Abstract
Objective
Many clinical trials of viscosupplementation have been conducted, although only the Gel-200 (primary) trial included a different patient population. A subgroup analysis of a multicenter, randomized controlled trial comparing the efficacy of single intra-articular injections of Gel-200 with phosphate buffered saline (PBS) was performed to demonstrate its benefit as treatment of osteoarthritis of the knee in a population similar to those of other reported trials of viscosupplementation.
Design
The subgroup population was defined as patients in the intention-to-treat (ITT) population who met the specified criteria. Changes from baseline in Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores following treatment with Gel-200 or PBS were derived from a longitudinal model and treatment differences compared between groups at weeks 12 and 26, and over 26 weeks.
Results
The subgroup included 311 subjects (152 Gel-200; 159 PBS). Mean improvements from baseline in WOMAC pain subscores in the Gel-200 over PBS groups were statistically significant at week 12 (P = 0.031) and week 26 (P = 0.019). Treatment group differences in WOMAC stiffness and total scores were statistically significant at week 26 (P = 0.023 and P = 0.036, respectively).
Conclusions
The efficacy of Gel-200 following a single injection for knee osteoarthritis was demonstrated in WOMAC pain, stiffness, and total scores as well as clinically important improvements in pain at 26 weeks in this subset of patients with comparable characteristics to populations evaluated in other viscosupplementation treatment trials.
Keywords: osteoarthritis, knee, intra-articular, cross-linked hyaluronan, Gel-200
Introduction
Osteoarthritis (OA) is the most common arthritis worldwide causing pain and physical dysfunction.1 A variety of intra-articular hyaluronan (IA-HA) injections, for example, viscosupplementation, have been studied as treatment for the symptoms of OA. In addition to effects of shock absorption, lubricating the joint, and protecting the cartilage from degradation, IA-HA treatment is believed to exert its therapeutic effect by providing chondroprotection and anti-inflammatory effects, stimulating endogenous synthesis of proteoglycans and HA, limiting subchondral bone changes, and reducing the action of joint nociceptors.2-4 In the United States, IA-HA products are approved for treatment of knee OA symptoms and have been widely used for almost 20 years despite that recent OA treatment guidelines by the American Academy of Orthopedic Surgeons (AAOS) and the American College of Rheumatology (ACR) have queried their therapeutic utility.5-9 There are many comparisons of IA-HA products from the point of view of their molecular weight yet reports of the efficacy of IA-HA is comparable between low- and high-molecular-weight products,10-12 despite differing results from the original clinical trials. The efficacy of IA-HA products was also reviewed from the point of view of differences in injection regimens: single injection versus multiple injections.13 In addition, differences in the enrolled patient populations in the original clinical trials of each IA-HA product should be considered.
One such product is Gel-200 (Gel-One, Seikagaku Corporation, Tokyo, Japan), which is a sterile, transparent, and viscoelastic hydrogel composed of a cross-linked HA, a derivative of a highly purified HA product.
Randomized controlled trials (RCTs) of Gel-200 have demonstrated its efficacy and safety over 26 weeks following a single injection. However, differences between inclusion criteria of the Gel-200 and other IA-HA RCTs confound comparative analyses.14-19 In most IA-HA trials, patients were enrolled with Kellgren-Lawrence (K-L) grades 2 or 3 by X-ray with >3 months duration of symptomatic non-posttraumatic OA. In the Gel-200 RCTs, patients were enrolled with K-L grades 1 to 3 and posttraumatic OA was not excluded provided they had symptomatic OA for >4 weeks. Such differences in the patient populations make difficult to compare results across the clinical trials.
For a valid comparison of data between trials, we focused on efficacy to assess the clinical benefit of Gel-200 over 26 weeks in a subgroup based on specific characteristics of the enrolled population comparable to other reported IA-HA trials.
Methods
Study Design
A multicenter RCT was conducted in the United States between August 2013 and February 2015, approved by a central institutional review board and registered with Clinical Trials.gov (identification numbers NTC 01934218).
Patients aged 40 to 80 years with diagnosed knee OA, K-L scores grade 1 to 3, and pretreatment pain scores of 50 to 90 mm on a 100-mm VAS in the target knee following a 50-foot walk test were enrolled. After screening, 817 subjects were randomized 1:1 to treatment with Gel-200 or phosphate buffered saline (PBS) balanced based on K-L grades and baseline scores. Screening was conducted 1 to 2 weeks prior to baseline (week 0) and patients returned for clinic evaluations at weeks 3, 6, 12, 18, and 26.
