Abstract
Background
Epidemiological evidence implies a link between heart disease and dementia. However, few prospective studies have assessed the association between serum NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) levels and dementia.
Methods and Results
A total of 1635 community‐dwelling Japanese elderly aged ≥60 years without dementia (57% women, mean age±SD 70.8±7.7 years) were followed up for 10 years. Serum NT‐proBNP levels were divided into 4 categories (≤54, 55‐124, 125‐299, and ≥300 pg/mL). The hazard ratios were estimated using a Cox proportional hazards model. During the follow‐up period, 377 subjects developed all‐cause dementia, 247 Alzheimer disease, and 102 vascular dementia. The age‐ and sex‐adjusted incidence of all‐cause dementia was 31.5 per 1000 person‐years and increased significantly with higher serum NT‐proBNP levels, being 16.4, 32.0, 35.7, and 45.5, respectively (P for trend <0.01). Subjects with serum NT‐proBNP levels of ≥300 pg/mL had a significantly higher risk of all‐cause dementia (hazard ratio=2.46, 95% CI 1.63‐3.71) than those with serum NT‐proBNP levels of ≤54 pg/mL after adjusting for confounders. Similar risks were observed for Alzheimer disease and vascular dementia. Incorporation of the serum NT‐proBNP level into a model with known risk factors for dementia significantly improved the predictive ability for incident dementia (c‐statistics 0.780‐0.787, P=0.02; net reclassification improvement 0.189, P=0.001; integrated discrimination improvement 0.011, P=0.003).
Conclusions
Higher serum NT‐proBNP levels were significantly associated with an increased risk of dementia. Serum NT‐proBNP could be a novel biomarker for predicting future risk of dementia in the general elderly population.
Keywords: Alzheimer disease, biomarker, N‐terminal pro–B‐type natriuretic peptide, prospective cohort study, vascular dementia
Subject Categories: Epidemiology, Risk Factors, Mental Health
Clinical Perspective
What Is New?
This prospective cohort study of a general Japanese population demonstrated a significant association between increased serum NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) levels and the development of dementia and its subtypes.
Incorporation of serum NT‐proBNP values into a model with known risk factors for dementia significantly improved the predictive ability for incident dementia.
What Are the Clinical Implications?
Serum NT‐proBNP may be a useful biomarker for evaluating future risk of dementia, especially Alzheimer disease.
Dementia is a major cause of disability among the elderly, and its medical and economic burdens on society have been increasing worldwide.1 Recent epidemiological studies have reported that lifestyle‐related diseases such as hypertension,2 diabetes mellitus,3 and obesity4 as well as lifestyle factors such as smoking habits,5 dietary patterns,6 and physical activity7 are associated with the risk of developing dementia. However, the influence of these factors on dementia, especially Alzheimer disease (AD), remains incompletely understood.
Heart disease is a major cause of disability and premature death among the elderly.8 Circulatory failure and vascular insufficiency that are caused by heart disease and its risk factors have the potential to impair function in various organs, including the brain. Several prospective studies have shown a close association between chronic heart failure and the risk of dementia.9, 10, 11 In addition, interventional studies revealed that treatment to increase cardiac output improved the cognitive function in patients with severe heart failure.12, 13 These findings imply a link between heart disease and dementia.
NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) is an inactive N‐terminal fragment of proBNP with 76 amino acids and is released as a prohormone from ventricular myocytes in response to mechanical stretch and ischemic injury.14 Serum NT‐proBNP is acknowledged to be a diagnostic biomarker for evaluating the severity of heart failure15, 16 and a predictive biomarker for cardiovascular events.17 It has also been associated with subclinical heart disease among asymptomatic individuals.18 Interestingly, several cross‐sectional studies reported that elevated serum NT‐proBNP levels are associated with cognitive impairment or brain microstructure change.19, 20 In addition, previous population‐based prospective studies conducted in Western populations showed a significant association between serum NT‐proBNP levels and the development of dementia.21, 22 However, there has been only 1 study addressing the association of NT‐proBNP levels with dementia subtypes, such as AD and vascular dementia (VaD).22 In addition, only 1 prospective cohort study has investigated whether serum NT‐proBNP could be a potential biomarker for predicting the future development of dementia.21 The objectives of this study were therefore to evaluate the association between serum NT‐proBNP levels and risk for the development of dementia in a general elderly Japanese population and to clarify whether the incorporation of serum NT‐proBNP levels into the predictive model can improve its ability to predict incident dementia.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
The Hisayama Study is an ongoing population‐based prospective cohort study in the town of Hisayama, a suburb of the Fukuoka metropolitan area in the southern part of Japan. Full community surveys of the residents have been repeated since 1961 to determine the prevalence and incidence of cerebro‐ and cardiovascular diseases and their risk factors in Japanese.23 In addition, since 1985, comprehensive surveys of dementia in the elderly of this town have been conducted every 6 or 7 years.24 In 2002 a total of 1760 residents aged 60 and older (participation rate 83.4%) underwent a screening examination for the present study. After exclusion of 122 subjects with dementia at baseline and 3 without available data on NT‐proBNP in serum, the remaining 1635 individuals (705 men and 930 women) were enrolled in this study.
Follow‐Up Survey
The subjects were followed prospectively from when they underwent a screening examination to November 2012 (median follow‐up period 10.2 years, interquartile range 7.2‐10.3 years). Detailed methods for screening potential dementia events have been reported in our previous studies.23, 24 In brief, information about new events, including stroke and dementia, was collected through a daily monitoring system established by the study team, local physicians, and members of the town's Health and Welfare Office. In this system the physicians in the study team visited clinics, hospitals, and the town's office regularly to collect information on events of stroke and dementia, including suspected cases. Regular health examinations were performed annually to obtain information on events. Letters or telephone calls were made to collect the health information of subjects who did not undergo regular examinations or who had moved out of town. In addition to the regular examination, follow‐up screening surveys of dementia were conducted in 2005 and 2012.24 When new neurological symptoms, including cognitive impairment, were suspected, the study physician and psychiatrist carefully evaluated the participant, conducting comprehensive investigations including interviews of the family or attending physician, physical and neurological examinations, and a review of the clinical records. In addition, when a subject died, we collected and fully examined all the available medical information, including data on cognitive function, activities of daily living, and brain imaging; we interviewed the family and attending physician of the deceased; and we tried to obtain permission for autopsy from the family. Causes of death were classified according to the International Classification of Diseases, 10th Revision (ICD‐10) as follows: cardiovascular death (ICD‐10 code of I00‐I99), cancer death (ICD‐10 code of C00‐C97), respiratory infection death (ICD‐10 codes of J00‐J22, J69, and J85‐J86), and death from other causes.25 During the follow‐up period, 370 subjects died: there were 98 (26.5%) deaths from cardiovascular causes, 124 (33.5%) from cancer, 59 (15.9%) from respiratory infection, and 89 (24.1%) from other causes (diseases of the digestive system, external causes, and other disease). Among the 370 decedents, 237 underwent brain examination at autopsy. Except for the deceased subjects, no subject was lost to follow‐up through November 2012.
