Abstract
Recently, the fungal sphingolipid glucosylceramide (GlcCer) synthesis has emerged as a highly promising new target for drug discovery of next-generation antifungal agents, and we found two aromatic acylhydrazones as effective inhibitors of GlcCer synthesis based on HTP screening. In the present work, we have designed libraries of new aromatic acylhydrazones, evaluated their antifungal activities (MIC80 and time-kill profile) against C. neoformans and performed an extensive SAR study, which led to the identification of five promising lead compounds, exhibiting excellent fungicidal activities with very large selectivity index. Moreover, two compounds demonstrated broad spectrum antifungal activity against six other clinically relevant fungal strains. These five lead compounds were examined for their synergism/cooperativity with five clinical drugs against seven fungal strains and very encouraging results were obtained, e.g., the combination of all five lead compounds with voriconazole exhibited either synergistic or additive effect to all seven fungal strains.
Graphical Abstract
INTRODUCTION
Invasive fungal infections (IFIs), such as cryptococcosis, aspergillosis and candidiasis, are a serious threat to human health, as IFIs are associated with a large number of deaths which is similar in number to that of tuberculosis or malaria.1 Recent statistics suggest that more than 150 million people suffer from serious fungal infections and it is estimated that annually around 1.5–2 million deaths occur as a result of these invasive fungal infections.2, 3 IFIs are highly prevalent among individuals with low immunity such as HIV positive patients, organ transplant and cancer patients receiving immunosuppressants, as well as pediatric and geriatric patients.4–8 Cryptococcus neoformans (C. neoformans) that causes meningoencephalitis is responsible for 600,000 deaths per year, accounting for 15% AIDS-related deaths globally.9, 10 Candida species can cause invasive candidiasis that includes blood-derived and deep-tissue infections in hospitalized individuals who are treated for various conditions.11 Candida species are also a major concern for immunocompromised patients.12, 13 Candidemia caused by Candida species is associated with poor prognosis and contributes to ~30–60% mortality rate.14 Aspergillus species was recently estimated to cause ca, 250,000 cases of invasive aspergillosis.2
Current treatment options for IFIs consist of three major classes of drugs which include azoles (e.g. fluconazole), polyenes (e.g. amphotericin B) and echinocandins (e.g. caspofungin).15 These drugs are associated with serious side effects such as nephrotoxicity, narrow spectrum of activity and drug resistance.16–19 Amphotericin B, the last resort anti-fungal agent, is associated with adverse drug-drug interactions with anticancer agents and azoles.20, 21 In spite of all those drawbacks, the same three classes of drugs have been used to date, because no newer and more efficacious anti-fungal drugs have been approved by FDA for some time. Hence, there is a dire need for new, efficacious antifungal drugs that can overcome drug resistance with novel mechanism of action.
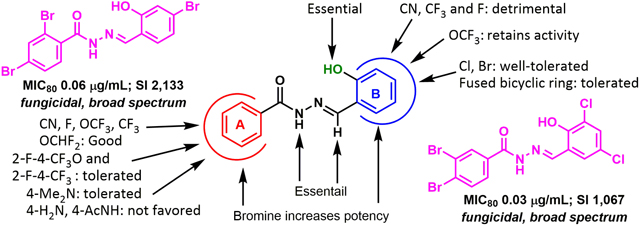
In this context, the fungal sphingolipid glucosylceramide (GlcCer) synthesis has emerged as a highly promising new target for the development of next-generation antifungal agents.22–24 GlcCer is essential for the cell division of pathogenic fungi such as C. neoformans, Candida albicans (C. albicans) and Aspergillus fumigatus (A. funigatus), and responsible for their virulence.23–27 It has been shown that fungal cells lacking GlcCer cannot replicate in neutral or alkaline environments.23, 24, 27 This finding clearly indicates the importance of GlcCer for virulence in alveolar spaces, cerebrospinal fluid or bloodstream of the host wherein the pH is neutral or alkaline, and thus makes GlcCer a promising target for drug discovery.23, 24 As shown in Figure 1A, aromatic acylhydrazone 1, bearing a salicylaldehyde-hydrazone moiety, selectively inhibited the synthesis of fungal GlcCer in a dose-dependent manner without affecting the synthesis of mammalian GlcCer.23, 24 When the efficacy of 1 and 2 was evaluated in vivo, using mice infected with C. neoformans, both compounds substantially improved the survival of mice, compared to the untreated ones (Figure 1B).22 Moreover, 2 exhibited better antifungal activity than fluconazole which is a clinically used drug to treat fungal infections.
Figure 1.
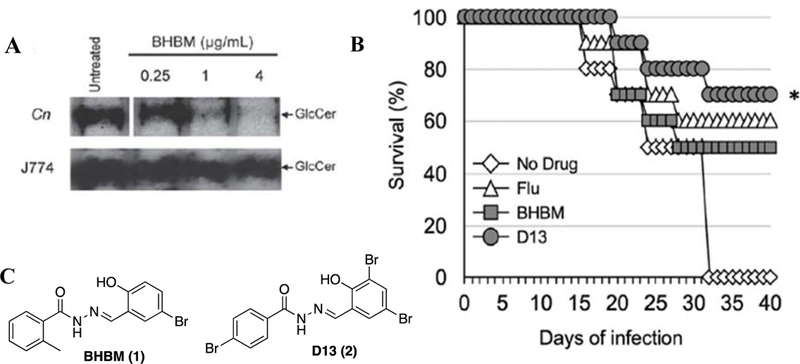
(A) Analysis of the synthesis of glucosylceramide (GlcCer) in C. neoformans or J774 cells labelled with [3H] palmitate and treated with 1 at the indicated concentrations; (B) Survival studies of mice infected intranasally with 5 × 105 C. neoformans cells and treated via i.p. injection on the day of infection with 1.2 mg/kg/day of fluconazole (Flu), 1 or 2. *Compound 2 versus no drug, P value of 0.0018; (C) Structures of 1 (BHBM) and 2 (D13).
Furthermore, 2 was effective against A. fumigatus in vivo, and more efficacious than the clinically used antifungal drug, voriconazole, with very high “selectivity index” (SI = LD50/MIC80) (>500).22 Although aromatic acylhydrazones are known to be pan assay interference compounds (PAINS), our initial hits possessed promising antifungal activity not only in vitro, but also in vivo.22–24, 28 Moreover, the fact that these aromatic acylhydrazones, bearing a salicylaldehyde-hydrazone moiety, were also highly fungus-selective (vide supra), gave us confidence to further explore this class of compounds as next-generation antifungal agents.29 We describe here the library synthesis, biological evaluations and SAR study of new aromatic acylhydrazones, bearing a salicylaldehyde-hydrazone moiety, against C. neoformans and six other pathogenic fungi. Our study has led to the identification of five highly potent and selective lead compounds, which have been further examined for their synergy/cooperativity with five antifungal drugs currently used in clinic against seven pathogenic fungal strains.
RESULTS AND DISCUSSION
Library synthesis.
For the synthesis of initial library of aromatic acylhydrazones 5.0~5.7, commercially available benzoyl chlorides or benzoic acids (1.0~1.3) were converted to their methyl esters (2.0~2.3), which were reacted with excess hydrazine monohydrate under reflux to give the corresponding hydrazides 3.0~3.3. Hydrazides 3, thus obtained, were condensed with different salicylaldehydes 4a-g in the presence of an arenesulfonic acid resin as a catalyst in DMSO. Excess unreacted aldehyde 4 was removed from the reaction mixture by treating with an aminomethylated resin. The reaction mixture was then filtered to afford the corresponding aromatic acylhydrazones 5.0~5.4 (Scheme 1, Table 1).
Scheme 1.
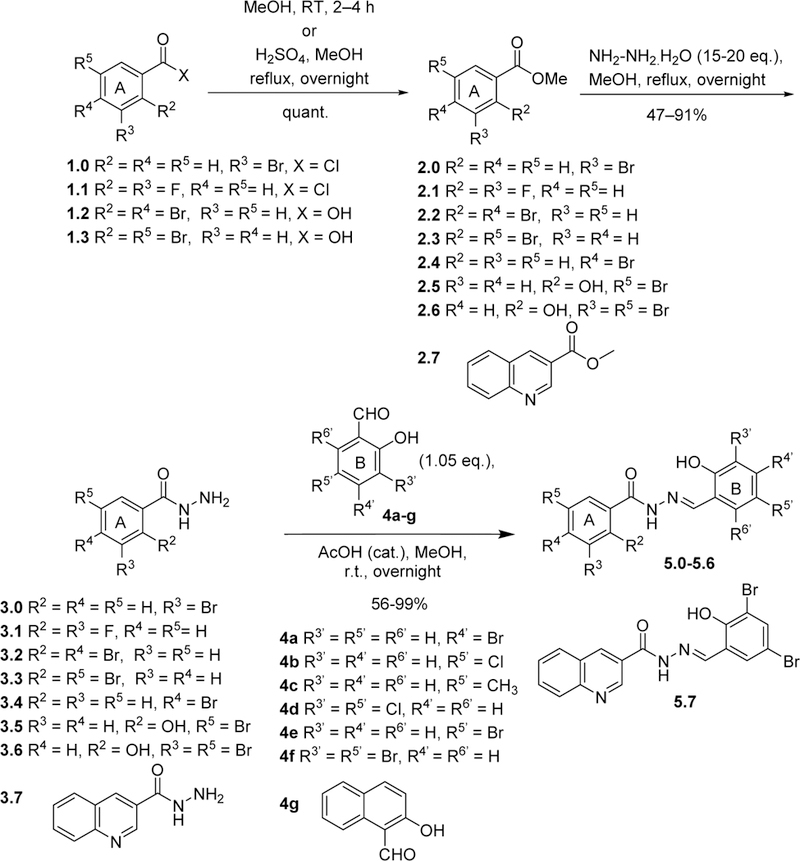
Synthesis of the initial library of acylhydrazones 5.0~5.7
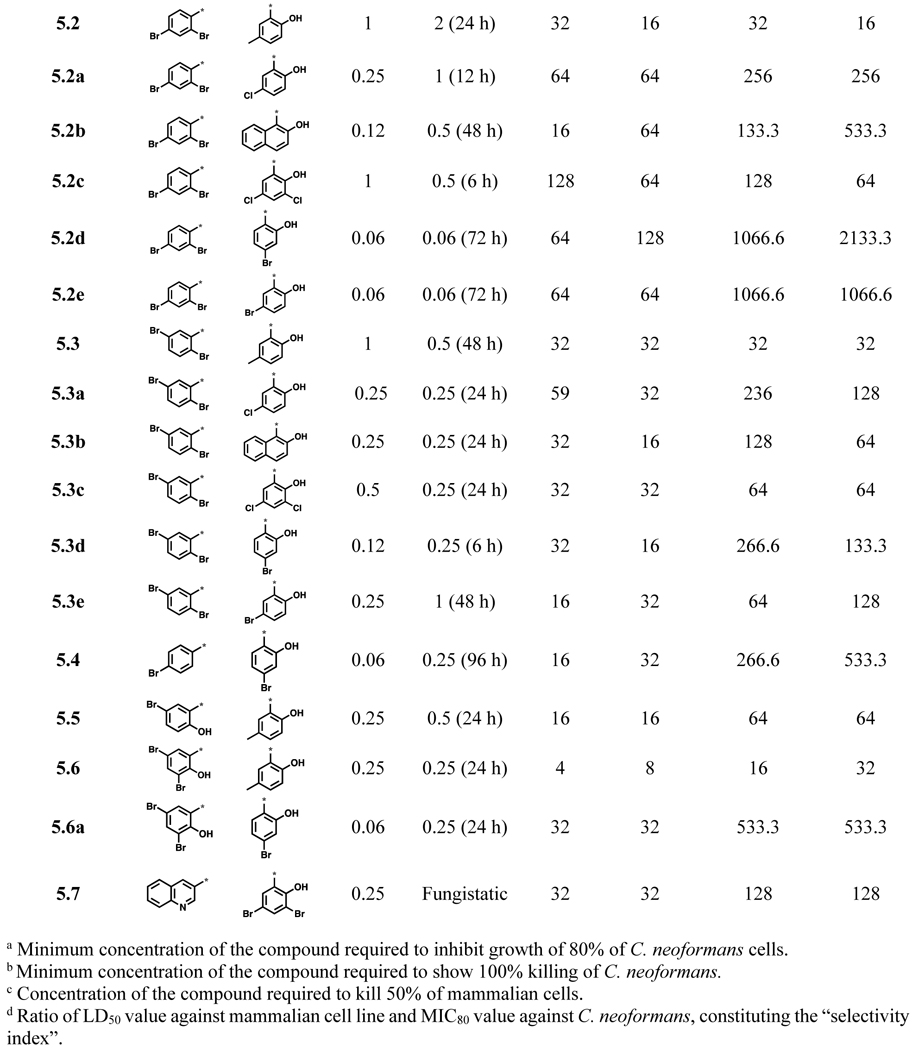
Table 1.
Antifungal activity, time-kill activity (K100) and cytotoxicity of acylhydrazones 5.0~5.7 in the initial 20 compounds library (μg/mL)
 | ||||||||
 |
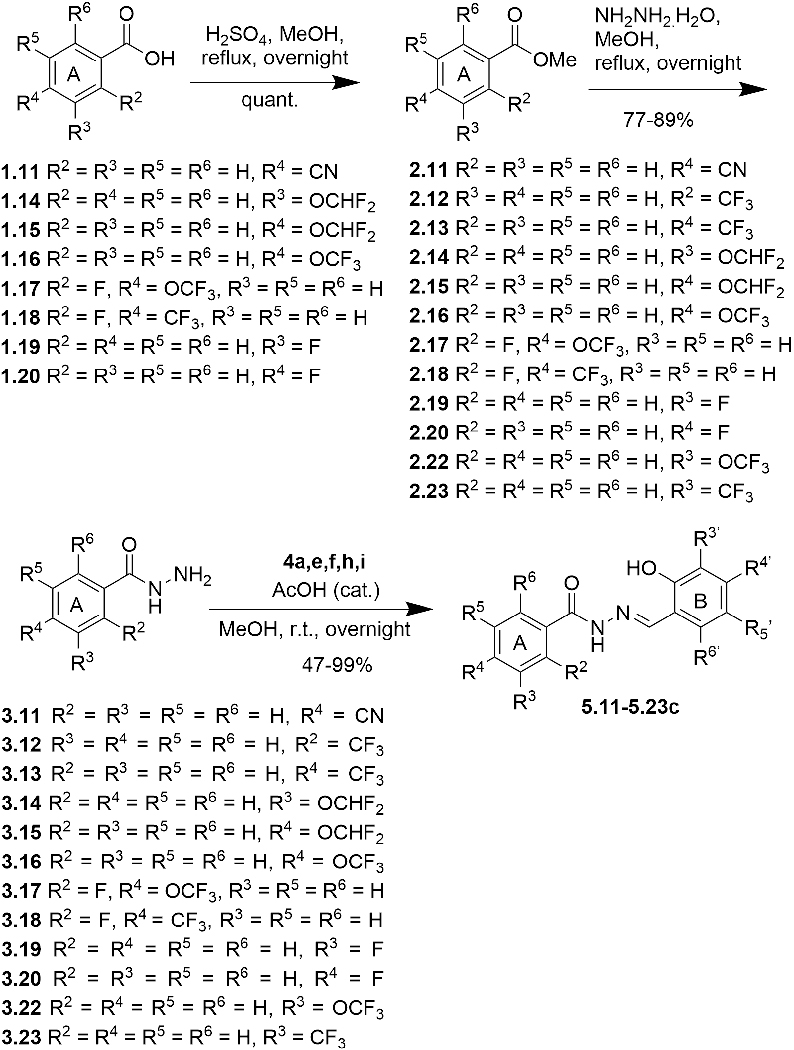
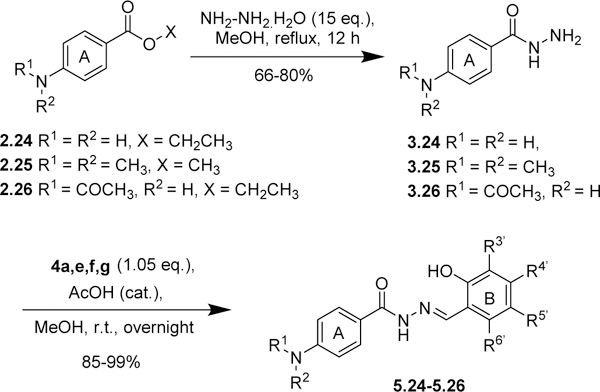
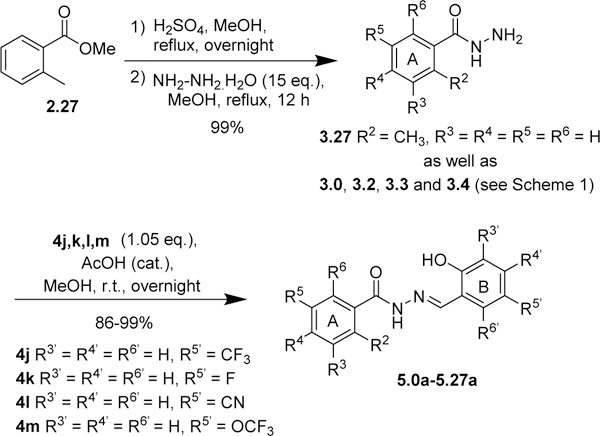
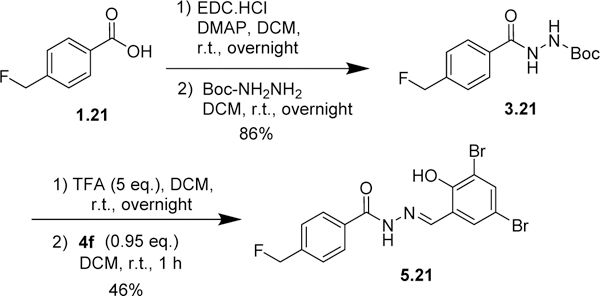
The same protocol was used for the synthesis of other aromatic acylhydrazones. In some cases (2.4~2.7, Scheme 1; 2.19~2.23, Scheme 3; 2.24~2.26, Scheme 5; 2.27, Scheme 6), the methyl or ethyl esters of substituted benzoic acids were commercially available. A variety of hydrazides 3 were condensed with different salicylaldehydes 4 to give the corresponding acylhydrazones 5 (57 compounds) (Schemes 2~5). For the synthesis of 5.21 (Scheme 4), p-fluoromethylbenzoic acid was condensed with Boc-hydrazine in the presence of EDC.HCl and DMAP to give Boc-protected hydrazide 3.21. Then, the Boc group was removed by TFA, followed by the condensation with salicylaldehyde 4 in the same pot to give 5.21.
Scheme 3.
Scheme 5.
Scheme 6.
Scheme 2.
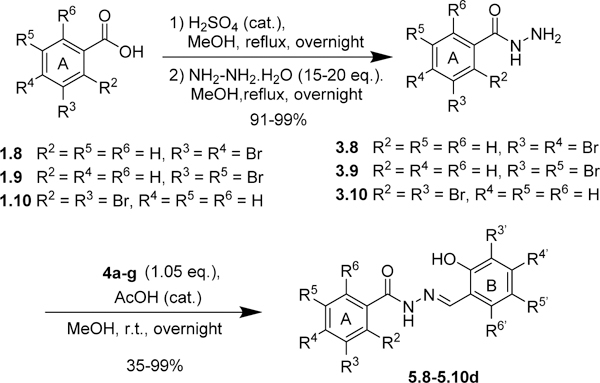
Scheme 4.
