Abstract
Objective
Recent studies showed a potential of magnetic resonance imaging (MRI), which can be used as an additional tool for diagnosing cartilage degeneration in the early stage. We designed a cross-sectional study in order to evaluate knee joint cartilage adaptation to running, using 3.0-T MRI equipped with the 3-dimensional turbo spin echo (VISTA = Volume ISotropic Turbo spin echo Acquisition) software. By this thickness (mm) and signal intensity (mean pixel value) can be quantified, which could be closely related to the fluid content of the knee joint cartilage, before and after running.
Methods
A total of 22 males, aged 18 to 35 years, dominant (right) and nondominant (left) knees were assessed before and after 30 minutes of running. Cartilage thickness and signal intensity of surfaces of the patella, medial and lateral femoral and tibial condyles were measured.
Results
Cartilage thickness of the lateral condyle decreased at the dominant knee, while it increased at the medial tibial plateau. Signal intensity decreased at all locations, except the lateral patella in both knees. The most obvious decrease in signal intensity (10.6%) was at the medial tibial plateau from 949.8 to 849.0 of the dominant knee.
Conclusion
There was an increase in thickness measurements and decrease in signal intensity in medial tibial plateau of the dominant knee after 30 minutes of running. This outcome could be related to fluid outflow from the tissue. Greater reductions in the medial tibial plateau cartilage indicate greater load sharing by these areas of the joint during a 30-minute running.
Keywords: running, magnetic resonance imaging, diagnostics, joint cartilage, signal intensity, thickness
Introduction
Knee joint cartilage adaptation to running has been studied extensively.1-3 Some studies4,5 reveal that running is harmful to knee joint cartilage while others indicated no adverse effects.1,3,6 A study even presented the chondroprotective outcome of running that was recently supported by a meta-analysis.7,8 Thirty minutes running at 80% of the maximal heart rate is a common fitness training that decreases intra-articular pro-inflammatory cytokine concentration9; however, little is known on its effects over knee joint cartilage. These controversial findings necessitate further research on running with advanced methods.
Experimental studies indicated cartilage erosion,10 degeneration,11 as well as decrease in cartilage thickness, chondrocyte number, and glycosaminoglycan content12 after strenous running. A recent review reported that mechanical overloading decreases proteoglycan content of joint cartilage and causes collagen network damage.13 Serum cartilage oligomatrix protein (COMP) accumulation increased immediately after running14 while lubricin and femoral cartilage thickness measured by ultrasound followed the same pattern.15
Magnetic resonance imaging (MRI), which is a precise16 and reproducible17,18 method shows that running exercise may alter thickness and volume of the knee joint cartilage.19,20 Two studies, on the other hand, reveal that a transient decrease in cartilage volume after 20 km or 1 hour running, respectively.21,22 High-field MR can recently measured changes in T1ρ and T2 responses at the medial compartment of the knee joint cartilage.23 Other studies confirmed decrease24,25 or increase26 in T2 response in the superficial femoral and tibial cartilage. Three-tesla MR studies25,27 mostly focused on limited numbers of nonprofessional marathon runners.5,20 Four 3-T MR studies assessed knee joint cartilage of recreational athletes before and after 15 to 30 minutes of running.26,28 Diverse findings on increase and/or decrease of cartilage thickness, volume and signal intensity T2 response at the medial and lateral femoral and tibial condyles and the joint surface of the patella necessitated indepth evaluation of these knee joint cartilage surfaces with advanced technology.
We asked whether 3-T MR equipped with the 3-dimensional turbo spin echo (VISTA = Volume ISotropic Turbo spin echo Acquisition) software could quantify thickness (mm) and signal intensity (SI; mean pixel value), which could be closely related to the fluid content of the knee joint cartilage, before and after 30 minutes running at 80% of the maximal heart rate. Therefore, this study protocol aimed to evaluate the effects of running in recreational subjects on the patellofemoral and femorotibial plateau cartilage in terms of water content, thickness, and signal intensity via a high-quality (3.0 T) imaging system.
Materials and Methods
Design
We designed a study where right dominant knee joint cartilage was evaluated before and after a 30-minutes heart rate–controlled running on a treadmill using MR, and the left nondominant knee was evaluated 30 minutes after the end of exercise. Independent and dependent variables were cartilage thicknesses (mm)—measured at 3 locations at the medial and lateral femoral and tibial condyles (K1-K6) and patella (K1-K5)—and signal intensities (mean pixel value)—measured at 3 locations at the medial and lateral femoral and tibial condyles (A1-A8) and patella (A1-A6)—respectively.