Diagnosis of Dementia
The diagnosis of dementia was made based on the guidelines of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised.26 Subjects diagnosed as having AD met the diagnostic criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association,27 and subjects diagnosed with VaD met the criteria of the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l'Enseignement en Neurosciences.28 Clinical information, including neuroimaging, was used to determine possible and probable dementia subtypes. Definite dementia subtypes were also determined based on clinical and neuropathological information in subjects with dementia who underwent brain autopsy. The diagnostic procedure for autopsy cases was reported previously.29 Every dementia case was adjudicated by expert stroke physicians and psychiatrists.
Measurement of NT‐proBNP
At the screening examination, serum samples were collected and frozen at −80°C. In 2009 we thawed these serum samples to measure the NT‐proBNP levels. NT‐proBNP levels were quantified with a second‐generation commercial kit, the Elecsys proBNP Immunoassay,30 on an Elecsys 2010 platform (Roche Diagnostics, Risch, Switzerland). Serum NT‐proBNP levels were divided into 4 categories according to the current guidelines and prior reports: ≤54, 55 to 124, 125 to 299, and ≥300 pg/mL.15, 16, 31
Risk Factor Measurements
At the baseline examination each subject completed a self‐administered questionnaire regarding smoking habits, alcohol intake, regular exercise, educational status, medical history, and treatment of hypertension, diabetes mellitus, and hypercholesterolemia. The questionnaire was checked by trained interviewers. Smoking habits and alcohol intake were classified as either current habitual use or not. Subjects engaging in sports at least 3 times a week during their leisure time were defined as the regular exercise group. A low education level was defined as ≤9 years of formal education. Cardiovascular disease was defined as coronary heart disease and stroke. History of cancer was defined by using a self‐reported questionnaire. Body height and weight were measured in light clothing without shoes, and body mass index (BMI) was calculated. Obesity was defined as a BMI level of ≥25 kg/m2 according to the criteria of the Japan Society for the Study of Obesity.32 Blood pressure was obtained 3 times using an automated sphygmomanometer after rest for at least 5 minutes in the sitting position. The mean of the 3 measurements was used in the present analysis. Hypertension was defined as blood pressure ≥140/90 mm Hg and/or current use of antihypertensive agents. Use of heart disease agents was defined as taking any of the following: diuretics, nitrates, antiarrhythmic agent, or digitalis. A total of 585 subjects used antihypertensive agents: 490 used calcium channel blockers, 133 used angiotensin‐converting enzyme inhibitors, 141 used angiotensin II receptor blockers, 128 used β‐blockers, and 86 used α‐blockers. Among the 181 subjects administered heart disease medications, 68 used diuretics, 75 used nitrates, 35 used antiarrhythmic agents, and 44 used digitalis. Plasma glucose levels were measured by the hexokinase method, and diabetes mellitus was determined by plasma glucose levels (fasting glucose level ≥7.0 mmol/L or postprandial glucose level ≥11.1 mmol/L), or a 75‐g oral glucose tolerance test according to the 1998 World Health Organization criteria33 and/or by the use of oral hypoglycemic agents or insulin (n=136). Serum total cholesterol levels were measured enzymatically, and hypercholesterolemia was defined as a total cholesterol level of ≥5.69 mmol/L and/or use of lipid‐lowering drugs. Serum creatinine concentrations were measured using an enzymatic method, and the estimated glomerular filtration rate (eGFR) was calculated using the Japanese coefficient‐modified Chronic Kidney Disease Epidemiology Collaboration equation.34 Chronic renal failure was defined as eGFR <15 mL/min per 1.73 m2. Atrial fibrillation was defined as the presence of Minnesota Code 8‐3 on electrocardiogram. History of stroke was defined on the basis of all clinical data available in the Hisayama Study. Serum HS‐CRP (high‐sensitivity C‐reactive protein) was measured in the frozen serum portion thawed in 2004 using a modified version of the Behring Latex‐Enhanced CRP assay on a Behring Nephelometer BN‐100 (Behring Diagnostics, Westwood, MA). High HS‐CRP was defined as a serum HS‐CRP level ≥1.0 mg/L.35
Statistical Analysis
Serum NT‐proBNP and serum HS‐CRP were transformed into logarithms to improve the skewed distribution for the analysis. The linear trends in the proportions of risk factors across serum NT‐proBNP levels were tested using a Cochran‐Armitage test. To examine the linear trends in the mean values, a linear regression analysis was used, where the variable of serum NT‐proBNP levels assigned ordinal numbers (ie, 1, 2, 3, 4) was included in the relevant model. The age‐ and sex‐adjusted cumulative incidence of outcomes across serum NT‐proBNP levels was estimated on the basis of regression estimates from a Cox proportional hazards model including age and sex.36 The assumption of the proportional hazards was checked graphically using the log cumulative hazard plots for each dementia subtype according to serum NT‐proBNP levels. The incidence rate of dementia was calculated by the person‐year method with adjustment for age and sex by the direct method. The hazard ratios (HRs) with their 95% CIs according to serum NT‐proBNP levels for the development of dementia and its subtypes were estimated using a Cox proportional hazards model. We evaluated 3 different models: (1) model 1, adjusted for age and sex; (2) model 2, adjusted for age, sex, education level, systolic blood pressure, use of antihypertensive agents, use of heart disease agents, diabetes mellitus, hypercholesterolemia, BMI, eGFR, atrial fibrillation, history of stroke, smoking habit, alcohol intake, and regular exercise; and (3) model 3, adjusted for the covariates included in model 2 plus log‐transformed serum HS‐CRP.
A 1‐SD increment of the log‐transformed serum NT‐proBNP was used to estimate the association between NT‐proBNP taken as a continuous variable and the risk of dementia. The heterogeneity in the association among subgroups was tested by adding multiplicative interaction terms to the relevant Cox model, where interaction terms were calculated as log‐transformed serum NT‐proBNP levels multiplied by each term of covariates. To compare the discrimination ability for the development of dementia between the models adjusted for potential risk factors with and without serum NT‐proBNP levels, the consistency in the Harrell's c‐statistics37 among models was estimated using a crossfold validation approach, as described by Newson.38 The increased predictive ability of serum NT‐proBNP was further examined with 2 measures: continuous and categorical net reclassification improvement (NRI)39, 40 and integrated discrimination improvement (IDI).39 For the evaluation of categorical NRI, we classified the probability of the risk of dementia over 10 years into clinically meaningful categories of <25%, 25 to 50%, and >50%. The individual probabilities were estimated by using the Cox proportional hazards model. A 2‐sided value of P<0.05 was considered to be statistically significant in all analyses. All statistical analyses were performed with the SAS statistical software program, version 9.4 (SAS Institute, Cary, NC), and Stata version 14.0 (StataCorp, College Station, TX).
