Abstract
Objective
The CC chemokine family member eotaxin-1, also named chemokine C-C motif ligand 11 (CCL11), has been detected in knee osteoarthritis (OA) and could induce breakdown of cartilage matrix. This study was performed to investigate the plasma and synovial fluid eotaxin-1 levels with the disease progression in elderly Han Chinese with primary knee OA.
Design
A total of 143 elderly primary knee OA patients and 135 healthy controls were enrolled in the study. The Western Ontario and McMaster Universities Arthritis Index (WOMAC) was performed to evaluate the clinical severity. The radiographic severity was assessed by Kellgren-Lawrence (K-L) grading. Plasma and synovial fluid (SF) eotaxin-1 levels were explored using enzyme-linked immunosorbent assay. The SF levels of matrix metalloproteinase–3 (MMP-3) and interleukin-6 (IL-6) were also examined.
Results
Elevated plasma eotaxin-1 levels were found in knee OA patients compared with healthy controls. Eotaxin-1 levels in SF of knee OA patients with K-L grade 4 were significantly elevated compared with those with K-L grades 2 and 3. Meanwhile, knee OA patients with K-L grade 3 had significantly increased SF levels of eotaxin-1 compared with those with K-L grade 2. Plasma eotaxin-1 levels in different K-L grading did not reach significant difference. Eotaxin-1 levels in SF of knee OA patients were significantly associated with disease severity evaluated by KL grading criteria. In addition, eotaxin-1 levels in SF were positively related to clinical severity illustrated by WOMAC as well as biochemical markers MMP-3 and IL-6.
Conclusions
Eotaxin-1 levels in SF instead of plasma, were independently and positively related to the disease severity in elderly knee OA patients. The inhibition of eotaxin-1 and its related signaling pathways may serve as a novel therapeutic approach for OA progression.
Keywords: elderly knee osteoarthritis, eotaxin-1, disease progression
Introduction
Osteoarthritis (OA) is a chronic joint disease characterized by progressive damage of articular cartilage, sclerosis of subchondral bone, and secondary synovial inflammation.1 The pathophysiology of knee OA is complex, and the probability of developing OA increases with age. The prevalence of knee OA is 9%, increasing to 30% in individuals older than 60 years, and 90% at the age between 70 and 74 years.2 Till the present, OA has been identified as 1 of the 5 diseases responsible for the greatest proportion of physical disability in noninstitutionalized elderly men and women. Together, disability and cost of treatment such as pain medication and total knee replacement surgery represent a great economic burden.3 OA occurs mainly in weightbearing joints, and the knee is the most frequently involved joint site in OA.3 So far, diagnosis of OA depends on patient-reported pain and disability, followed by imaging (usually plain X-ray, magnetic resonance imaging, etc.).4 However, these radiographic measures are late-stage indicators of disease, and studies using these outcomes become lengthy and costly because they require follow-up times that extend over many years.5 Therefore, a more simple, noninvasive method for early diagnosis of knee OA is urgently needed.
