Abstract
Age-related decreases in Quality of Life (QoL) are often compounded by comorbidities, including cancer. This study aimed to examine QoL changes before and after a new cancer diagnosis using data from the National Health and Aging Trend Study (NHATS), linked to Medicare claims (N = 136). There was a significant increase in the relative odds of fair/poor self-reported health and needing help with Activities of Daily Living. There was also a marginal increase in depression, but no change in anxiety or pain scores. Results underscore importance of considering pre-cancer QoL when making treatment decisions for older adult cancer patients.
Keywords: Neoplasms, quality of life, aging, mental health
Introduction
Increasing life expectancy in the United States (US) has contributed to an unprecedented growth in the population of older Americans. By the year 2030, all baby boomers will be older than 65 and the US Census Bureau’s 2017 National Population Projections estimate that older adults will outnumber children by 2035 (1). As individuals in the US live longer, quality of life (QoL) among older individuals warrants increased attention. Older adults face declines in physical functioning, including unintentional weight loss, exhaustion, weakness and decreased physical activity as a product of age (2). Cognitive decline (3,4) and mental health concerns, including depression (5,6) and anxiety (6) may also impact quality of life among older adults. These physical and cognitive declines often limit the activities of daily living (ADLs) of older individuals, including getting dressed or getting out of bed, further impacting quality of life (7–10). Some research, however, indicates that older adults have differing trajectories and that declines in mental health and QoL are not necessarily synonymous with aging. For example, socioemotional selectivity theory posits that older adults are more likely to place increasing value on the achievement of emotionally meaningful goals as compared to younger adults and that this assists with emotion regulation, potentially leading to better QoL (11). It is unclear, however, how these potential pockets of resilience for older adults are impacted when faced with a life-threatening illness, such as cancer.
Age-related decreases in QoL, including both mental health, physical distress and difficulties later in life are often compounded by other comorbid conditions, including cancer (12,13). As a disease primarily of older adults, it is projected that the aging of the US population will contribute to a 67% increase in the incidence of cancer among older adults versus 11% for younger adults (14). In general, a diagnosis of cancer is associated with higher rates of distress even as compared to diagnoses of non-neoplastic diseases with worse prognoses (15). Further, the rate of depression in cancer patients is significantly higher than in the general population and is associated with higher mortality rates, with one meta-analysis conferring up to a 39% increased mortality rate (16–20). Older patients face unique challenges following a cancer diagnosis given pre-diagnostic poorer quality of life. Older adult cancer patients are at an increased risk of psychiatric disorders including depression and anxiety (16,21–24). Risk of suicide may also increase in certain subpopulations of older adults following a cancer diagnosis (25,26). In addition, an estimated 80% of older cancer patients experience pain (27) and up to 70% experience fatigue (28) due to their disease. Measures of patient-reported QoL among older adult cancer patients may provide important information that could impact treatment preferences and decision making (28). QoL measures have also been established as significant prognostic indicators of survival (18,19,29–31).
While the increased risk of physical and cognitive impairment in older age and the impacts of a cancer diagnosis on QoL in older adults are well documented, there is a paucity of research examining changes in QoL among older individuals before and after a cancer diagnosis. A systematic review of unmet needs of newly diagnosed cancer patients undergoing active treatment found that a significant portion of older cancer patients reported unmet psychological, informational and physical needs (32). As the number of Americans diagnosed with cancer grows, the US health system and individual providers’ ability to address QoL among older cancer patients will play a vital role in the well-being and survivorship of older cancer patients. As such, this study sought to examine changes in QoL outcomes before and after a new cancer diagnosis among community dwelling adults 65 years and older, using a longitudinal data set of a nationally representative sample of Medicare beneficiaries. We expect that physical and mental health components of QoL will decrease after a cancer diagnosis.
Materials and methods
Study design and sample
This study used annual survey data collected from the National Health and Aging Trend Study (NHATS), linked to Medicare claims. NHATS is a longitudinal, population-based survey of late life disability trends and trajectories, drawn from a random sample of Medicare beneficiaries as of 30 September 2010 who were at least 65 years of age and living in the contiguous US, with an oversampling of those over age 90 years and non-Hispanic blacks. The enrollment file represents 96% of all older adults in the United States. Enrollment interviews were completed between May and November 2011 (33,34). Annual follow up interviews were completed through 2016. In-person interviews collect detailed self-reported information on participants’ physical capacity, function, chronic health conditions, and socio-economic status. Physical and cognitive performance batteries are also conducted. For the current study, we used the NHATS cohort enrolled in 2011 (with surveys from 2011 to 2016), and their corresponding Medicare claims from the inpatient, outpatient, and national claims history (NCH) files from 2009 to 2016. NHATS was conducted via Johns Hopkins School of Public Health with approval from the Institutional Review Board (JHSPH IRB # 00002083). All subjects provided written informed consent. The present study was conducted with approval from The Icahn School of Medicine at Mount Sinai Review Board for Health Sciences Research.