Participants
Twenty-two physically active (at least 150 minutes aerobic exercise regularly in a week) males participated. The long-form of the International Physical Activity Questionnaire (IPAQ) was applied to assess physical activity levels of participants.29 Additional question, according to the literature “If you would shoot a ball on a target, which leg would you use to shoot the ball?” were asked to determine leg dominance of the participants.30 Exclusion criteria included a history of knee pain or stiffness during the previous 6 months, prior knee trauma, joint disorders, or a history of orthopedic surgery that affects the meniscus and cartilage of the knee joint. Participants height and weight were measured using a digital scale (Seca 769, Hamburg, Germany) and body mass index (BMI) was calculated by dividing body weight in kilograms by square of the height in meters (kg/m2).
Running Protocol
MR data of the right dominant knees was obtained a day before of testing and recorded as baseline evaluation. Participants arrived at the hospital at 7:00 am after 24 hours of rest to prevent interference of daily activities on thickness and SI. They rested in supine position for 30 minutes before MR. MR data were acquired in less than 3 minutes after the run the other day. The left nondominant knees were scanned 30 minutes after the right knees. Participants run 30 minutes at 80% of their maximal heart rate on a treadmill after 10 minutes of warm-up. Maximum heart rate was measured with a heart rate monitor (Forerunner 305, Garmin, KS, USA), and speed and heart rate was monitored throughout the run.
Magnetic Resonance Data Acquisition
MR images of the knee joints were obtained with a 3.0-T MR (Ingenia, Philips Electronics, the Netherlands) with constant magnetic field strength. Eight channels knee wrap was used and 4 series of images (sagittal fat-suppressed turbo spin echo T2-weighted, sagittal fat-suppressed proton density–weighted, axial and coronal 3D turbo spin echo (VISTA = Volume Isotropic Turbo spin echo Acquisition) were acquired. Sagittal images were obtained to diagnose a possible additional pathology. The slice thickness is selected 3 mm in all series. Total acquisition time was 22 minutes. The position of the knee coils was determined in such way that the images could be obtained from the same points of the participants’ knee and remained the same before and after running. All participants were asked not to move their legs and their knee joints were also stabilized to the coil by bandages while scanning.
Image Analysis and Quantification
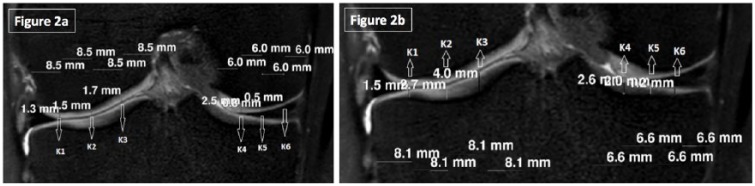
All images were transferred to a workstation (Philips Workstation, Version 12.0, the Netherlands) for manual segmentation. A musculoskeletal radiology expert (BD) identified images and graded cartilage lesions, meniscal, ligamentous and other knee abnormalities. A total of 9152 regions of interrest (ROIs); 40 femur, 40 tibia, 40 patella per knee) and 7040 thickness measurements (30 femur, 30 tibia, 20 patella per knee) were obtained from both knees of 22 participants before and after the running MR sessions. The femur and tibia load-bearing regions were selected from the point where the fibula was first seen on the anterior-posterior segment and every fifth successive coronal image was evaluated ( Fig. 1 ) Medial and lateral femoral and tibial condyles were divided into 3 equal parts (K1-K6) and thicknesses were measured in triplicate ( Fig. 2a and b ). Subsequently, area under meniscus was measured separately from the rest. With these measurements we obtain more precise results in the area of patellofemoral and femorotibial cartilage and determine if there were regional differences in response after running.
Figure 1.
Sagittal plane: fat-suppressed T2 weighted image areas for thickness and signal intensity measurements on the femoral and tibial condyles.
Figure 2.
(a) Coronal plane 3-dimensional isotropic T2-weighted fast spin echo (VISTA) was used to evaluate the thickness measurements of femoral condyles. (b) Coronal plane 3-dimensional isotropic T2-weighted fast spin echo (VISTA) was used to evaluate the thickness measurements of tibial condyles.