Biochemical markers of joint metabolism require only blood or synovial fluid collection and thus can be assessed more quickly and at a lower cost than radiographic or magnetic resonance imaging methods. In addition, application of biomarkers may be more sensitive for detecting the development and progression of OA than current imaging methods,6 particularly at early “molecular” stages of the disease.7
Chemokines are composed of a family of small heparin binding cytokines, originally identified by their chemotactic activity.8 So far, 4 subfamilies of chemokines have been identified according to the juxtaposition of cysteine residues in the protein’s N-terminus. These families have been named C, C-C, C-X-C, and C-X3-C.9 Among them, the C-C and C-X-C chemokines represent 2 major subgroups. There is accumulating evidence suggesting the involvement of chemokines and their receptors in OA.10 In addition, chemokines and chemokine receptors are expressed by chondrocytes and, as mentioned earlier, interaction between chemokine receptors and their ligands may cause matrix metalloproteinase–3 (MMP-3) induction, a key cartilage damage marker, in chondrocytes as well as a major cause of cartilage deterioration.11
The chemokine CCL11, also known as eotaxin (eotaxin-1 is used as alternative name in this article), was first identified in the peripheral immune system as a potent eosinophil chemoattractant that is produced by a variety of cell types.12 CCL11 binds to the chemokine receptors CCR2, CCR3, and CCR5, with highest affinity to CCR313,14 High levels of CCL11 have been described in several chronic inflammatory diseases, such as atopic dermatitis,15 gastrointestinal disease,16 and rheumatoid arthritis.17 In recent years, the role of eotaxin-1 in knee OA has been investigated. Hsu et al.18 found that eotaxin-1 was produced in cytokine-activated chondrocytes. Further stimulation with eotaxin-1 could upregulate its receptor CCR3, CCR5 as well as MMP-3 and MMP-13, which are related to joint inflammation and cartilage breakdown.18 Meanwhile, pretreatment of anti-eotaxin-1 antibody significantly decreased the MMP-3 expression induced by interleukin-1β (IL-1β).18 In another study, Chao et al.19 discovered that eotaxin-1 not only induces MMP-3 gene expression but also promotes MMP-3 protein secretion through G protein–coupled eotaxin-1 receptor activities. One recent report shows that OA fibroblast-like synoviocytes express CCR3 and respond to eotaxin-1 with production of catabolic proteases.20
All the previous works suggest that eotaxin-1 may play an important role in the pathogenesis and progression of knee OA. However, there are no studies available investigating the relationship between eotaxin-1 levels and disease severity of knee OA. Therefore, the scope of our study was to explore whether circulating and SF eotaxin-1 levels are altered in knee OA patients. We further intensively examine the correlation of the concentrations of plasma and SF eotaxin-1 with the radiographic progression as well as the symptomatic severity in elderly knee OA patients.
Methods
Study Subjects
From May 2016 to July 2017, 143 patients older than 60 years diagnosed with primary knee OA according to 1986 classification of osteoarthritis of knee were enrolled in the current study.21 Meanwhile, 135 age- and sex-matched healthy volunteers receiving regular body check at the Department of Physical Examination were selected as healthy controls (HCs). The exclusive criteria were as follows: inflammatory knee disease, rheumatoid arthritis, malignant tumors, systemic diseases, autoimmune diseases, and corticosteroids treatment during the past 3 months. The protocol of this study was approved by our Ethical Committee. Written informed consents were taken from all the subjects at the time of enlistment.
Statistical Power Analysis
Statistical analyses were carried out using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). Data are presented as mean ± standard deviation or median (interquartile range). The Kolmogorov-Smirnov test was used to analyze normal distribution. The statistical significance between the 2 groups was determined by the Student t test or Mann-Whitney U test, and the statistical significance between 3 or more groups was determined with 1-way analysis of variance or Kruskal-Wallis test. Bartlett’s test was used to test the homogeneity of group variances, followed with Tukey or Tamhane post hoc tests, where appropriate. Statistical significance of the correlation of eotaxin-1 levels in plasma and SF with disease severity was determined using Pearson or Spearman analysis. The multivariate statistical analysis was performed to determine the overall effect of independent factors on SF or plasma eotaxin-1 levels. All tests were 2-sided and P values less than 0.05 were considered to be statistically significant.
Statistical Power Calculation
The post hoc statistical power (1 − β) calculation was performed by PASS (Power Analysis and Sample Size) 2008 Statistical Software (Kaysville, UT, USA). The formula below was carried out on the basis of the obtained data of different mean eotaxin-1 concentrations, standard error, and number of patients enrolled in each group.22 Statistical power was regarded strong when >0.8. The formula is
Radiographic Assessment
Anteroposterior extended-view weightbearing radiographs of the knees were carryout out for each patient. The radiographic progression was determined using the Kellgren-Lawrence (K-L) scale. K-L grading scale: grade 1, doubtful narrowing of joint space and possible osteophytic lipping; grade 2, definite osteophytes and possible narrowing of joint space; grade 3, moderate multiple osteophytes, definite narrowing of joints space, some sclerosis and possible deformity of bone contour; grade 4, large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour.23 The results were read by 2 experienced radiologists who were blinded to the pairing or clinical information. The grade used for analysis was the higher of the 2 knees. The kappa value was calculated for intraobserver agreement for side-by-side readings.