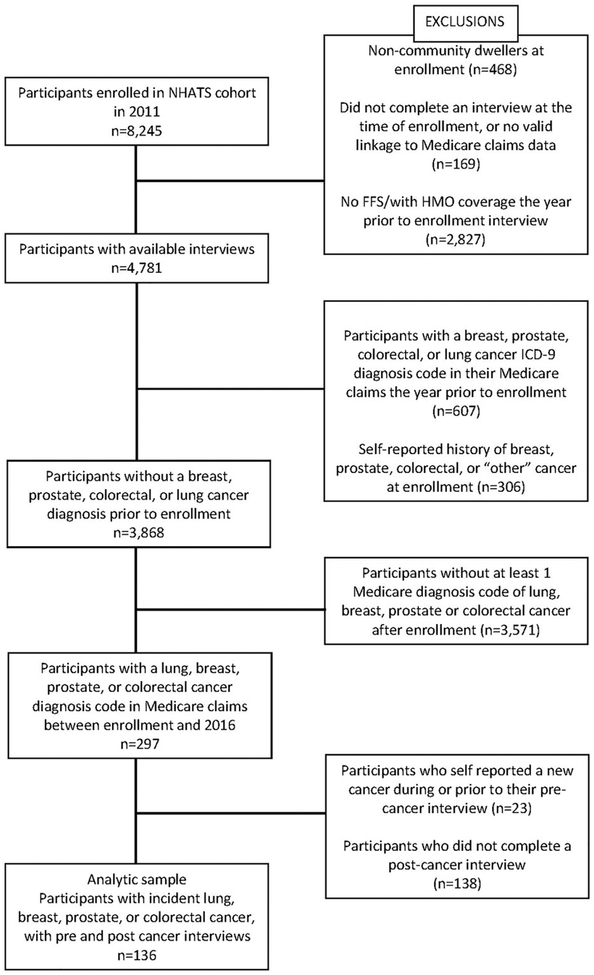
Of the 8245 participants enrolled in NHATS in 2011 (71% response rate), we included only community dwellers (i.e. not in a nursing home facility) at the time of enrollment who completed enrollment interviews (n=7608). Participants were excluded if they did not have continuous Part A and B Medicare coverage, or had HMO coverage in the 12 months prior to their enrollment in NHATS (n=2827), if they had breast, colorectal, lung, or prostate cancer diagnoses in their Medicare claims within 1 year prior to their enrollment interview (n=607), or a prior self-reported history of cancer in their enrollment interview (n=306), as these would indicate prevalent cancers. Self-reported history of cancer was identified if participants answered “yes” to the question “Please tell me if a doctor ever told you that you had cancer”, and “yes” to the subsequent question about specific cancer types as “breast”, “prostate” or “colon”. Since NHATS does not ask specifically about a history of lung cancer, participants who report an “other” cancer type at their enrollment interview were also excluded, as these could have included lung cancer. For participants without a history of cancer (n=3868), the Medicare fee for service (FFS) inpatient, outpatient, and NCH files were queried from the time of enrollment in NHATS through the end of 2016. Participants who had at least one ICD-9 code (until October 2015) or ICD-10 (from October 2015 onward) for breast, colorectal, lung, or prostate cancer in that time frame (see Supplemental Table 1 for list of ICD-9 and ICD-10 codes) were identified as incident cancer cases (n=297). As ICD-9/ICD-10 codes are more detailed than the cancer questions in the NHATS survey, we were able to differentiate between each of the 4 major types of cancer for analysis. The date of the first appearance of a diagnosis code was used as the date of diagnosis and to categorize the type of cancer. The NHATS interview that most closely preceded the incident cancer diagnosis was designated the pre-cancer interview, and the nearest interview after the diagnosis was designated the post-cancer-interview. Participants who self-reported a new cancer at or before their pre-cancer interview (prior to the diagnosis date based on claims) were excluded (n=23). Participants with a pre-cancer interview and a post-cancer interview were included in the analysis, resulting in an analytic sample of 136 subjects (Figure 1). For a post-hoc analysis comparing the change in QoL indicators over time between our cancer group and a non-cancer group, we queried the group of NHATS participants with no history of cancer at their enrollment interview (n=3868), and who were not recorded as developing cancer. From these participants, we selected a sample who were enrolled in NHATS in 2011 and had a second interview in 2012 (n=2794) as our non-cancer comparison group.
Figure 1.
Selection criteria used to identify eligible NHATS cases.
Measures
Quality of life-related outcome measures were assessed at the time of the pre-cancer diagnosis interview and of the post-cancer diagnosis interview, in order to evaluate the change over time. While NHATS does not include standardized measures of health-related QoL (e.g., SF-12), we assessed five indicators that constitute overall QoL including mental health (i.e., anxiety and depression symptoms), self-reported physical health, pain, and functional limitations. These indicators directly map on to five of the SF-12 subscales, substantiating their use as QoL indicators for the current study.
Mental health
Participants were categorized as having probable depression if they scored ≥3 on the PHQ-2 scale (range: 0–6) (35) and as having probable anxiety if they scored ≥3 on the GAD-2 scale (range: 0–6), indicating depressive and/or anxiety symptoms within the last month (36).
Physical health
Participants were asked to self-report their health as “poor”, “fair”, “good”, “very good”, or “excellent”, and to indicate whether or not they were bothered by pain in the last month.
Functional limitations
Participants were asked to indicate whether they received help within the last month with any of the following activities of daily living (ADLs); eating, getting cleaned up, using the toilet, dressing, walking around inside, and getting out of bed.