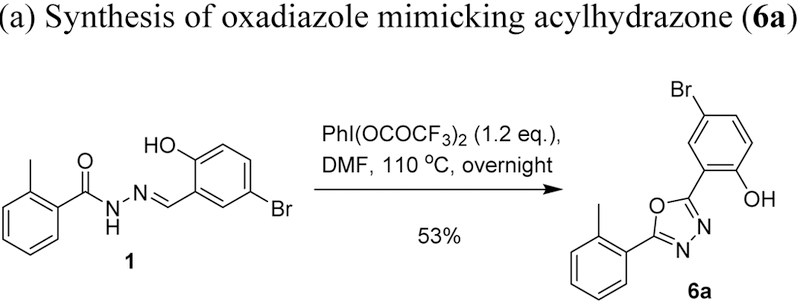
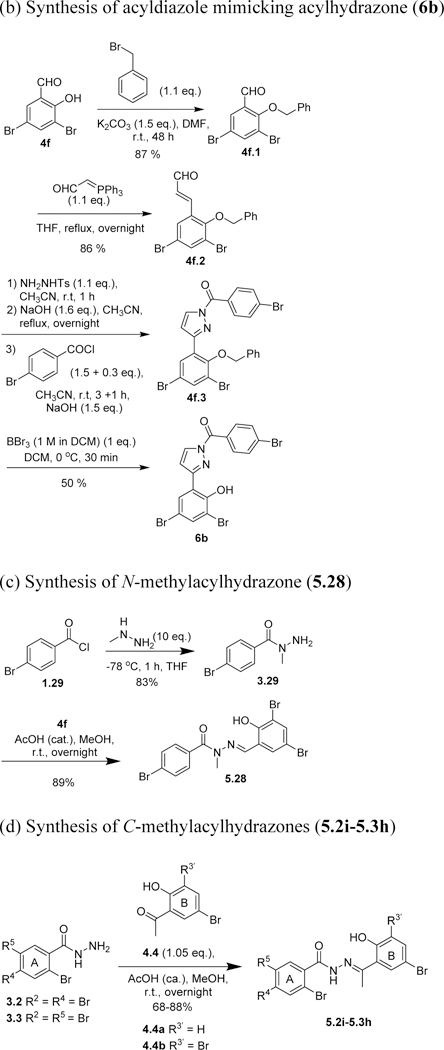
An oxadiazole mimic of acylhydrazone, 6a, was synthesized by treating 1 with hypervalent iodine (PhI(OCOCF3)2 (Scheme 7a). Another diazole mimic of acylhydrazone, 6b, was synthesized through diazole formation from 3,5-dibromo-1-benzyloxycinnamaldehyde (4f.2) and tosylhydrazine, followed by deprotection, 4-bromobenzoylation and debenzylation (Scheme 7b). Cinnamaldehyde 4f.2 was prepared from 3,5-dibromosalicylaldehyde (4f) in two steps (Scheme 7b). N-Methylacylhydrazone 5.28 was synthesized through the condensation of 3,5-dibromosalicylaldehyde (4f) with N-methylhydrazide 3.29, which prepared by reacting 4-bromobenzoyl chloride (1.29) with N-methylhydrazine at −78 °C (Scheme 7c). C-Methylacylhydrazones 5.2i~5.3h were synthesized by condensing hydrazides 3.2 and 3.3 with 2-acetylphenols 4.4a and 4.4b (Scheme 7d). Bis-acylhydrazones 5.29 and 5.30 were synthesized through the condensation of salicylaldehydes with isophthaloyl bishydrazide (3.28), which was prepared from dimethyl isophthalate (2.28) with hydrazine monohydrate (Scheme 7e).
Scheme 7.
Evaluation of antifungal activities against C. neoformans.
All aromatic acylhydrazones 5 and their mimics 6 thus synthesized were evaluated for their antifungal activity against C. neoformans. The antifungal activities of the compounds are indicated by MIC80 values. Compounds that displayed MIC80 ≤1 μg/mL were further examined for their fungicidal activities by a time-kill assay in vitro, indicated by K100 values. Results are summarized in Tables 1~6.
Activities of diarylacylhydrazones 5.0~5.7 in the initial library.
Nineteen out of the 20 compounds shown in Table 1 are found to be fungicidal in the time-kill assay in vitro, except for compound 5.7, which is fungistatic. It is worthy of note that compounds 5.2d, 5.2e and 5.6a are highly potent and selective to fungus with a selectivity index (SI) (vide supra) of ≥500 based on their LD50 values for HepG2 and A549.
Modifications of ring A: Introduction of 2,3-, 3,4- and 3,5-diboromophenyl groups.
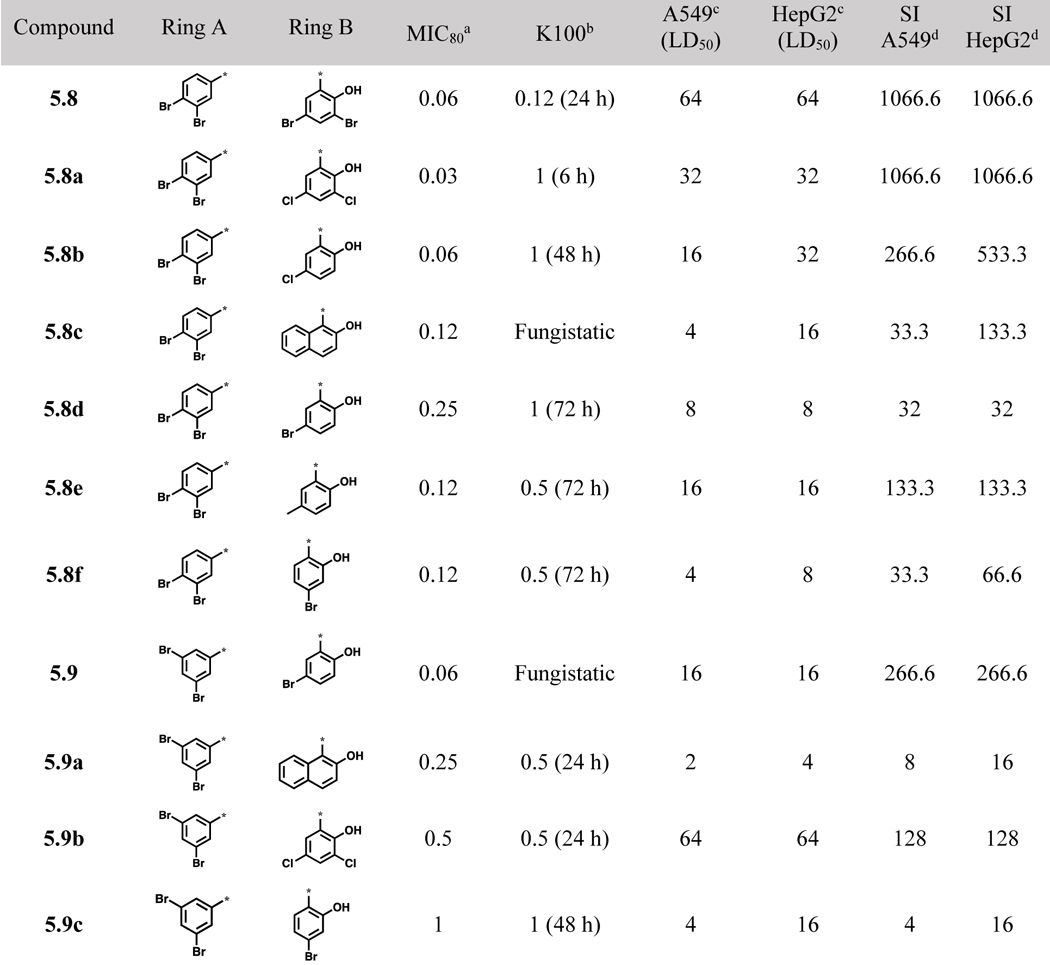
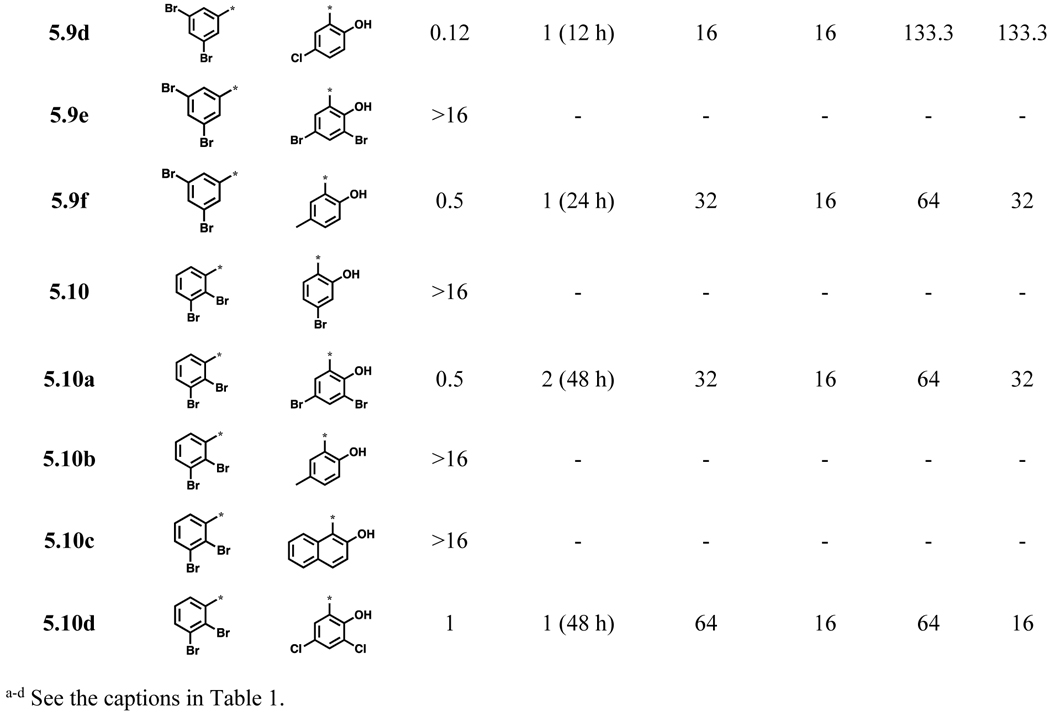
Since the majority of compounds with high potency from the initial library was bearing either a 2,4- or 2,5-dibromophenyl group as the ring A, other dibromobenzoylhydrazones bearing a 2,3-, 3,4- or 3,5-diboromophenyl group as ring A were synthesized (Scheme 2) and examined for their activities against C. neoformans (Table 2). Compounds 5.8 and 5.8a were not only highly potent (MIC80 0.03–0.06 μg/mL), but also showed very low toxicity in mammalian cell lines (SI >1,000). Most of acylhydazones bearing 2,3-dibromophenyl group as ring A (5.10, 5.10b and 5.10c) were not potent (MIC80 >16 μg/mL) against C. neoformans except 5.10a (Table 2)
Table 2.
Acylhydrazones 5.8~5.10 bearing dibromophenyl groups in ring A and their biological activities (μg/mL)
 | ||||||||
 |
Modifications of ring A: Introduction of bioisosteres of bromine and fluorine-containing groups.
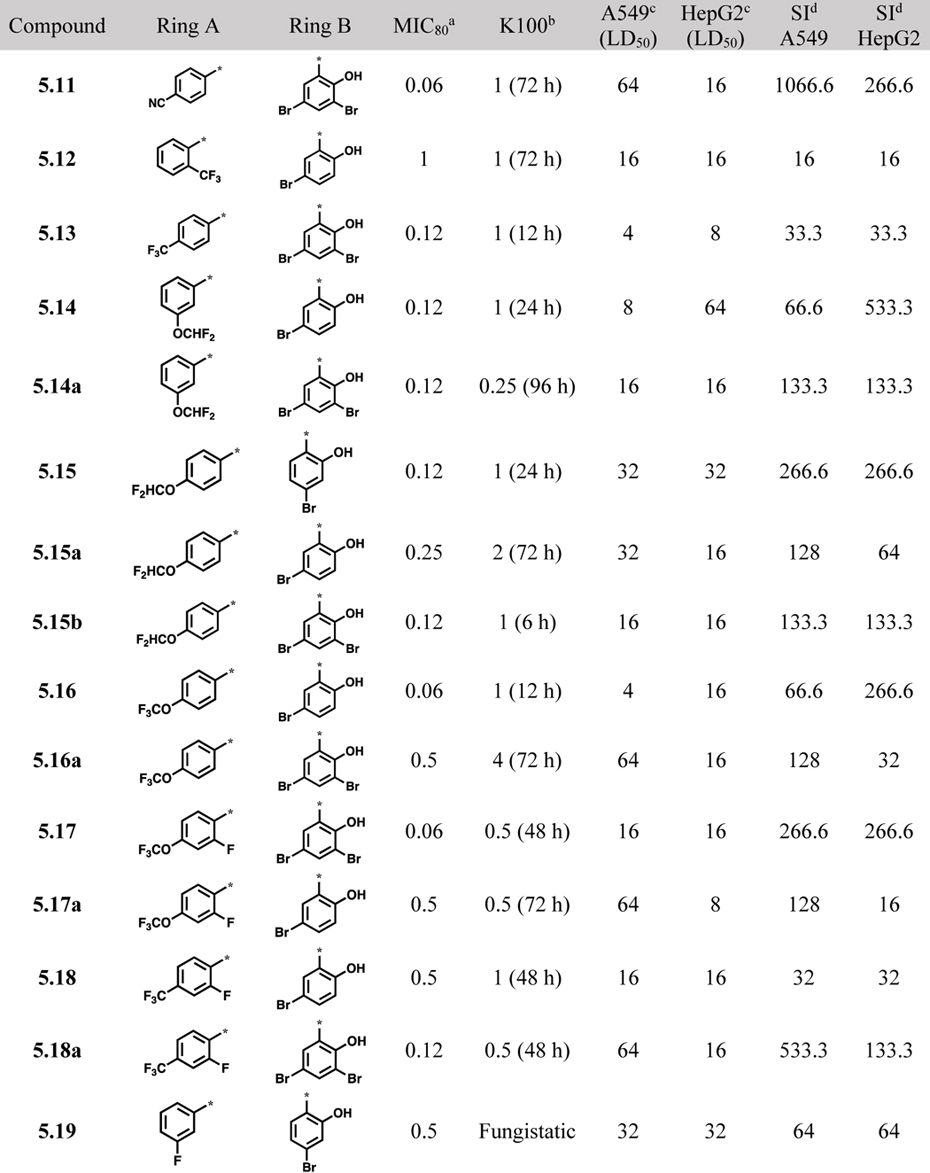
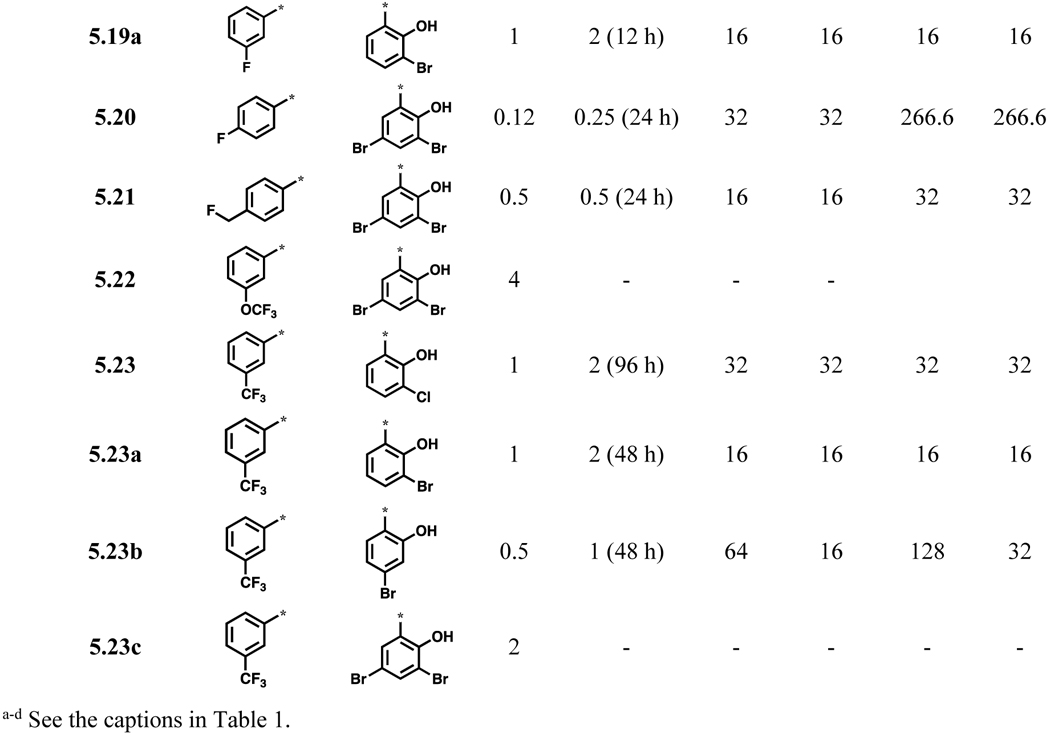
Since the vast majority of active compounds contained one or more bromine atoms on ring A, bromine appears to have a favorable size and polarity as substituent(s) in ring A. However, it is rather rare to have many bromines in a pharmaceutical drug candidate. Thus, we examined if bromine could be replaced with its bioisosters such as CN and CF3 groups (Scheme 3), and found that indeed 4-cyanobenzoyl- and 4-trifluoromethylbenzoylhydrazones (5.11 and 5.13) exhibited good MIC80 values (Table 3). Accordingly, other acylhydrazones containing F, OCF3 or OCHF2 group, as well as 2-F-4-OCF3 or 2-F-4-CF3 groups in ring A, were synthesized (Schemes 3 and 4) and their biological activities examined. As Table 3 shows, all compounds, except 5.22 and 5.23c, in this series exhibited ≤1 μg/mL MIC80 values against C. neoformans. Compounds 5.11, 5.14a, 5.15, 5.15b, 5.17, 5.18a and 5.20 possess good selectivity index (SI >100). Except for compound 5.19, all compounds with ≤1μg/mL MIC80 values are fungicidal.
Table 3.
Acylhydrazones bearing bromine-bioisosteres or fluorine-contaning groups in ring A and their biological activities (μg/mL)
 | ||||||||
 |
Modifications of ring A: Introduction of 4-aminobenzoyl groups.
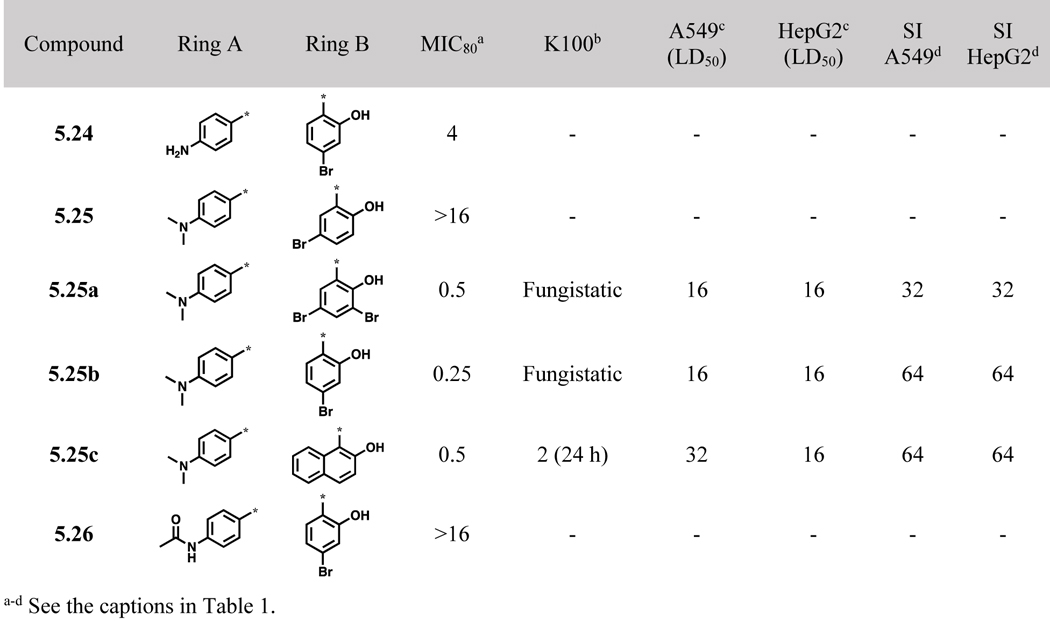
Several benzoylhydrazones bearing an amino-, N,N-dimethylamino or N-acetamido group were synthesized and examined for their biological activities (Scheme 5, Table 4). As Table 4 shows, the introduction of a 4-aminobenzoyl group as ring A reduced the potency (5.24, MIC80 4 μg/mL) and that of a 4-acetamidobenzoyl group was detrimental to the potency (5.26, MIC80 16 μg/mL). Compounds with a 4- N,N-dimethylaminobenzoyl group exhibited mixed results, i.e., 5.25 did not show appreciable potency, while 5.25a, 5.25b and 5.25c exhibited good potency (MIC80 0.25–0.5) with modest SI values. However, 5.25a and 5.25b were fungistatic. Compound 5.25c, bearing a 2-hydroxynaphthyl group as ring B, was found to be fungicidal with a good time-kill profile.
Table 4.
4-Aminobenzoylhydrazones 5.24~5.26 and their biological activities (μg/mL)
 |
Modifications of ring B. Introduction of bioisosteres of bromine and fluorine-containing groups.