Ethical Considerations
This study was conducted with the approval of the Kyushu University Institutional Board for Clinical Research. Written informed consent was obtained from all subjects.
Results
The baseline characteristics of the study population according to serum NT‐proBNP levels are summarized in Table 1. The mean age, systolic blood pressure, and serum HS‐CRP and the proportions of female sex, low education, hypertension, use of antihypertensive agents, use of heart disease agents, chronic renal failure, atrial fibrillation, history of stroke, history of cardiovascular disease, and history of cancer increased significantly with elevated serum NT‐proBNP levels. Conversely, the mean values of BMI and eGFR and the proportions of hypercholesterolemia, obesity, smoking habits, and alcohol intake decreased significantly with higher serum NT‐proBNP levels. During the follow‐up period, 377 subjects (138 men and 239 women) developed all‐cause dementia; 336 were evaluated by brain imaging, 94 by brain autopsy (the agreement rate between the clinical and the neuropathological diagnosis was 0.79), and 83 by both. Thus, 347 subjects (92.0%) underwent some form of morphological examination. Among dementia subjects, 17 subjects had a mixed type of AD and VaD, and these cases were counted as events in the analysis for each subtype. Including these subjects, 247 subjects had AD and 102 had VaD.
Table 1.
Clinical Characteristics of Study Subjects According to Serum NT‐proBNP Levels at Baseline, 2002
| NT‐proBNP Levels (pg/mL) | P for Trend | ||||
|---|---|---|---|---|---|
| ≤54 (n=514) | 55 to 124 (n=595) | 125 to 299 (n=336) | ≥300 (n=190) | ||
| Age, mean (SD), y | 67 (5) | 70 (7) | 74 (8) | 77 (9) | <0.001 |
| Female sex, % | 46.9 | 62.9 | 63.7 | 53.2 | 0.002 |
| Education ≤9 y, % | 42.0 | 52.5 | 65.5 | 60.5 | <0.001 |
| Systolic BP, mean (SD), mm Hg | 133 (18) | 137 (20) | 142 (23) | 143 (23) | <0.001 |
| Diastolic BP, mean (SD), mm Hg | 79 (11) | 79 (11) | 80 (13) | 78 (12) | 0.34 |
| Hypertension, % | 49.2 | 57.5 | 66.7 | 75.8 | <0.001 |
| Use of antihypertensive agents, % | 28.2 | 34.5 | 38.4 | 55.8 | <0.001 |
| Use of heart disease agents, % | 5.6 | 7.6 | 11.6 | 35.8 | <0.001 |
| Diabetes mellitus, % | 20.8 | 22.5 | 22.0 | 23.2 | 0.52 |
| Hypercholesterolemia, % | 43.4 | 44.0 | 37.5 | 29.0 | <0.001 |
| BMI, mean (SD), kg/m2 | 23.6 (2.9) | 23.2 (3.3) | 22.5 (3.4) | 22.0 (3.3) | <0.001 |
| Obesity, % | 30.7 | 27.4 | 23.5 | 20.0 | 0.001 |
| eGFR, mean (SD), mL/min per 1.73 m2 | 75 (9) | 73 (9) | 69 (12) | 60 (19) | <0.001 |
| Chronic renal failure, % | 0.0 | 0.0 | 0.0 | 4.7 | <0.001 |
| Atrial fibrillation, % | 0.0 | 0.0 | 0.9 | 16.3 | <0.001 |
| History of stroke, % | 3.3 | 3.9 | 7.1 | 13.7 | <0.001 |
| History of cardiovascular disease, % | 5.1 | 5.4 | 9.2 | 17.9 | <0.001 |
| History of cancer, % | 7.2 | 8.1 | 11.0 | 11.6 | 0.02 |
| Smoking habits, % | 17.3 | 14.6 | 14.6 | 10.5 | 0.03 |
| Alcohol intake, % | 42.0 | 33.8 | 33.3 | 29.0 | <0.001 |
| Regular exercise, % | 13.2 | 13.1 | 11.9 | 10.5 | 0.33 |
| Serum HS‐CRP, median (interquartile range), mg/L | 0.5 (0.3‐1.1) | 0.5 (0.3‐1.0) | 0.6 (0.3‐1.8) | 1.0 (0.4‐1.8) | <0.001 |
| Serum HS‐CRP ≥1.0 mg/L, % | 28.0 | 25.4 | 36.6 | 47.4 | <0.001 |
Hypertension was defined as blood pressure ≥140/90 mm Hg and/or current use of antihypertensive agents. Heart disease agents were defined as diuretics, nitrates, antiarrhythmic agents, and digitalis. Hypercholesterolemia was defined as serum total cholesterol ≥5.69 mmol/L and/or use of lipid‐lowering drugs. Obesity was defined as a BMI level of ≥25 kg/m2. Chronic renal failure was defined as eGFR <15 mL/min per 1.73 m2. Atrial fibrillation was defined as Minnesota Code 8‐3 on electrocardiography. Cardiovascular disease was defined as coronary heart disease and stroke. Smoking habits and alcohol intake were classified as current use or not. Regular exercise was defined as engaging in sports at least 3 times per week during leisure time. BMI indicates body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HS‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide.
Figure demonstrates the age‐ and sex‐adjusted cumulative incidence of dementia and its subtypes according to serum NT‐proBNP levels. The incidences of all‐cause dementia, AD, and VaD increased significantly with higher serum NT‐proBNP levels (all P for trend <0.01). Table 2 shows the estimated HRs and 95% CIs for the development of dementia and its subtypes according to serum NT‐proBNP levels. After adjustment for age, sex, educational level, systolic blood pressure, use of antihypertensive agents, use of heart disease agents, diabetes mellitus, hypercholesterolemia, BMI, eGFR, atrial fibrillation, history of stroke, smoking habits, alcohol intake, and regular exercise (model 2), the risk of developing all‐cause dementia, AD, and VaD increased significantly with higher serum NT‐proBNP levels (all P for trend <0.01). These associations did not change substantially even after adjustment for HS‐CRP in addition to the abovementioned confounding factors (model 3): the multivariable‐adjusted HRs of all‐cause dementia, AD, and VaD were significantly higher in subjects with serum NT‐proBNP levels of ≥300 pg/mL than in those with serum NT‐proBNP levels of ≤54 pg/mL (HR=2.46, 95% CI=1.63‐3.71 for all‐cause dementia; HR=2.43, 95% CI=1.41‐4.16 for AD; and HR=3.55, 95% CI=1.64‐7.72 for VaD). When estimating the risk of developing dementia per 1‐SD increment in log‐transformed serum NT‐proBNP levels, the HRs of all‐cause dementia, AD, and VaD increased significantly with higher serum NT‐proBNP levels (HR=1.38, 95% CI=1.21‐1.58, P<0.001 for all‐cause dementia; HR=1.35, 95% CI=1.14‐1.59, P<0.001 for AD; and HR=1.51, 95% CI=1.19‐1.93, P<0.001 for VaD) after adjustment for confounding factors.
Figure 1.