At all visits, patients reported their VAS pain scores following a 50-foot walk test and completed the Western Ontario McMaster Universities Arthritis Index (WOMAC), and physician and patient global evaluations. A blinded evaluating physician or a back-up blinded evaluating physician, conducted the assessments, including K-L scores and an unblinded injecting physician administered treatment of Gel-200 or PBS.
Patients who met all 4 conditions below in the intention-to-treat (ITT) population of the Gel-200 RCT were defined as the subgroup for comparisons with other IA-HA trials. The criteria included
Non-posttraumatic OA
K-L grade 2 or 3
WOMAC pain during walking (A1) and WOMAC pain subscores of 40 to 80 mm
≥3 months’ duration of OA pain
Inclusion criteria for pain differed across IA-HA trials: major pain inclusion of 41 to 90 mm scores following the 50-foot walk test17; 50 to 90 mm scores following the 50-foot walk test14; 40 to 80 mm on the WOMAC pain A1 score18; knee pain ≥20 mm for at least 1 item of the WOMAC VAS pain subscores.19 As the WOMAC A1 pain score measures pain during walking, it is similar to the 50-foot walk test. To define an inclusive range among the reported criteria, subgroup analysis included only subjects reporting WOMAC pain A1 scores and WOMAC pain subscores of 40 to 80 mm.
Primary Outcome
The primary efficacy objective was treatment difference in mean changes from baseline in WOMAC pain subscores between Gel-200 and PBS over 26 weeks.
Secondary Outcome
Secondary efficacy endpoints included other WOMAC scores, physician and patient global evaluations, and Outcome Measures in Rheumatology and Osteoarthritis Research Society International (OMERACT-OARSI) strict responders.20-22
Statistical Analysis
The primary efficacy analysis was change in WOMAC pain subscores reported by patients in the subgroup population, using longitudinal analyses. A repeated-measures model was prespecified that expressed the pain score as a linear function of treatment, time, treatment-by-time interaction, clinically relevant covariates, and a fixed site effect.
The primary efficacy objective was tested using the null hypothesis of no difference in mean changes from baseline in WOMAC pain subscores between Gel-200 and PBS over 26 weeks, and the group comparison was defined as the average effect over weeks 3, 6, 12, 18, and 26. Analyses were repeated for the secondary efficacy endpoints. All analyses were post hoc and specified following database lock and unblinding.
Results
Patient Population
In the Gel-200 trial, the ITT population included 809 patients (402 Gel-200; 407 PBS). The subgroup population included 311 patients (152 Gel-200; 159 PBS).
Demographic and Baseline Characteristics
Table 1 summarizes the baseline characteristics and demographics in the subgroup population, which were similar between treatment groups both in original ITT and subgroup populations.14
Table 1.
Baseline Characteristics and Demographics.a
| Parameter | PBS (n = 159) | Gel-200 (n = 152) |
|---|---|---|
| Gender, n (%) | ||
| Male | 60 (37.7) | 64 (42.1) |
| Female | 99 (62.3) | 88 (57.9) |
| Age, years (mean ± SD) | 62.8 ± 8.85 | 61.0 ± 9.38 |
| K-L grade, n (%) | ||
| Grade 2 | 92 (57.9) | 85 (55.9) |
| Grade 3 | 67 (42.1) | 67 (44.1) |
| Duration of OA knee disease, months (mean ± SD) | 7.83 ± 6.07 | 7.43 ± 6.75 |
| Baseline WOMAC pain subscores (mean ± SD) | 63.45 ± 9.20 | 63.42 ± 9.12 |
PBS = phosphate buffered saline; K-L = Kellgren-Lawrence; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
No statistically significant differences were identified between treatment groups.
Primary Efficacy
Table 2 and Figure 1 summarize changes from baseline in WOMAC pain subscores in the subgroup population. Treatment group differences and mean (95% confidence intervals [CI]) changes from baseline were −4.5 mm (−8.7, −0.4) over 26 weeks and −6.2 mm (−11.4, −1.0) at week 26, statistically significantly different (P = 0.032 and P = 0.019, respectively). Treatment differences were evident and statistically significant from week 12.
Table 2.