Other covariates
Older adults’ demographic characteristics included age (at each interview and at diagnosis), gender, race, education level, marital status, income quartile, and number of chronic conditions (including heart disease, hypertension, arthritis, osteoporosis, diabetes, lung disease, stroke, and hip fracture).
Data analysis
Differences in demographics and quality of life were compared across cancer groups at the time of the pre-cancer interview, using χ2 or Fisher’s exact tests for categorical variables and Kruskal–Wallis for continuous variables. Univariate changes in each QoL-related item from pre-cancer interview to post-cancer interview were assessed using McNemar’s test, overall and by cancer type. A generalized linear model (GLM), applying a logit link function, with a generalized estimating equation (GEE) was used to estimate odds ratios (OR) and 95% confidence intervals (CI) of QoL-related items post diagnosis, compared to pre-diagnosis to examine the overall effect of time. An additional model including an interaction between time and cancer type was used to assess differences across cancer types over time. All analyses were adjusted for cancer type, age at diagnosis, gender, race, education level, marital status, income, and number of chronic conditions. Sensitivity analyses were conducted on continuous measures of depression score, anxiety score, and number of ADLs requiring assistance, as well as for all categories of self-reported health. Analyses were also conducted without gender as a covariate, as there is strong correlation between gender and cancer type. Lastly, to account for the complex sampling strategy of NHATS, a sensitivity analysis with the survey procedures in SAS was performed using multivariable logistic regression with an indicator variable for survey time as the main predictor of interest. A post-hoc analysis comparing the overall cancer group to a non-cancer group with interviews in 2011 and 2012 was done to assess whether the population in general experienced similar changes in QoL indicators over time, by examining the interaction between time and cancer status. All analyses were conducted using SAS software v9.4 (SAS Institute, Cary, NC).
Results
Description of sample
There were 136 participants with an incident diagnosis of breast, colorectal, lung, or prostate cancer and both pre-cancer and post-cancer interviews completed in NHATS; 37 with breast cancer, 26 with colorectal, 26 with lung, and 47 with prostate cancer. The pre-cancer interview was conducted an average of 7.5 (SE: 0.3) months before diagnosis (range: 0.1–15.5 months) and the post-cancer interview was conducted an average of 5.5 (SE: 0.3) months after diagnosis (range: 0.1–15.15 months). On average, the pre- and post-cancer interviews were 13 (SE: 0.1) months apart (range: 9.0–17.0 months). At the time of their diagnosis, participants were, on average, 80.0 years old (SE: 0.6), with similar ages across cancer groups. The sample was approximately evenly split between men and women, and about 70% non-Hispanic White. Those with prostate cancer were significantly more likely than those with other cancers to be married (p=.0346), while individuals with breast cancer were more likely to have attained more than a high school education than those with other cancer types (p=.0030) (Table 1).
Table 1.
Demographics of sample at the time of pre-cancer interview, overall and according to cancer type.
| %a, mean (SE) | ||||||
|---|---|---|---|---|---|---|
| Overall | Breast | Colorectal | Lung | Prostate | ||
| Variable | N = 136 | n = 37 (27.2%) | n = 26 (19.1%) | n = 26 (19.1%) | n = 47 (34.6%) | p-valuec |
| Demographics | ||||||
| Age at diagnosis (years) | 80.0 (0.6) | 81.5 (1.5) | 80.2 (1.4) | 79.0 (1.2) | 79.3 (1.0) | .6578 |
| Age at pre-cancer interview (years) | 78.9 (0.6) | 80.4 (1.5) | 79.1 (1.4) | 77.9 (1.2) | 78.3 (1.0) | .6603 |
| Gender | ||||||
| Male | b | – | <42.3b | 46.2 | 100.0 | – |
| Female | b | 100.0 | ≥42.3b | 53.9 | – | |
| Race | .3061 | |||||
| Non-Hispanic White | 69.4 | 67.6 | ≥42.3b | ≥42.3b | 65.2 | |
| Black/Hispanic/Other | 30.6 | 32.4 | <42.3b | <42.3b | 34.8 | |
| Marital status | .0346 | |||||
| Married/living with partner | 51.1 | 38.9 | 42.3 | 46.2 | 68.1 | |
| Unmarried | 48.9 | 61.1 | 57.7 | 53.9 | 31.9 | |
| Education | .0030 | |||||
| ≤High school | 53.7 | 29.7 | ≥42.3b | ≥42.3b | 55.3 | |
| >High school | 46.3 | 70.3 | <42.3b | <42.3b | 44.7 | |
| Household income (2016 $) | ||||||
| Below median (<$28,808) | 44.9 | 46.0 | 53.9 | 46.2 | 38.3 | .6364 |
| At or above median (≥$28,808) | 55.2 | 54.0 | 46.2 | 53.9 | 61.7 | |
| Number of chronic conditions | 2.6 (0.1) | 2.8 (0.2) | 2.7 (0.3) | 2.5 (0.3) | 2.4 (0.2) | .5761 |
| Quality of life indicators | ||||||
| Depression Score | ||||||
| Mean (SE) | 1.0 (0.1) | 0.7 (0.2) | 0.9 (0.3) | 0.9 (0.2) | 1.2 (0.2) | .5473 |
| Anxiety Score | ||||||
| Mean (SE) | 0.8 (0.1) | 0.8 (0.2) | 1.0 (0.3) | 0.7 (0.2) | 0.7 (0.2) | .7775 |
| Number of ADLs requiring help | ||||||
| Mean (SE) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.2) | 0.3 (0.2) | 0.4 (0.2) | .6487 |
Percentages presented out of non-missing values.