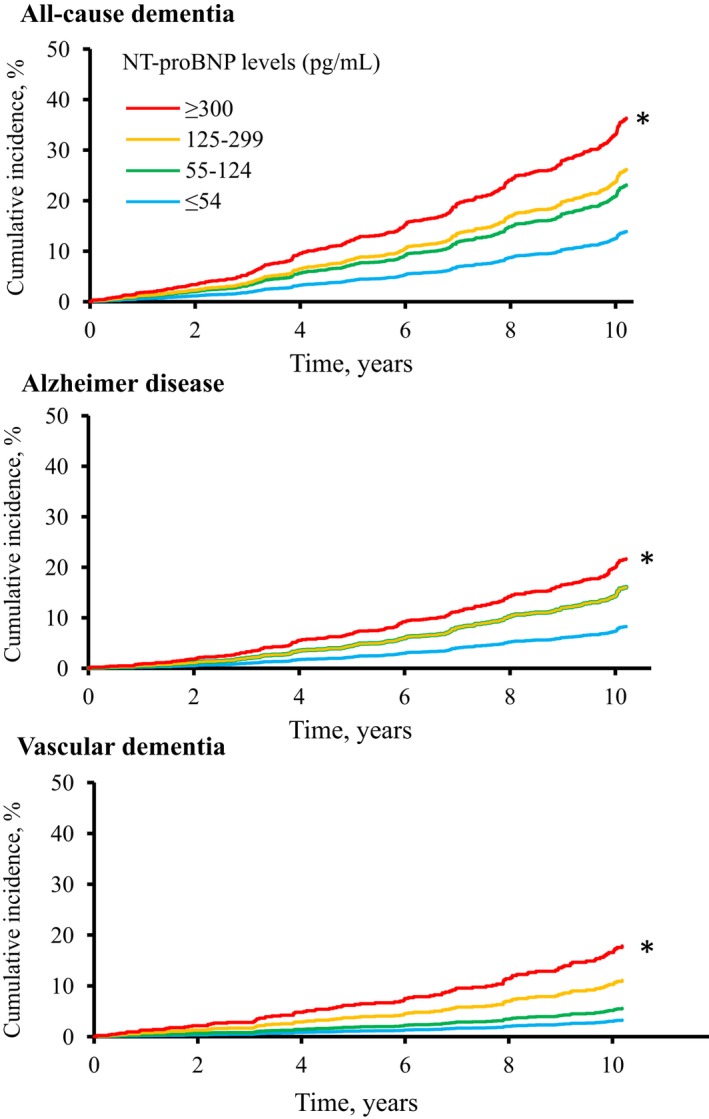
Age‐ and sex‐adjusted cumulative incidence of all‐cause dementia, Alzheimer disease, and vascular dementia according to serum NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide) levels. *P values for trend <0.01.
Table 2.
Risk of Dementia and Its Subtypes According to Serum NT‐proBNP Levels, 2002‐2012
| NT‐proBNP (pg/mL) | People at Risk | No. of Events | Crude Incidence Per 1000 Person‐Years | Hazard Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| All‐cause dementia | ||||||
| ≤54 | 514 | 55 | 11.2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 55 to 124 | 595 | 141 | 27.0 | 1.76 (1.28‐2.41) | 1.66 (1.21‐2.29) | 1.66 (1.21‐2.29) |
| 125 to 299 | 336 | 111 | 42.1 | 2.03 (1.44‐2.85) | 1.65 (1.16‐2.34) | 1.69 (1.19‐2.39) |
| ≥300 | 190 | 70 | 61.0 | 3.02 (2.08‐4.37) | 2.38 (1.58‐3.58) | 2.46 (1.63‐3.71) |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| 1‐SD increment in log (serum NT‐proBNP levels) | 1635 | 377 | 27.1 | 1.43 (1.29‐1.59) | 1.36 (1.20‐1.55) | 1.38 (1.21‐1.58) |
| Alzheimer disease | ||||||
| ≤54 | 514 | 32 | 6.5 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 55 to 124 | 595 | 104 | 19.9 | 2.03 (1.36‐3.04) | 2.00 (1.33‐3.00) | 2.00 (1.33‐2.99) |
| 125 to 299 | 336 | 75 | 28.5 | 2.03 (1.32‐3.13) | 1.82 (1.17‐2.84) | 1.90 (1.22‐2.96) |
| ≥300 | 190 | 36 | 31.4 | 2.41 (1.47‐3.96) | 2.23 (1.31‐3.82) | 2.43 (1.41‐4.16) |
| P for trend | 0.001 | 0.01 | 0.003 | |||
| 1‐SD increment in log (serum NT‐proBNP levels) | 1635 | 247 | 17.8 | 1.30 (1.13‐1.49) | 1.30 (1.11‐1.53) | 1.35 (1.14‐1.59) |
| Vascular dementia | ||||||
| ≤54 | 514 | 13 | 2.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 55 to 124 | 595 | 28 | 5.4 | 1.72 (0.88‐3.36) | 1.57 (0.81‐3.07) | 1.57 (0.81‐3.07) |
| 125 to 299 | 336 | 35 | 13.3 | 3.53 (1.81‐6.88) | 2.51 (1.26‐4.99) | 2.52 (1.26‐5.03) |
| ≥300 | 190 | 26 | 22.6 | 5.92 (2.94‐11.90) | 3.54 (1.63‐7.68) | 3.55 (1.64‐7.72) |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| 1‐SD increment in log (serum NT‐proBNP levels) | 1635 | 102 | 7.3 | 1.71 (1.42‐2.05) | 1.51 (1.18‐1.92) | 1.51 (1.19‐1.93) |
The SD of log‐transformed NT‐proBNP levels (pg/mL) was 1.058. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, education level, systolic blood pressure, use of antihypertensive agents, use of heart disease agents, diabetes mellitus, hypercholesterolemia, body mass index, estimated glomerular filtration rate, atrial fibrillation, history of stroke, smoking habit, alcohol intake, and regular exercise. Model 3: adjusted for the covariates included in model 2 plus high‐sensitivity C‐reactive protein. NT‐proBNP indicates N‐terminal pro–B‐type natriuretic peptide.
The sensitivity analysis by categorizing serum NT‐proBNP levels into quartiles also showed a significant positive association of serum NT‐proBNP levels with the risk of all‐cause dementia, AD, and VaD (Table S1). In addition, we investigated the association of serum NT‐proBNP levels with the risk of dementia with and without incident cardiovascular disease before the onset of dementia during the follow‐up period among subjects without a history of cardiovascular disease and/or dementia at baseline. The results showed that higher serum NT‐proBNP levels were associated with increased risk of dementia, regardless of the presence or absence of the onset of cardiovascular disease during follow‐up (Table S2). Similar significant associations were also observed between serum NT‐proBNP levels and risk of dementia with and without incident diabetes mellitus before the onset of dementia during the follow‐up period among subjects without a history of diabetes mellitus and/or dementia at baseline (Table S3). A supplementary analysis focused on mortality risk showed that a 1‐SD increment in log‐transformed serum NT‐proBNP levels was significantly associated with a higher risk of all‐cause death (multivariable‐adjusted HR=1.37, 95% CI=1.21‐1.56, P<0.001).