Changes from Baseline in WOMAC Pain Subscores.
| Time Points | Estimated Change from Baseline (mm) |
Difference (mm) | 95% CI | P a | |
|---|---|---|---|---|---|
| PBS (n = 159) | Gel-200 (n = 152) | ||||
| Over 26 weeks | −15.9 | −20.4 | −4.5 | −8.7, −0.4 | 0.032 |
| Week 3 | −15.5 | −14.8 | 0.7 | −4.2, 5.6 | 0.788 |
| Week 6 | −16.3 | −20.3 | −4.1 | −9.2, 1.1 | 0.121 |
| Week 12 | −16.5 | −22.1 | −5.6 | −10.7, −0.5 | 0.031 |
| Week 18 | −16.4 | −23.9 | −7.5 | −12.9, −2.0 | 0.007 |
| Week 26 | −14.8 | −21.0 | −6.2 | −11.4, −1.0 | 0.019 |
PBS = phosphate buffered saline; K-L = Kellgren-Lawrence; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Estimated change from baseline, differences, and P value based on repeated measures longitudinal model with fixed effects for treatment, time, treatment-by-time interaction, baseline pain measurement, K-L grade, and fixled site effect.
Figure 1.
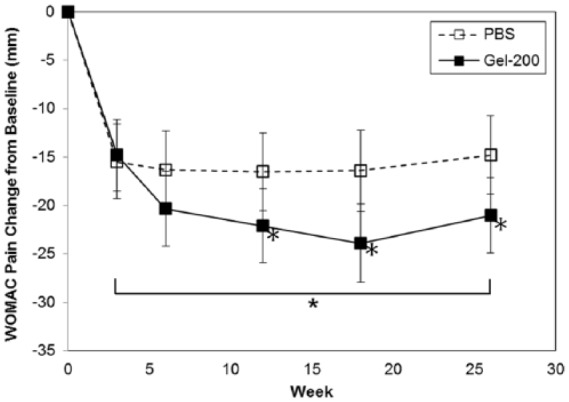
Change from baseline in Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain subscores.
*P < 0.05.
Secondary Efficacy
Gel-200 showed a statistically significant difference over PBS at Week 26 in WOMAC stiffness subscores, total scores, and physician global evaluation ( Table 3 ).
Table 3.
Secondary Efficacy Results at Week 26.
| Measurements | Difference (mm) | 95% CI | P |
|---|---|---|---|
| WOMAC function | −5.0 | −10.1, 0.1 | 0.056 |
| WOMAC stiffness | −6.6 | −12.2, −0.9 | 0.023 |
| WOMAC total | −5.4 | −10.4, −0.4 | 0.036 |
| Physician global evaluation | −7.3 | −13.1, −1.6 | 0.013 |
| Patient global evaluation | −5.1 | −10.7, 0.6 | 0.078 |
WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Discussion
We assessed the 26-week efficacy of Gel-200 according to this subgroup analysis from the original clinical trial, defined by criteria consistent with other RCTs of IA-HA products. To align with the study populations in other IA-HA trials, our subgroup population was identified by baseline reported pain, duration of OA symptoms, and K-L scores. The subgroup analysis demonstrated that a single injection of Gel-200 resulted in statistically significant mean changes from baseline in WOMAC pain and stiffness subscores and total scores at 26 weeks as well as physician global assessments compared with a single injection of PBS.
In our analyses, the treatment difference between Gel-200 and PBS was 6.2 mm on 100-mm VAS in WOMAC pain subscores. This is the largest treatment difference at week 26 reported among the IA-HA products; compared with approximately 3 mm treatment differences in WOMAC VAS pain subscores16,17,23 reported other IA-HA products; as well as others without statistical significance although conducted in similar patient populations to this defined subgroup.18,24
Moreover, Gel-200 demonstrated efficacy in secondary endpoints such as WOMAC stiffness subscores, total scores, and physician global assessments but did not demonstrate statistically significant differences in WOMAC physical function subscores. Other IA-HA products evaluated similar scores in RCTs, however no other products showed statistically significant differences over PBS treatment except in WOMAC pain scores when evaluating similar patient populations.16-19,24
In certain treatment guidelines regarding use of IA-HA products, between group treatment differences were misinterpreted as clinically meaningful—raising questions whether they were based on appropriate decisions.8,25 Clinically meaningful between group differences should not be considered similar to clinically meaningful within group changes from baseline, for example, in individual patients, such as the percentage reporting improvements ≥MCID (minimum clinically important differences).26,27 Gel-200 demonstrated >20 mm clinically important improvement in WOMAC 100-mm VAS pain subscores, which exceeds minimum clinically important improvements (MCII) reported to be 19.9 mm on 100-mm pain scale for knee OA.28
Especially for IA treatments, placebo responses to IA PBS should be carefully considered when interpreting treatment differences in efficacy measures. The network meta-analysis showed that IA injections are the most effective treatments for knee OA symptoms compared with pharmacologic interventions.29 Another systematic meta-analysis also reported that IA PBS injections provide pain relief in RCTs.30-33 Considering the nature of treatment effects observed with IA PBS injections, Gel-200 demonstrated statistically larger treatment benefits.