Exact percentages masked to protect against identification of patients.
χ2 test (or Fisher’s exact test) for categorical variables and Kruskal–Wallis test for continuous variables.
ADL: Activity of Daily Living; SE: Standard Error.
Changes from pre- to post-cancer diagnosis
Univariable
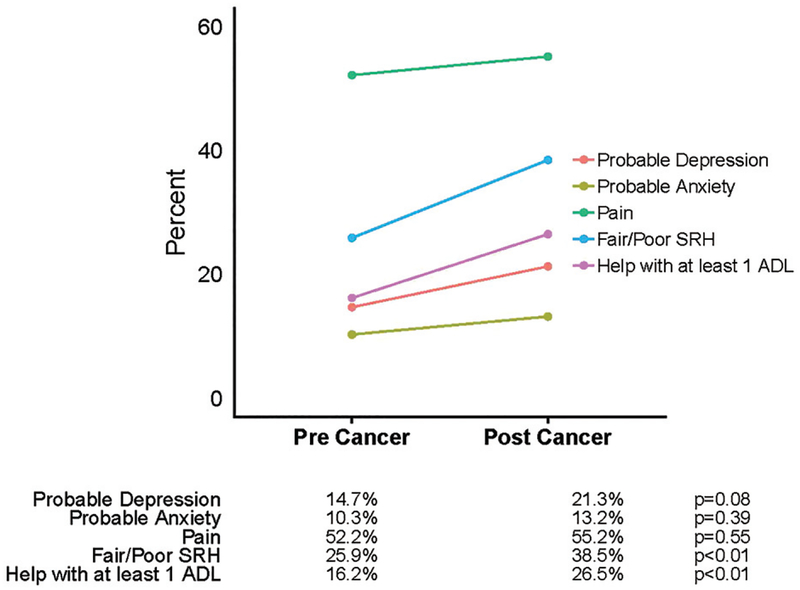
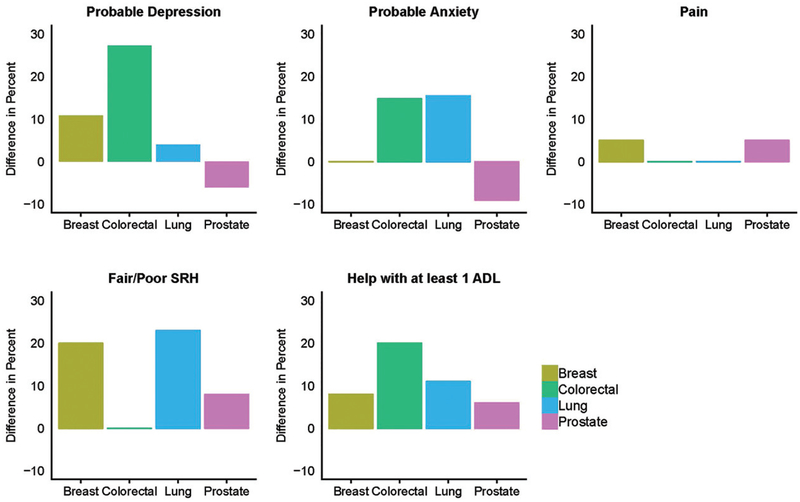
At the pre-cancer interview, there was no significant difference in QoL-related items between cancer types. From pre to post cancer diagnosis, the proportion of those with probable depression increased from 14.7 to 21.3% (p=.0833); the proportion of those with self-reported fair/poor health increased from 25.9 to 38.5% (p=.0079) (those reporting poor health more than doubled from the pre-cancer to post-cancer interview); and the proportion of those needing help with ≥1 ADL increased from 16.2 to 26.5% (p=.0028). Among those receiving help with ≥1 ADL, the most common areas of help at the pre-cancer interview were dressing (>50%), followed by bathing, walking around inside, and eating. At the post-cancer interview, there was an increase across all areas of ADLs. Dressing remained the most common (64%), followed by walking around inside and bathing (53%), getting out bed (44%), and eating and using the toilet (33%). There was no significant increase in the percent of people with probable anxiety (10.3 to 13.2%, p=.3938) or those reporting pain (52.2 to 55.2%, p=.5465). Changes over time overall are shown in Figure 2, and differences (post-cancer minus pre-cancer) by cancer type are shown in Figure 3.
Figure 2.
Change in quality of life indicators from pre- to post-cancer diagnosis.
Figure 3.
Difference in percent (post- minus pre-cancer diagnosis) of respondents reporting quality of life indicators, according to cancer type. SRH: Self-Reported Health; ADL: Activity of Daily Living. Difference is shown to mask small cell sizes at both time points.