Next, we estimated the multivariable‐adjusted HRs of dementia per 1‐SD increment in log‐transformed NT‐proBNP levels in subgroups of potential risk factors for dementia (Table 3). There was no evidence of heterogeneity in the association of serum NT‐proBNP levels with the risk of dementia between subjects with and those without other risk factors.
Table 3.
Multivariable‐Adjusted Hazard Ratios for Development of Dementia Owing to a 1‐SD Increment in Log‐Transformed Serum NT‐proBNP Levels by Presence or Absence of Other Cardiovascular Risk Factors, 2002‐2012
| Variables | People at Risk | No. of Events | Hazard Ratio (95% CI) | P for Heterogeneity |
|---|---|---|---|---|
| Age | ||||
| <75 y | 1137 | 155 | 1.46 (1.21‐1.76) | 0.43 |
| ≥75 y | 498 | 222 | 1.47 (1.25‐1.73) | |
| Sex | ||||
| Men | 705 | 138 | 1.42 (1.20‐1.69) | 0.43 |
| Women | 930 | 239 | 1.51 (1.26‐1.81) | |
| Education | ||||
| ≤9 y | 840 | 247 | 1.47 (1.26‐1.73) | 0.87 |
| >9 y | 750 | 125 | 1.37 (1.11‐1.69) | |
| Hypertension | ||||
| No | 672 | 127 | 1.44 (1.13‐1.82) | 0.27 |
| Yes | 963 | 250 | 1.50 (1.29‐1.74) | |
| Use of heart disease agents | ||||
| No | 1454 | 316 | 1.41 (1.22‐1.63) | 0.53 |
| Yes | 181 | 61 | 1.70 (1.29‐2.24) | |
| Diabetes mellitus | ||||
| No | 1276 | 296 | 1.49 (1.30‐1.72) | 0.43 |
| Yes | 359 | 81 | 1.48 (1.11‐1.99) | |
| Hypercholesterolemia | ||||
| No | 969 | 228 | 1.45 (1.24‐1.69) | 0.71 |
| Yes | 666 | 149 | 1.44 (1.15‐1.79) | |
| Obesity | ||||
| No | 1197 | 296 | 1.42 (1.23‐1.63) | 0.90 |
| Yes | 438 | 81 | 1.67 (1.25‐2.24) | |
| Estimated glomerular filtration rate | ||||
| ≥60 mL/min per 1.73 m2 | 1405 | 288 | 1.36 (1.17‐1.59) | 0.41 |
| <60 mL/min per 1.73 m2 | 230 | 89 | 1.51 (1.21‐1.87) | |
| Atrial fibrillation | ||||
| No | 1601 | 370 | 1.55 (1.38‐1.74)a | 0.11 |
| Yes | 34 | 7 | 0.83 (0.28‐2.46)a | |
| History of stroke | ||||
| No | 1545 | 344 | 1.50 (1.31‐1.71) | 0.47 |
| Yes | 90 | 33 | 1.09 (0.67‐1.79) | |
| Smoking habits | ||||
| No | 1390 | 324 | 1.49 (1.30‐1.71) | 0.29 |
| Yes | 245 | 53 | 1.30 (0.88‐1.94) | |
| Alcohol intake | ||||
| No | 1051 | 268 | 1.53 (1.31‐1.78) | 0.61 |
| Yes | 584 | 109 | 1.34 (1.08‐1.67) | |
| Regular exercise | ||||
| No | 1429 | 334 | 1.46 (1.28‐1.66) | 0.78 |
| Yes | 206 | 43 | 1.94 (1.18‐3.21) | |
| Serum HS‐CRP | ||||
| <1.0 mg/L | 1127 | 256 | 1.61 (1.32‐1.97) | 0.19 |
| ≥1.0 mg/L | 508 | 121 | 1.35 (1.15‐1.59) | |
Hazard ratios and their 95% CIs represent the risk of all‐cause dementia per 1‐SD increment in log‐transformed NT‐proBNP levels, where the SD of log‐transformed NT‐proBNP levels (pg/mL) was 1.058. Except in the atrial fibrillation subgroups, the model was adjusted for age, sex, education level, hypertension, use of heart disease agents, diabetes mellitus, hypercholesterolemia, obesity, estimated glomerular filtration rate, atrial fibrillation, history of stroke, smoking habit, alcohol intake, regular exercise, and HS‐CRP. The variables relevant to the subgroup were excluded from the corresponding model. HS‐CRP indicates high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide.
Adjusted only for age and sex due to the small number of events in the subgroup with atrial fibrillation.
To evaluate the influence of serum NT‐proBNP on the accuracy of risk assessment for the development of dementia, we evaluated the Harrell's c‐statistics, categorized NRI, continuous NRI, and IDI between models with and without serum NT‐proBNP values (Table 4 and Table S4). The Harrell's c‐statistics increased significantly when serum NT‐proBNP values were added to the relevant model (from 0.780 [95% CI 0.758‐0.803] to 0.787 [95% CI 0.765‐0.809], P=0.02). Moreover, adding serum NT‐proBNP values to the model also significantly improved the predictive ability of categorized NRI, continuous NRI, and IDI; categorized NRI was estimated as 0.062 (ZNRI=3.21, P=0.001), continuous NRI was estimated as 0.189 (ZNRI=3.22, P=0.001), and the IDI was estimated as 0.011 (ZIDI=3.03, P=0.003). When serum NT‐proBNP values were added to the models by using the Framingham or CAIDE (Cardiovascular Risk Factors, Aging, and Incidence of Dementia) risk score,41, 42 the Harrell's c‐statistics, continuous NRI, and IDI of each model were also improved significantly (Table S5).
Table 4.
Predictive Ability, Reclassification, and Discrimination of Dementia by Serum NT‐proBNP, 2002‐2012
| Harrell's c‐Statistics | P Value for Harrell's c‐Statistics Difference | Continuous NRI (95% CI) | P Value for NRI | IDI (95% CI) | P Value for IDI | |
|---|---|---|---|---|---|---|
| Basic modela | 0.780 | 0.02 | 0.189 (0.075‐0.304) | 0.001 | 0.011 (0.004‐0.019) | 0.003 |
| Basic modela+log (serum NT‐proBNP levels) | 0.787 |
IDI indicates integrated discrimination improvement; NRI, net reclassification improvement; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide.
The basic model included age, sex, education level, systolic blood pressure, use of antihypertensive agents, use of heart disease agents, diabetes mellitus, hypercholesterolemia, body mass index, estimated glomerular filtration rate, atrial fibrillation, history of stroke, smoking habit, alcohol intake, regular exercise, and serum high‐sensitivity C‐reactive protein.