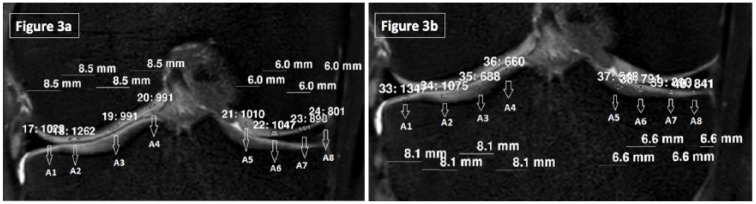
ROI not less than 1 mm2 without reaching the superficial and deep layers was manually selected from the middle half of the joint cartilage for SI evaluation. The largest ROI was found as 3 mm2 (8 pixels), the smallest was 1 mm2 (2 pixels), and the average size was 2 ± 1 mm2 (5 ± 3 pixels). Average pixel values were recorded for each ROI and the changes in signal intensity were evaluated over these values. Medial and lateral femoral and tibial condyles were again divided into four equal parts (A1-A8) and SI was assessed again in triplicate ( Fig. 3a and b ).
Figure 3.
(a) Signal intensity measurements of the femoral condyles. Coronal plane 3-dimensional isotropic T2-weighted fast spin echo (VISTA) was used for evaluation. (b) Signal intensity measurements of the tibial condyles. Coronal plane 3-dimensional isotropic T2-weighted fast spin echo (VISTA) was used for evaluation.
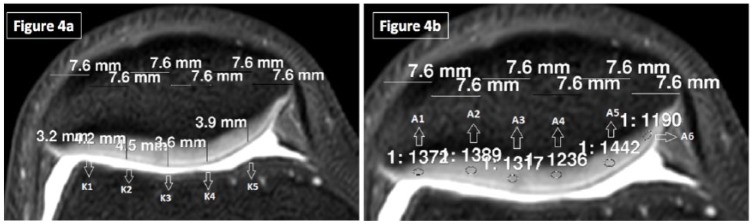
A1-A2 and A7-A8 regions were assessed separately than A3-A4 and A5-A6 regions in order to compare the protective effect of the meniscus on joint cartilage after running. Thickness and SI of the patellar cartilage were assessed in axial images at 5 (K1-K5) and 6 (A1-A6) locations, respectively ( Fig. 4a and b ). Before and after running thickness and SI values of the right and the left knees of each individual was compared separately.
Figure 4.
(a) Axial plane 3-dimensional isotropic T2-weighted fast spin echo (VISTA) was used to evaluate the thickness measurements of the patellar cartilage. (b) Signal intensity measurements of the patella at axial plane.
Statistical Analysis
Mean, standard deviation, minimum value, and maximum value are presented as descriptive statistics. Wilcoxon test was used to compare thickness and SI data of before and after running. P values less than 0.05 were considered as statistically significant.
Results
The 3T MR equipped with the 3D turbo spin echo (VISTA) software could quantify thickness (mm) and SI of the knee joint cartilage before and after 30 minutes of running. Thickness did not change before and after running in the right dominant and left nondominant knees ( Table 1 ). Signal intensity decreased in all but not the lateral cartilage of the patella (LP) in the right knees, and even increased in all joint cartilage compartments of the patella of both knees 30 minutes after running ( Table 2 ). The decrease in SI in both the femoral and tibial condyles was less in the areas under the menisci (A1-A2 and A7-A8). Signal intensity of the subchondral bone did not change after running.
Table 1.
Thickness Measurements of Lateral Femoral Condyle (LFC), Medial Femoral Condyle (MFC), Lateral Tibial Plateau (LTP), Medial Tibial Plateau (MTP), and Patella.
| Thickness (mm) | Right Knee Before Running | Right Knee After Running | Change % | P | Left Knee Before Running | Left Knee After Running | Change % | P |
|---|---|---|---|---|---|---|---|---|
| LFC (K1-K3) | 1.77 | 1.65 | −6.7 | <0.001* | 1.73 | 1.70 | −1.73 | 0.120 |
| MFC (K4-K6) | 1.46 | 1.46 | — | 0.400 | 1.49 | 1.49 | — | 0.601 |
| LTP (K1-K3) | 1.81 | 1.87 | +3.31 | 0.072 | 1.78 | 1.82 | −0.25 | 0.056 |
| MTP (K4-K6) | 1.40 | 1.49 | +6.42 | <0.001* | 1.51 | 1.47 | −2.64 | 0.105 |
| Patella (K1-K5) | 3.52 | 3.53 | — | 0.890 | 3.59 | 3.59 | — | 0.398 |
Statistically significant (P < 0.05).