Multivariable
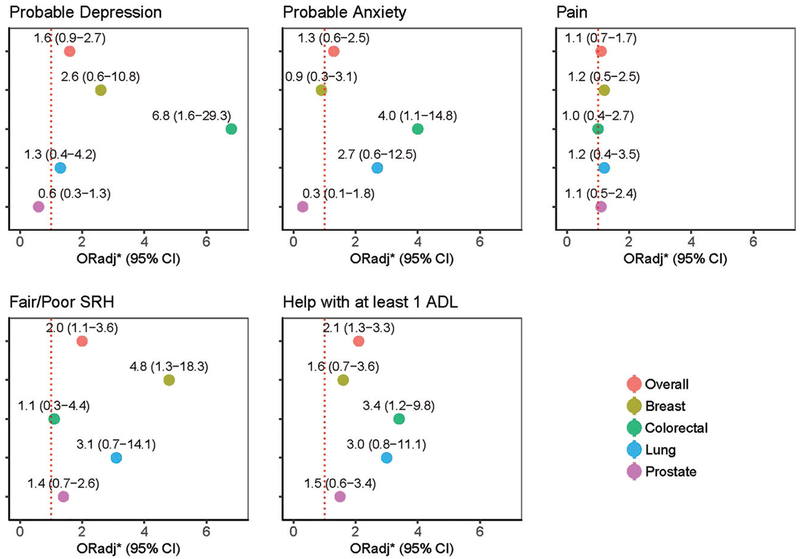
Among the entire sample, there was a significant increase in the relative odds of fair/poor self-reported health (ORadj: 2.0, 95% CI: 1.1–3.6), and help with ≥1 ADL (ORadj: 2.1, 95% CI: 1.3–3.3) at the post-cancer interview, compared to the pre-cancer interview, while the increase in odds of probable depression was marginally significant (ORadj: 1.6, 95% CI: 0.9–2.7). There was no significant increase in odds of probable anxiety or pain in the overall group. When including a time-*cancer type interaction, there was a significant increase in the relative odds of probable depression and probable anxiety at the post- compared to the pre-cancer interview for those with colorectal cancer (ORadj: 6.8, 95% CI: 1.6–29.3; ORadj: 4.0, 95% CI: 1.1–14.8, respectively). There was no significant change for breast, lung, or prostate cancer for either outcome. The odds of fair/poor self-reported health at the post cancer interview significantly increased for breast (ORadj: 4.8, 95% CI: 1.3–18.3) with no significant change for colorectal, lung, or prostate cancer. Those with colorectal cancer also had a significant increase in help with ≥1 ADL, compared to the pre-cancer interview (ORadj: 3.4, 95% CI: 1.2–9.8); while there was no significant change for the other cancer types. The odds of pain at the post cancer interview, compared to the pre-cancer interview, were similar across all cancer types, with no significant change over time. Although results are not all significant across the cancer subtypes, we saw a general trend of worsening quality of life after a cancer diagnosis, with more muted changes in the prostate cancer subgroup (Figure 4).
Figure 4.
Adjusted odds of poor quality of life indicators post-cancer diagnosis, compared to pre-cancer diagnosis.
Covariates in the model were examined to determine whether they were associated with QoL-related items. There was a significant inverse association between income and fair/poor self-reported health (ORadj: 0.2, 95% CI: 0.1–0.5), and help with ≥1 ADL (ORadj: 0.2, 95% CI: 0.1–0.6) for those with ≥median income, compared to those with < median income. Increased age at diagnosis was significantly associated with help with ≥1 ADL (ORadj: 1.1, 95% CI: 1.0–1.1). An increased number of chronic conditions was significantly associated with pain (ORadj:1.6, 95% CI: 1.3–2.1), fair/poor self-reported health (ORadj:2.0, 95% CI: 1.4–2.6), and help with 1 ADL (ORadj: 1.5, 95% CI: 1.1–1.9).
Post-hoc analysis
When comparing the non-cancer group (n=2794) to our overall cancer group, the cancer group was significantly less female. At baseline, there were no significant differences in age, race, marital status, education, income, number of chronic conditions, or in any of the QoL-related indicators. In a model adjusted for all covariates, we found a significant interaction between time and presence of cancer for fair/poor self-reported health (p=.0102) and marginally significant interactions for help with ADLs (p=.0696) and probable depression (p=.1054). Although not all significant, those without cancer generally had minimal declines, while those with cancer had more extreme changes over time (Table 2).
Table 2.
Adjusted odds of poor quality of life indicators at the second interview, compared to the first interview, by cancer status (non-cancer vs. cancer).
| Quality of life indicator | Non-cancer group ORadja (95% CI) | Cancer group ORadja (95% CI) | p-value (time*cancer status interaction) |
|---|---|---|---|
| Probable depression | 1.0 (0.8–1.1) | 1.5 (0.9–2.7) | .1054 |
| Probable anxiety | 1.0 (0.8–1.1) | 1.3 (0.6–2.5) | .4817 |
| Pain | 1.0 (0.9–1.1) | 1.1 (0.7–1.7) | .6163 |
| Fair/poor SRH | 0.9 (0.8–1.0) | 1.9 (1.1–3.0) | .0102 |
| Help with ≥1 ADL | 1.2 (1.1–1.3) | 1.8 (1.1–2.8) | .0696 |
Model of cancer status (yes/no) + time indicator (1st or 2nd interview) + cancer status* time, adjusted for age at interview, gender, race, education, marital status, income, and number of chronic conditions.
ADL: Activity of daily living; SRH: Self-reported health.