Clinical Severity Assessment
The Western Ontario and McMaster Universities Arthritis Index (WOMAC) was used for the clinical evaluation of pain and function of knee OA, respectively. The WOMAC OA index is a health status scale evaluating the deficiency related to the condition in knee and/or hip osteoarthritis.24 The WOMAC index is a scale of 24 items in 3 sections. Pain is evaluated in the first section with 5 items, stiffness in the second section with 2 items, and physical function in the third section with 17 items. A 10-point scale is used in the scoring of the items. The total WOMAC score is obtained as the sum of the scores of each section. High WOMAC scores show increased pain and stiffness as well as disruption in physical function. Its validity and reliability have been evaluated in previous studies.25,26
Laboratory Examination
For plasma sample, 10 mL aliquot of blood was obtained from all participants directly into sodium citrate tubes at fasting time at 8:00 a.m. Synovial fluid was collected from each patient via arthrocentesis immediately before the first injection in the current course of sodium hyaluronate treatment or before a scheduled operation. All SF and blood samples were immediately centrifuged, aliquoted and stored at −80°C until use. Double-blind quantitative detection of eotaxin-1 in plasma and synovial fluid was performed by sandwich enzyme-linked immunosorbent assays (ELISA) using commercially available test kits in accordance to the manufacturer’s protocol (Abcam, Cambridge, UK). The intra-assay variation of the method was 4.4% and the interassay variation was 8.8%. The detection range was 0.41 to 300 pg/mL. SF MMP-3 and IL-6 levels were also examined (R&D Systems, Minneapolis, MN, USA). The manufacturer-reported intra-assay precision was 5.7% to 6.4% for MMP-3 and 1.6% to 4.2% for IL-6, whereas interassay precision was 7.9% to 8.6% for MMP-3 and 3.3% to 6.4% for IL-6. The sensitivity of these assays was 0.045 ng/mL for MMP-3 and 0.7 pg/mL for IL-6.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). Data are presented as mean ± standard deviation or median (interquartile range). The Kolmogorov-Smirnov test was used to analyze normal distribution. The statistical significance between the 2 groups was determined by the Student t test or Mann-Whitney U test, and the statistical significance between 3 or more groups was determined with the 1-way analysis of variance or Kruskal-Wallis test. Statistical significance of the correlation of eotaxin-1 levels in plasma and SF with disease severity was determined using Pearson or Spearman analysis. All tests were 2-sided and P values less than 0.05 were considered to be statistically significant.
Results
Basic Clinical Data
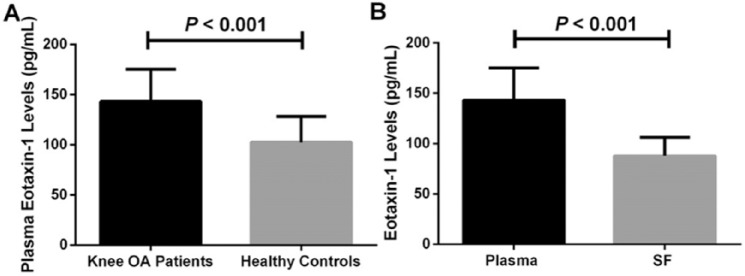
As displayed in Table 1 , No significant differences were found in age, sex, and body mass index (BMI) between patients with elderly knee OA and healthy control. OA patients had higher plasma eotaxin-1 concentrations than in healthy controls (143.3 ± 31.9 vs. 102.5 ± 25.7 pg/mL, P < 0.001) ( Fig. 1A ). Eotaxin-1 level in plasma was significantly higher than in paired SF samples (143.3 ± 31.9 vs. 87.8 ± 18.3 pg/mL, P < 0.001) ( Fig. 1B ). The post hoc statistical analysis showed that the statistical power was nearly 1.0 following calculation ( Fig. 2 ).
Table 1.