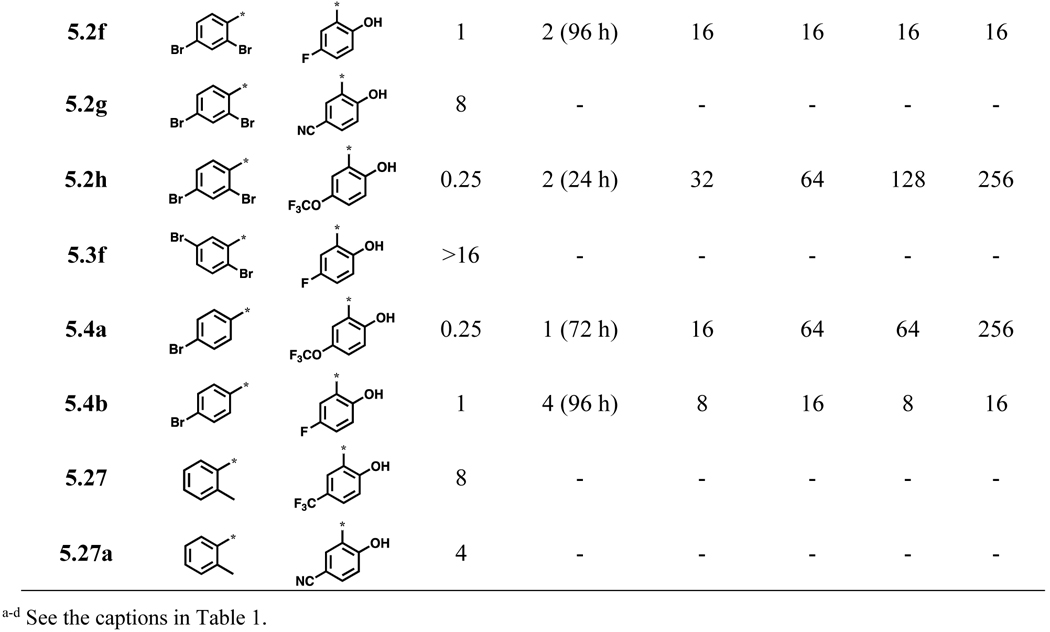
In a manner similar to that performed on ring A, a bromine substituent on ring B was replaced with a CN, CF3, F or OCF3 group and examined for their activity against C. neoformans (Scheme 6, Table 5). As Table 5 shows, 5.2f, 5.2h, 5.4a and 5.4b exhibited good potency (MIC80 ≤1 μg/mL) and were fungicidal in the time-kill assay. Compound 5.2g and 5.27a were weakly active (MIC80 8 and 4 μg/mL, respectively), while compound 5.3f did not show appreciable activity (MIC80 >16 μg/mL).
Table 5.
Acylhydrazones with CN, CF3, F or OCF3 substitution in ring B and their biological activities (μg/mL)
 | ||||||||
 |
Modifications of the central acylhydrazone moiety.
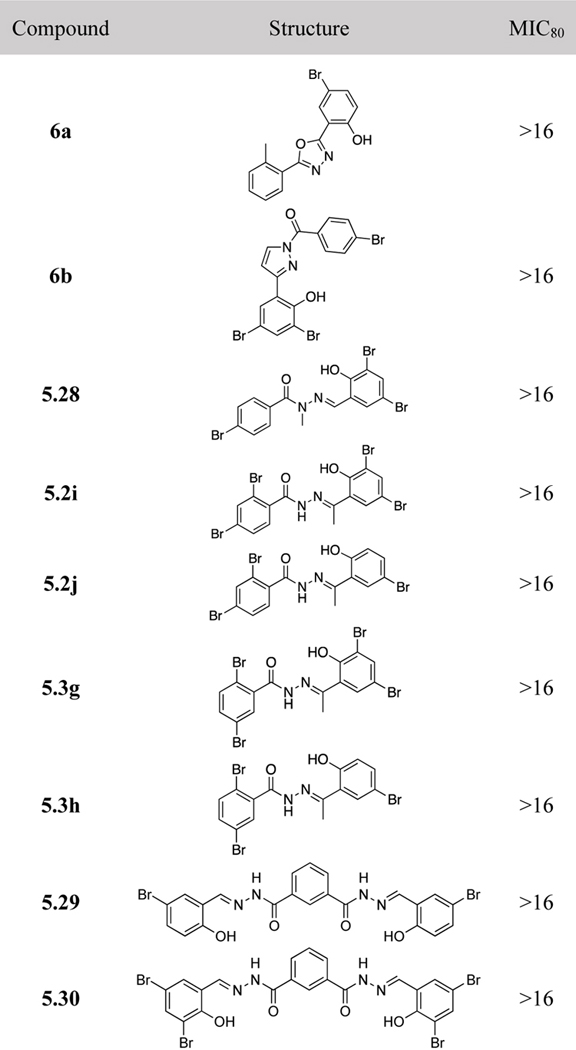
In order to explore structural variations, as well as examine the effects of conformational rigidification on antifungal activity, heterocyclic mimics of aromatic acylhydrazones, 6a and 6b, were synthesized (Scheme 7a and 7b) and their activity against C. neoformans was examined. Rather unexpectedly, these two mimics did not show appreciable activity (MIC80 >16 μg/mL, Table 6). The results indicate that the acylhydrazone structure appears to be essential for antifungal activity. It is noteworthy that N-methylated (5.28) and C-methylated (5.2i, 5.2j, 5.3g and 5.3h) acylhydrazones also did not exhibit appreciable activity (MIC80 >16 μg/mL, Table 6). The results clearly indicate that the NH and CH groups in the acylhydrazone moiety are essential for their antifungal activity, which is also consistent with the lack of appreciable activity in the heterocyclic mimics, 6a and 6b, wherein both NH and CH groups are eliminated by the introduction of oxadiazole and diazole moieties. Bis-acylhydrazones, 5.29 and 5.30 (Scheme 7e) did not exhibit appreciable activity (MIC80 >16 μg/mL, Table 6). Thus, the double units of the acylhydrazone moieties did not enhance binding, but deteriorated the affinity. The result may indicate that the binding site of aromatic acylhydrazones 5, especially around the ring A, is rather compact.
Table 6.
Triclyclic, N-methylated and C-methylated acylhydrazones, bis-hydrazones and their MIC80 values (μg/mL)
 |
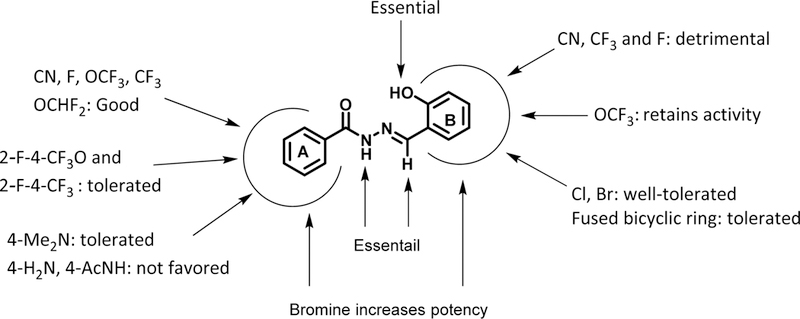
Structure-activity relationship (SAR) analysis.
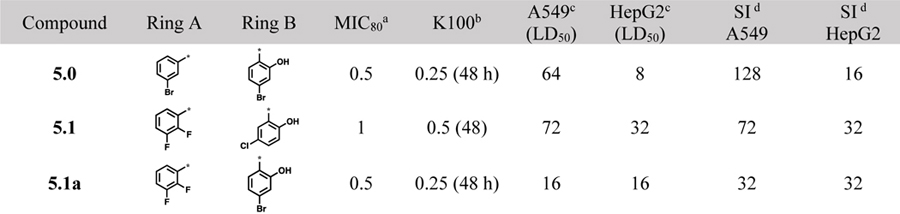
A library of acylhydrazones (192 compounds) was synthesized and screened for activity against C. neoformans H99. Out of the 192 compounds, 42 of them exhibited strong potency (MIC80 ≤1 μg/mL) against C. neoformans and 20 of these hit compounds have not been reported in literature (Scheme 1, Table 1). The SAR of these early hit compounds shown in Table 1 indicated that bromine was well-tolerated on both rings A and B. 2-Hydroxyl group on ring B was found to be essential for antifungal activity. Other halogens such as chlorine and iodine were tolerated on ring B, as well. Most of aromatic acylhydrazones with 2-hydroxyl-5-methylphenyl as ring B showed lower selectivity indices. Compounds 5.1 and 5.1a with 2,3-difluorophenyl as ring A displayed low SI, as well. Compounds, bearing 2,4-dibromophenyl as ring A and 2-hydroxy-4-bromophenyl (5.2d) and 2-hydroxy-5-bromophenyl (5.2e) as ring B were not only highly selective (SI >1,000), but also fungicidal at a very low concentration (0.06 μg/mL). Compounds 5.2c and 5.3d exhibited an excellent time-kill profile by completely eradicating C. neoformans in 6 h at very low concentrations, i.e., 0.5 and 0.25 μg/mL, respectively. Compound 5.4 needed a much longer incubation time (96 h) to show fungicidal activity. Even though compound 5.7, bearing a quinolin-3-yl group as ring A, displayed a low MIC80 value (0.25 μg/mL) and fairly good SI (>100), it was found to be fungistatic in the time-kill assay.
Compounds, bearing 3,4-dibromophenyl as ring A and 2-hydroxy-3,5-dibromophenyl (5.8) or 2-hydroxy-3,5-dichlorophenyl (5.8a) as ring B exhibited excellent antifungal activity (MIC80 0.06 and 0.03 μg/mL, respectively) as well as very high selectivity to the fungus (SI >1,000) (Table 2). However, compounds, bearing 2-hydroxy-5-bromophenyl (5.8d) or 2-hydroxy-4-bromophenyl (5.8f, 5.9c, and 5.10) as ring B, were less selective to the fungus. Compound 5.9, bearing 3,5-dibromophenyl as ring A and 2-hydroxy-5-bromophenyl as ring B displayed good selectivity (SI 266.6), but was found to be fungistatic. Compounds, bearing 2-hydroxy-4-bromophenyl (5.9c) and 2-hydroxy-3,5-dibromophenyl (5.9e) as ring B did not show good selectivity and appreciable activity, respectively. In contrast, compound, bearing 2-hydroxy-5-chlorophenyl (5.9d) or 2-hydroxy-3,5-dichlorophenyl (5.9b) as ring B was fungicidal with fairly good selectivity (SI >100). Accordingly, for compounds with 3,5-dibromophenyl as ring A, chlorine substitutions on ring B were better tolerated than the bromine counterparts. Having 2,3-dibromophenyl as ring A appears to impair aromatic acylhydrazone’s antifungal activity, since 3 out of 5 compounds in this series (5.10, 5.10b and 5.10c) (Table 2) did not exhibit appreciable activity (MIC80 >16) and even the remaining 2 compounds (5.10a and 5.10d, Table 2) exhibited low selectivity (SI = 32 and 16, respectively).
Replacement of 4-bromophenyl with 4-CN-phenyl in ring A was well tolerated (5.11, Table 3), showing activity equivalent to that of 2.22 Replacement of bromine with CF3 in ring A resulted in acylhydrazones that displayed good MIC80 values and time-kill activities, but with low to modest selectivity. Replacement of 3- or 4-bromophenyl with 3- or 4-CHF2O-phenyl was relatively well-tolerated. Use of 3-CF3O-phenyl as ring A significantly reduced the antifungal activity (5.22, Table 3). Compounds with 2-F, 4-OCF3 or 2-F, 4-CF3 substitutions on the phenyl group as ring A and 2-hydroxy-3,5-dibromophenyl as ring B possessed better selectivity than that of the counterparts bearing 2-hydroxy-5-bromophenyl as ring B (Table 3). Compounds with a 3-fluorophenyl as ring A were either fungistatic or not selective. However, compound bearing a 4-fluorophenyl as ring A exhibited good antifungal activity, good selectivity and excellent time-kill activity at a very low concentration (5.20, Table 3). Replacement of 4-fluorophenyl with 4-FCH2-phenyl resulted in drastic reduction of selectivity (5.21, Table 3).
The use of 4-amino- and 4-acetamidophenyl as ring A was found to be detrimental to antifungal activity (Table 4). Compounds with 4-dimethylaminophenyl as ring A were either fungistatic or lacking appreciable activity, except for a compound bearing 2-hydroxynaphthyl as ring B (5.25c, Table 4).
Ring B was found to be more sensitive to the replacement of bromine with its bioisosteres and fluorine-containing groups, as compared to ring A (Table 5). The replacement of bromine at the 5-poisiton of the phenyl group in ring B with CN, CF3 or F drastically affected either the antifungal activity or selectivity, whereas replacing bromine with OCF3 on ring B was well-tolerated.
As described above, tricyclic analogs of 1 or 2, bearing either an oxadiazole or diazole moiety as the third ring, did not show appreciable antifungal activity (Table 6). Also, acylhydrazone derivatives with N-methylation of the hydrazone’s NH moiety or C-methylation of the hydrazone’s methylidene CH moiety did not exhibit appreciable activity, either (Table 6). Furthermore, the bis-acylhydrazone skeleton was detrimental to the antifungal activity (Table 6).
Based on the findings and analysis described above, a summary of the SAR of aromatic acylhydrazones in this study is illustrated in Figure 2.
Figure 2.
SAR of aromatic acylhydrazones
Five most potent and selective acylhydrazones.
Among the 83 new aromatic acylhydrazones evaluated, 5 of them exhibited excellent potency and time-kill profile against C. neoformans with very high selectivity indices (SI >500). These 5 lead compounds are summarized in Table 7.
Table 7.
Five most potent and selective acylhydrazones and their biological activities (μg/mL)
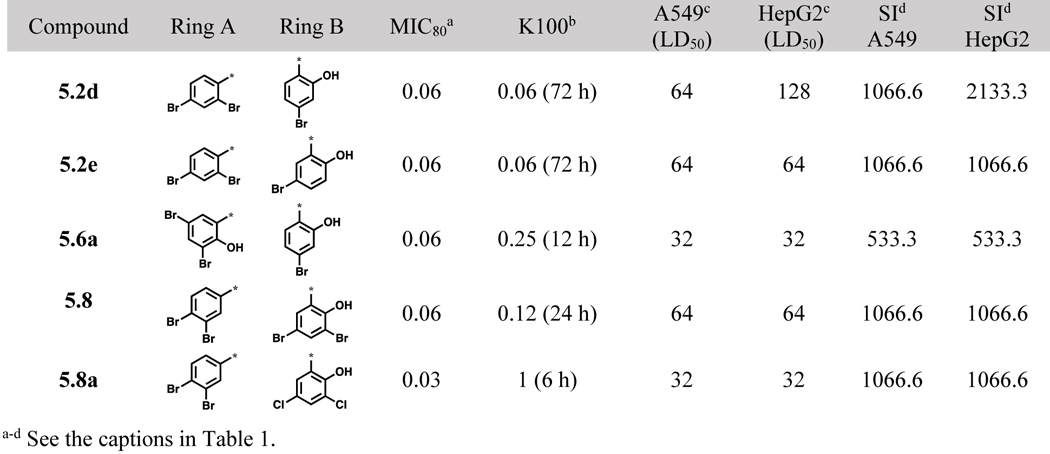 |
Also, the time-kill activities of the 5 compounds are shown in Figure 3. All 5 compounds were fungicidal at very low concentrations. Compounds 5.2d and 5.2e were fungicidal at extremely low concentrations (0.06 μg/mL). Compound 5.8 even though displayed low MIC80 and good selectivity, it behaved slightly erratic in the time-kill assay (Figure 3D). Compound 5.8a showed fungicidal activity at a slightly higher concentration (1 μg/mL), but was able to completely eradicate the fungi in a short span of 6 h.
Figure 3.
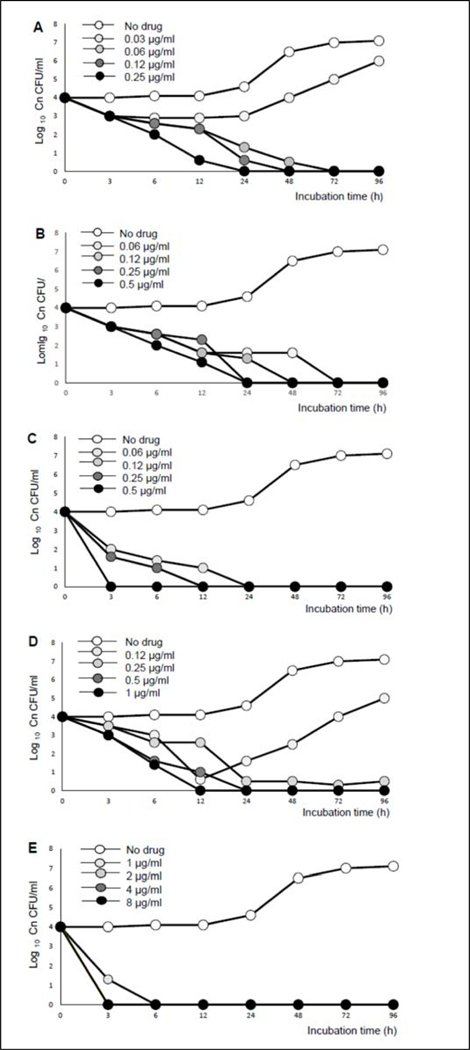
In vitro time-kill activities of (A) 5.2d, (B) 5.2e, (C) 5.6a, (D) 5.8 and (E) 5.8a. Time-kill activity was determined using an in vitro time-kill kinetics assay in which the compounds were co-incubated with C. neoformans cells at 37°C, 5% CO2, pH 7.4. The number of CFU is counted during 96 h of incubation. All of the compounds displayed antifungal activity in a dose-dependent manner.
Evaluation of antifungal activities against other pathogenic fungal strains.
These 5 lead compounds were evaluated for their antifungal activities against other pathogenic strains of fungi such as C. albicans, C. auris, C. krusei, C. krusei R (resistant to a majority of current antifungal agents), C. parapsilosis and A. fumigatus. As Table 8 shows, compounds 5.6a and 5.8a exhibited broad spectrum antifungal activity. It should be noted that 5.6a and 5.8a were active against C. auris, an emerging pathogen that is resistant to all currently available antifungal agents.30 Compound 5.2d was only modestly active against C. parapsilosis, but did not show appreciable activity to other fungi. Compound 5.2e showed modest activity against C. krusei and C. parapsilosis, while 5.8 exhibited moderate activity against the resistant strain of C. krusei and weak activity against C. auris and C. parapsilosis. It is worthy of note that each fungal strain appears to be very sensitive to rather small variations in the substitution patterns in ring A and ring B.
Table 8.
MIC80 (μg/mL) values of lead compounds against different pathogenic strains of fungi
| Compound |
C. neoformans |
C. albicans |
C. auris |
C. krusei |
C. krusei R |
C. parapsilosis |
A. fumigatus |
|---|---|---|---|---|---|---|---|
| 5.2d | 0.06 | >16 | >16 | >16 | >16 | 8 | >16 |
| 5.2e | 0.06 | >16 | >16 | 8 | >16 | 8 | >16 |
| 5.6a | 0.06 | 2 | 0.5 | 0.25 | 0.25 | 2 | 4 |
| 5.8 | 0.06 | >16 | 16 | >16 | 4 | 16 | >16 |
| 5.8a | 0.03 | 2 | 0.5 | 0.25 | 0.25 | 1 | 8 |
Drug combination studies.
Since the next-generation antifungal agents are likely to be used in combination with antifungal drugs that are currently available in clinic, it is important to examine possible drug-drug interactions or synergy/antagonism between our lead compounds and clinically used antifungal drugs. Accordingly, the 5 lead compounds, 5.2d, 5.2e, 5.6a, 5.8 and 5.8a (Tables 7 and 8) were examined for their potential synergism/antagonism with 5 clinical drugs, i.e., fluconazole (Flu), voriconazole (Vori), itraconazole (Itra), caspofungin (Caspo); amphotericin B (AB) against 7 fungal strains, i.e., C. neoformans, C. albicans, fluconazole-resistant C. krusei (Ck Flu R), C. krusei 6258 (Ck6258), C. auris, C. parapsilosis and A. fumigatus. Results are shown in Tables 9,10,11, 12, and 13, in which each Table summarizes the activity of each of 5 lead compounds against 7 fungal strains when combined with clinically used drugs. The level of cooperativity was estimated based on the fractional inhibitory concentration index (FICI). The FICI is defined as (MICcombined/MICdrugA) + (MICcombined/MICdrugB).31 The level of cooperativity is categorized as follows: strongly synergistic: FICI <0.5; synergistic: FICI <1; autonomous: FICI = 1; additive: FICI 1 ~ 4; antagonistic: FICI >4.31, 32
Table 9.
FICI values of 5.2d combined with clinical drugs against 7 fungal strains
| Fungal strain | 5.2d/Flu* | 5.2d/Vori* | 5.2d/Itra* | 5.2d/Caspo* | 5.2d/AB* |
|---|---|---|---|---|---|
| C. neoformans | 0.065 | 0.072 | 0.072 | ND | 0.086 |
| C. albicans | 0.625 | 0.256 | 0.128 | 0.502 | ND |
| Ck Flu R | 2 | 0.281 | 0.252 | 2 | ND |
| Ck6258 | 2 | 1.250 | 1.063 | 2 | ND |
| C. auris | 2 | 1.016 | 0.508 | 2 | ND |
| C. parapsilosis | 1.002 | 0.076 | 0.124 | 2 | ND |
| A. fumigatus | 2 | 0.515 | 0.484 | ND | ND |
Flu: fluconazole; Vori: voriconazole; Itra/itraconazole; Caspo: caspofungin; AB: amphotericin B.