Table 2.
Signal Intensity Measurements of Lateral Femoral Condyle (LFC), Medial Femoral Condyle (MFC), Lateral Tibial Plateau (LTP), and Medial Tibial Plateau (MTP).
| Signal Intensity (Mean Pixel Value) | Right Knee Before Running | Right Knee After Running | Change % | P | Left Knee Before Running | Left Knee After Running | Change % | P |
|---|---|---|---|---|---|---|---|---|
| LFC (A1-A4) | 1256.0 | 1176.5 | −6.32 | <0.001* | 1293.9 | 1255.9 | −2.93 | <0.001* |
| MFC (A5-A8) | 993.5 | 903.2 | −9.09 | <0.001* | 998.9 | 955.4 | −4.34 | <0.001* |
| LTP (A1-A4) | 1092.4 | 1009.3 | −7.60 | <0.001* | 1086.8 | 1029.8 | −5.24 | <0.001* |
| MTP (A5-A8) | 949.8 | 849.0 | −10.61 | <0.001* | 956.9 | 892.7 | −6.71 | <0.001* |
| LP (A1-A3) | 1249.0 | 1218.1 | −2.48 | 0.094 | 1290.4 | 1320.2 | +2.32 | 0.002* |
| MP (A4-A6) | 1081.3 | 1048 | −3.03 | 0.039* | 1170.4 | 1204.0 | +2.90 | 0.001* |
Statistically significant (P < 0.05).
Discussion
In recent years, it was shown that 2 quantitative techniques, T2 and T2* relaxation times and 3.0 T MRI, appeared to be valuable parameters in the evaluation of immediate changes in the cartilage ultrastructure after running.24 Sensitivity and specificity levels were also said to be acceptable for detecting articular cartilage defects using 3-T MRI.24 The original aspect of the current study is that the 3D turbo spin echo (VISTA) MRI protocol was used to evaluate joint cartilage. Changes in T2 values and cartilage thickness in the tibiofemoral joint of both dominant and nondominant knees were observed by the VISTA MRI technique in healthy individuals following running. The main finding of our study was that 30 minutes of aerobic running exercise caused T2 values to decrease significantly in the tibial plateau and femoral condyle of both the dominant and nondominant knees. This result is therefore in line with previous studies evaluating T2 values.31,32 Nishii et al.32 found a reduction in T2 values only in areas where the femur was in direct contact with the opposing tibial cartilage, whereas a significant decrease in T2 values of the tibia were observed in every region. It is noteworthy that there were no changes in the femur in regions under the meniscus. Similarly, another study showed reduction in T2 values after axial loading and this decrease was returned to normal immediately after the application of axial loading.31 We noticed that the decrease was more obvious in the dominant knee compared to the nondominant knee. However, there was a different situation with the patellar cartilage. In the dominant knee, T2 values in the medial patellar cartilage decreased, while in the nondominant knee, T2 values for both LP and MP increased. It is possible that this difference between knees was the 30 minutes of time difference between the 2 MRI sessions. During these 30 minutes, the nondominant knee was at rest; thus, T2 values started to return to their original values. In this situation, the increase of T2 values in the nondominant knee and patella might be due to water rushing back into the patella cartilage. Another possible reason may be that there was a difference between kinetics and kinematics of the dominant and nondominant knees during running. However, there are different considerations for this second reason. The most recent studies show no evidence of any significant kinetic or kinematic differences between the dominant and nondominant lower limbs of female runners,33 while the dominant and nondominant lower limbs of male runners show greater maximal ground reaction force (stance phase) and swing phase differences the dominant limb.34 In this case, the reduction in T2 values in the dominant tibiofemoral joint of the knee can be explained by the excess burden on the dominant side, although there is no response to T2 changes in the patella. Cha et al.26 further compared T2 relaxation on 3.0-T MR images of the knee articular cartilage before and after running and compared these data between young and old amateur athletes. These authors did not find any significant changes in global cartilage T2 values after running between the age groups in response to exercise. However, they did report relatively higher T2 values in the older group, mainly in the superficial layers of the femoral and tibial cartilage (P < 0.05) where collagen matrix degeneration was primarily initiated. In our comprehensive review of the literature, we failed to find any study relating to the 2 dominant and nondominant legs.35 The current study is therefore the first to evaluate the severity effects of running on both dominant and nondominant knees.