Basic Clinical Data.
| OA Patients (n = 143), Mean ± SD | Healthy Controls (n = 135), Mean ± SD | P | |
|---|---|---|---|
| Age (years) | 65.8 ± 3.4 | 66.1 ± 3.6 | 0.577 |
| Sex (female/male), n | 80/63 | 77/58 | 0.854 |
| BMI (kg/m2) | 24.3 ± 2.7 | 23.5 ± 2.4 | 0.239 |
| WOMAC pain score | 9.5 ± 3.2 | — | |
| WOMAC stiffness score | 4.1 ± 1.4 | — | |
| WOMAC physical function score | 34.6 ± 9.6 | — | |
| WOMAC total score | 48.5 ± 11.9 | — | |
| K-L grading(2/3/4), n | 50/52/41 | — | |
| Plasma eotaxin-1 levels (pg/mL) | 143.3 ± 31.9 | 102.5 ± 25.7 | <0.001 |
| SF eotaxin-1 levels (pg/mL) | 87.8 ± 18.3 | — |
BMI = body mass index; K-L = Kellgren-Lawrence; OA = osteoarthritis; SD = standard deviation; SF = synovial fluid; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Figure 1.
(A) Comparison of plasma eotaxin-1 levels between knee osteoarthritis (OA) patients and healthy controls. (B) Comparison of eotaxin-1 levels between plasma and synovial fluid (SF) in knee OA patients.
Figure 2.
Post hoc statistical power calculated by mean eotaxin-1 concentrations, standard error, and sample size. Statistic power: Green line for 0.9, purple line for 1.0, and blue line for 0.7.
Association of Eotaxin-1 Levels With Radiographic Severity
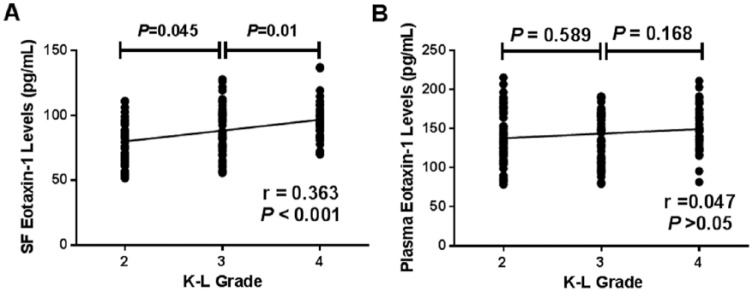
The characteristics of the OA subgroups on the basis of K-L classification are depicted in Table 1 . Collectively, 50 patients were K-L grade 2, 52 patients were K-L grade 3, and 41 patients were K-L grade 4 OA. The SF eotaxin-1 concentrations in knee OA patients with K-L grade 4 were significantly elevated compared with those with K-L grades 3 and 2 (97.2 ± 16.0 vs. 87.5 ± 18.9 pg/mL, P < 0.001; 97.2 ± 16.0 vs. 80.4 ± 16.3 pg/mL, P < 0.001). In addition, knee OA patients with K-L grade 3 had higher SF concentrations of eotaxin-1 compared with those with K-L grade 2 (87.5 ± 18.9 vs. 80.4 ± 16.3 pg/mL, P = 0.045) ( Fig. 3A ). SF eotaxin-1 levels were positively associated with K-L grades (r = 0.363, P < 0.001) ( Fig. 3A and Table 2 ). However, there were no significant differences between plasma eotaxin-1 levels in different K-L grades ( Fig. 3B ). Moreover, no significant differences in the plasma levels of eotaxin-1 were found among patients with different K-L grades ( Fig. 3B ).
Figure 3.
(A) Comparison of synovial fluid (SF) eotaxin-1 levels among different Kellgren-Lawrence (K-L) grades in knee osteoarthritis (OA) patients. and correlation of SF eotaxin-1 levels with K-L grades in knee OA patients. (B) Comparison of plasma eotaxin-1 among different K-L grades in knee OA patients and correlation of plasma eotaxin-1 levels with K-L grades in knee OA patients.
Table 2.