Table 10.
FICI values of 5.2e combined with clinical drugs against 7 fungal strains
| Fungal strain | 5.2e/Flu | 5.2e/Vori | 5.2e/Itra | 5.2e/Caspo | 5.2e/AB |
|---|---|---|---|---|---|
| C. neoformans | 0.065 | 0.072 | 0.072 | ND | 0.086 |
| C. albicans | 2 | 0.256 | 0.128 | 0.502 | ND |
| Ck Flu R | 2 | 1.063 | 0.375 | 2 | ND |
| Ck6258 | 1.002 | 1.060 | 2.015 | 1.002 | ND |
| C. auris | 2 | 1.125 | 1.016 | 2 | ND |
| C. parapsilosis | 1.015 | 0.135 | 2.098 | 1.5 | ND |
| A. fumigatus | 2 | 0.515 | 0.484 | ND | ND |
For abbreviation of drug names, see the captions in Table 9.
Table 11.
FICI values of 5.6a combined with clinical drugs against 7 fungal strains
| Fungal strain | 5.6a/Flu | 5.6a/Vori | 5.6a/Itra | 5.6a/Caspo | 5.6a/AB |
|---|---|---|---|---|---|
| C. neoformans | 0.240 | 0.240 | 0.247 | ND | 0.261 |
| C. albicans | 2 | 0.375 | 0.188 | 0.508 | ND |
| Ck Flu R | 0.00016 | 0.018 | 0.023 | 0.0005 | ND |
| Ck6258 | 0.501 | 0.129 | 0.183 | 2.002 | ND |
| C. auris | 0.531 | 0.252 | 0.180 | 2 | ND |
| C. parapsilosis | 1.015 | 0.135 | 0.508 | 2 | ND |
| A. fumigatus | 2 | 0.188 | 1.016 | ND | ND |
For abbreviation of drug names, see the captions in Table 9.
Table 12.
FICI values of 5.8 combined with clinical drugs against 7 fungal strains
| Fungal strain | 5.8/Flu | 5.8/Vori | 5.8/Itra | 5.8/Caspo | 5.8/AB |
|---|---|---|---|---|---|
| C. neoformans | 0.237 | 0.247 | 0.247 | ND | 0.247 |
| C. albicans | 0.563 | 0.271 | 0.370 | 0.500 | ND |
| Ck Flu R | 0.501 | 0.310 | 0.528 | 0.121 | ND |
| Ck6258 | 1 | 0.254 | 0.153 | 0.125 | ND |
| C. auris | 1.001 | 1.001 | 1.12 | 0.501 | ND |
| C. parapsilosis | 2.015 | 1.03 | 2.007 | 1.001 | ND |
| A. fumigatus | 2 | 1.031 | 0.281 | ND | ND |
For abbreviation of drug names, see the captions in Table 9.
Table 13:
FICI values of 5.8a combined with clinical drugs against 7 fungal strains
| Fungal strain | 5.8a/Flu | 5.8a/Vori | 5.8a/Itra | 5.8a/Caspo | 5.8a/AB |
|---|---|---|---|---|---|
| C. neoformans | 0.237 | 0.247 | 0.247 | ND | 0.247 |
| C. albicans | 0.252 | 0.077 | 0.091 | 1.00 | ND |
| Ck Flu R | 0.501 | 1.030 | 1.240 | 1.004 | ND |
| Ck6258 | 0.250 | 0.560 | 0.530 | 1.001 | ND |
| C. auris | 0.500 | 0.500 | 1.12 | 0.500 | ND |
| C. parapsilosis | 0.258 | 1.500 | 1.250 | 0.501 | ND |
| A. fumigatus | 2 | 1.031 | 1.008 | ND | ND |
For abbreviation of drug names, see the captions in Table 9.
All 5 lead compounds showed synergistic effect (i.e., FICI <1) with these clinical drugs against C. neoformans, while they were not tested in combination with caspofungin against C. neoformans, since caspofungin is known to be inactive against this fungal strain.33 In all other fungal strains, drug combinations with amphotericin B were not studied since Amphotericin B is the first line treatment only for cryptococcosis clinically.34 For C. albicans, 5.2d, 5.8 and 5.8a showed very to fairly strong synergistic effects (FICI = 0.077~0.625) with only exception of 5.8a/Caspo combination, which was autonomous (FICI = 1). Compound 5.2e exhibited strong synergistic effects with voriconazole, itraconazole and caspofungin, but the 5.2e/Flu and 5.6/Flu combinations were additive (FICI = 2). For fluconazole-resistant C. krusei (Ck Flu R), 5.6a demonstrated exceptionally strong synergism with all 4 clinical drugs (FICI = 0.00016~0.018), especially with fluconazole, restoring and enhancing the drug sensitivity (Table 11). Compound 5.8 also exhibited strong synergism with all 4 drugs (FICI = 0.121~0.528) and 5.8a showed synergistic and additive effects. For C. krusei 6258 (Ck6258), 5.8 and 5.8a exhibited strong synergism (FICI <0.5) in most combinations, except for two combinations that were autonomous (FICI = 1). Compounds 5.6a showed strong synergism with 2 clinical drugs, but became additive with fluconazole and caspofungin, depending on fungal strains. Other two compounds, 5.2d and 5.2e did not show synergistic effects in all combinations. For C. auris, 5.8a and 5.8 exhibited strongly synergistic and autonomous effects in all drug combinations, while 5.6a showed strong synergism with 3 drugs, but the combination with caspofungin was additive. For C. parapsilosis, only 5.8a exhibited strong synergistic and additive effects with two drugs each. All other drug combinations resulted in mixed results, although the 5.2d/Vori combination showed extremely strong synergism (FICI = 0.076). Finally, for A. fumigatus, combinations with caspofungin in addition to amphotericin B were not studied because caspofungin is not active against this fungal strain. For the combinations with remaining three drugs, only 5.2d/Vori, 5.2d/Itra and 5.6a/Vori showed synergistic effects, i.e., all other combinations were additive.
CONCLUSIONS
The SAR study on the 83 new aromatic acylhydrazones, designed, synthesized and their antifungal activities examined against C. neoformans, resulted in several critical findings: (i) 2-Hydroxyl group in the phenyl or naphthyl moiety as ring B is essential for good antifungal activity; (ii) Bromine substituent in both ring A and ring B is found to have significantly positive effect on the antifungal activity, as well as selectivity index (SI); (iii) Bromine isosteres, such as CN and CF3, are well-tolerated in ring A, but mostly detrimental in ring B to potency and/or SI; (iv) Other fluorine-based bromine surrogates, F, CF3O, CHF2O are well-tolerated in ring A, while F was detrimental in ring B, especially to SI, but CF3O was well-tolerated in ring B; (v) Ring B is much more sensitive to the replacement of bromine, compared to ring A; (vi) Free NH and CH in the hydrazine moiety are found to be essential to antifungal activity.
Our SAR study has led to the identification of 5 lead compounds, 5.2d, 5.2e, 5.6a, 5.8 and 5.8a, that exhibit excellent antifungal activity against C. neoformans with very low toxicity on mammalian cells, resulting in a very high SI. These 5 compounds were examined for their activities against a panel of 7 pathogenic fungal strains, i.e., C. neoformans, C. albicans, fluconazole-resistant C. krusei, C. krusei 6258, C. auris, C. parapsilosis and A. fumigatus. Then, 5.6a and 5.8a demonstrated broad spectrum antifungal activity against all 7 fungal strains. Finally, the 5 lead compounds were examined for their synergism/cooperativity with 5 clinical drugs, i.e., fluconazole, voriconazole, itraconazole, caspofungin and amphotericin B against the 7 fungal strains. Notable findings are (i) the combination of all 5 lead compounds with voriconazole exhibited either synergistic or additive effect to all 7 fungal strains and no antagonism was observed and (ii) the combinations of 5.2d, 5.6a and 5.8a with itraconazole showed a very similar profile. These are noteworthy findings for drug discovery and development of the next-generation antifungal agents. Further studies on in vivo efficacy, PK/PD, ADME profiles of these lead compounds are actively ongoing in these laboratories.
EXPERIMENTAL SECTION
General Methods.
Melting points were measured on a Thomas Hoover Capillary melting point apparatus and are uncorrected. NMR spectra were recorded on a Bruker Ascend 700 spectrometer operating at 700 MHz for 1H and 175 MHz for 13C, a Bruker 500 Advance spectrometer operating at 500 MHz and 125 MHz for 1H and 13C,respectively, or a Bruker 400 Nanobay spectrometer operating at 400 MHz, 100 MHz, and 376 MHz for 1H, 13C, and 19F, respectively or a Bruker 300 Nanobay spectrometer operating at 300 MHz of 1H. Chemical shifts were referenced to the residual proton and carbon-13 peaks of solvents used for 1H and 13C NMRs, respectively (1H: CDCl3, δ7.26; 13C: CDCl3, δ77.23; 1H: DMSO-d6, δ 2.50; 13C: DMSO-d6, δ 39.51). Signals are listed in ppm, and multiplicity identified as s ¼ singlet, br ¼ broad, d ¼ doublet, dd ¼ doublet of doublets, t ¼ triplet, q ¼ quartet, m ¼ multiplet; J -coupling constants in Hz, and integration. High resolution mass spectrometry (HRMS) analysis was carried out on an Agilent LC-UV-TOF mass spectrometer at the Institute of Chemical Biology and Drug Discovery, Stony Brook. Purity of the synthesized compounds was determined by Shimadzu LC-2010A HT series HPLC assembly. Three analytical conditions were used and noted as a part of the characterization data for synthesized compounds. HPLC (A): Kinetex PFP 2.6 μM, 100 × 2.1 mm, 100 Å column, acetonitrile and water, flow rate of 0.2 mL/min, t = 0–30 min, gradient of 40–95% acetonitrile. HPLC (B): Kinetex PFP 2.6 μM, 100 × 2.1 mm, 100 Å column, acetonitrile and water, flow rate of 0.2 mL/min, t = 0–30 min, gradient of 50–95% acetonitrile. HPLC (C): Kinetex PFP 2.6 μM, 100 × 2.1 mm, 100 A column, acetonitrile and water, flow rate of 0.2 mL/min, t = 0–30 min, gradient of 55–95% acetonitrile. Measurements were made at 220 and 254 nm.
Materials.
All air- and moisture-insensitive reactions were carried out under an ambient atmosphere, magnetically stirred, and monitored by thin layer chromatography (TLC) using Agilent Technologies TLC plates pre-coated with 250 μm thickness silica gel 60 F254 plates and visualized by fluorescence quenching under UV light. Flash chromatography was performed on SiliaFlash® Silica Gel 40–63 μm 60 Å particle size using a forced flow of eluent at 0.3–0.5 bar pressure. All air- and moisture-sensitive manipulations were performed using oven-dried glassware using the standard Schlenk and glovebox techniques under nitrogen. Diethyl ether and THF were distilled from deep purple sodium benzopheone ketyl. Dichloromethane, chloroform and acetonitrile were dried over calcium hydride and distilled. Dichloromethane was degassed via three freeze-pump-thaw cycles. All other chemicals were used as received. All deuterated solvents were purchased from Cambridge Isotope Laboratories. 3-Bromobenzohydrazide (3.0),35 4-Bromobenzohydrazide (3.4),36 2-Hydroxy-5-bromobenzohydrazide (3.5),37 2-Hydroxy-3,5-dibromobenzohydrazide (3.6),37 3-Quinolinecarbohydrazide (3.7),38 3,5-Dibromobenzohydrazide (3.9),39 4-Cyanobenzohydrazide (3.11),40 4-Trifluoromethylbenzohydrazide (3.13),41 4-Fluorobenzohydrazide (3.20),42 4-Aminobenzohydrazide (3.24),43 4-Acetamidobenzohydrazide (3.26),44 2-Methylbenzohydrazide (3.27),45 2-Benzyloxy-3,5-dibromobenzaldehyde (4.1),46 were synthesized by the literature methods.
Synthesis and characterization of benzohydrazides (3): 2,3-Difluorobenzohydrazide (3.1)
To a solution of 2,3-Difluromehtylbenzoate in methanol hydrazine monohydrate (15 eq.) was added. The reaction mixture was refluxed overnight. After the completion of reaction, the reaction mixture was concentrated in a rotary evaporator under pressure, followed by the addition of ice cold water (30 mL). This resulted in the precipitation of the product, which was filtered and dried to yield the product as a white solid (67% yield); mp 139–141 °C; 1H NMR (500 MHz, DMSO-d6) δ 4.56 (s, 2 H), 7.24 – 7.28 (m, 1 H), 7.32 – 7.35 (m, 1 H), 7.50 – 7.56 (m, 1 H), 9.65 (s, 1 H); 13C NMR (100 MHz DMSO-d6) δ 119.0, 119.2, 124.9, 124.9, 124.97, 125.04, 125.4, 125.5, 145.7, 145.9, 148.2, 148.36, 148.4, 148.5, 150.8, 151.0, 162.15, 162.17; HRMS (TOF) m/z calcd for C7H6F2N2OH+: 173.0521, found: 173.0524 (Δ= −2.06 ppm).
All other benzohydrazides were prepared in the same manner. Characterization data for new benzohydrazides are shown below.
2,4-Dibromobenzohydrazide (3.2).
White solid (83 % yield); mp 176–177 °C; 1H NMR (500 MHz, DMSO- d6) δ 4.48 (s, 2 H), 7.29 (d, 1 H, J = 8.1 Hz), 7.63 d, 1 H, J = 8.1 Hz), 7.91 (s, 1 H), 9.57 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 120.6, 123.0, 130.55, 130.63, 134.7, 137.0, 165.7; HRMS (TOF) calcd for C7H6Br2N2O2H+: 292.89197, found 292.89284 (Δ = −2.98 ppm).
2,5-Dibromobenzohydrazide (3.5).
White solid (91 % yield); mp 186 – 188 °C; 1H NMR (300 MHz, DMSO- d6) δ 4.49 (s, 2 H), 7.28 (d, 1 H, J = 8.2 Hz), 7.63 (d, 1 H, J = 8.2 Hz), 7.92 (s, 1 H), 9.58 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 118.6, 120.4, 131.6, 133.7, 134.7, 139.6, 165.0; HRMS (TOF) calcd for C7H6Br2N2OH+ 292.8920, found 292.8922 (Δ = −0.8 ppm).
3,4-Dibromobenzohydrazide (3.8).
White solid (91% yield); mp 160–162 °C; 1H NMR (500 MHz, DMSO- d6) δ 4.57 (s, 2 H), 7.72 (dd, 1 H, J = 8.3, 2.0 Hz), 7.84 (d, 1 H, J = 8.3 Hz), 8.13 (d, 1 H, J = 2.0 Hz), 9.94 (s, 1 H); 13C NMR (125 MHz, DMSO- d6) δ 124.0, 126.9, 127.7, 131.9, 133.8, 134.1, 163.5; HRMS (TOF) m/z calcd for C7H6Br2N2OH+: 292.89197, found 292.89211 (Δ = −0.49 ppm).
2,3-Dibromobenzohydrazide (3.10).
White solid (77% yield); mp 219–221 °C; 1H NMR (400 MHz, DMSO- d6) δ 4.30 (s, 2 H), 7.29 – 7.36 (m, 2 H), 7.80 (dd, 1 H, J = 9.5, 1.8 Hz), 9.57 (s, 1 H); 13C NMR (100 MHz, DMSO- d6) δ 121.8, 125.2, 127.8, 129.2, 134.2, 140.7, 166.2, HRMS (TOF) m/z calcd for C7H6Br2N2OH+: 292.8919, found: 292.8924(Δ= −1.57 ppm).
2-Trifluoromethylbenzohydrazide (3.12).
White solid (70% yield); mp 123–125 °C; 1H NMR (500 MHz DMSO- d6) δ 4.47 (s, 2 H), 7.46 (d, 1 H, J = 7.5 Hz), 7.62 – 7.65 (m, 1 H), 7.68 – 7.71 (m, 1 H), 7.77 (d, 1 H, J = 6.8 Hz), 9.59 (s, 1 H); 13C NMR (125 MHz, DMSO- d6) δ 122.6, 124.8, 125.9, 126.16, 126.22, 126.26, 126.4, 128.9, 129.8, 132.4, 135.1, 166.4; HRMS (TOF) m/z calcd for C8H7F3N2OH+: 205.0583, found: 205.0589 (Δ = −2.86 ppm).
3-Difluoromethoxybenzohydrazide (3.14).
White solid (89% yield); mp 98–99 °C; 1H NMR (300 MHz DMSO- d6) δ 4.61 (s, 2 H, 100%), 7.03 (s, 1 H, 25%), 7.27 – 7.32 (m, 2 H, 100%, 50%), 7.47 – 7.52 (m, 2 H, 100%, 25%), 7.58 (s, 1 H, 100%), 7.67 (d, 1 H, 100%, J = 7.5 Hz), 9.87 (s, 1 H, 100%); 13C NMR (125 MHz, DMSO- d6) δ 114.3, 116.3, 117.2, 118.4, 121.3, 123.6, 130.2, 135.2, 150.9, 164.7; HRMS (TOF) m/z calcd for C8H8F2N2O2H+: 203.0626, found: 203.0636 (Δ = −5.05 ppm).
4-Difluoromethoxybenzohydrazide (3.15).
White solid (85 % yield); mp 108–110 °C; 1H NMR (400 MHz DMSO- d6) δ 4.50 (s, 2 H), 7.14 (s, 1 H, 25%), 7.23 (d, 2 H, J = 8.8 Hz), 7.33 (s, 1 H, 50%), 7.51 (s, 1 H, 25%), 7.89 (d, 2 H, J = 8.8 Hz), 9.78 (s, 1 H); 13C NMR (100 MHz, DMSO- d6) δ 113.5, 116.1, 117.9, 118.6, 129.0, 129.9, 152.93, 152.96, 153.0, 164.9; HRMS (TOF) m/z calcd for C8H8F2N2O2H+: 203.0626, found: 203.0632 (Δ = −2.82 ppm).
4-Trifluoromethoxybenzohydrazide (3.16).
White solid (79% yield); mp 112 – 113 °C; 1H NMR (500 MHz DMSO- d6) δ 4.53 (s, 2 H), 7.43 (d, 2 H, J = 8.6 Hz), 7.93 (d, 2 H, J = 8.8 Hz), 9.87 (s, 1 H); 13C NMR (125 MHz, DMSO- d6) δ 118.9, 120.6, 121.0, 129.2, 132.4, 150.1, 164.6; HRMS (TOF) m/z calcd for C8H7F3N2O2H+: 221.0534, found: 221.0540 (Δ = −3.54 ppm).
2-Fluoro-4-trifluoromethoxybenzohydrazide (3.17).
White solid (77% yield); mp 93–95 °C; 1H NMR (400 MHz DMSO- d6) δ 4.19 (s, 2 H), 7.0 (d, 1 H, J = 10.9 Hz), 7.13 (d, 1 H, J = 8.8 Hz), 7.93 (d, 1 H, J = 10 Hz), 8.15 (t, 1 H, J = 8.6 Hz); 13C NMR (100 MHz, DMSO- d6) δ 108.8, 109.0, 116.5, 117.0, 117.9, 118.1, 119.1, 121.6, 133.56, 133.60, 152.3, 152.5, 159.5, 161.9, 163.5, 163.6; HRMS (TOF) m/z calcd for C8H6F4N2O2H+: 239.0438, found: 239.0440 (Δ = −0.97 ppm).
2-Fluoro-4-trifluoromethylbenzohydrazide (3.18).
White solid (79% yield); mp 108–110 °C; 1H NMR (400 MHz DMSO- d6) δ 4.64 (s, 2 H), 7.66 (d, 1 H, J = 8.0 Hz), 7.75 – 7.81 (m, 2 H), 9.74 (s, 1 H); 13C NMR (100 MHz, DMSO- d6) δ 113.59, 113.63, 113.85, 113.88, 121.4, 121.7, 124.4, 127.3, 127.5, 131.20, 131.23, 131.85, 131.93, 132.2, 132.3, 157.5, 160.0, 162.1; HRMS (TOF) m/z calcd for C8H6F4N2OH+: 223.0489, found: 223.0493 (Δ = −1.83 ppm).
3-Fluorobenzohydrazide (3.19).
White solid (81% yield); mp 135 – 136 °C; 1H NMR (700 MHz, DMSO-d6) δ 4.54 (s, 2H), 7.36 (m, 1H), 7.50 (m, 1H), 7.61 (m, 1H), 7.68 (m, 1H), 9.88 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 113.7, 113.8, 117.9, 118.0, 123.10, 123.11, 130.5, 130.6, 135.60, 135.64, 161.2, 162.6, 164.4; HRMS (TOF) calcd for C7H7FN2OH+: 155.0615, found 155.0617 (Δ = −1.0 ppm).