Various studies have investigated structural knee changes after running, but with conflicting results. In studies measuring thickness and volume, Mosher et al.20 observed thinning of the tibial and femoral cartilage thickness after running, whereas Subburaj et al.27 did not detect any difference. Mosher et al.20 calculated that the cartilage thickness and T2 values of 22 marathon runners, and 15 sedentary controls, from the central femoral and tibial cartilage, using a 3.0-T scanner before and after 30 minutes of running. After running, MR T2 values decreased in the superficial femoral and tibial cartilage, along with a decrease in cartilage thickness (femoral, 4%-8%; tibial, 0%-12%). A smaller decrease in cartilage T2 values was observed in the middle zone of the cartilage, and no change was observed in the deepest layer.20 The study by Kessler et al.36 on 30 healthy men showed a significant reduction in patella, tibia, and meniscal volumes after a 5-km run, and as the running distance increased to 10 and 20 km, only the medial meniscal volume continued to decrease. In their second study, Kessler and colleagues reported that these changes returned to normal within one hour, even after a 20-km run. In our study, we observed a reduction in the thickness of the joint cartilage on the lateral femoral condyle on the dominant leg and an increase in thickness of the joint cartilage on the medial tibial plateau. In the nondominant knee, no statistically significant difference was found. The absence of a change in the thickness of the nondominant knee after 30 minutes is similar to the findings of Kessler and colleagues. Joint cartilage thickness and signal intensity has the potential to recover to their preexercise state rapidly in young healthy males. Fluid circulation during and after exercise can explain this volume and thickness change.
There are, to our knowledge, no previously published studies investigating both the dominant and the nondominant knees following running and how load such as a 30-minute running changes the thickness and signal intensity of both knee joints.
Furthermore, there is no standardization of running exercise intensity (80% of the maximal heart rate) in the literature. Another original aspect of the current study is that the 3-dimensional turbo spin echo (VISTA) MRI protocol was used to evaluate knee joint cartilage. Changes in T2 values and cartilage thickness in the tibiofemoral joint of both the dominant and the nondominant knees were observed by the VISTA MRI technique in healthy individuals following running. Thus, the results suggest that 3-dimensional turbo spin echo (VISTA) MRI protocol might be helpful to identify early cartilage degeneration and a potential progression. Our overall results could help understand how exercise can influence cartilage volume and thickness changes.
A limitation of our study is that no biomechanical evaluation was conducted on participants during running and we did not measure alignment of the lower extremities. If such data were collected, we may have been able to interpret different outcomes in different regions of the joint cartilage in a more substantial manner. The fact that females were not included in this study also created a lack of data for the female gender. Similarly, we cannot arrive at any conclusions for healthy older subjects or osteoarthritic patients. These limitations, however, give a sound background for our future studies. Our MR measurements furthermore were very detailed and thus required a significant period of time for evaluation. Because of this, only the load-bearing sections of the knee joint cartilage were mapped. There was a 30-minute time difference between MR imaging of the 2 knees. This gave us some time lapse information form the contralateral joint. In the future, however, it might be better a good idea to assess recovery of joint cartilage changes by obtaining later MR images. Results of this study should be therefore considered as early joint changes after 30 minutes moderate running on a treadmill.
Conclusion
Joint cartilage thicknesses changed after 30 minutes of moderate intensity running in healthy participants. The right but not the left lateral femoral condyle thickness decreased while it increased at the medial tibial plateau. Signal intensity decreased at all locations but not at the lateral patella in both knees after running. The most obvious decrease in signal intensity (10.6%) was at the medial tibial plateau from 949.8 to 849.0 at the right knees. Decrease in signal intensity was less in the femoral condyles and tibial plateau under the menisci that may enhance the protective effect of meniscus on joint cartilage.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The ethical approval was obtained from the Ethics Committee of Hacettepe University (26.08.2015/16969557-1066).