Correlation of SF Eotaxin-1 Levels With K-L Grade, WOMAC Index, and Biochemical Factors in Elderly Knee OA Patients Adjusted by Age and BMI.
| SF Eotaxin-1 Levels (pg/mL) |
SF Eotaxin-1 Levels (pg/mL)a |
|||
|---|---|---|---|---|
| Variables | r | P | r | P |
| BMI | 0.092 | >0.05 | — | — |
| Age | 0.144 | >0.05 | — | — |
| K-L grade | 0.363 | <0.001 | 0.310 | 0.009 |
| WOMAC pain index | 0.433 | <0.001 | 0.378 | <0.001 |
| WOMAC stiffness index | >0.05 | >0.05 | — | — |
| WOMAC function index | 0.430 | <0.001 | 0.365 | <0.001 |
| WOMAC total index | 0.395 | <0.001 | 0.332 | 0.005 |
| SF IL-6 levels | 0.382 | <0.001 | 0.327 | 0.004 |
| SF MMP-3 levels | 0.395 | <0.001 | 0.334 | 0.005 |
BMI = body mass index; IL-6 = interleukin-6; K-L = Kellgren-Lawrence; MMP-3 = matrix metalloproteinase–3; OA = osteoarthritis; SD = standard deviation; SF = synovial fluid; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Adjusted by age and BMI.
Association of SF Eotaxin-1 Levels With Clinical Severity
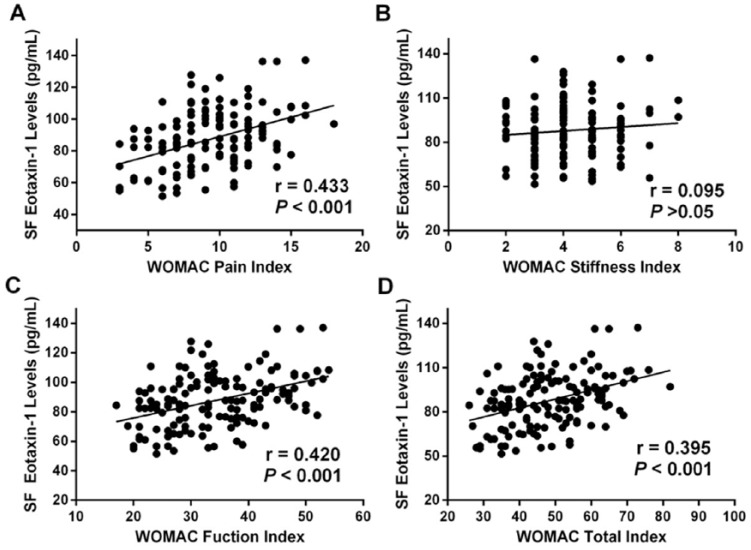
SF eotaxin-1 levels were positively related to WOMAC pain score (r = 0.433, P < 0.001) ( Fig. 4A and Table 2 ), WOMAC function scores (r = 0.420, P < 0.001) ( Fig. 4B ), and WOMAC total score (r = 0.395, P < 0.001) ( Fig. 4D ). However, relationship between SF eotaxin-1 and WOMAC stiffness scores did not achieve statistical significance (r = 0.095, P > 0.05) ( Fig. 4C ). Multivariate linear regression showed that eotaxin-1 could serve as an independent factor for assess K-L grades and clinical severity (Table 3).
Figure 4.
(A) Correlation of synovial fluid (SF) eotaxin-1 levels with Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain index in knee osteoarthritis (OA) patients. (B) Correlation of SF eotaxin-1 levels with WOMAC stiffness index in knee OA patients. (C) Correlation of SF eotaxin-1 levels with WOMAC function index in knee OA patients. (D) Correlation of SF eotaxin-1 levels with WOMAC total index in knee OA patients.
Table 3.
Multivariate Linear Regression.
