:165–171. [DOI] [PubMed] [Google Scholar]
- 20. Nishikimi T, Kuwahara K, Nakagawa Y, Kangawa K, Minamino N, Nakao K. Complexity of molecular forms of B‐type natriuretic peptide in heart failure. Heart. 2013;99:677–679. [DOI] [PubMed] [Google Scholar]
- 21. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 22. Imazu M, Takahama H, Asanuma H, Funada A, Sugano Y, Ohara T, Hasegawa T, Asakura M, Kanzaki H, Anzai T, Kitakaze M. Pathophysiological impact of serum fibroblast growth factor 23 in patients with nonischemic cardiac disease and early chronic kidney disease. Am J Physiol Heart Circ Physiol. 2014;307:H1504–H1511. [DOI] [PubMed] [Google Scholar]
- 23. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. [DOI] [PubMed] [Google Scholar]
- 24. Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. [DOI] [PubMed] [Google Scholar]
- 25. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stolfo D, Stenner E, Merlo M, Porto AG, Moras C, Barbati G, Aleksova A, Buiatti A, Sinagra G. Prognostic impact of BNP variations in patients admitted for acute decompensated heart failure with in‐hospital worsening renal function. Heart Lung Circ. 2017;26:226–234. [DOI] [PubMed] [Google Scholar]
- 27. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 28. Damman K, Ng Kam Chuen MJ, MacFadyen RJ, Lip GY, Gaze D, Collinson PO, Hillege HL, van Oeveren W, Voors AA, van Veldhuisen DJ. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol. 2011;57:2233–2241. [DOI] [PubMed] [Google Scholar]
- 29. Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, Nishino K, Yoshimasa T, Nakao K. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy: evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995;96:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. [DOI] [PubMed] [Google Scholar]
- 31. Jensen J, Ma LP, Fu ML, Svaninger D, Lundberg PA, Hammarsten O. Inflammation increases NT‐proBNP and the NT‐proBNP/BNP ratio. Clin Res Cardiol. 2010;99:445–452. [DOI] [PubMed] [Google Scholar]
- 32. Hamada M, Shigematsu Y, Takezaki M, Ikeda S, Ogimoto A. Plasma levels of atrial and brain natriuretic peptides in apparently healthy subjects: effects of sex, age, and hemoglobin concentration. Int J Cardiol. 2017;228:599–604. [DOI] [PubMed] [Google Scholar]
- 33. van Kimmenade RR, Januzzi JL Jr, Bakker JA, Houben AJ, Rennenberg R, Kroon AA, Crijns HJ, van Dieijen‐Visser MP, de Leeuw PW, Pinto YM. Renal clearance of B‐type natriuretic peptide and amino terminal pro‐B‐type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol. 2009;53:884–890. [DOI] [PubMed] [Google Scholar]
- 34. Tsutamoto T, Wada A, Sakai H, Ishikawa C, Tanaka T, Hayashi M, Fujii M, Yamamoto T, Dohke T, Ohnishi M, Takashima H, Kinoshita M, Horie M. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2006;47:582–586. [DOI] [PubMed] [Google Scholar]
- 35. Horii M, Matsumoto T, Uemura S, Sugawara Y, Takitsume A, Ueda T, Nakagawa H, Nishida T, Soeda T, Okayama S, Somekawa S, Ishigami K, Takeda Y, Kawata H, Kawakami R, Saito Y. Prognostic value of B‐type natriuretic peptide and its amino‐terminal proBNP fragment for cardiovascular events with stratification by renal function. J Cardiol. 2013;61:410–416. [DOI] [PubMed] [Google Scholar]
- 36. Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O'Connor CM, Felker GM, Hernandez AF. Admission, discharge, or change in B‐type natriuretic peptide and long‐term outcomes: data from organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF) linked to Medicare claims. Circ Heart Fail. 2011;4:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]