References
- 1. Miller RH. Joint loading in runners does not initiate knee osteoarthritis. Exerc Sport Sci Rev. 2017;45(2):87-95. doi: 10.1249/JES.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 2. Hoessly ML, Wildi LM. Magnetic resonance imaging findings in the knee before and after long-distance running—documentation of irreversible structural damage? A systematic review. Am J Sports Med. 2017;45(5):1206-17. doi: 10.1177/0363546516656180. [DOI] [PubMed] [Google Scholar]
- 3. Hansen P, English M, Willick SE. Does running cause osteoarthritis in the hip or knee? PM R. 2012;4(5 Suppl):S117-21. doi: 10.1016/j.pmrj.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 4. Harkey MS, Blackburn JT, Davis H, Sierra-Arévalo L, Nissman D, Pietrosimone B. Ultrasonographic assessment of medial femoral cartilage deformation acutely following walking and running. Osteoarthritis Cartilage. 2017;25(6):907-13. doi: 10.1016/j.joca.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 5. Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776-86. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krampla WW, Newrkla SP, Kroener AH, Hruby WF. Changes on magnetic resonance tomography in the knee joints of marathon runners: a 10-year longitudinal study. Skeletal Radiol. 2008;37:619-26. doi: 10.1007/s00256-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 7. Van Ginckel A, Baelde N, Almqvist KF, Roosen P, Mcnair P, Witvrouw E. Functional adaptation of knee cartilage in asymptomatic female novice runners compared to sedentary controls. A longitudinal analysis using delayed gadolinium enhanced magnetic resonance imaging of cartilage (dGEMRIC). Osteoarthritis Cartilage. 2010;18(12):1564-9. doi: 10.1016/j.joca.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 8. Timmins KA, Leech RD, Batt ME, Edwards KL. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45(6):1447-57. doi: 10.1177/0363546516657531. [DOI] [PubMed] [Google Scholar]
- 9. Hyldahl RD, Evans A, Kwon S, Ridge ST, Robinson E, Hopkins JT, et al. Running decreases knee intra-articular cytokine and cartilage oligomeric matrix concentrations: a pilot study. Eur J Appl Physiol. 2016;116(11-12):2305-14. doi: 10.1007/s00421-016-3474-z. [DOI] [PubMed] [Google Scholar]
- 10. Li Z, Liu SY, Xu L, Xu SY, Ni GX. Effects of treadmill running with different intensity on rat subchondral bone. Sci Rep. 2017;7(1):1977. doi: 10.1038/s41598-017-02126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ni GX, Zhou YZ, Chen W, Xu L, Li Z, Liu SY, et al. Different responses of articular cartilage to strenuous running and joint immobilization. Connect Tissue Res. 2016;57(2):143-51. doi: 10.3109/03008207.2015.1117457. [DOI] [PubMed] [Google Scholar]
- 12. Ni GX, Liu SY, Lei L, Li Z, Zhou YZ, Zhan LQ. Intensity-dependent effect of treadmill running on knee articular cartilage in a rat model. Biomed Res Int. 2013;2013:172392. doi: 10.1155/2013/172392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jørgensen AEM, Kjær M, Heinemeier KM. The effect of aging and mechanical loading on the metabolism of articular cartilage. J Rheumatol. 2017;44(4):410-7. doi: 10.3899/jrheum.160226. [DOI] [PubMed] [Google Scholar]
- 14. Celik O, Salci Y, Ak E, Kalaci A, Korkusuz F. Serum cartilage oligomeric matrix protein accumulation decreases significantly after 12 weeks of running but not swimming and cycling training—a randomised controlled trial. Knee. 2013;20(1):19-25. doi: 10.1016/j.knee.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 15. Roberts HM, Moore JP, Griffith-McGeever CL, Fortes MB, Thom JM. The effect of vigorous running and cycling on serum COMP, lubricin, and femoral cartilage thickness: a pilot study. Eur J Appl Physiol. 2016;116(8):1467-77. doi: 10.1007/s00421-016-3404-0. [DOI] [PubMed] [Google Scholar]
- 16. Hunter DJ, Altman RD, Cicuttini F, Crema MD, Duryea J, Eckstein F, et al. OARSI clinical trials recommendations: knee imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):698-715. doi: 10.1016/j.joca.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 17. Hinterwimmer S, Feucht MJ, Steinbrech C, Graichen H, von Eisenhart-Rothe R. The effect of a six-month training program followed by a marathon run on knee joint cartilage volume and thickness in marathon beginners. Knee Surg Sport Traumatol Arthrosc. 2014;22(6):1353-9. doi: 10.1007/s00167-013-2686-6. [DOI] [PubMed] [Google Scholar]