| K-L Grade |
WOMAC Pain Index |
WOMAC Function Index |
WOMAC Total Index |
|||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Age | −0.073 | 0.334 | −0.047 | 0.605 | −0.027 | 0.950 | −0.023 | 0.894 |
| BMI | 0.130 | 0.122 | 0.116 | 0.140 | 0.177 | 0.088 | 0.170 | 0.902 |
| SF eotaxin-1 | 1.222 | 0.001 | 1.154 | 0.002 | 1.173 | 0.002 | 1.068 | 0.045 |
BMI = body mass index; K-L = Kellgren-Lawrence; SF = synovial fluid; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Association of SF Eotaxin-1 Levels With Biochemical Indices
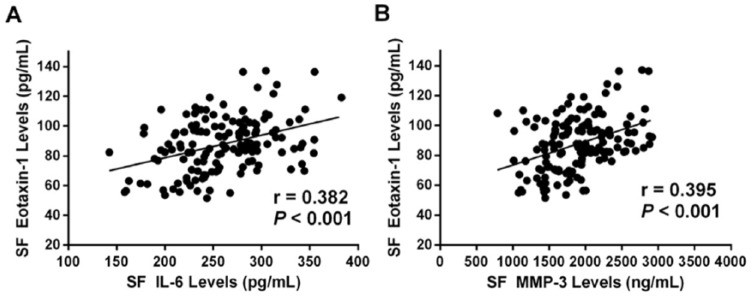
To further explore the role of eotaxin-1 in the pathogenesis in OA progression, we last examined the relationship between SF eotaxin-1 levels and cartilage breakdown marker MMP-3 as well as inflammatory factor IL-6. Interestingly, we found that SF eotaxin-1 levels were positively associated with IL-6 (r = 0.382, P < 0.001) ( Fig. 5A ) and MMP-3 (r = 0.395, P < 0.001) ( Fig. 5B ) levels.
Figure 5.
(A) Correlation of synovial fluid (SF) eotaxin-1 levels with SF interleukin-6 (IL-6) levels. (B) Correlation of SF eotaxin-1 levels with SF matrix metalloproteinase–3 (MMP-3) levels.
Discussion
To the best of our knowledge, this is the first study to show elevated SF eotaxin-1 levels were positively related to the disease progression of elderly knee OA as evaluated by K-L grade, WOMAC index, and biochemical factors that are involved in knee OA pathogenesis, including IL-6 and MMP-3. These correlations still remain significant after adjusted for age and BMI. Our findings implicated that SF eotaxin-1 levels may serve as a potential biomarker for knee OA.
A growing body of evidence suggests that development of OA, even at the early stages, is often accompanied by inflammation.27 As disease modulators, chemokines exert in a wide variety of inflammatory and immune responses via the chemoattractive effects of innate and adaptive immune cells, participating in the pathogenesis or progression in various disease.28 In knee OA, chemokines released by mononuclear cells present in the synovial membrane or by the chondrocytes themselves can induce an autocrine or paracrine stimulation, leading to extracellular matrix degradation.29
As one of the most studied chemokines, eotaxin-1 has been detected in knee OA and identified as a crucial factor in the development of knee OA along with other inflammatory cytokines. One previous study has shown that elevated eotaxin-1 plasma concentrations have been detected in OA compared with controls,18 which is consistent with our findings. In addition, the authors also observed that pretreatment with eotaxin-1 upregulate the surface expression of its corresponding receptor CCR3 on chondrosarcoma cells and increase the production of CCR3 mRNA in a dose-dependent manner.18 We next observed that SF eotaxin-1 levels were positively related to K-L grade classification and WOMAC index, and found knee OA patients with elevated SF eotaxin-1 levels exhibited severe radiographic changes and higher WOMAC indices. However, this correlation was not significant in plasma, although the correlation was slightly positive. This phenomenon indicates that knee OA is a local disease rather than the systematic reaction.