Sensitivity analyses
In a sensitivity analysis incorporating the complex sampling design, the associations follow the trends found in the unweighted analysis (see Supplemental Table 2), particularly for increases in probable depression, fair/poor self-reported health, and help with ≥1 ADL. Additionally, the sensitivity analysis found a significant increase in probable anxiety at the post cancer interview. As was seen in the original analyses, there was no significant change on any of the indicators for those with prostate cancer. When treating depression score, anxiety score, and number of ADLs requiring assistance as continuous outcomes, there was a significant increase over time in mean depression score (βadj: 0.29, 95% CI: 0.04–0.55) and mean number of ADLs requiring assistance (βadj: 0.52, 95% CI: 0.20–0.84), while there was no significant change in anxiety score (βadj: 0.28, 95% CI: −0.003–0.56). An analysis using all 5 categories of self-reported health found no significant change over time (ORadj: 1.14, 95% CI: 0.75–1.74) (Supplemental Table 3). Although significance varied from the original analyses, particularly for the analyses of cancer subgroups, the general trends remained similar. A sensitivity analysis excluding gender as a covariate yielded extremely similar results to the initial analysis (data not shown).
Discussion
The present analysis examines the changes in measures associated with QoL from pre- to post-cancer diagnosis in a sample of community-dwelling older adult participants in NHATS. Our final analytic sample consisted of 136 participants with a diagnosis of breast, colorectal, lung or prostate cancer who had completed an interview both pre and post cancer diagnosis. From pre- to post-diagnosis, when adjusting for demographics, there was a significant increase in the relative odds of fair/poor self-reported health and needing help with ADLs and a marginal increase in depression. There was no notable change in probable anxiety or pain from pre- to post-diagnosis. Although differences across cancer types was not the main objective of study, we found that baseline QoL-related measures were similar across cancer types. When examining the change over time, although many results were not statistically significant, we did see consistent trends of worsening QoL indicators after a cancer diagnosis, particularly among those with breast, colorectal, and lung cancer. There was no significant difference in any of the outcomes over time for those with prostate cancer, and the measured change was consistently less extreme for these patients. This is not surprising given the potentially better prognosis of prostate cancer compared to the other cancers that were examined in analyses (37).
Overall, QoL indicators among participants were low even prior to diagnosis regardless of future cancer status. A post-hoc comparison of the NHATS population sample without cancer and the analytical sample indicated that at the pre-diagnosis interview our sample did not differ significantly from those without a history of one of the four cancers, who had interviews at enrollment and 1 year later (n=2794). However, at the post-diagnosis interview, individuals with cancer were more likely to report fair/poor self-reported health as well as help with ADLs and probable depression, although the interaction effect was only statistically significant for the decline in self-reported health for the population with cancer as compared to those with no cancer history. These results indicate that while older age may contribute to overall declines in measures associated with QoL, a cancer diagnosis can accelerate those declines among older adults and suggests that as the population ages, greater attention to factors associated with QoL among older adults, particularly those with a cancer diagnosis, is warranted.
Among the entire sample, there was a significant increase in the relative odds of fair/poor self-reported health and help with ADLs at the post-cancer interview, compared to the pre-cancer interview. Because cancer is primarily a disease of older adults, its impacts on factors associated with QoL specifically among older Americans has significant implications for the treatment of older cancer patients. Our research suggests that a diagnosis of cancer can have serious negative impacts on the functioning of older cancer patients, which in turn may impact mortality. Thus, baseline QoL among older adult cancer patients and potential declines in QoL post-diagnosis should factor into treatment decisions as well as interventions during and after treatment (16–20,24). For example, it would be important to assess a patient’s existing social supports in order to best prepare for potential declines in ADLs and needs regarding overall support in order to attenuate declines in function.
There was no notable change in probable anxiety or pain from pre- to post-diagnosis among the overall sample and the change in depression was marginal, but these changes were observed in models which included interactions by cancer type. It is possible that cancer-specific factors such as staging and prognosis have greater impact on these specific variables as opposed to ADLs and overall health (38,39). Also, because timing of treatment is not factored into analyses, it is possible that post-surgery and/or post-chemotherapy changes in anxiety, depression and pain would be easier to detect should treatment timing be the point at which change is assessed. Our group found that pain, for example, increases after surgery for lung cancer specifically (40,41). Further research should examine the nuances between changes in QoL factors between pre-diagnosis, diagnosis/pre-treatment, and post-treatment. Further, tailoring post-treatment psychosocial interventions for particular patients to address particular needs is important as clinicians start to think about personalized approaches to care. It is possible that support groups or mind-body interventions will be particularly beneficial for patients with specific cancer types at specific stages of treatment.
Notably, several demographic characteristics were significantly associated with increased odds of poor QoL-related outcomes post-diagnosis. There was a significant inverse association between income and fair/poor self-reported health and help with ≥1 ADL. This relationship could reflect a lack of access to higher-quality cancer care among lower-income older cancer patients or a lack of access to other resources that may could improve QoL, like in-home care or assistance. The relationship between socioeconomic status and self-reported health among older cancer patients is a potential point of intervention and should be examined in future research. In addition, increased age at diagnosis was significantly associated with help with ≥1 ADL. This may reflect the fact that increased age contributes to lower QoL even before a cancer diagnosis which, in turn, could contribute to greater changes in QoL-related factors post-diagnosis. This points to the need to for providers to pay particular attention to much older patients’ needs and existing support systems after a cancer diagnosis is made.