Discussion
In this prospective study of a general Japanese elderly population, we clearly demonstrated that the risks for the development of all‐cause dementia, AD, and VaD increased significantly with elevated serum NT‐proBNP levels. These associations remained unchanged even after adjustment for confounding factors. Moreover, the incorporation of serum NT‐proBNP values into a model with potential risk factors significantly improved the predictive ability for incident dementia. These findings suggest that serum NT‐proBNP is a novel biomarker for the future development of dementia.
Several population‐based prospective studies have shown that chronic heart failure and left ventricular dysfunction are associated with an increased risk of developing dementia.9, 10, 11, 43, 44 In addition, 2 prospective studies have assessed the association between serum NT‐proBNP levels and the risk of developing dementia. The National FINRISK Study21 and the Rotterdam Study22 found that elevation of serum NT‐proBNP levels was significantly associated with the incidence of all‐cause dementia, and our findings were in agreement with these studies. With regard to subtypes of dementia, the Rotterdam Study revealed significant associations of serum NT‐proBNP levels with the risk of all‐cause dementia, but not AD and VaD.22 However, the association tended to be positive for both subtypes, which may have been due to the lack of sufficient statistical power in the analysis, as the authors have discussed.22 In contrast, the present study showed a significant association between higher serum NT‐proBNP levels and the development of AD and VaD. Considering the limited evidence on the association between serum NT‐proBNP levels and dementia subtypes, this association should be confirmed by further prospective investigations and/or meta‐analyses of the relevant studies.
One possible explanation for our observation of a significant association between serum NT‐proBNP and the risk of dementia is that an elevation of serum NT‐proBNP levels may reflect the accumulation of vascular risk factors, which in turn could indicate brain and heart injury.45 Vascular risk factors are known to increase the risk of both heart diseases46 and dementia.47 The Rotterdam Study19 and the AGES (Age, Gene/Environment Susceptibility) –Reykjavik Study20 have reported that higher serum NT‐proBNP levels were closely associated with white matter microstructural damage and brain atrophy in community‐dwelling subjects without cerebrocardiovascular disease and dementia. Certainly, the present study found that higher serum NT‐proBNP was linked with a higher risk of dementia in subjects with prior onset of cardiovascular disease or diabetes mellitus. However, it has also been shown that subjects with increased serum NT‐proBNP levels have a greater risk of dementia, even without prior onset of cardiovascular disease and diabetes mellitus, which may suggest that other explanations exist. Long‐term decline in cardiac function may lead to hypoperfusion of the brain and thereby development of dementia via microvascular disease and neurodegeneration. Although cerebral blood flow is usually preserved by cerebral autoregulation, clinical and experimental studies have reported that cerebral blood flow is decreased in subjects with chronic heart failure.48, 49 Chronic cerebral hypoperfusion induces oxidative stress, impairs the clearance of amyloid‐β, and thereby accelerates neuronal dysfunction and apoptosis via deposition of amyloid‐β or neurovascular damage.50 Moreover, the biomarkers of systemic inflammation have been reported to be linked to higher serum NT‐proBNP levels,51 as was also observed in the present study. Because there is growing evidence that inflammatory response could play an important role in the early stage of the pathological cascade of dementia,52 we investigated the influence of systemic inflammation on the association between serum NT‐proBNP levels and dementia risk, but we found that adjusting the model for the serum HS‐CRP levels did not attenuate the significant associations. Nevertheless, these results can not completely exclude the potential role of types of neuroinflammation, such as microglial inflammation,53 on the association between serum NT‐proBNP level and dementia risk because the serum HS‐CRP level is not a good indicator of inflammatory responses in the brain. Further investigations are needed to clarify the pathogenesis of elevated serum NT‐proBNP levels in the development of dementia and its subtypes.
In this study we showed significant improvement of the predictive abilities of c‐statistics, NRI, and IDI by adding serum NT‐proBNP values to the relevant models. The National FINRISK Study also investigated this issue and found significant improvements in the predictive abilities of continuous NRI and IDI, but not c‐statistics, between the models with and without serum NT‐proBNP values.21 Our findings support these previous studies, although the results for c‐statistics were somewhat different. However, the NRI and the IDI have been acknowledged to provide better estimations of the change in predictive ability across models than c‐statistics, which are relatively insensitive to such changes.54, 55 Therefore, these findings highlight that serum NT‐proBNP is a useful biomarker for estimating the risk of the future development of dementia in clinical practice.
The strengths of our study include its population‐based prospective cohort study design, its low selection bias of population selection, perfect follow‐up, and accuracy in the diagnosis of dementia and its subtypes. Several limitations should also be noted. First, serum NT‐proBNP levels were based on a single measurement at baseline. During the follow‐up, the serum NT‐proBNP levels and other risk factors may have changed, and, thus, misclassification was possible. This could have weakened the association found in this study, biasing the results toward the null hypothesis. Second, prodromal dementia cases might have been included at baseline. However, a sensitivity analysis excluding subjects who developed dementia during the first 3 years of the follow‐up period did not alter the findings (Table S6). Third, we could not access the influence of severe cardiac dysfunction on the risk of dementia due to the limited number of subjects with very high levels of serum NT‐proBNP in our general populations. Fourth, we did not have any data on morphological and functional cardiac information at baseline. Fifth, the NT‐proBNP level might be increased by cancer, chronic renal failure, or hormone replacement therapy. However, sensitivity analyses excluding subjects with cancer and chronic heart failure or hormone replacement therapy did not alter these findings (Tables S7 and S8). Finally, we could not exclude the influence of residual confounding factors (eg, sleep apnea) on the association of serum NT‐proBNP levels with the risk of dementia.
Conclusions
The present study clearly demonstrated that elevated serum NT‐proBNP levels were significantly associated with the development of all‐cause dementia, AD, and VaD. This study also indicated the potential applicability of serum NT‐proBNP as a novel biomarker for predicting future risks of dementia. These results should be confirmed by other large‐scale, population‐based observational studies in different populations and settings. Finally, it is not clear whether serum NT‐proBNP levels would be a suitable target for therapeutic intervention. Further elucidation of the exact mechanisms underlying the association between serum NT‐proBNP levels and the risk of developing dementia will also be needed to reduce the future burden of dementia.
Sources of Funding
This study was supported in part by Grants‐in‐Aid for Scientific Research (A) (JP16H02644 and JP16H02692) and (B) (JP16H05850, JP16H05557, JP17H04126, and JP18H02737) and (C) (JP16K09244, JP17K09114, JP17K09113, JP17K01853, JP18K07565, and JP18K09412) and (Early‐Career Scientists) (JP18K17925 and JP18K17382) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (H29‐Junkankitou‐Ippan‐003 and H30‐Shokuhin‐[Sitei]‐005); and by the Japan Agency for Medical Research and Development (JP18dk0207025, JP18ek0210082, JP18gm0610007, JP18ek0210083, JP18km0405202, JP18ek0210080, JP18fk0108075).
Disclosures
None.