3-Trifluoromethoxybenzohydrazide (3.22).
White solid (89 % yield); mp 92 – 93 °C; 1H NMR (700 MHz, DMSO-d6) δ 4.57 (s, 1H), 7.53 (br d, J = 8.1 Hz, 1H), 7.60 (t, J = 8.1 Hz, 1H), 7.77 (s, 1H), 7.86 (d, J = 8.1 Hz, 1H), 9.97 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 119.3, 119.5, 120.8, 123.6, 126.0, 130.6, 135.5, 148.3, 164.1; HRMS (TOF) calcd for C8H7F3N2O2H+: 221.0532, found 221.0537 (Δ = −2.0 ppm).
3-Trifluoromethylbenzohydrazide (3.23).
White solid (83 % yield); mp 109 – 110 °C; 1H NMR (700 MHz, DMSO-d6) δ 4.64 (s, 1H), 7.71 (t, J = 7.8 Hz, 1H), 7.89 (d, J = 7.8 Hz, 1H), 8.12 (d, J = 7.8 Hz, 1H), 8.15 (s, 1H), 10.05 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 123.2, 123.56, 123.58, 124.7, 127.7, 129.1, 129.2, 129.7, 131.0, 134.2, 164.2; HRMS (TOF) calcd for C8H7F3N2OH+ 205.0583, found 205.0586 (Δ = −1.1 ppm).
4-Bromo-N-methylbenzohydrazide (3.29).
White solid (83 % yield); mp 115 – 116 °C; 1H NMR (500 MHz, CDCl3) δ 3.20 (s, 3 H), 4.62 (s, 2 H), 7.34 (s, 2 H), 7.54 (d, 2 H, J = 8.0 Hz); 13C NMR (125 MHz, CDCl3) δ 40.7, 124.6, 129.3, 130.2, 131.8, 134.0, 169.2; HRMS (TOF) m/z calcd for C8H9BrN2OH+: 228.9971, found: 228.9969 (Δ = 0.61 ppm).
tert-Butyl 2-(4-(fluoromethyl)benzoyl)hydrazine-1-carboxylate (3.21).
To a solution of 4-Fluoromethylbenzoic acid (0.65 mmol) in dichloromethane (5 mL) was added N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (1.1 eq.), 4-Dimethylaminopyridine (1.1 eq.) and tert- Butyl carbazate (1.1 eq.). The reaction mixture was stirred at room temperature overnight. After completion, the reaction mixture was washed with brine, water and extracted with dichloromethane. The organic layer was collected, dried over magnesium sulfate and concentrated using rotary evaporator. Recrystallization of the crude product using dichloromethane: hexanes (2:8) resulted in pure product as white crystals (86% yield); mp 143 – 144 °C; 1H NMR (500 MHz DMSO- d6) δ 1.4 (s, 9 H), 5.43 (s, 1 H), 5.53 (s, 1 H), 7.50 (d, 2 H, 8.1 Hz), 7.88 (d, 2 H, J = 7.8 Hz), 8.91 (s, 1 H), 10.22 (s, 1 H); 13C NMR (100 MHz, DMSO-d6) δ 28.1, 79.2, 82.9, 84.2, 127.2, 127.3, 132.6, 139.7, 139.9, 155.4, 165.6; HRMS (TOF) m/z calcd for C13H17FN2O3H+: 269.1296, found: 269.1293 (Δ = 1.02 ppm).
Synthesis and characterization of acylhydrazones (5): 3-Bromo-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.0).
To a solution of 3-Bromobenzohydrazide (0.26 mmol) and 4-Bromosalicylaldehyde (1.1 eq.) in methanol (3 mL), 2 drops of glacial acetic acid were added. The reaction mixture was stirred at room temperature overnight. Addition of water to the reaction mixture resulted in precipitation of the product, which was filtered, washed with water and dried to give pure product as a white solid (98 % yield); mp 223–225 °C; 1H NMR (500 MHz, DMSO- d6) δ 7.11 (dd, 1 H, J = 8.3, 1.9 Hz), 7.14 (d, 1 H, J = 1.9 Hz), 7.51 (t, 1 H, J = 7.9 Hz), 7.58 (d, 1 H, J = 8.3 Hz), 7.81 (dd, 1 H, J = 8.0, 1.0 Hz), 7.93 (d, 1 H, J = 7.9 Hz), 8.11 (t, 1 H, J = 1.7 Hz), 8.62 (s, 1 H), 11.39 (s, 1 H), 12.17 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 118.6, 119.1, 121.8, 122.5, 124.0, 126.9, 130.2, 130.8, 134.7, 135.0, 146.7, 158.0, 161.3; HRMS (TOF) m/z calcd for C14H10Br2N2O2H+: 396.9181, found: 396.9188 (Δ = −1.77 ppm). HPLC (A): t = 12.56 min, purity >98%.
All other acylhydrazones were prepared in the same manner. Characterization data for new acylhydrazones are shown below.
2,3-Difluoro-N’-(5-chloro-2-hydroxybenzylidene)benzohydrazide (5.1).
Brown solid (56% yield); mp 171–173 °C; 1H NMR (400 MHz DMSO-d6) δ 6.85 (d, 1 H, 25%, J = 8.6 HZ), 6.94 (d, 1 H, 75%, J = 8.8HZ), 7.20 (d, 1 H, 15%, J = 2.6 Hz), 7.21 (s, 1 H, 40%), 7.30 – 7.37 (m, 4 H, 85%, 61%, 25%, 20%), 7.48 – 7.52 (m, 1 H, 75%), 7.55 – 7.64 (m, 1 H, 100%), 7.67 (d, 1 H, 80%, J = 2.6 Hz), 8.29 (s, 1 H, 25%), 8.51 (s, 1 H, 75%), 10.25 (s, 1 H, 25%), 11.01 (s, 1 H, 75%), 12.22 (s, 1 H, 100%); 13C NMR (100 MHz DMSO-d6) δ 118.1, 118.2, 118.9, 119.1, 119.9, 120.1, 121.4, 123.0, 123.1, 124.6, 124.8, 124.9, 125.3, 125.4, 127.2, 130.7, 131.1, 141.1, 146.1, 155.3, 156.0, 159.16, 159.18; 19F NMR (376 MHz DMSO-d6) δ −137.95 (d, 1 F, J = 23 Hz), −138.73 (d, 1 F, J = 23 Hz), −139.07 (d, 1 F, J = 23 Hz), −139.92 (d, 1 F, J = 23 Hz); HRMS (TOF) m/z calcd for C14H9OF2N2O2H+: 311.0393, found: 311.0387 (Δ= 1.75 ppm). HPLC (B): t = 5.33 min, purity >95%.
2,3-Difluoro-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.2).
White solid (87% yield); mp 219–221 °C; 1H NMR (400 MHz DMSO-d6) δ 6.97 (d, 1 H, 25%, J = 8.3 Hz), 7.03 (s, 1 H, 25%), 7.10 (d, 1 H, 75%, 8.3 Hz), 7.13 (s, 1 H, 75%), 7.19 (d, 1 H, 25%, J = 8.4 Hz), 7.30 – 7.37 (m, 3 H, 75%, 25%, 22%), 7.48 – 7.51 (m, 1 H, 75%), 7.58 (d, 1 H, 100%, J = 8.3 Hz), 7.60 – 7.66 (m, 1 H, 75%), 8.30 (s, 1 H, 26%), 8.52 (s, 1 H, 74%), 10.41 (s, 1 H, 25%), 11.22 (s, 1 H, 75%), 12.16 (s, 1 H, 100%); 13C NMR (100 MHz DMSO-d6) δ 118.6, 118.8, 118.9, 119.1, 119.9, 120.0, 122.5, 123.7, 124.2, 124.6, 124.1, 124.9, 125.27, 125.31, 127.9, 129.9, 141.8, 146.7, 148.4, 157.2, 158.0, 159.1; 19F NMR (376 MHz DMSO-d6) δ −137.95(d, 1 F, J = 23 Hz), −138.74 (d, 1 F, J = 23 Hz), −139.00 (d, 1 F, 23 Hz), −139.94 (d, 1 F, J = 23 Hz); HRMS (TOF) m/z calcd for C14H9BrF2N2O2H+: 354.9888, found: 354.9887 (Δ= 0.26 ppm). HPLC (A): t = 7.35 min, purity >95%.
2,4-Dibromo-N’-(2-hydroxy-5-methylbenzylidene)benzohydrazide (5.3).
White solid (84% yield); mp 181 – 183 °C; 1H NMR (500 MHz, DMSO-d6) δ 2.14 (s, 1H, 33%), 2.24 (s, 1H, 67%), 6.70 (d, 1H, 33%, J = 8.4 Hz), 6.83 (d, 1H, 67%, J = 8.4 Hz), 7.02 (dd, 1H, 33%, J = 8.4 Hz, J = 2.0 Hz), 7.07 (d, 1H, 33%, J = 1.6 Hz), 7.11 (dd, 1H, 67%, J = 8.4 Hz, J = 2.0 Hz), 7.39 (d, 1H, 67%, J = 1.8 Hz), 7.41 (d, 1H, 33%, J = 8.2 Hz), 7.54 (d, 1H, 67%, 8.2 Hz), 7.72 (m, 1H), 8.00 (d, 1H, 33%, J = 1.8 Hz), 8.02 (d, 1H, 67%, J = 1.8 Hz), 8.25 (s, 1H, 33%), 8.44 (s, 1H, 67%), 9.61 (s, 1H, 33%), 10.69 (s, 1H, 67%), 12.10 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 19.9, 116.0, 116.2, 118.30, 118.34, 119.7, 120.7, 122.7, 123.7, 127.9, 128.0, 128.4, 128.9, 130.0, 130.8, 130.9, 132.0, 132.4, 133.5, 134.2, 134.8, 136.2, 137.1, 144.7, 148.1, 154.5, 155.2, 162.3, 168.0; HRMS (TOF) calcd for C15H12Br2N2O2H+: 410.9338, found 410.9338 (Δ = −1.0 ppm). HPLC (B): t = 4.0 min, purity >95%.
2,4-Dibromo-N’-(5-chloro-2-hydroxybenzylidene)benzohydrazide (5.2a).
White solid (72% yield); mp 187 – 190 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.94 (d, 1H, 75%, J = 8.8 Hz), 6.98 (d, 1H, 25%, J = 8.8 Hz), 7.30 (dd, 1H, 75%, J = 8.8 Hz, J = 2.7 Hz), 7.40 (dd, 1H, 25%, J = 8.8 Hz, J = 2.7 Hz), 7.66 (d, 1H, 78%, J = 2.7 Hz), 7.75 (d, 1H, 22%, J = 2.7 Hz),7.71 (m, 2H, 83%), 8.00 (d, 1H, 35%, J = 1.8 Hz), 8.02 (d, 1H, 65%, 1.8 Hz), 8.27 (s, 1H, 35%), 8.47 (s, 1H, 65%),10.20 (br s, 1H, 35%), 11.01 (br s, 1H, 65%), 12.19 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 118.0, 118.2, 119.8, 120.67, 120.68, 121.0, 122.7, 123.0, 123.1, 123.8, 126.0, 127.1, 130.2, 130.69, 130.71, 130.8, 130.9, 131.1, 134.2, 134.9, 136.1, 137.2, 141.6, 145.7, 155.3, 155.9, 162.5, 168.3; HRMS (TOF) calcd for C14H9Br2ClN2O2H+: 430.8792, found 430.8794 (Δ = −0.5 ppm). HPLC (B): t1 = 3.2, t2 = 4.6 min, purity >95%.
2,4-Dibromo-N’-((2-hydroxynaphthalen-1-yl)methylene)benzohydrazide (5.2b).
White solid (68% yield); mp > 220 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.12 (d, 1H, 40%, J = 8.9 Hz), 7.28 (m, 2H, 70%), 7.41 (t, 1H, 60%, J = 7.3 Hz), 7.47 (d, 1H, 40%, J = 8.2 Hz), 7.60 (m, 2H, 60%), 7.80 (m, 3H, 60%), 8.06 (m, 1H), 8.14 (d, 1H, 40%, 8.5 Hz), 8.31 (d, 1H, 60%, 8.5 Hz), 8.95 (s, 1H, 40%), 9.28 (s, 1H, 60%), 10.66 (s, 1H, 40%), 12.11 (s, 1H, 40%), 12.24 (s, 1H, 60%), 12.36 (s, 1H, 60%); 13C NMR (125 MHz, DMSO-d6) δ 108.5, 109.6, 118.2, 118.8, 119.7, 120.8, 121.1, 122.5, 122.8, 123.4, 123.6, 123.9, 127.3, 127.88, 127.92, 128.0, 128.7, 129.0, 129.8, 130.95, 131.03, 131.5, 132.6, 133.1, 134.3, 135.0, 135.9, 137.7, 143.3, 147.2, 157.0, 158.1, 162.1, 168.1; HRMS (TOF) calcd for C18H13Br2N2O2H+: 446.9338, found 446.9344 (Δ = −1.3 ppm). HPLC (B): t1 = 3.0, t2 = 3.5 min, purity >96%.
2,4-Dibromo-N’-(3,5-dichloro-2-hydroxybenzylidene)benzohydrazide (5.2c).
White solid (68% yield); 197 – 199 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.41 (d, 1H, 30%, J = 2.5 Hz), 7.45 (d, 1H, 30%, J = 8.2 Hz), 7.55 (d, 1H, 30%, J = 2.5 Hz), 7.58 (d, 1H, 70%, J = 8.2 Hz), 7.65 (d, 1H, 70%, 2.5 Hz), 7.69 (d, 1H, 70%, J = 2.5 Hz), 7.75 (m, 1H), 8.05 (d, 1H, J = 1.6 Hz), 8.27 (s, 1H, 30%), 8.44 (s, 1H, 70%), 10.34 (s, 1H, 30%), 12.12 (s, 1H, 70%), 12.45 (s, 1H, 30%), 12.55 (s, 1H, 70%); 13C NMR (125 MHz, DMSO-d6) δ 119.5, 120.6, 120.8, 121.5, 121.6, 121.7, 123.06, 123.10, 123.4, 124.1, 127.2, 128.3, 130.0, 130.3, 130.6, 130.8, 130.9, 131.0, 134.4, 134.9, 135.5, 136.6, 143.4, 147.3, 151.0, 152.1, 162.6, 168.2; HRMS (TOF) calcd for C14H8Br2Cl2N2O2H+: 464.8402, found 464.8407 (Δ = −1.1 ppm). HPLC (B): t = 3.4 min, purity >96%.
2,4-Dibromo-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.2d).
White solid (83% yield); mp 187 – 188 °C; 1H NMR (700 MHz, DMSO-d6) δ 6.99 (dd, 1H, 37%, J = 8.3 Hz, J = 1.8 Hz), 7.03 (d, 1H, 37%, J = 1.8 Hz), 7.12 (dd, 1H, 63%, J = 8.3 Hz, J = 1.8 Hz), 7.14 (d, 1H, 63%, J = 1.8 Hz), 7.18 (d, 1H, 37%, J = 8.4 Hz), 7.40 (d, 1H, 37%, J = 8.2 Hz), 7.54 (d, 1H, 63%, J = 8.2 Hz), 7.59 (d, 1H, 63%, J = 8.4 Hz), 7.69 (dd, 1H, 37%, J = 8.1 Hz, J = 1.8 Hz), 7.74 (dd, 1H, 63%, J = 8.1 Hz, J = 1.8 Hz), 7.98 (d, 1H, 37%, J = 1.8 Hz), 8.02 (d, 1H, 63%, J = 1.8 Hz), 8.28 (s, 1H, 37%), 8.46 (s, 1H, 63%), 10.36 (s, 1H, 37%), 11.20 (s, 1H, 63%), 12.14 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 118.6, 118.8, 118.97, 119.05, 119.8, 120.7, 122.6, 122.7, 123.7, 123.8, 124.2, 128.5, 129.9, 130.2, 130.7, 130.8, 130.9, 134.2, 134.9, 136.1, 137.2, 142.1, 146.3, 157.2, 157.9, 162.4, 168.2; HRMS (TOF) calcd for C14H9Br3N2O2H+: 474.8287, found 474.8286 (Δ = 0.2 ppm). HPLC (B): t = 3.8 min, purity >96%.
2,4-Dibromo-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.2e).
White solid (91% yield); mp : > 220 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.80 (d, 1H, 35%, J = 8.7 Hz), 6.90 (d, 1H, 65%, 8.7 Hz), 7.35 (m, 1H, 65%), 7.43 (m, 1H), 7.54 (d, 1H, 65%, J =8.2 Hz), 7.71 (dd, 1H, 35%, J = 8.2 Hz, J = 1.8 Hz), 7.73 (dd, 1H, 65%, J = 8.2 Hz, J = 1.8 Hz), 7.81 (d, 1H, 65%, J = 2.5 Hz), 8.26 (s, 1H, 35%), 8.46 (s, 1H, 65%), 10.22 (br s, 1H, 35%), 11.00 (br s, 1H, 65%), 12.18 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 110.51, 110.52, 118.5, 118.7, 119.8, 120.6, 121.3, 121.6, 122.7, 123.8, 129.0, 130.0, 130.2, 130.7, 130.8, 130.9, 133.5, 133.9, 134.1, 134.8, 136.1, 137.2, 141.5, 145.6, 155.7, 156.4, 162.5, 168.3; HRMS (TOF) calcd for C14H9Br3N2O2H+: 474.8287, found 474.8292 (Δ = −1.1 ppm). HPLC (A): t = 7.8 min, purity >98%.
2,5-Dibromo-N’-(2-hydroxy-5-methylbenzylidene)benzohydrazide (5.3).
White solid (71% yield); mp 190 – 192 °C; 1H NMR (700 MHz, DMSO-d6) δ 2.14 (s, 3H, 33%), 2.24 (s, 3H, 67%), 6.70 (d, 1H, 33%, J = 8.3 Hz), 6.83 (d, 1H, 67%, J = 8.3 Hz), 7.02 (d, 1H, 33%, J = 8.4 Hz), 7.07 (s, 1H, 33%), 7.12 (d, 1H, 67%, J = 8.4 Hz), 7.40 (s, 1H, 67%), 7.60 (dd, 1H, 33%, J = 8.5 Hz, J = 2.2 Hz), 7.67 (m, 2H), 7.84 (d, 1H, 67%, J = 2.2 Hz), 8.25 (s, 1H, 33%), 8.44 (s, 1H, 67%), 9.60 (s, 1H, 33%), 10.67 (s, 1H, 67%), 12.13 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 20.0, 116.1, 116.3, 117.8, 118.3, 118.4, 118.8, 120.67, 120.73, 127.9, 128.0, 128.3, 128.8, 131.0, 131.9, 132.0, 132.4, 133.5, 134.2, 134.3, 134.9, 138.8, 139.9, 144.6, 148.1, 154.5, 155.2, 161.6, 167.2; HRMS (TOF) calcd for C15H12Br2N2O2H+: 410.9338, found 410.9330 (Δ = 1.9 ppm). HPLC (B): t = 4.8 min, purity >95%.
2,5-Dibromo-N’-(5-chloro-2-hydroxybenzylidene)benzohydrazide (5.3a).
White solid (72% yield); mp 185 – 187 °C; 1H NMR (700 MHz, DMSO-d6) δ 6.85 (m, 1H, 35%), 6.96 (d, 1H, 65%, J = 8.8 Hz), 7.23 (m, 1H, 65%), 7.33 (dd, 1H, 65%, J = 8.8 Hz, J = 2.2 Hz), 7.64 (m, 2H, 68%), 7.69 (m, 2H, 83%), 7.84 (d, 1H, 65%, J = 1.5 Hz), 8.27 (s, 1H, 35%), 8.46 (s, 1H, 65%), 10.19 (br s, 1H, 35%), 10.99 (br s, 1H, 65%), 12.22 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 117.9, 118.0, 118.2, 118.7, 120.6, 120.69, 120.73, 121.1, 123.0, 123.1, 126.0, 127.0, 130.7, 131.09, 131.12, 131.9, 133.5, 134.2, 134.4, 134.9, 138.7, 139.8, 141.5, 145.8, 155.3, 156.0, 161.8, 167.5; HRMS (TOF) calcd for C14H9Br2ClN2O2H+: 430.8792, found 430.8789 (Δ = 0.7 ppm). HPLC (B): t = 4.2 min, purity >95%.
2,5-Dibromo-N’-((2-hydroxynaphthalen-1-yl)methylene)benzohydrazide (5.3b).