We also found synovial eotaxin-1 levels were positively associated with MMP-3 and IL-6 levels. MMPs are a family of structurally related calcium- and zinc-dependent proteolytic enzymes, which play important roles in the degradation of many different components of extracellular matrix.30 Upregulation of MMPs has been shown to be involved in the progression of OA.31 During OA, chondrocytes in the joint cartilage produce increased amount of MMPs, causing cartilage destruction.32 Among the family of MMPs, MMP-3 which is the most widely studied. MMP-3 could be secreted by fibroblasts, synovial cells, and chondrocytes, performs as a pivotal role in cartilage destruction: It degrades proteoglycan and collagen types II, IX, and XI33 and activates other MMPs.34 Previous studies have shown that in human chondrocyte cell line, eotaxin-1 could significantly upregulate the expressions of MMP-3 in a dose-dependent manner, indicating that eotaxin-1 and MMP-3 act as 2 important factors to damage the cartilage.18,19 In patients with knee OA, the expression of IL-6 by the synovium is enhanced by synovitis, which is induced by the degradation of articular cartilage.35 In chondrocytes and cartilage explants, IL-6 treatment reduced proteoglycan content with increased production of MMP-3.36 In addition, blockade of IL-6 could alleviate experimental arthritis in mice.36 Previous study has shown that IL-6 levels are almost 100-fold higher in OA SF than in serum in patients with OA37 and IL-6 was the strongest predictor of severe cartilage lesions.38
Our study findings must be interpreted considering study limitations. First, although the sample size was large enough to illustrate the results, the current study was carried out only in our affiliated hospital among Han Chinese Elderly knee OA patients, our findings should be further identified in multicenter. Second, we only examined the eotaxin-1 levels in OA patients; the investigation of other chemokines or inflammatory cytokines would provide more information on the pathogenesis of chemokines in OA. Third, we did not obtain the SF fluid in healthy controls because of ethical reasons. Previous studies have compared SF eotaxin-1 levels between knee OA and healthy controls. Beekhuizen et al.27 found SF Eotaxin-1 in knee OA patients were significantly lower than in healthy controls. Hampel et al.29 used a 2 step dot sandwich ELISA and found that eotaxin-1 levels in knee OA patients were significantly lower compared with rheumatoid arthritis patients, but the authors also did not collect SF samples from healthy controls.29 In another study, eotaxin-1 in knee OA patients did not reach significance with healthy controls (detailed data were not shown).38 These different findings may be attributed to ethnic differences between the studied populations, differences of enrolled patients as well as different investigative methods used. In addition, all these works above did not explore the relationship between eotaxin-1 and disease severity with knee OA.
In conclusion, the current study has revealed a significant increase in plasma eotaxin-1 of elderly knee OA patients and illustrated a pronounced positive correlation of SF eotaxin-1 with disease severity with primary elderly knee OA. The results of our study postulate that SF eotaxin-1 may be used as a prognostic indicator to reflect the disease progression of primary knee OA. Further studies are needed to define the mechanisms underlying this association. Additional investigations should be considered to elucidate a possible role of eotaxin-1 in the pathogenesis of chronic degenerative joint disorder, aiming to the development of effective therapeutic methods to block OA progression.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The 12th five-year plan project of Philosophy and Social Science in Guangdong province (GD13XGL18) and the project of School of Health Services Management of Southern Medical University (WG2016019).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The protocol of this study was approved by our Ethical Committee SMU201619.
Informed Consent: Written informed consent was taken from all the subjects at the time of enlistment.
Trial Registration: Not applicable.
References
- 1. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115-26. [DOI] [PubMed] [Google Scholar]
- 2. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-55. [DOI] [PubMed] [Google Scholar]
- 3. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boesen M, Ellegaard K, Henriksen M, Gudbergsen H, Hansen P, Bliddal H, et al. Osteoarthritis year in review 2016: imaging. Osteoarthritis Cartilage. 2017;25:216-26. [DOI] [PubMed] [Google Scholar]
- 5. Takahashi M, Naito K, Abe M, Sawada T, Nagano A. Relationship between radiographic grading of osteoarthritis and the biochemical markers for arthritis in knee osteoarthritis. Arthritis Res Ther. 2004;6:R208-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723-7. [DOI] [PubMed] [Google Scholar]