Supporting information
Table S1. Risk of Dementia and Its Subtypes According to Quartiles of Serum NT‐proBNP, 2002‐2012
Table S2. Risk of Dementia With and Without Incident Cardiovascular Disease During Follow‐Up Period According to Serum NT‐proBNP Levels Among Subjects Without History of Cardiovascular Disease and/or Dementia at Baseline, 2002‐2012
Table S3. Risk of Dementia With and Without Incident Diabetes Mellitus During Follow‐Up Period According to Serum NT‐proBNP Levels Among Subjects Without History of Diabetes Mellitus and/or Dementia at Baseline, 2002‐2012
Table S4. Reclassification of the 10‐Year Predicted Absolute Risk of Development of Dementia, 2002‐2012
Table S5. Predictive Ability, Reclassification, and Discrimination of Dementia by Serum NT‐proBNP, 2002‐2012
Table S6. Risk of Dementia and Its Subtypes According to Serum NT‐proBNP Levels After Exclusion of Subjects Whose Events Occurred Within 3 Years After Baseline, 2002‐2012
Table S7. Risk of Dementia and Its Subtypes According to Serum NT‐proBNP Levels After Exclusion of Subjects With Cancer or Chronic Renal Failure, 2002‐2012
Table S8. Risk of Dementia and Its Subtypes According to Serum NT‐proBNP LevelsAfter the Exclusion of Subjects With Hormone Replacement Therapy, 2002‐2012
Acknowledgments
The authors thank the residents of the town of Hisayama for their participation in the survey and the staff of the Division of Health and Welfare of Hisayama for their cooperation with this study. We are also grateful to Professor Yoshinao Oda, Professor Toru Iwaki, and their colleagues in the Department of Anatomic Pathology and Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, who provided expertise and insight into the autopsy findings that greatly assisted our research. The statistical analyses were carried out using the computer resource offered under the category of General Projects by Research Institute for Information Technology, Kyushu University.
(J Am Heart Assoc. 2019;8:e011652 DOI: 10.1161/JAHA.118.011652.)
References
- 1. World Alzheimer Report 2015. The Global Impact of Dementia. Available at: https://www.alz.co.uk/research/world-report-2015. Accessed June 30, 2018.
- 2. Iadecola C. Hypertension and dementia. Hypertension. 2014;64:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta‐analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, Egan K. Body mass index in midlife and dementia: systematic review and meta‐regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement. 2017;8:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anstey KJ, von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta‐analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. [DOI] [PubMed] [Google Scholar]
- 6. Lourida I, Soni M, Thompson‐Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479–489. [DOI] [PubMed] [Google Scholar]
- 7. Blondell SJ, Hammersley‐Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta‐analysis of longitudinal studies. BMC Public Health. 2014;14:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. [DOI] [PubMed] [Google Scholar]
- 9. Adelborg K, Horvath‐Puho E, Ording A, Pedersen L, Toft Sorensen H, Henderson VW. Heart failure and risk of dementia: a Danish nationwide population‐based cohort study. Eur J Heart Fail. 2017;19:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population‐based cohort study. Arch Intern Med. 2006;166:1003–1008. [DOI] [PubMed] [Google Scholar]
- 11. Rusanen M, Kivipelto M, Levalahti E, Laatikainen T, Tuomilehto J, Soininen H, Ngandu T. Heart diseases and long‐term risk of dementia and Alzheimer's disease: a population‐based CAIDE Study. J Alzheimers Dis. 2014;42:183–191. [DOI] [PubMed] [Google Scholar]
- 12. Petrucci RJ, Rogers JG, Blue L, Gallagher C, Russell SD, Dordunoo D, Jaski BE, Chillcott S, Sun B, Yanssens TL, Tatooles A, Koundakjian L, Farrar DJ, Slaughter MS. Neurocognitive function in destination therapy patients receiving continuous‐flow vs pulsatile‐flow left ventricular assist device support. J Heart Lung Transplant. 2012;31:27–36. [DOI] [PubMed] [Google Scholar]
- 13. Duncker D, Friedel K, König T, Schreyer H, Lüsebrink U, Duncker M, Oswald H, Klein G, Gardiwal A. Cardiac resynchronization therapy improves psycho‐cognitive performance in patients with heart failure. Europace. 2015;17:1415–1421. [DOI] [PubMed] [Google Scholar]
- 14. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. [DOI] [PubMed] [Google Scholar]
- 15. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135:e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart F ailure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 17. Natriuretic Peptides Studies Collaboration , Willeit P, Kaptoge S, Welsh P, Butterworth AS, Chowdhury R, Spackman SA, Pennells L, Gao P, Burgess S, Freitag DF, Sweeting M, Wood AM, Cook NR, Judd S, Trompet S, Nambi V, Olsen MH, Everett BM, Kee F, Ärnlöv J, Salomaa V, Levy D, Kauhanen J, Laukkanen JA, Kavousi M, Ninomiya T, Casas JP, Daniels LB, Lind L, Kistorp CN, Rosenberg J, Mueller T, Rubattu S, Panagiotakos DB, Franco OH, de Lemos JA, Luchner A, Kizer JR, Kiechl S, Salonen JT, Goya Wannamethee S, de Boer RA, Nordestgaard BG, Andersson J, Jørgensen T, Melander O, Ballantyne ChM, DeFilippi Ch, Ridker PM, Cushman M, Rosamond WD, Thompson SG, Gudnason V, Sattar N, Danesh J, Di Angelantonio E. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual‐participant‐data meta‐analysis. Lancet Diabetes Endocrinol. 2016;4:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luers C, Wachter R, Kleta S, Uhlir M, Koschack J, Scherer M, Binder L, Herrmann‐Lingen C, Zapf A, Kulle B, Kochen MM, Pieske B. Natriuretic peptides in the detection of preclinical diastolic or systolic dysfunction. Clin Res Cardiol. 2010;99:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zonneveld HI, Ikram MA, Hofman A, Niessen WJ, van der Lugt A, Krestin GP, Franco OH, Vernooij MW. N‐terminal pro‐B‐type natriuretic peptide and subclinical brain damage in the general population. Radiology. 2017;283:205–214. [DOI] [PubMed] [Google Scholar]
- 20. Sabayan B, van Buchem MA, de Craen AJ, Sigurdsson S, Zhang Q, Harris TB, Gudnason V, Arai AE, Launer LJ. N‐terminal pro‐brain natriuretic peptide and abnormal brain aging: the AGES‐Reykjavik Study. Neurology. 2015;85:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tynkkynen J, Laatikainen T, Salomaa V, Havulinna AS, Blankenberg S, Zeller T, Hernesniemi JA. NT‐proBNP and the risk of dementia: a prospective cohort study with 14 years of follow‐up. J Alzheimers Dis. 2015;44:1007–1013. [DOI] [PubMed] [Google Scholar]
- 22. Mirza SS, de Bruijn RF, Koudstaal PJ, van den Meiracker AH, Franco OH, Hofman A, Tiemeier H, Ikram MA. The N‐terminal pro B‐type natriuretic peptide, and risk of dementia and cognitive decline: a 10‐year follow‐up study in the general population. J Neurol Neurosurg Psychiatry. 2016;87:356–362. [DOI] [PubMed] [Google Scholar]