White solid (68% yield); mp > 220 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.13 (d, 1H, 40%, 8.9 Hz), 7.26 (d, 1H, 60%, 8.9 Hz), 7.30 (m, 2H, 40%), 7.42 (t, 1H, 60%, J = 7.4 Hz), 7.61 (t, 1H, 60%, J = 7.6 Hz), 7.66 (td, 1H, 60%, J = 10.4 Hz, J = 1.8 Hz), 7.69 (d, 1H, 40%, J = 1.8 Hz), 7.72 (t, 1H), 7.79 (m, 2H, 40%), 7.83 (d, 1H, 40%, J = 8.9 Hz), 7.90 (d, 1H, 60%, J = 8.1 Hz), 7.92 (d, 1H, 60%, J = 1.6 Hz), 7.96 (d, 1H, 60%, 8.9 Hz), 8.20 (d, 1H, 40%, J = 8.3 Hz), 8.33 (d, 1H, 60%, J = 8.6 Hz), 8.94 (s, 1H, 40%), 9.28 (s, 1H, 60%), 10.65 (s, 1H, 40%), 12.15 (s, 1H, 40%), 12.28 (s, 1H, 60%), 12.35 (s, 1H, 60%); 13C NMR (175 MHz, DMSO-d6) δ 108.5, 109.6, 117.7, 118.1, 118.76, 118.84, 120.78, 120.80, 121.1, 123.0, 123.3, 123.6, 127.4, 127.88, 127.94, 128.0, 128.6, 129.0, 130.7, 131.0, 131.5, 132.0, 132.6, 133.2, 133.4, 134.3, 134.5, 135.0, 138.5, 140.4, 143.3, 147.4, 157.0, 158.1, 161.3, 167.3; HRMS (TOF) calcd for C18H12Br2N2O2H+: 446.9338, found 446.9333 (Δ = 1.1 ppm). HPLC (B): t = 3.6 min, purity >97%.
2,5-Dibromo-N’-(3,5-dichloro-2-hydroxybenzylidene)benzohydrazide (5.3c).
White solid (49% yield); mp 196 – 198 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.41 (d, 1H, 28%, J = 2.5 Hz) 7.55 (d, 1H, 28%, J = 2.5 Hz), 7.64 (m, 1H), 7.69 (m, 3H, 72%), 7.75 (d, 1H, 28%, J = 2.5 Hz), 7.88 (d, 1H, 72%, J = 2.2 Hz), 8.27 (s, 1H, 28%), 8.44 (s, 1H, 72%), 10.33 (br s, 1H, 28%), 12.10 (br s, 1H, 72%), 12.48 (s, 1H, 28%), 12.58 (s, 1H, 72%); 13C NMR (100 MHz, DMSO-d6) δ 118.7, 120.7, 120.8, 120.9, 121.6, 121.7, 121.8, 123.1, 127.2, 128.3, 130.6, 131.9, 134.4, 134.6, 134.9, 143.4, 147.4, 151.1, 152.2, 161.8; HRMS (TOF) calcd for C14H8Br2Cl2N2O2H+: 464.8402, found 464.8394 (Δ = 1.7 ppm). HPLC (B): t = 4.5 min, purity >95%.
2,5-Dibromo-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.3d).
White solid (87% yield); mp 186 – 187 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.98 (dd, 1H, 33%, J = 8.4 Hz, J = 1.6 Hz), 7.03 (d, 1H, 33%, J = 1.7 Hz), 7.11 (dd, 1H, 67%, J = 8.4 Hz, J = 1.6 Hz), 7.14 (d, 1H, 67%, J = 1.7 Hz), 7.18 (d, 1H, 33%, J = 8.4 Hz), 7.59 (m, 1H), 7.66 (m, 2H), 7.83 (d, 1H, 67%, J = 2.3 Hz), 8.28 (s, 1H, 33%), 8.47 (s, 1H, 67%), 10.34 (s, 1H, 33%), 11.19 (s, 1H, 67%), 12.17 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 117.8, 118.6, 118.7, 118.8, 118.9, 119.0, 120.6, 120.7, 122.5, 123.7, 124.2, 128.5, 129.8, 131.0, 131.8, 133.5, 134.2, 134.4, 134.8, 138.7, 139.9, 142.3, 146.5, 157.2, 157.9, 161.6, 167.4; HRMS (TOF) calcd for C14H9Br3N2O2H+: 474.8287, found 474.8279 (Δ = 1.7 ppm). HPLC (B): t = 4.2 min, purity >95%.
2,5-Dibromo-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.3e).
White solid (87% yield); mp >220 °C; 1H NMR (700 MHz, DMSO-d6) δ 6.80 (d, 1H, 35%, J = 8.7 Hz), 6.91 (d, 1H, 65%, J = 8.7 Hz), 7.34 (dd, 1H, 35%, J = 8.7 Hz, J = 2.3 Hz), 7.36 (d, 1H, 35%, J = 2.3 Hz), 7.44 (dd, 1H, 65%, J = 8.7 Hz, J = 2.3 Hz), 7.60 (dd, 1H, 35%, J = 8.7 Hz, J = 2.3 Hz), 7.66 (m, 3H, 55%), 7.70 (d, 1H, 35%, J = 2.0 Hz), 7.82 (d, 1H, 65%, J = 2.3 Hz), 7.84 (d, IH, 65%, J = 2.0 Hz), 8.26 (s, 1H, 35%), 8.46 (s, 1H, 65%), 10.20 (s, 1H, 35%), 10.99 (s, 1H, 65%), 12.22 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 110.56, 110.59, 117.9, 118.5, 118.68, 118.72, 120.6, 120.7, 121.3, 121.6, 129.0, 139.9, 131.1, 131.9, 133.50, 133.53, 133.9, 134.2, 134.4, 134.9, 138.7, 139.8, 141.5, 145.6, 155.7, 156.3, 161.8, 167.5; HRMS (TOF) calcd for C14H9Br3N2O2H+ 474.8287, found 474.8281 (Δ = 1.3 ppm). HPLC (B): t1 = 3.9, t2 = 4.9 min, purity >95%.
4-Bromo-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.4).
White solid (88 % yield); mp >230 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.11 (d, 1 H, J = 8.3 Hz), 7.14 (s, 1 H), 7.57 (d, 1 H, J = 8.3 Hz), 7.76 (d, 2 H, J = 8.5 Hz), 7.88 (d, 2 H, J = 8.5 Hz), 8.62 (s, 1 H), 11.44 (s, 1 H), 12.17 (s, 1 H); 13C NMR (100 MHz DMSO-d6) δ 118.6, 119.1, 122.4, 124.0, 125.8, 129.7, 130.2, 131.6, 131.9, 146.7, 158.0, 161.9; HRMS (TOF) m/z calcd for C14H10Br2N2OH+: 396.9181, found: 396.9188 (Δ = −1.56 ppm). HPLC (A): t = 9.69 min, purity >97%.
3-Bromo-6-hydroxy-N’-(2-hydroxy-5-methylbenzylidene)benzohydrazide (5.5).
Light yellow solid (89 % yield); mp >230 °C; 1H NMR (500 MHz, DMSO-d6) δ 2.25 (s, 3 H), 6.84 (d, 1 H, J = 8.2 Hz), 6.96 (d, 1 H, J = 8.8 Hz), 7.12 (d, 1 H, J = 7.8 Hz), 7.38 (s, 1 H), 7.59 (d, 1 H, J = 8.5 Hz), 8.03 (s, 1 H), 8.62 (s, 1 H), 10.82 (s, 1 H), 11.88 (s, 1 H), 12.01 (s, 1 H); 13C NMR (100 MHz DMSO-d6) δ 19.9, 110.0, 116.3, 118.0, 118.3, 119.6, 128.0, 129.0, 130.7, 132.5, 136.2, 149.0, 155.3, 158.0, 163.0; HRMS (TOF) m/z calcd for C15H13BrN2O3H+: 349.0182, found: 349.0187 (Δ = −1.35 ppm). HPLC (B): t = 5.74 min, purity >95%.
3,5-Dibromo-2-hydroxy-N’-(5-methyl-2-hydroxyphenylmethylidene)benzohydrazide (5.6).
Yellow solid (88% Yield); mp 223 – 224 °C; 1H NMR (700 MHz DMSO-d6) δ 2.23 (s, 3 H), 6.83 (d, 1 H, J = 8.3 Hz), 7.12 (dd, 1 H, J = 8.3, 1.9 Hz), 7.43 (s, 1 H), 8.01 (d, 1 H, J = 2.2 Hz), 8.19, (d, 1 H, J = 2.2 Hz), 8.67 (s, 1 H), 10.63 (s, 1 H), 12.37 (s, 1 H), 13.09 (s, 1 H); 13C NMR (175 MHz DMSO-d6) δ 20.0, 109.8, 112.4, 116.3, 116.6, 118.4, 128.1, 128.5, 129.2, 132.8, 138.7, 149.5, 155.4, 156.8, 164.2; HRMS (TOF) m/z calcd for C15H12Br2N2O3H+: 426.9287, found: 426.9290 (Δ = −0.64 ppm). HPLC (C): t = 4.9 min, purity >96%.
3,5-Dibromo-2-hydroxy-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.6a).
Yellow solid (95% Yield); mp >230 °C; 1H NMR (700 MHz DMSO-d6) δ 7.10 (dd, 1 H, J = 8.3, 1.8 Hz), 7.12 (s, 1 H), 7.62 (d, 1 H, J = 8.3 Hz), 8.00 (d, 1 H, J = 2.2 Hz), 8.17 (d, 1 H, J = 2.2 Hz), 8.68 (s, 1 H), 11.17 (s, 1 H), 12.41 (s, 1 H); 13C NMR (175 MHz DMSO-d6) δ 109.8, 112.5, 116.6, 118.6, 119.1, 122.6, 124.6, 129.2, 129.5, 138.8, 147.8, 156.8, 158.1, 164.2; HRMS (TOF) m/z calcd for C14H9Br3N2O3H+: 490.8236, found: 490.8234 (Δ = 0.42 ppm). HPLC (A): t = 7.8 min, purity >95%.
N’-(3,5-Dibromo-2-hydroxybenzylidene)quinolinylhydrazide (5.7).
Beige solid (99 % yield); mp >215 °C; 1H NMR (700 MHz DMSO-d6) δ 7.73 (t, 1 H, J = 7.9 Hz), 7.85 (dd, 2 H, J = 13.4, 2.4 Hz), 7.91 (t, 1 H, J = 7.7 Hz), 8.12 (d, 1 H, J = 8.5 Hz), 8.16 (d, 1 H, J = 7.8 Hz), 8.57 (s, 1 H), 8.95 (d, 1 H, J = 2.0 Hz), 9.34 (d, 1 H, J = 2.2 Hz), 12.63 (s, 1 H), 12.85 (s, 1 H); 13C NMR (175 MHz DMSO-d6) δ 110.5, 111.3, 121.0, 125.1, 126.4, 127.7, 128.9, 129.3, 131.8, 132.2, 135.8, 136.5, 147.5, 148.8, 153.7, 161.8; HRMS (TOF) m/z calcd for C17H11Br2N3O2H+: 447.9290, found: 447.9036 (Δ = −3.47 ppm). HPLC (A): t = 8.3 min, purity >95%.
3,4-Dibromo-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.8).
Yellow solid (78 % yield); mp >230 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.81 – 7.86 (m, 3 H), 7.95 (d, 1 H, 8.3 Hz), 8.27 (d, 1 H, J = 1.8 Hz), 8.50 (s, 1 H), 12.61 (d, 2 H); 13C NMR (125 MHz, DMSO-d6) δ 110.5, 111.3, 120.9, 124.3, 128.4, 128.6, 132.1, 132.5, 132.9, 134.1, 135.8, 147.6, 153.6, 160.8; HRMS (TOF) m/z calcd for Cl4H8Br4N2O2H+: 552.7391, found: 552.7392 (Δ= 0.18 ppm). HPLC (A): t = 9.1 min, purity >98%.
3,4-Dibromo-N’-(3,5-dichloro-2-hydroxybenzylidene)benzohydrazide (5.8a).
Yellow solid (64 % yield); mp 212–214 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.61 (d, 1 H, J = 2.3 Hz), 7.67 (d, 1 H, J = 2.4 Hz), 7.84 (dd, 1 H, J = 8.1, 1.5 Hz), 7.95 (d, 1 H), 8.27 (s, 1 H), 8.54 (s, 1 H), 12.31 (s, 1 H), 12.59 (s, 1 H); 13C NMR (125 MHz, DMSO-d6) δ 120.8, 121.6, 123.0, 124.3, 128.36, 128.39, 128.6, 130.5, 132.5, 133.0, 134.1, 147.5, 152.2, 160.8; HRMS (TOF) m/z calcd for Cl4H8Br2Cl2N2O2H+: 464.8404, found: 464.8402 (Δ= −0.32 ppm). HPLC (B): t = 4.1 min, purity >98%.
3,4-Dibromo-N’-(5-chloro-2-hydroxybenzylidene)benzohydrazide (5.8b).
Light yellow solid (35% yield); mp >230 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.94 (d, 1 H, 75%, J = 8.8Hz), 6.98 (d, 1 H, 25%, J = 8.8 Hz), 7.30 (dd, 1 H, 75%, J = 8.8, 2.7 Hz), 7.40 (dd, 1 H, 25%, J = 8.8, 2.6 Hz), 7.66 (d, 1 H, 78%, J = 2.7 Hz), 7.75 (d, 1 H, 22%, J = 2.7 Hz), 7.84 (dd, 1 H, 80%, J = 8.3, 2.0 Hz), 7.93 (d, 1 H, 78%, J = 8.3 Hz), 8.28 (d, 1 H, 76%, J = 2.0 Hz), 8.60 (s, 1 H, 77%), 8.93 (s, 1 H, 23%), 11.10 (s, 1 H, 25%), 11.12 (s, 1 H, 75%), 12.24 (s, 1 H, 73%); 13C NMR (125 MHz, DMSO-d6) δ 118.2, 118.5, 119.9, 120.7, 123.0, 123.1, 124.2, 127.2, 128.0, 128.5, 128.6, 131.0, 132.4, 132.7, 133.6, 134.0, 146.1, 156.0, 157.2, 160.7, 160.9; HRMS (TOF) m/z calcd for Cl4H9Br2ClN2O2H+: 430.8812, found: 430.8792 (Δ= −4.51 ppm). HPLC (B): t = 8.1 min, purity >95%.
3,4-Dibromo-N’-(2-hydroxy-1-naphthylidene)benzohydrazide (5.8c).
Yellow solid (66 % yield); mp >230 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.23 (d, 1 H, J = 8.9 Hz), 7.40 (t, 1 H, J = 7.2 Hz), 7.60 (t, 1 H, J = 7.3 Hz), 7.87 – 7.99 (m, 4 H), 8.27 – 8.32 (m, 2 H), 9.44 (s, 1 H), 12.30 (s, 1 H), 12.57 (s, 1 H); 13C NMR (100 MHz, DMSO-d6) δ 108.6, 118.9, 120.91, 123.6, 124.3, 127.86, 127.88, 128.1, 128.5, 129.0, 131.6, 132.4, 133.1, 133.5, 134.2, 147.6, 158.1, 160.4; HRMS (TOF) m/z calcd for C18H12Br2N2O2H+: 446.9340, found: 446.9338 (Δ= −0.46 ppm). HPLC (B): t = 4.2 min, purity >96%.
3,4-Dibromo-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.8d).
Light yellow solid (53 % yield); mp >230 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.89 (d, 1 H, J = 8.8 Hz), 7.42 (dd, 1 H, J = 8.7, 2.5 Hz), 7.79 (d, 1 H, J = 2.5 Hz), 7.84 (dd, 1 H, J = 8.3, 2.0 Hz), 7.93 (d, 1 H, J = 8.4 Hz), 8.28 (d, 1 H, J = 1.9 Hz), 8.60 (s, 1 H), 11.13 (s, 1 H), 12.24 (s, 1 H); 13C NMR (125 MHz, DMSO-d6) δ 110.5, 118.7, 121.3, 124.2, 128.0, 128.5, 130.0, 132.5, 133.6, 133.8, 134.0, 145.9, 156.4, 160.7; HRMS (TOF) m/z calcd for C14H9Br3N2O2H+: 474.8288, found: 474.8287 (Δ= −0.19 ppm). HPLC (B): t = 7.5 min, purity >95%.
3,4-Dibromo-N’-(2-hydroxy-5-methylbenzylidene)benzohydrazide (5.8e).
White solid (94 % yield); mp 214–216 °C; 1H NMR (500 MHz DMSO-d6) δ 2.23 (s, 3 H, 78%), 2.24 (s, 3 H, 22%), 6.82 (d, 1 H, 77%, J = 8.3 Hz), 6.86 (d, 1 H, 23%, J = 8.3 Hz), 7.09 (dd, 1 H, 77%, J = 8.3, 1.6 Hz), 7.18 (dd, 1 H, 23%, J = 8.3, 1.7 Hz), 7.36 (s, 1 H, 78%), 7.45 (s, 1 H, 22%), 7.83 (dd, 1 H, 78%, J = 8.3, 1.9 Hz), 7.93 (d, 1 H, 78%, J = 8.3 Hz), 8.27 (d, 1 H, 77%, J = 1.9 Hz), 8.58 (s, 1 H, 78%), 8.90 (s, 1 H, 22%), 10.85 (s, 1 H, 75%), 10.87 (s, 1 H, 25%), 12.15 (s, 1 H, 77%); 13C NMR (100 MHz, DMSO-d6) δ 19.9, 116.3, 116.4, 117.9, 118.4, 124.2, 127.90, 127.94, 128.1, 128.5, 129.0, 130.5, 132.3, 132.4, 133.7, 133.9, 134.0, 148.5, 155.3, 156.5, 160.5, 162.5; HRMS (TOF) m/z calcd for C15H12Br2N2O2H+: 410.9343, found: 410.9338 (Δ= −1.25 ppm). HPLC (A): t = 9.5 min, purity >97%.
3,4-Dibromo-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.8f).
Off-white solid (63 % yield); m.p. >230 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.09 (dd, 1 H, J = 8.3, 1.8 Hz), 7.12 (d, 1 H, J = 1.8 Hz), 7.57 (d, 1 H, J = 8.4 Hz), 7.83 (dd, 1 H, J = 8.4, 2.0 Hz), 7.93 (d, 1 H, J = 8.4 Hz), 8.27 (d, 1 H, J = 2 Hz), 8.60 (s, 1 H), 11.33 (s, 1 H), 12.18 (s, 1 H); 13C NMR (100 MHz, DMSO-d6) δ 118.6, 119.0, 122.5, 124.1, 124.2, 128.0, 128.5, 130.0, 132.4, 133.6, 134.0, 146.8, 158.0, 160.6; HRMS (TOF) m/z calcd for C14H9Br3N2O2H+: 474.8286, found: 474.8287 (Δ= 0.16 ppm). HPLC (A): t = 7.6 min, purity >95%.
3,5-Dibromo-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.9).
White solid (58 % yield); m.p. >230 °C; 6.90 (d, 1 H, J = 8.8 Hz), 7.43 (dd, 1 H, J = 8.8, 2.6 Hz), 7.82 (d, 1 H, J = 2.5 Hz), 8.11 (s, 3 H), 8.61 (s, 1 H), 11.10 (s, 1 H), 12.25 (s, 1 H); 13C NMR (100 MHz, DMSO-d6) δ 110.5, 118.7, 121.3, 122.7, 129.6, 130.0, 133.9, 136.4, 136.6, 146.0, 156.4, 160.1; HRMS (TOF) m/z calcd for C14H9Br3N2O2H+: 474.8288, found: 474.8287 (Δ= −0.32 ppm). HPLC (A): t = 15.1 min, purity >95%.
3,5-Dibromo-N’-(2-hydroxy-1-naphthylidene)benzohydrazide (5.9a).
Yellow solid (42 % yield); m.p. >230 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.24 (d, 1 H, J = 8.9 Hz), 7.42 (t, 1 H, J = 7.4 Hz), 7.62 (t, 1 H, J = 7.6 Hz), 7.90 (d, 1 H, J = 8 Hz), 7.95 (d, 1 H, J = 8.9 Hz), 8.14 (t, 1 H, J = 1.6 Hz), 8.16 (d, 2 H, J = 1.6 Hz), 8.32 (d, 1 H, J = 8.6 Hz), 9.44 (s, 1 H), 12.31 (s, 1 H), 12.50 (s, 1 H); 13C NMR (175 MHz, DMSO-d6) δ 108.5, 118.8, 121.0, 122.9, 123.6, 127.86, 127.90, 129.0, 129.6, 131.6, 133.1, 136.3, 136.7, 147.8, 158.2, 159.7; HRMS (TOF) m/z calcd for C18H12Br2N2O2H+: 446.9340, found: 446.9338 (Δ= −0.34 ppm). HPLC (B): t = 8.1 min, purity >97%.
3,5-Dibromo-N’-(3,5-dichloro-2-hydroxybenzylidene)benzohydrazide (5.9b).