Study limitations
The findings should be interpreted in the context of the study limitations. Due to eligibility criteria, our final analytic sample is small in comparison to the larger sampling frame. Due to this small sample size, we may have lacked sufficient statistical power to capture changes over time in some of the QoL indicators, particularly among the cancer type subgroups. In addition, our analysis did not include information in regard to prognosis, staging and treatment, all data points pertinent to QoL following a cancer diagnosis. Prognosis and staging information cannot be found in Medicare claims, and inclusion of treatment information would have necessitated more stringent Medicare coverage requirements, which would have further limited our sample size. Furthermore, standard treatment differs by stage and cancer type, so treatment burdens likely would not have been comparable across groups, Also, bias may have been introduced by limiting to those who had a second interview since those who had a second interview generally lived longer than those who were identified with incident cancer, but did not have a follow up interview. Our analysis of differences indicated that the final analytic sample may have been healthier, had earlier stage cancer and a better prognosis and were part of demographic groups with higher QoL which could potentially bias the results to the null in that less change in QoL could be evident. Another limitation to note is the correlation between gender and cancer type, with prostate and breast each present in a single gender. We ran our analyses without the inclusion of gender and found very similar results, but due to sample size constraints, were unable to run analyses stratified by gender. Given this constraint, it is difficult to fully untangle the effects of gender on QoL.
This is one of the first studies, to our knowledge, to assess changes in patient-centered outcomes after a cancer diagnosis in an older adult population. The findings from this study can be used to help older cancer patients, their families and their providers to make treatment decisions regarding how to best maximize factors related to better QoL. The results suggest the importance of the consideration of baseline QoL-related indicators when making treatment decisions for older adult cancer patients. Furthermore, the results suggest the potential for interventions to target important QoL-related factors in order to prepare patients for post-treatment experience and ensure that there are supportive services in place to buffer/address the potential decreases in physical and mental health and functioning. Consistent with socioemotional selectivity theory (11), it is possible that maximizing emotion-related goals, such as increasing social interaction and support, will result in more positive outcomes even for older adults facing cancer. Finally, particular attention should be focused on vulnerable populations including low-income and older patients, who may be at a greater risk for declines in QoL.
Supplementary Material
Funding
NHATS is sponsored by NIA Grant U01AG032947 through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health.
Footnotes
Disclosure statement
The authors have no conflicts to disclose.
Supplemental data for this article can be accessed here.
References
- 1.Vespa J, Armstrong DM, Medina L. Demographic turning points for the United States: population projections for 2020 to 2060 Current population reports. Washington, D.C.: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; 2018. [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Hill NL, McDermott C, Mogle J, Munoz E, DePasquale N, Wion R, et al. Subjective cognitive impairment and quality of life: a systematic review. Int Psychogeriatr. 2017; 29(12):1965–1977. doi: 10.1017/S1041610217001636. [DOI] [PubMed] [Google Scholar]
- 4.Baernholdt M, Hinton I, Yan G, Rose K, Mattos M. Factors associated with quality of life in older adults in the United States. Qual Life Res. 2012;21(3): 527–534. doi: 10.1007/s11136-011-9954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steffens DC, Fisher GG, Langa KM, Potter GG, Plassman BL. Prevalence of depression among older Americans: the aging, demographics and memory study. Int Psychogeriatr. 2009;21(05):879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maddux RE, Delrahim KK, Rapaport MH. Quality of life in geriatric patients with mood and anxiety disorders. CNS Spectr. 2003;8(S3):35–47. [DOI] [PubMed] [Google Scholar]
- 7.Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sørensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2(1):52. doi: 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki M, Ohyama N, Yamada K, Kanamori M. The relationship between fear of falling, activities of daily living and quality of life among elderly individuals. Nurs Health Sci. 2002;4(4):155–161. doi: 10.1046/j.1442-2018.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 9.Shin KR, Byeon YS, Kang Y, Oak J. A study on physical symptom, activity of daily living, and health-related quality of life (HRQoL) in the community-dwelling older adults. Taehan Kanho Hakhoe Chi. 2008;38(3):437–444. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen J, Neyens JCL, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11(1):33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LL Carstensen, Mikels J. At the intersection of emotion and cognition aging and the positivity effect. Curr Dir Psychol Sci. 2005;14(3):117–121. doi: 10.1111/j.0963-7214.2005.00348.x. [DOI] [Google Scholar]