- 23. Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T, Kiyohara Y. Secular trends in cardiovascular disease and its risk factors in Japanese: half‐century data from the Hisayama Study (1961–2009). Circulation. 2013;128:1198–1205. [DOI] [PubMed] [Google Scholar]
- 24. Ohara T, Hata J, Yoshida D, Mukai N, Nagata M, Iwaki T, Kitazono T, Kanba S, Kiyohara Y, Ninomiya T. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology. 2017;88:1925–1932. [DOI] [PubMed] [Google Scholar]
- 25. Umehara K, Mukai N, Hata J, Hirakawa Y, Ohara T, Yoshida D, Kishimoto H, Kitazono T, Hoka S, Kiyohara Y, Ninomiya T. Association between serum vitamin D and all‐cause and cause‐specific death in a general Japanese population—the Hisayama Study. Circ J. 2017;81:1315–1321. [DOI] [PubMed] [Google Scholar]
- 26. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 27. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 28. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, Moody DM, O'Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 29. Fujimi K, Sasaki K, Noda K, Wakisaka Y, Tanizaki Y, Matsui Y, Sekita A, Iida M, Kiyohara Y, Kanba S, Iwaki T. Clinicopathological outline of dementia with Lewy bodies applying the revised criteria: the Hisayama Study. Brain Pathol. 2008;18:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yeo KT, Wu AH, Apple FS, Kroll MH, Christenson RH, Lewandrowski KB, Sedor FA, Butch AW. Multicenter evaluation of the Roche NT‐proBNP assay and comparison to the Biosite Triage BNP assay. Clin Chim Acta. 2003;338:107–115. [DOI] [PubMed] [Google Scholar]
- 31. Doi Y, Ninomiya T, Hata J, Hirakawa Y, Mukai N, Ikeda F, Fukuhara M, Iwase M, Kiyohara Y. N‐terminal pro‐brain natriuretic peptide and risk of cardiovascular events in a Japanese community: the Hisayama Study. Arterioscler Thromb Vasc Biol. 2011;31:2997–3003. [DOI] [PubMed] [Google Scholar]
- 32. Examination Committee of Criteria for “Obesity Disease” in Japan . New criteria for “obesity disease” in Japan. Circ J. 2002;66:987–992. [DOI] [PubMed] [Google Scholar]
- 33. Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 34. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD‐EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. [DOI] [PubMed] [Google Scholar]
- 35. Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High‐sensitivity C‐reactive protein and coronary heart disease in a general population of Japanese: the Hisayama Study. Arterioscler Thromb Vasc Biol. 2008;28:1385–1391. [DOI] [PubMed] [Google Scholar]
- 36. SAS/STAT 14.3 User's Guide CTPP. Cary, NC: SAS Institute Inc; 2017:7139–7145. Available at: https://support.sas.com/documentation/onlinedoc/stat/143/phreg.pdf. Accessed June 30, 2018. [Google Scholar]
- 37. Pencina MJ, D'Agostino RB Sr, Song L. Quantifying discrimination of Framingham risk functions with different survival c statistics. Stat Med. 2012;31:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Newson RB. Comparing the predictive power of survival models using Harrell's C or Somers’ D. Stata J. 2010;10:339–358. [Google Scholar]
- 39. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 40. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li J, Ogrodnik M, Devine S, Auerbach S, Wolf PA, Au R. Practical risk score for 5‐, 10‐, and 20‐year prediction of dementia in elderly persons: Framingham Heart Study. Alzheimers Dement. 2018;14:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population‐based study. Lancet Neurol. 2006;5:735–741. [DOI] [PubMed] [Google Scholar]
- 43. Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O'Donnell CJ, Manning WJ, Wolf PA, Au R, Benjamin EJ. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation. 2015;131:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Bruijn RF, Portegies ML, Leening MJ, Bos MJ, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW, Franco OH, Koudstaal PJ, Ikram MA. Subclinical cardiac dysfunction increases the risk of stroke and dementia: the Rotterdam Study. Neurology. 2015;84:833–840. [DOI] [PubMed] [Google Scholar]
- 45. van der Velpen IF, Feleus S, Bertens AS, Sabayan B. Hemodynamic and serum cardiac markers and risk of cognitive impairment and dementia. Alzheimers Dement. 2017;13:441–453. [DOI] [PubMed] [Google Scholar]
- 46. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta‐analysis. Open Heart. 2015;2:e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimers Dement. 2015;11:718–726. [DOI] [PubMed] [Google Scholar]
- 48. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roy B, Woo MA, Wang DJJ, Fonarow GC, Harper RM, Kumar R. Reduced regional cerebral blood flow in patients with heart failure. Eur J Heart Fail. 2017;19:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cermakova P, Eriksdotter M, Lund LH, Winblad B, Religa P, Religa D. Heart failure and Alzheimer's disease. J Intern Med. 2015;277:406–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jensen J, Ma LP, Fu ML, Svaninger D, Lundberg PA, Hammarsten O. Inflammation increases NT‐proBNP and the NT‐proBNP/BNP ratio. Clin Res Cardiol. 2010;99:445–452. [DOI] [PubMed] [Google Scholar]
- 52. Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs. 2012;44:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12. [DOI] [PubMed] [Google Scholar]
- 54. Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. [DOI] [PubMed] [Google Scholar]
- 55. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Risk of Dementia and Its Subtypes According to Quartiles of Serum NT‐proBNP, 2002‐2012
Table S2. Risk of Dementia With and Without Incident Cardiovascular Disease During Follow‐Up Period According to Serum NT‐proBNP Levels Among Subjects Without History of Cardiovascular Disease and/or Dementia at Baseline, 2002‐2012
Table S3. Risk of Dementia With and Without Incident Diabetes Mellitus During Follow‐Up Period According to Serum NT‐proBNP Levels Among Subjects Without History of Diabetes Mellitus and/or Dementia at Baseline, 2002‐2012
Table S4. Reclassification of the 10‐Year Predicted Absolute Risk of Development of Dementia, 2002‐2012
Table S5. Predictive Ability, Reclassification, and Discrimination of Dementia by Serum NT‐proBNP, 2002‐2012
Table S6. Risk of Dementia and Its Subtypes According to Serum NT‐proBNP Levels After Exclusion of Subjects Whose Events Occurred Within 3 Years After Baseline, 2002‐2012
Table S7. Risk of Dementia and Its Subtypes According to Serum NT‐proBNP Levels After Exclusion of Subjects With Cancer or Chronic Renal Failure, 2002‐2012
Table S8. Risk of Dementia and Its Subtypes According to Serum NT‐proBNP LevelsAfter the Exclusion of Subjects With Hormone Replacement Therapy, 2002‐2012