Tan solid (99% yield); mp >230 °C; 1H NMR (500 MHz, DMSO-d6) δ 7.63 (d, 1 H, J = 2.4 Hz), 7.69 (d, 1 H, J = 2.4 Hz), 8.11 (s, 2 H), 8.12 (d, 1 H, J = 1.4 Hz), 8.55 (s, 1 H), 12.24 (s, 1 H), 12.610 (s, 1 H); 13C NMR (100 MHz, DMSO-d6) δ 120.8, 121.6, 122.8, 123.1, 128.3, 129.7, 130.5, 135.8, 136.9, 147.7, 152.2, 160.2; HRMS (TOF) m/z calcd for C14H8Br2Cl2N2O2H+: 464.8402, found: 464.8427 (Δ = −5.33 ppm). HPLC (A): t = 9.6 min, purity >95%.
3,5-Dibromo-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.9c).
White solid (84% yield); mp >230 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.15 (d, 1 H, J = 8.3 Hz), 7.14 (s, 1 H), 7.59 (d, 1 H, J = 8.3 Hz), 8.10 (s, 3 H), 8.62 (s, 1 H), 11.30 (s, 1 H), 12.20 (s, 1 H); 13C NMR (175 MHz, DMSO-d6) δ 118.6, 119.1, 122.5, 122.8, 124.2, 129.6, 129.9, 136.5, 136.6, 146.8, 158.0, 160.0; HRMS (TOF) m/z calcd for C14H9Br3N2O2H+: 474.8289, found: 474.8287 (Δ= −0.42 ppm). HPLC (C): t = 5.2 min, purity >96%.
3,5-Dibromo-N’-(5-chloro-2-hydroxybenzylidene)benzohydrazide (5.9d).
White solid (92% yield); mp >230 °C; 1H NMR (700 MHz, DMSO-d6) δ 6.95 (d, 1 H, J = 8.8Hz), 7.33 (dd, 1 H, J = 8.8, 2.7 Hz), 7.69 (s, 1 H), 8.11 (s, 3 H), 8.62 (s, 1 H), 11.09 (s, 1 H), 12.25 (s, 1 H); 13C NMR (175 MHz, DMSO-d6) δ 118.3, 120.7, 122.8, 123.1, 127.1, 129.6, 131.1, 136.4, 136.6, 146.2, 156.0, 160.1; HRMS (TOF) m/z calcd for C14H9Br2ClN2O2H+: 430.8802, found: 430.8792 (Δ= −2.34ppm). HPLC (B): t = 5.3 min, purity >95%.
3,5-Dibromo-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.9e).
Yellow solid (69% yield); mp >230 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.84 (d, 2 H, J = 3.9 Hz), 8.11 (d, 2 H, J = 1.6 Hz), 8.13 (s, 1 H), 8.51 (s, 1 H), 12.49 (s, 1 H), 12.64 (s, 1 H); 13C NMR (175 MHz, DMSO-d6) δ 110.5, 111.4, 120.9, 122.8, 129.7, 132.2, 135.8, 135.9, 136.9, 147.9, 153.7, 160.3; HRMS (TOF) m/z calcd for C14H8Br4N2O2H+: 552.7392, found: 552.7389 (Δ = 0.43 ppm). HPLC (A): t = 8.7 min, purity >95%.
3,5-Dibromo-N’-(2-hydroxy-5-methylbenzylidene)benzohydrazide (5.9f).
White solid (92% yield); mp >230 °C; 1H NMR (400 MHz, DMSO-d6) δ 2.25 (s, 3 H), 6.82 (d, 1 H, J = 8.2 Hz), 7.10 (dd, 1 H, J = 8.3, 1.8 Hz), 7.38 (s, 1 H), 8.09 (s, 3 H), 8.91 (s, 1 H), 10.78 (s, 1 H), 12.15 (s, 1 H); 13C NMR (175 MHz, DMSO-d6) δ 19.9, 116.2, 118.4, 122.7, 127.9, 128.8, 129.5, 132.4, 136.5, 148.5, 155.3, 159.9; HRMS (TOF) m/z calcd for C15H12Br2N2O2H+: 410.9345, found: 410.9338 (Δ = −1.65 ppm). HPLC (A): t = 8.8 min, purity >99%.
2,3-Dibromo-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.10).
White solid (99% yield); mp >230 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.96 (dd, 1 H, 36%, J = 8.4, 1.7 Hz), 7.01 (d, 1 H, 35%, J = 1.8 Hz), 7.09 (dd, 1 H, 64%, J = 8.4, 1.7 Hz), 7.12 (s, 1 H, 70%), 7.14 (s, 1 H, 30%), 7.37 – 7.44 (m, 3 H, 65%, 35%, 35%), 7.54 (dd, 1 H, 65%, J = 7.6, 1.4 Hz), 7.58 (d, 1 H, 65%, J = 8.4 Hz), 7.81 – 7.84 (m, 1 H, 36%), 7.88 (dd, 1 H, 64%, J = 8.0, 1.4 Hz), 8.26 (s, 1 H, 35%), 8.44 (s, 1 H, 65%), 10.33 (s, 1 H, 30%), 11.19 (s, 1 H, 70%), 12.13 (s, 1 H, 100%); 13C NMR (100 MHz, DMSO-d6) δ 118.6, 118.8, 119.0, 120.9, 121.8, 122.5, 123.7, 124.2, 124.8, 125.4, 127.2, 128.0, 128.6, 129.3, 129.4, 129.8, 133.9, 134.8, 139.7, 140.5, 142.3, 146.4, 157.2, 157.9, 162.8, 168.4; HRMS (TOF) m/z calcd for C14H9Br3N2O2H+: 474.8286, found: 474.8285 (Δ = 0.26 ppm). HPLC (A): t = 11.7 min, purity >96%.
2,3-Dibromo-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.10a).
White solid (98% yield); m.p. > 230 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.42 – 7.46 (m, 2 H, 30%, 100%), 7.56 – 7.58 (m, 2 H, 70%, 30%), 7.74 (s, 1 H, 25%), 7.82 (s, 1 H, 75%), 7.84 (s, 1 H, 70%), 7.88 (d, 1 H, 20%), 7.90 (dd, 1 H, 80%, J = 7.9, 1.4 Hz), 8.21 (s, 1 H, 25%), 8.38 (s, 1 H, 75%), 10.30 (s, 1 H, 25%), 12.32 (s, 1 H, 75%), 12.47 (s, 1 H, 35%), 12.60 (s, 1 H, 65%); 13C NMR (175 MHz, DMSO-d6) δ 110.6, 111.5, 120.8, 120.9, 121.5, 121.8, 125.2, 125.5, 127.2, 128.1, 129.5, 129.7, 131.3, 132.2, 134.5, 135.2, 135.7, 135.9, 139.2, 139.7, 144.0, 147.8, 152.6, 153.6, 163.1, 168.3; HRMS (TOF) m/z calcd for C14H8Br4N2O2H+: 552.7393, found: 552.7392 (Δ= −0.19 ppm). HPLC (A): t1 = 10.3, t2 = 13.1 min, purity >97%.
2,3-Dibromo-N’-(2-hydroxy-5-methylbenzylidene)benzohydrazide (5.10b).
White solid (41% yield); mp 202–203 °C; 1H NMR (500 MHz, DMSO-d6) δ 2.12(s, 1 H, 33%), 2.23(s, 1 H, 66%), 6.67 (d, 1 H, 33%, J = 8.3 Hz), 6.82 (d, 1 H, 67%, J = 8.3 Hz), 7.0 (dd, 1 H, 34%, J = 8.4, 1.9 Hz), 7.03 (s, 1 H, 32%), 7.10 (dd, 1 H, 66%, J = 8.4, 1.9 Hz), 7.38 (s, 1 H, 68%), 7.40 – 7.44 (m, 2 H, 100%, 30%), 7.54 (dd, 1 H, 70%, J = 7.5, 1.5 Hz), 7.83 – 7.85 (m, 1 H, 34%), 7.88 (dd, 1 H, J = 8.0, 1.5 Hz), 8.22 (s, 1 H, 32%), 8.41 (s, 1 H, 68%), 9.54 (s, 1 H, 33%), 10.66 (s, 1 H, 67%), 12.10 (s, 1 H, 100%); 13C NMR (100 MHz, DMSO-d6) δ 19.9, 116.1, 116.3, 118.3, 118.4, 120.9, 121.8, 124.8, 125.4, 127.1, 127.9, 128.0, 128.1, 128.4, 128.9, 129.4, 129.5, 132.0, 132.4, 133.9, 134.8, 139.8, 140.5, 144.5, 148.1, 154.5, 155.2, 162.7, 168.2; HRMS (TOF) m/z calcd for C15H12Br2N2O2H+: 410.9338, found: 410.9346 (Δ = −1.97 ppm). HPLC (A): t = 10.5 min, purity >97%.
2,3-Dibromo-N’-(2-hydroxy-1-naphthylidene)benzohydrazide (5.10c).
Light yellow solid (67% yield); mp >230 °C; 1H NMR (400 MHz DMSO-d6) δ 7.10 (d, 1 H, 45%, J = 8.9 Hz), 7.22 – 7.30 (m, 2 H, 55%, 95%), 7.38 – 7.41 (m, 1 H, 63%), 7.45 – 7.48 (m, 2 H, 100%, 37%), 7.56 – 7.62 (m, 2 H, 100%, 10%), 7.75 – 7.80 (m, 1 H, 90%), 7.84 – 7.94 (m, 2 H, 100%, 100%), 8.15 (d, 1 H, 45%, J = 8.3 Hz), 8.30 (d, 1 H, 55%, J = 8.6 Hz), 8.91 (s, 1 H, 45%), 9.24 (s, 1 H, 55%), 10.58 (s, 1 H, 40%), 12.10 (s, 1 H, 52%), 12.32 (s, 1 H, 100%); 13C NMR (125 MHz DMSO-d6) δ 108.5, 109.7, 118.1, 118.7, 120.9, 121.1, 121.9, 123.0, 123.3, 123.6, 124.9, 125.5, 126.9, 127.3, 127.87, 127.91, 128.0, 128.1, 128.6, 129.0, 129.48, 129.51, 131.0, 131.5, 132.6, 133.1, 133.8, 134.9, 139.6, 141.0, 143.2, 147.2, 157.0, 158.1, 162.5, 168.4; HRMS (TOF) m/z calcd for C18H12Br2N2O2H+: 446.9338, found: 446.9350 (Δ = −2.79 ppm). HPLC (A): t = 9.5 min, purity >95%.
2,3-Dibromo-N’-(3,5-dichloro-2-hydroxybenzylidene)benzohydrazide (5.10d).
Product washed with water and ~1 mL ethyl acetate, filtered, and washed with DCM and hexanes. Tan solid (43% yield); mp 229–230 °C; 1H NMR (500 MHz DMSO-d6) δ 7.37 (d, 1 H, 30%, 2.6 Hz), 7.42 – 7.46 (m, 3 H, 30%, 70%, 30%), 7.53 (d, 1 H, 30%, J = 2.6 Hz), 7.57 (dd, 1 H, 70%, J = 7.6, 1.5 Hz), 7.64 (d, 1 H, J = 2.6 Hz), 7.68 (d, 1 H, 70%, J = 2.6 Hz), 7.89 (dd, 1 H, 40%, J = 6.7, 2.9 Hz), 7.91 (dd, 1 H, 60%, J = 8. 1.5 Hz), 8.25 (s, 1 H, 30%), 8.42 (s, 1 H, 70%), 10.27 (s, 1 H, 30%), 12.08 (s, 1 H, 70%), 12.45 (s, 1 H, 30%), 12.56 (s, 1 H, 70%); 13C NMR (125 MHz, DMSO-d6) δ 120.79, 120.84, 121.57, 121.66, 121.74, 121.8, 123.2, 123.4, 125.0, 125.5, 127.1, 127.3, 128.1, 128.3, 129.5, 129.6, 130.3, 130.6, 134.3, 135.1, 139.2, 139.9, 143.4, 147.4, 151.1, 152.2, 163.0, 168.4; HRMS (TOF) m/z calcd for C14H8Br2Cl2N2O2H+ : 464.8402, found: 464.8398 (Δ= 0.76 ppm). HPLC (A): t = 9.6 min, purity >95%.
4-Cyano-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.11).
White solid (47 % yield); mp >215 °C; 1H NMR (500 MHz DMSO-d6) δ 7.83 (s, 2 H), 8.05 (d, 2 H, J = 8.6 Hz), 8.09 (d, 2 H, J = 8.6 Hz), 8.53 (s, 1 H), 12.64 (s, 2 H); 13C NMR (125 MHz DMSO-d6) δ 110.5, 111.3, 114.5, 118.2, 120.9, 128.6, 132.2, 132.7, 135.8, 136.2, 147.9, 153.7, 161.8; HRMS (TOF) m/z calcd for C15H9Br2N3O2H+: 421.9134, found: 421.9150 (Δ = −3.67 ppm). HPLC (A): t = 8.0 min, purity >99%.
2-Trifluoromethyl-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.12).
Beige solid (91 % yield); m.p. 98–100 °C; 1H NMR (500 MHz DMSO-d6) δ 6.75 (d, 1 H, 35%, J = 8.6 Hz), 6.89 (d, 1 H, 65%, J = 8.8 Hz), 7.27 – 7.30 (m, 2 H, 38%, 30%), 7.43 (dd, 1 H, 62%, J = 8.8, 2.6 Hz), 7.54 (d, 1 H, 35%, J = 7.6 Hz), 7.68 – 7.86 (m, 5 H, 100%, 100%, 85%, 70%, 65%), 8.22 (s, 1 H, 35%), 8.45 (s, 1 H, 65%), 10.12 (s, 1 H, 35%), 11.00 (s, 1 H, 65 %), 12.15 (s, 1 H, 35%), 12.22 (s, 1 H, 63%); 13C NMR (125 MHz DMSO-d6) δ 110.47, 110.54, 118.4, 118.7, 121.3, 121.6, 126.07, 126.11, 126.14, 126.3, 126.48, 126.52, 126.6, 128.1, 128.9, 129.0, 129.6, 130.0, 130.6,132.4, 132.6, 133.8, 134.0, 134.5, 140.9, 145.4, 155.6, 156.3, 163.0, 169.1;19F NMR (376 MHz DMSO-D6) δ −57.85, −58.58; HRMS (TOF) m/z calcd for C15H10BrF3N2O2H+: 386.9951, found: 386.9957 (Δ = −1.69 ppm). HPLC (A): t = 11.2 min, purity >96%.
4-Trifluoromethyl-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.13).
Yellow solid (98 % yield); mp >215 °C; 1H NMR (500 MHz DMSO-d6) δ 7.83 (s, 2 H); 7.93 (d, 2 H, J = 8.3 Hz), 8.14 (d, 2 H, J = 8.1 Hz), 8.55 (s, 1 H), 12.61 (s, 1 H), 12.68 (s, 1 H); 13C NMR (100 MHz DMSO-d6) δ 110.5, 111.3, 120.9, 125.59, 125.62, 128.7, 132.2, 135.8, 136.0, 147.8, 153.7, 161.9; 19F NMR (376 MHz DMSO-d6) δ −61.44 (s, 3 F); HRMS (TOF) m/z calcd for C15H9Br2F3N2O2H+: 464.9056, found: 464.9059 (Δ = −0.73 ppm). HPLC (C): t = 6.7 min, purity >96%.
3-Difluoromethoxy-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.14).
Yellow solid (90 % yield); mp 166–168 °C; 1H NMR (500 MHz DMSO-d6) δ 6.91 (d, 1 H, 90%, J = 8.8 Hz), 6.95 (d, 1 H, 10%, J = 8.8 Hz), 7.18 (s, 1 H, 25%), 7.33 (s, 1 H, 50%), 7.42 – 7.44 (m, 2 H, 100%, 80%), 7.48 (s, 1 H, 25%), 7.54 – 7.56 (m, 2 H, 20%), 7.61 (t, 1 H, 100%, J = 8 Hz), 7.72 (s, 1 H, 90%), 7.81 – 7.83 (m, 2 H, 100%, 80%), 7.90 (s, 1 H, 10%), 8.63 (s, 1 H, 90%), 8.93 (s, 1 H, 10%), 11.13 (s, 1 H, 10%), 11.20 (s, 1 H, 90%), 12.22 (s, 1 H, 87%); 13C NMR (125 MHz DMSO-d6) δ 110.5, 110.6, 114.3, 117.9, 118.4, 118.7, 118.9, 120.6, 121.3, 122.3, 122.5, 124.5, 130.2, 130.4, 131.6, 133.7, 134.7, 135.5, 145.8, 150.9, 151.0, 156.4, 157.7, 160.8, 161.8; 19F NMR (376 MHz DMSO-d6) δ −82.16 (s, 2 F); HRMS (TOF) m/z calcd for C15H11Br2F2N2O3H+: 384.9994, found: 384.9996 (Δ = −0.49 ppm). HPLC (B): t = 3.5 min, purity >95%.
3-Difluoromethoxy-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.14a).
Yellow solid (99 % yield); m.p. 187–188 °C; 1H NMR (500 MHz DMSO-d6) δ 7.19 (s, 1 H, 25%), 7.34 (s, 1 H, 50%), 7.46(d, 1 H, 100%, J = 8 Hz), 7.49 (s, 1 H, 25%), 7.63 (t, 1 H, 100%, J = 8 Hz), 7.73 (s, 1H, 100%), 7.83 −7.96 (m, 3 H, 100%, 100%, 90%), 7.96 (d, 1 H, 10%, J = 2.3 Hz), 8.54 (s, 1 H, 90%), 9.06 (s, 1 H, 10%), 10.04 (s, 1 H, 3%), 11.99 (s, 1 H, 10%), 12.60 (s, 1 H, 90%), 12.64 (s, 1 H, 100%); 13C NMR (125 MHz DMSO-d6) δ 110.4, 110.8, 111.3, 111.6, 114.2, 116.3, 118.1, 118.3, 120.4, 120.9, 122.6, 124.5, 130.5, 132.1, 133.5, 134.0, 135.7, 137.8, 147.5, 150.98, 151.00, 153.7, 154.7, 161.9, 164.0; 19F NMR (376 MHz DMSO-d6) δ −82.22 (s, 2 F); HRMS (TOF) m/z calcd for C15H10Br2F2N2O3H+: 462.9099, found: 462.9100 (Δ = −0.2 ppm). HPLC (B): t = 7.6 min, purity >95%.
4-Difluoromethoxy-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.15).
Yellow solid (96% yield); mp 208–209 °C; 1H NMR (400 MHz DMSO-d6) δ 7.10 – 7.14 (m, 2 H, 100%), 7.20 (s, 1 H, 30%), 7.33 (d, 2 H, 100%, J = 8.5 Hz), 7.39, (s, 1 H, 50%), 7.56 (d, 2 H, 20%, 100%, J = 7.4 Hz), 8.01 (d, 2 H, 100% J = 8.5 Hz), 8.62 (s, 1 H, 100%), 11.49 (s, 1 H, 100%), 12.14 (s, 1 H, 100%); 13C NMR (100 MHz, DMSO-d6) δ 113.5, 116.0, 118.1, 118.56, 118.59, 119.1, 122.4, 123.9, 129.3, 129.9, 130.3, 146.5, 153.7, 158.0, 161.8; 19F NMR (376 MHz DMSO-d6) δ −82.94 (s, 1 F); HRMS (TOF) m/z calcd for C15H11BrF2N2O3H+: 384.9994, found: 385.0007 (Δ = −3.49 ppm). HPLC (C): t = 3.6 min, purity >95%.
4-Difluoromethoxy-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.15a).
Beige solid (94% yield); mp 194–196 °C; 1H NMR (400 MHz DMSO-d6) δ 6.90 (d, 1 H, 100%, J = 8.8 Hz), 7.20 (s, 1 H, 25%), 7.33 (d, 2 H, 100%, J = 8.6), 7.39 (s, 1 H, 50%), 7.43 (dd, 1 H, 100%, J = 8.8, 2.2 Hz), 7.57 (s, 1 H, 25%), 7.80 (s, 1 H, 100%), 8.01 (d, 2 H, 100%, J = 8.6 Hz), 8.61 (s, 1 H, 100%), 11.26 (s, 1 H, 100%), 12.19 (s, 1 H, 100%); 13C NMR (100 MHz, DMSO-d6) δ 110.5, 113.5, 116.0, 118.1, 118.6, 118.7, 121.3, 129.3, 129.9, 130.4, 133.6, 145.6, 153.7, 156.4, 161.9; 19F NMR (376 MHz DMSO-d6) δ −82.94 (s, 1 F); HRMS (TOF) m/z calcd for C15H11BrF2N2O3H+: 384.9993, found: 385.0011 (Δ = −4.62 ppm). HPLC (B): t = 8.9 min, purity >96%.
4-Difluoromethoxy-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.15b).