- 18. Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737-44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 19. Hanna F, Teichtahl AJ, Bell R, Davis SR, Wluka AE, O’Sullivan R, et al. The cross-sectional relationship between fortnightly exercise and knee cartilage properties in healthy adult women in midlife. Menopause. 2007;14(5):830-4. doi: 10.1097/gme.0b013e31802f316b. [DOI] [PubMed] [Google Scholar]
- 20. Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18(3):358-64. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Recovery of the menisci and articular cartilage of runners after cessation of exercise. Am J Sports Med. 2008;36(5):966-70. doi: 10.1177/0363546507313093. [DOI] [PubMed] [Google Scholar]
- 22. Kersting UG, Stubendorff JJ, Schmidt MC, Brüggemann GP. Changes in knee cartilage volume and serum COMP concentration after running exercise. Osteoarthritis Cartilage. 2005;13(10):925-34. doi: 10.1016/j.joca.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23. Luke AC, Stehling C, Stahl R, Li X, Kay T, Takamoto S, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running. Am J Sports Med. 2010;38(11):2273-80. doi: 10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- 24. Behzadi C, Welsch GH, Laqmani A, Henes FO, Kaul MG, Schoen G, et al. The immediate effect of long-distance running on T2 and T2* relaxation times of articular cartilage of the knee in young healthy adults at 3.0 T MR imaging. Br J Radiol. 2016;89(1064):20151075. doi: 10.1259/bjr.20151075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hesper T, Miese FR, Hosalkar HS, Behringer M, Zilkens C, Antoch G, et al. Quantitative T2* assessment of knee joint cartilage after running a marathon. Eur J Radiol. 2015;84(2):284-89. doi: 10.1016/j.ejrad.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 26. Cha JG, Lee JC, Kim HJ, Han JK, Lee EH, Kim YD, et al. Comparison of MRI T2 relaxation changes of knee articular cartilage before and after running between young and old amateur athletes. Korean J Radiol. 2012;13(5):594-601. doi: 10.3348/kjr.2012.13.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subburaj K, Kumar D, Souza RB, Alizai H, Li X, Link TM, et al. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40(9):2134-41. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gatti AA, Noseworthy MD, Stratford PW, Brenneman EC, Totterman S, Tamez-Pena J, et al. Acute changes in knee cartilage transverse relaxation time after running and bicycling. J Biomech. 2017;53:171-7. doi: 10.1016/j.jbiomech.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 29. Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-62. [DOI] [PubMed] [Google Scholar]
- 30. van Melick N, Meddeler BM, Hoogeboom TJ, Nijhuis-van der, Sanden MWG, van Cingel REH. How to determine leg dominance: the agreement between self-reported and observed performance in healthy adults. PLoS One. 2017;12(12):e0189876. doi: 10.1371/journal.pone.0189876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schoenbauer E, Szomolanyi P, Shiomi T, Juras V, Zbýň Š, Zak L, et al. Cartilage evaluation with biochemical MR imaging using in vivo knee compression at 3T-comparison of patients after cartilage repair with healthy volunteers. J Biomech. 2015;48(12):3349-55. doi: 10.1016/j.jbiomech.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 32. Nishii T, Kuroda K, Matsuoka Y, Sahara T, Yoshikawa H. Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging. 2008;28(1):175-80. doi: 10.1002/jmri.21418. [DOI] [PubMed] [Google Scholar]
- 33. Brown AM, Zifchock RA, Hillstrom HJ. The effects of limb dominance and fatigue on running biomechanics. Gait Posture. 2014;39(3):915-9. doi: 10.1016/j.gaitpost.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 34. Pappas P, Paradisis G, Vagenas G. Leg and vertical stiffness (a)symmetry between dominant and non-dominant legs in young male runners. Hum Mov Sci. 2015;40:273-83. doi: 10.1016/j.humov.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 35. Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33(1):1-13. [DOI] [PubMed] [Google Scholar]
- 36. Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Volume changes in the menisci and articular cartilage of runners. Am J Sports Med. 2006;34(5):832-6. doi: 10.1177/0363546505282622. [DOI] [PubMed] [Google Scholar]