- 7. Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19:515-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91-104. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469-99. [DOI] [PubMed] [Google Scholar]
- 10. Yuan GH, Masuko-Hongo K, Sakata M, Tsuruha J, Onuma H, Nakamura H, et al. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001;44:1056-70. [DOI] [PubMed] [Google Scholar]
- 11. Scanzello CR. Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J Orthop Res. 2017;35:735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adar T, Shteingart S, Ben Ya’acov A, Bar-Gil Shitrit A, Goldin E. From airway inflammation to inflammatory bowel disease: eotaxin-1, a key regulator of intestinal inflammation. Clin Immunol. 2014;153:199-208. [DOI] [PubMed] [Google Scholar]
- 13. Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, et al. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996;271:7725-30. [DOI] [PubMed] [Google Scholar]
- 14. Ye J, Kohli LL, Stone MJ. Characterization of binding between the chemokine eotaxin and peptides derived from the chemokine receptor CCR3. J Biol Chem. 2000;275:27250-7. [DOI] [PubMed] [Google Scholar]
- 15. Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syversen SW, Goll GL, Haavardsholm EA, Bøyesen P, Lea T, Kvien TK. A high serum level of eotaxin (CCL 11) is associated with less radiographic progression in early rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu YH, Hsieh MS, Liang YC, Li CY, Sheu MT, Chou DT, et al. Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J Cell Biochem. 2004;93:929-39. [DOI] [PubMed] [Google Scholar]
- 19. Chao PZ, Hsieh MS, Cheng CW, Lin YF, Chen CH. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J Biomed Sci. 2011;18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang X, Shen J, Yang H, Xu Y, Gao W, Wang J, et al. Upregulated expression of CCR3 in osteoarthritis and CCR3 mediated activation of fibroblast-like synoviocytes. Cytokine. 2016;77:211-9. [DOI] [PubMed] [Google Scholar]
- 21. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039-49. [DOI] [PubMed] [Google Scholar]
- 22. Chow S, Shao J, Wang H. Sample size calculations in clinical research. 2nd ed. London, England: Chapman and Hall/CRC Biostatistics Series; 2008:58. [Google Scholar]
- 23. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heintjes EM, Bierma-Zeinstra SM, Berger MY, Koes BW. Lysholm scale and WOMAC Index were responsive in prospective cohort of young general practice patients. J Clin Epidemiol. 2008;61:481-8. [DOI] [PubMed] [Google Scholar]
- 25. Roos EM, Klässbo M, Lohmander LS. WOMAC Osteoarthritis Index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28:210-5. [DOI] [PubMed] [Google Scholar]
- 26. Basaran S, Guzel R, Seydaoglu G, Guler-Uysal F. Validity, reliability, and comparison of the WOMAC Osteoarthritis Index and Lequesne Algofunctional Index in Turkish patients with hip or knee osteoarthritis. Clin Rheumatol. 2010;29:749-56. [DOI] [PubMed] [Google Scholar]
- 27. Beekhuizen M, Gierman LM, van Spil WE, Van Osch GJ, Huizinga TW, Saris DB, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis Cartilage. 2013;21:918-22. [DOI] [PubMed] [Google Scholar]
- 28. Nomiyama H, Osada N, Yoshie O. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev. 2010;21:253-62. [DOI] [PubMed] [Google Scholar]
- 29. Hampel U, Sesselmann S, Iserovich P, Sel S, Paulsen F, Sack R. Chemokine and cytokine levels in osteoarthritis and rheumatoid arthritis synovial fluid. J Immunol Methods. 2013;396:134-9. [DOI] [PubMed] [Google Scholar]
- 30. Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207-14. [DOI] [PubMed] [Google Scholar]
- 31. Yang CC, Lin CY, Wang HS, Lyu SR. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS One. 2013;8:e79662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585-94. [DOI] [PubMed] [Google Scholar]
- 33. Dodge GR, Jimenez SA. Glucosamine sulfate modulates the levels of aggrecan and matrix metalloproteinase-3 synthesized by cultured human osteoarthritis articular chondrocytes. Osteoarthritis Cartilage. 2003;11:424-32. [DOI] [PubMed] [Google Scholar]
- 34. Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford study. Arthritis Rheum. 2009;60:2037-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Latourte A, Cherifi C, Maillet J, Ea HK, Bouaziz W, Funck-Brentano T, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76:748-55. [DOI] [PubMed] [Google Scholar]
- 37. Tsuchida AI, Beekhuizen M, Rutgers M, van Osch GJ, Bekkers JE, Bot AG, et al. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model. Arthritis Res Ther. 2012;14:R262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuéllar VG, Cuéllar JM, Kirsch T, Strauss EJ. Correlation of synovial fluid biomarkers with cartilage pathology and associated outcomes in knee arthroscopy. Arthroscopy. 2016;32:475-85. [DOI] [PubMed] [Google Scholar]