There are various reports about correlations between treatment responses and radiographic evidence. Conrozier et al.34 reported no correlation between them. On the other hand, Altman et al.35 reported larger changes from baseline in patients with K-L grade 2, compared with those with K-L grade 3. No evidence is presented about the population with or without K-L grade 1. Since our objective was to assess the efficacy of Gel-200 in a subgroup population defined by similar baseline pain levels, OA symptom duration and K-L scores to other IA-HA clinical trials we matched selection criteria to compare accordingly. We have not assessed treatment responses by stratifying each K-L grade. However, Gel-200 demonstrated significant improvements from baseline in the total population, including K-L grade 1 to grade 3,14 and a larger treatment difference when focusing on the K-L grade 2 and 3 subpopulation to be consistent with other IA-HA studies. These results suggest that patients with K-L grade 1 experience pain improvements with both Gel-200 and placebo, but higher placebo responses may affect treatment differences. Further subgroup analysis by stratifying each K-L grade would be considered to identify the most responsible patient population for Gel-200 treatment in the future study.
There are limitations to the conclusion that Gel-200 demonstrates a larger treatment effect versus PBS than other IA-HA products since no formal meta-analysis has been conducted with other IA-HA products.
In conclusion, this subgroup analyses demonstrated that Gel-200 treatment provides benefit to patients with knee OA by K-L scores including those at an early OA symptom stage with shorter disease duration in the entire patient populations of the original RCT as well as those with moderate to severe radiographic damage and longer disease duration in the subgroup, with the latter patient group gaining greater therapeutic benefit from Gel-200 over PBS treatment at Week 26 measured by WOMAC pain, stiffness and total scores with clinically meaningful improvement in pain relief.
Footnotes
Acknowledgments and Funding: We wish to thank Sooyeol Lim for help with editing the manuscript, Benjamin Vaughn for help with the statistical analyses, and Yuko Shibata, Taiki Osato, and Taku Hosaka for help with reviewing the study data. This project was funded by Seikagaku Corporation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JT and TS are employees of Seikagaku Corporation. JT is listed as an inventor of a hyaluronate product. VS has served as a consultant to Seikagaku Corporation as well as Bioventus, Carbylan, Eupraxia, Flexion, Iroko, and Sanofi.
Ethical Approval: Ethical approval was not sought for the present study because all data was obtained from a previously conducted trial.
Informed Consent: Informed consent was not sought for the present study because all data was obtained from a previously conducted trial.
Trial Registration: This trial is registered with ClinicalTrials.gov (identification number NTC 01934218).