- 12.Garin N, Olaya B, Moneta MV, Miret M, Lobo A, Ayuso-Mateos JL, et al. Impact of multimorbidity on disability and quality of life in the spanish older population. Plos One. 2014;9(11):e111498. doi: 10.1371/journal.pone.0111498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brettschneider C, Leicht H, Bickel H, Dahlhaus A, Fuchs A, Gensichen J, et al. Relative impact of multi-morbid chronic conditions on health-related quality of life – results from the multicare cohort study. Plos One. 2013;8(6):e66742. doi: 10.1371/journal.pone.0066742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. JCO. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 15.Mishel MH, Hostetter T, King B, Graham V. Predictors of psychosocial adjustment in patients newly diagnosed with gynecological cancer. Cancer Nurs. 1984;7(4):291. [PubMed] [Google Scholar]
- 16.Okamura M, Yamawaki S, Akechi T, Taniguchi K, Uchitomi Y. Psychiatric disorders following first breast cancer recurrence: prevalence, associated factors and relationship to quality of life. J Clin Oncol. 2005;35(6):302–309. doi: 10.1093/jjco/hyi097. [DOI] [PubMed] [Google Scholar]
- 17.Hyphantis T, Almyroudi A, Paika V, Degner LF, Carvalho AF, Pavlidis N. Anxiety, depression and defense mechanisms associated with treatment decisional preferences and quality of life in non-metastatic breast cancer: a 1-year prospective study. Psychooncology. 2013;22(11):2470–2477. doi: 10.1002/pon.3308. [DOI] [PubMed] [Google Scholar]
- 18.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40(11): 1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, Goldhirsch A. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet. 2000;356(9238): 1326–1327. doi: 10.1016/S0140-6736(00)02821-X. [DOI] [PubMed] [Google Scholar]
- 20.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 21.Ravi P, Karakiewicz PI, Roghmann F, Gandaglia G, Choueiri TK, Menon M, et al. Mental health outcomes in elderly men with prostate cancer. Urol Oncol. 2014;32(8):1333–1340. doi: 10.1016/j.urolonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Parpa E, Tsilika E, Gennimata V, Mystakidou K. Elderly cancer patients’ psychopathology: a systematic review: aging and mental health. Arch Gerontol Geriatr. 2015;60(1):9–15. doi: 10.1016/j.archger.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. 2009;50(4):383–391. doi: 10.1176/appi.psy.50.4.383. [DOI] [PubMed] [Google Scholar]
- 24.Linden W, Yi D, Barroetavena MC, MacKenzie R, Doll R. Development and validation of a psychosocial screening instrument for cancer. Health Qual Life Outcomes. 2005;3(1):54. doi: 10.1186/1477-7525-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llorente MD, Burke M, Gregory GR, Bosworth HB, Grambow SC, Horner RD,et al. Prostate cancer: a significant risk factor for late-life suicide. Am J Geriatr Psychiatr. 2005;13(3):195–201. doi: 10.1176/appi.ajgp.13.3.195. [DOI] [PubMed] [Google Scholar]
- 26.Rasic DT, Belik S-L, Bolton JM, Chochinov HM, Sareen J. Cancer, mental disorders, suicidal ideation and attempts in a large community sample. Psychooncology. 2008;17(7):660–667. doi: 10.1002/pon.1292. [DOI] [PubMed] [Google Scholar]
- 27.Rao A, Cohen HJ. Symptom management in the elderly cancer patient: fatigue, pain, and depression. JNCI Monogr. 2004;2004(32):150–157. doi: 10.1093/jncimonographs/lgh031. [DOI] [PubMed] [Google Scholar]
- 28.Giacalone A, Quitadamo D, Zanet E, Berretta M, Spina M, Tirelli U. Cancer-related fatigue in the elderly. Support Care Cancer. 2013;21(10):2899–2911. doi: 10.1007/s00520-013-1897-1. [DOI] [PubMed] [Google Scholar]
- 29.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. JCO. 2008;26(8): 1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 30.Montazeri A Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7(1): 102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedding U, Pientka L, Höffken K. Quality-of-life in elderly patients with cancer: a short review. Eur J Cancer. 2007;43(15):2203–2210. doi: 10.1016/j.ejca.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Puts MTE, Papoutsis A, Springall E, Tourangeau AE. A systematic review of unmet needs of newly diagnosed older cancer patients undergoing active cancer treatment. Support Care Cancer. 2012;20(7): 1377–1394. doi: 10.1007/s00520-012-1450-7. [DOI] [PubMed] [Google Scholar]
- 33.Montaquila J, Freedman V, Edwards B, Kasper JD. National Health and Aging Trends Study Round 1 Sample Design and Selection. NHATS Technical Paper #1. Johns Hopkins Univ Sch Public Health. 2012. [Google Scholar]
- 34.Kasper JD, Freedman V. National Health and Aging Trends Study User Guide: Rounds 1, 2, 3, 4 & 5 Beta Release. 2016.
- 35.Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 36.Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 2008;46(3): 266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 37.American Cancer Society. Cancer Factors & Figures 2017. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 38.Cardoso G, Graca J, Klut C, Trancas B, Papoila A. Depression and anxiety symptoms following cancer diagnosis: a cross-sectional study. Psychol Health Med. 2016;21(5):562–570. doi: 10.1080/13548506.2015.1125006. [DOI] [PubMed] [Google Scholar]
- 39.Johanes C, Monoarfa RA, Ismail RI, Umbas R. Anxiety level of early- and late-stage prostate cancer patients. Prostate Int. 2013;1(4):177–182. doi: 10.12954/PI.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz RM, Yip R, Olkin I, Sikavi D, Taioli E, Henschke C. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14(1):37–44. doi: 10.12788/jcso.0205. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz RM, Yip R, Flores RM, Olkin I, Taioli E, Henschke C, et al. The impact of resection method and patient factors on quality of life among stage IA non-small cell lung cancer surgical patients. J Surg Oncol. 2017;115(2):173–180. doi: 10.1002/jso.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.