References
- 1. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanism of action. Arthritis Res Ther. 2003;5:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Migliore A, Procopio S. Effectiveness and utility of hyaluronic acid in osteoarthritis. Clin Cases Miner Bone Metab. 2015;12:31-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldberg VM, Goldberg L. Intra-articular hyaluronans: the treatment of knee pain in osteoarthritis. J Pain Res. 2010;3:51-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strand V, Conaghan PG, Lohmander LS, Koutsoukos AD, Hurley FL, Bird H, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:859-66. [DOI] [PubMed] [Google Scholar]
- 7. Migliore A, Bizzi E, Herrero-Beaumont J, Petrella RJ, Raman R, Chevalier X. The discrepancy between recommendations and clinical practice for viscosupplementation in osteoarthritis: mind the gap! Eur Rev Med Pharmacol Sci. 2015;19(7):1124-9. [PubMed] [Google Scholar]
- 8. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline 2nd Edition 2013. May 18 [cited 2018 Jan 24]. Available from: https://www.aaos.org/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf [DOI] [PubMed]
- 9. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465-74. [DOI] [PubMed] [Google Scholar]
- 10. Gigis I, Fotiadis E, Nenopoulos A, Tsitas K, Hatzokos I. Comparison of two different molecular weight intra-articular injections of hyaluronic acid for the treatment of knee osteoarthritis. Hippokratia. 2016;20:26-31. [PMC free article] [PubMed] [Google Scholar]
- 11. Papalia R, Russo F, Torre G, Albo E, Grimaldi V, Papalia G, et al. Hybrid hyaluronic acid versus high molecular weight hyaluronic acid for the treatment of osteoarthritis in obese patients. J Biol Regul Homeost Agents. 2017;31(4 Suppl 2):103-9. [PubMed] [Google Scholar]
- 12. Shewale AR, Barnes CL, Fischbach LA, Ounpraseuth ST, Painter JT, Martin BC. Comparison of low-, moderate-, and high-molecular-weight hyaluronic acid injections in delaying time to knee surgery. J Arthroplasty. 2017;32:2952-2957.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Concoff A, Sancheti P, Niazi F, Shaw P, Rosen J. The efficacy of multiple versus single hyaluronic acid injections: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED); Gel-One®–P080020/S020. 2016 [cited 2018 Jan 24]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf8/P080020S020B.pdf.
- 15. Strand V, Baraf HSB, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20:350-6. [DOI] [PubMed] [Google Scholar]
- 16. Chevalier X, Jerosch J, Goupille P, Dijk NV, Luyten FP, Scott DV, et al. Single, intra-articular treatment with 6 mL of hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomized, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA® for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum. 2009;39:1-9. [DOI] [PubMed] [Google Scholar]
- 18. Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED); HYMOVIS®–P150010. 2015. [cited 2018 Jan 24]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150010B.pdf.
- 19. Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED); Hyalgan®–P950027. 1997. [cited 2018 Jan 24]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/P950027a.pdf.
- 20. Dougados M, Leclaire P, van der Heijde D, Bloch DA, Bellamy N, Altman RD. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000;8:395-403. [DOI] [PubMed] [Google Scholar]
- 21. Pham T, van der Heijde D, Lassare M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30:1648-54. [PubMed] [Google Scholar]
- 22. Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389-99. [DOI] [PubMed] [Google Scholar]
- 23. Food and Drug Administration. FDA Summary for Synvisc-One Orthopaedic and Rehabilitation Devices Panel. 2008. Dec 9 [cited 2018 Jan 24]. Available from: https://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4404b1-09-FDA%20Executive%20Summary.pdf.
- 24. Food and Drug Administration. Summary of Safety and Effectiveness Data (SSED); MONOVISC™–P090031. 2014. [cited 2018 Jan 24]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf9/P090031b.pdf.
- 25. Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? J Arthroscopy. 2014;30:86-9. [DOI] [PubMed] [Google Scholar]
- 26. Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238-44. [DOI] [PubMed] [Google Scholar]
- 27. Altman RD, Devji T, Bhandari M, Fierlinger A, Niazi F, Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum. 2016;46:151-9. [DOI] [PubMed] [Google Scholar]
- 28. Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systemic review and network meta-analysis. Ann Intern Med. 2015;162:46-54. [DOI] [PubMed] [Google Scholar]
- 30. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doherty M, Dieppe P. The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage. 2009;17:1255-62. [DOI] [PubMed] [Google Scholar]
- 32. Strand V, Kelman A. Outcome measures in osteoarthritis: randomized controlled trials. Curr Rheumatol Rep. 2004;6:20-30. [DOI] [PubMed] [Google Scholar]
- 33. Strand V, Boers M, Inzerada L, Kirwan JR, Kvien TK, Tugwell PS, et al. Its’ good to feel better but it’s better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol. 2011;38(8):1720-7. [DOI] [PubMed] [Google Scholar]
- 34. Conrozier T, Mathieu P, Schott AM, Laurent I, Hajri T, Crozes P, et al. Factors predicting long-term efficacy of Hylan GF-20 viscosupplementation in knee osteoarthritis. Joint Bone Spine. 2003;70(2):128-33. [DOI] [PubMed] [Google Scholar]
- 35. Altman RD, Farrokhyar F, Fierlinger A, Niazi F, Rosen J. Analysis for prognostic factors from a database for the intra-articular hyaluronic acid (Euflexxa) treatment for osteoarthritis of the knee. Cartilage. 2016;7:229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]