Beige solid (89% yield); mp >220 °C; 1H NMR (400 MHz DMSO-d6) δ 7.21 (s, 1 H, 25%), 7.35 (d, 2 H, 100%, J = 8.6 Hz), 7.40 (s, 1 H, 50%), 7.58 (s, 1H, 25%), 7.83 (s, 2 H, 100%), 8.03 (d, 2 H, 100% J = 8.7 Hz), 8.53 (s, 1 H, 100%), 12.56 (s, 1 H, 100%), 12.70 (s, 1 H, 100%); 13C NMR (100 MHz, DMSO-d6) δ 110.4, 111.2, 113.4, 116.0, 118.1, 121.0, 128.7, 130.1, 132.1, 135.6, 147.1, 153.7, 162.0; 19F NMR (376 MHz DMSO-d6) δ −83.03 (s, 1 F); HRMS (TOF) m/z calcd for C15H10Br2F2N2O3H+: 462.9099, found: 462.9103 (Δ = −0.92 ppm). HPLC (A): t = 11.3 min, purity >95%.
4-Trifluoromethoxy-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.16).
Beige solid (88 % yield); mp 199–201 °C; 1H NMR (500 MHz DMSO-d6) δ 6.91 (d, 1 H, J = 8.8 Hz), 7.43 (dd, 1 H, J = 8.7, 2.5 Hz), 7.54 (d, 2 H, J = 8.3 Hz), 7.81 (s, 1 H), 8.07 (d, 2 H, J = 8.8 Hz), 8.61 (s, 1 H), 11.21 (s, 1 H), 12.25 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 110.5, 116.9, 118.7, 118.9, 120.8, 121.0, 121.31, 123.0, 130.1, 130.3, 131.9, 133.7, 145.8, 150.7, 156.4, 161.8; 19F NMR (376 MHz DMSO-d6) δ −56.64; HRMS (TOF) m/z calcd for C15H10BrF3N2O3H+: 402.9900, found: 402.9902 (Δ = −0.63 ppm). HPLC (B): t = 6.6 min, purity >95%.
4-Trifluoromethoxy-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.16a).
Beige solid (79 % yield); mp >215 °C; 1H NMR (500 MHz DMSO-d6) δ 7.55 (d, 2 H, J = 8.3 Hz), 7.82 (s, 2 H), 8.05 (d, 2 H, J = 8.7 Hz), 8.52 (s, 1 H), 12.63 (s, 2 H); 13C NMR (125 MHz DMSO-d6) δ 110.4, 111.2, 118.9, 120.86, 120.91, 130.2, 131.2, 132.2, 135.7, 147.4, 150.9, 153.7, 161.9; 19F NMR (376 MHz DMSO-d6) δ −56.65 (s, 3 F); HRMS (TOF) m/z calcd for C15H9Br2F3N2O3H+:480.9005, found: 480.9000 (Δ = 0.9 ppm). HPLC (A): t = 12.9 min, purity >95%.
2-Fluoro-4-trifluoromethoxy-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.17).
Yellow solid (62 % yield); mp 206–207 °C; 1H NMR (500 MHz DMSO-d6) δ 6.80 (dd, 1H, 30%, J = 7.4, 1.8 Hz), 6.89 (d, 1 H, 73%, J = 8.8 Hz), 7.31 – 7.33 (m, 2 H, 27%, 23%), 7.35 (d, 1 H, 31%, J = 8.8 Hz), 7.39 (d, 1 H, 73%, J = 8.6 Hz), 7.43 (dd, 1 H, 70%, J = 8.8, 2.6 Hz), 7.53 (d, 1 H, 29%, J = 10.0 Hz), 7.58 (d, 1 H, 71%, J = 10.3 Hz), 7.67 (t, 1 H, 31%, J = 8.0 Hz), 7.80 (d, 1 H, 77%, J = 2.6 Hz), 7.84 (t, 1 H, 69%, J = 8.2 Hz), 8.27 (s, 1 H, 28%), 8.49, (s, 1 H, 72%), 10.27 (s, 1 H, 26%), 11.03 (s, 1 H, 74%), 12.15 (s, 1 H, 34%), 12.15 (s, 1 H, 66%); 13C NMR (125 MHz DMSO-d6) δ 109.9, 110.1, 110.5, 111.1, 111.3, 111.8, 117.2, 117.4, 118.8, 120.9, 121.3, 121.4, 122.2, 122.9, 130.6, 130.9, 132.07, 132.10, 132.2, 135.6, 135.9, 143.3, 147.8, 150.6, 150.7, 153.6, 158.6, 159.4, 160.6; 165.6; 19F NMR (376 MHz DMSO-d6) δ −56.94 (s, 3 F), −57.02 (s, 3 F), −109.09 (s, 1 F), −109.94 (s, 1 F); HRMS (TOF) m/z calcd for C15H8Br2F4N2O3H+: 498.8911, found: 498.8923 (Δ = −2.57 ppm). HPLC (A): t = 12.7 min, purity >95%.
2-Fluoro-4-trifluoromethoxy-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.17a).
Yellow solid (57 % yield); m.p. 151–152 °C; 1H NMR (500 MHz DMSO-d6) δ 7.40 (d, 1 H, 100%, J = 8.5 Hz), 7.54 (d, 1 H, 15%, J = 2.3 Hz), 7.60 (d, 1 H, 100%, J = 8.9 Hz), 7.70 (t, 1 H, 15%, J = 8.0 Hz), 7.74 (d, 1 H, 15%, J = 2.3 Hz), 7.82 (d, 1 H, 85%, J = 2.3 Hz), 7.83 (d, 1 H, 85%, J = 2.3 Hz), 7.87 (t, 1 H, 85%, J = 8.2 Hz), 8.25 (s, 1 H, 15%), 8.45 (s, 1 H, 85 %), 12.40 (s, 1 H, 100%), 12.56 (s, 1 H, 100%); 13C NMR (125 MHz DMSO-d6) δ 109.9, 110.1,110.5, 111.3, 117.2, 118.8, 120.9, 121.3, 121.4, 132.07, 132.1, 132.2, 135.9, 147.8, 150.6, 150.7, 153.6, 158.6, 159.4, 160.6; 19F NMR (376 MHz DMSO-d6) δ −56.95 (s, 3 F), −57.04 (s, 3 F), −109.09 (s, 1 F), 109.47 (s, 1 F); HRMS (TOF) m/z calcd for C15H9BrF4N2O3H+: 420.9805, found: 420.9820 (Δ = −3.47 ppm). HPLC (A): t = 10.9 min, purity >97%.
2-Fluoro-4-trifluoromethyl-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.18).
Yellow solid (61 % yield); mp 180–182 °C; 1H NMR (400 MHz DMSO-d6) δ 6.80 (d, 1 H, 30%, J = 8.5 Hz), 6.90 (d, 1 H, 70% J = 8.8 Hz), 7.30 – 7.34 (m, 2 H, 26%, 31%), 7.44 (dd, 1 H, 69%, J = 8.8, 2.5 Hz), 7.70 – 7.94 (m, 4 H, 100%, 100%, 100%, 74%), 8.30 (s, 1 H, 30%), 8.52 (s, 1 H, 70%), 10.27 (s, 1 H, 30%), 11.01 (s, 1 H, 70%), 12.28 (s, 1 H, 100%); 13C NMR (125 MHz DMSO-d6) δ 110.6, 113.87, 113.9, 114.0, 114.1, 118.5, 118.7, 121.2, 121.62, 121.65, 121.7, 121.9, 126.7, 126.8, 128.2, 130.0, 130.7, 131.55, 131.58, 133.5, 134.0, 141.0, 146.0, 155.6, 156.4, 158.0, 159.2, 160.0, 165.6; 19F NMR (376 MHz DMSO-d6) δ −61.33 (s, 3 F), −61.46 (s, 3 F), −111.26 (s, 1 F), −111.49 (s, 1 F); HRMS (TOF) m/z calcd for C15H9BrF4N2O2H+: 404.9856, found: 404.9864 (Δ = −1.84 ppm). HPLC (B): t = 6.5 min, purity >95%.
2-Fluoro-4-trifluoromethyl-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.18a).
Yellow solid (74 % yield); mp 193–195 °C; 1H NMR (400 MHz DMSO-d6) δ 7.52 (d, 1 H, 17%), 7.74 – 7.97 (m, 5 H, 100%, 100%, 100%, 100%, 69% ), 8.27 (s, 1 H, 18%), 8.46 (s, 1 H, 82%), 12.34 (s, 1 H, 81%), 12.53 (s, 1 H, 19%), 12.67 (s, 1 H, 81%); 13C NMR (100 MHz DMSO-d6) δ 110.6, 111.4, 114.0, 114.2, 120.9, 121.69, 121.73, 126.0, 126.2, 131.66, 131.69, 132.2, 136.0, 143.6, 148.1, 152.3, 153.6, 157.8, 159.4, 160.3; 19F NMR (376 MHz DMSO-d6) δ −61.34 (s, 3 F), −61.50 (s, 3 F), −111.16 (s, 1 F), −111–93 (s, 1 F); HRMS (TOF) m/z calcd for C15H8Br2F4N2O2H+: 482.8961, found: 482.8958 (Δ = 0.69 ppm). HPLC (A): t = 9.4 min, purity >95%.
3-Fluoro-N’-(3-bromo-2-hydroxybenzylidene)benzohydrazide (5.19).
White solid (68% yield); mp 154 – 155 °C; 1H NMR (700 MHz, DMSO-d6) δ 6.92 (t, J = 7.8 Hz, 1H, 75%), 6.99 (t, J = 7.8 Hz, 1H, 25%), 7.50 (td, J = 8.4 Hz, J = 2.2 Hz, 1H, 75%), 7.54 (d, J = 7.6 Hz, 1H, 75%), 7.63 (m, 2H, 88%), 7.76 (m, 1H), 7.81 (d, J = 7.8 Hz, 1H, 75%), 8.58 (s, 1H, 75%), 9.13 (s, 1H, 25%), 12.08 (m, 2H, 25%), 12.48 (m, 2H, 75%); 13C NMR (175 MHz, DMSO-d6) δ 110.0, 110.1, 114.5, 114.6, 118.8, 119.1, 119.27, 119.33, 120.6, 121.1, 123.96, 123.97, 130.5, 130.9, 130.95, 132.2, 134.5, 134.6, 134.7, 136.4, 149.1, 154.2, 155.3, 161.3, 161.6, 162.7, 165.1; 19F NMR (376 MHz, DMSO-d6) δ −112.26 (m, 1F); HRMS (TOF) calcd for C14H10BrFN2O2H+ 336.9983, found 336.9988 (Δ = 1.2 ppm). HPLC (B): t = 5.2 min, purity >95%.
3-Fluoro-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.19a).
White solid (72% yield); mp 171 – 172 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.12 (d, J = 8.3 Hz, 1H, 80%), 7.16 (m, 1H), 7.19 (s, 1H, 20%), 7.47 (m, 1H, 80%), 7.60 (m, 2H, 80%), 7.67 (d, J = 8.3 Hz, 1H, 20%), 7.74 (d, J = 9.7 Hz, 1H, 80%), 7.79 (d, J = 7.7 Hz, 1H, 80%), 8.63 (s, 1H, 80%), 8.96 (s, 1H, 20%), 11.42 (s, 1H), 12.17 (s, 1H, 80%); 13C NMR (175 MHz, DMSO-d6) δ 114.4, 114.5, 118.0, 118.6, 118.9, 119.0, 119.1, 119.3, 122.5, 122.8, 123.89, 123.90, 124.1, 126.1, 130.2, 130.79, 130.84, 131.5, 135.1, 135.2, 146.7, 158.0, 159.2, 161.26, 161.33, 161.6, 162.6; 19F NMR (376 MHz, DMSO-d6) δ −112.41 (m, 1F); HRMS (TOF) calcd for C14H10BrFN2O2H+ 336.9983, found 336.9985 (Δ = −0.8 ppm). HPLC (A): t = 9.4 min, purity >95%.
4-Fluoro-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.20).
Yellow solid (97 % yield); mp 216–217 °C; 1H NMR (500 MHz DMSO-d6) δ 7.40 (t, 2 H, J = 8.8 Hz), 7.82 (s, 2 H), 8.02 – 8.04 (m, 2 H), 8.52 (s, 1 H), 12.55 (s, 1 H), 12.70 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 110.4, 111.2, 115.6, 115.8, 120.9, 128.60, 128.62, 130.5, 130.6, 132.1, 135.6, 147.1, 153.6, 162.0, 163.5, 165.5; 19F NMR NMR (376 MHz DMSO-d6) δ −107.36 (s, 1 F); HRMS (TOF) m/z calcd for C14H9Br2FN2O2H+: 414.9087, found: 414.9123 (Δ = −1.57 ppm). HPLC (A): t = 10.1 min, purity >97%.
3-Trifluoromethoxy-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.22).
White solid (65% yield); mp 158 – 160 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.66 (d, J = 8.1 Hz, 1H), 7.72 (t, J = 8.1 Hz, 1H), 7.84 (s, 2H), 7.91 (s, 1H), 8.00 (d, J = 8.1 Hz, 1H), 8.55 (s, 1H), 12.63 (s, 2H); 13C NMR (175 MHz, DMSO-d6) δ 110.4, 111.3, 119.3, 120.3, 120.8, 120.9, 124.8, 127.0, 130.9, 132.2, 134.4, 135.8, 147.7, 148.4, 153.7, 161.5; 19F NMR (376 MHz, DMSO-d6) δ - 56.79 (s, 3F); HRMS (TOF) calcd for C15H9Br2F3N2O3H+ 480.9005, found 480.9008 (Δ = −0.7 ppm). HPLC (A): t = 7.7 min, purity >97%.
3-Trifluoromethyl-N’-(3-chloro-2-hydroxybenzylidene)benzohydrazide (5.23).
White solid (71% yield); mp 168 – 170 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.98 (t, J = 7.8 Hz, 1H), 7.51 (m, 2H), 7.82 (t, J = 7.8 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.26 (d, J = 7.8 Hz, 1H), 8.29 (s, 1H), 8.64 (s, 1H), 12.28 (s, 1H), 12.55 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 119.6, 120.1, 120.4, 124.2, 128.7, 129.2, 129.5, 130.0, 131.5, 131.9, 133.3, 149.2, 153.3, 161.5. 19F NMR (376 MHz, DMSO-d6) δ −61.14 (s, 3F); HRMS (TOF) calcd for C15H10ClF3N2O2H+ 343.0456, found 343.0457(Δ = −0.3 ppm). HPLC (B): t = 5.9 min, purity >95%.
3-Trifluoromethyl-N’-(3-bromo-2-hydroxybenzylidene)benzohydrazide (5.23a).
White solid (86% yield); mp 173 – 175 °C; 1H NMR (400 MHz, DMSO-d6) δ 6.93 (t, J = 7.8 Hz, 1H), 7.55 (dd, J = 7.8 Hz, J = 1.4 Hz, 1H), 7.65 (dd, J = 7.8 Hz, J = 1.4 Hz, 1H), 7.82 (t, 7.8 Hz, 1H), 8.02 (d, J = 7.8 Hz, 1H), 8.26 (d, J = 7.8 Hz, 1H), 8.29 (s, 1H), 8.60 (s, 1H), 12.49 (br s, 1H), 12.55 (br s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 110.0, 119.3, 120.6, 128.8, 130.0, 130.4, 131.9, 133.3, 134.5, 149.3, 154.2, 161.5; 19F NMR (376 MHz, DMSO-d6) δ −61.14 (s, 3F); HRMS (TOF) m/z calcd for C15H10BrF3N2O2H+: 386.9951, found 386.9955 (Δ = −1.1 ppm). HPLC (B): t = 5.2 min, purity >95%.
3-Trifluoromethyl-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.23b).
White solid (69% yield); mp 178 – 180 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.13 (m, 2H), 7.60 (d, J = 8.1 Hz, 1H), 7.80 (t, J = 7.8 Hz, 1H), 7.99 (d, J = 7.8 Hz, 1H), 8.24 (d, J = 8.1 Hz, 1H), 8.28 (s, 1H), 8.66 (s, 1H), 11.37 (s, 1H), 12.28 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ 118.6, 119.1, 122.5, 124.1, 129.9, 130.0, 131.9, 133.8, 146.8, 158.0, 161.4; 19F NMR (376 MHz, DMSO-d6) δ −61.12 (s, 3F); HRMS (TOF) m/z calcd for C15H10BrF3N2O2H+: 386.9951, found 386.9954 (Δ = −0.9 ppm). HPLC (B): t = 5.0 min, purity >95%.
3-Trifluoromethyl-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.23c).
White solid (63% yield); mp 136 – 137 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.82 (m, 3H), 8.02 (d, J = 7.3 Hz, 1H), 8.26 (d, J = 7.3 Hz, 1H), 8.28 (s, 1H), 8.55 (s, 1H), 12.60 (s, 1H), 12.73 (s, 1H); 13C NMR (175 MHz, DMSO-d6) δ 110.5, 111.3, 120.9, 123.1, 124.3, 124.6, 128.9, 129.2, 129.4, 130.0, 132.0, 132.2, 133.1, 135.8, 147.7, 153.7, 161.7; 19F NMR (376 MHz, DMSO-d6) δ −61.14 (s, 3F); HRMS (TOF) C15H9Br2F3N2O2H+: 464.9056, found 464.9062 (Δ = −1.4 ppm). HPLC (A): t = 9.4 min, purity >95%.
4-Amino-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.24).
Yellow solid (85 % yield); mp 226–227 °C; 1H NMR (500 MHz DMSO-d6) δ 5.83 (s, 2 H), 6.59 (2 H, J = 8.7 Hz), 7.08 (dd, 1 H, J = 8.3, 1.9 Hz), 7.12 (d, 1 H, J = 1.9 Hz), 7.46 (d, 1 H, J = 8.3 Hz), 7.66 (d, 2 H, J = 8.7 Hz), 8.52 (s, 1 H), 11.77 (s, 2 H); 13C NMR (125 MHz DMSO-d6) δ 112.6, 118.55, 118.59, 119.1, 122.3, 123.3, 129.4, 130.7, 145.3, 152.6, 158.0, 162.7; HRMS (TOF) m/z calcd for C14H12BrN3O2H+: 334.0817, found: 334.01945 (Δ = −2.63 ppm). HPLC (B): t = 7.9 min, purity >96%.
4-Dimethylamino-N’-(5-bromo-2-hydroxybenzylidene)benzohydrazide (5.25).
Yellow solid (99 % yield); mp >230 °C; 1H NMR (400 MHz DMSO-d6) δ 2.99 (s, 6 H), 6.75 (d, 2 H, J = 9 Hz), 6.88 (d, 1 H, J = 8.8 Hz), 7.39 (dd, 1 H, J = 8.72, 2.52 Hz), 7.73 (d, 1 H, J = 2.5 Hz), 7.82 (d, 2 H, J = 8.9 Hz), 8.54 (s, 1 H), 11.51 (s, 1 H), 11.90 (s, 1 H) ); 13C NMR (100 MHz DMSO-d6) δ 110.3, 110.8, 118.5, 118.6, 121.3, 129.2, 130.7, 133.1, 144.5, 152.6, 156.4, 162.7; HRMS (TOF) m/z calcd for C16H16BrN3O2H+: 362.0487, found: 362.0500 (Δ = −0.43 ppm). HPLC (C): t = 3.7 min, purity >95%.sssssss
4-Dimethylamino-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.25a).
Yellow solid (99% yield); mp >215 °C; 1H NMR (400 MHz DMSO-d6) δ 3.0 (s, 6 H), 6.76 (d, 2 H, J = 9 Hz), 7.76 (dd, 2 H, J = 13.1, 2.3 Hz), 7.83 (d, 2 H, J = 9 Hz), 8.46 (s, 1 H), 12.21 (s, 1 H), 12.96 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 110.2, 110.9, 111.0, 117.9, 121.1, 129.3, 131.9, 135.1, 145.3, 152.8, 153.6; HRMS (TOF) m/z calcd for C16H15Br2N3O2H+: 439.9604, found: 439.9603 (Δ = −0.19 ppm). HPLC (B): t = 6.5 min, purity >95%.
4-Dimethylamino-N’-(4-bromo-2-hydroxybenzylidene)benzohydrazide (5.25b).
Yellow solid (93 % yield); m.p. 195–196 °C; 1H NMR (400 MHz DMSO-d6) δ 2.99 (s, 6 H), 6.75 (d, 2 H, J = 9 Hz), 7.09 (dd, 1 H, J = 8.24, 1.8 Hz), 7.12 (s, 1 H), 7.47 (d, 1 H, J = 8.3 Hz), 7.81 (d, 2 H, J = 9 Hz), 8.55 (s, 1 H), 11.76 (s, 1 H), 11.85 (s, 1 H); 13C NMR (100 MHz DMSO-d6) δ 110.8, 118.6, 119.0, 122.3, 123.4, 129.1, 130.7, 145.4, 152.6, 158.0, 162.6; HRMS (TOF) m/z calcd for C16H16BrN3O2H+: 362.0499, found: 362.0504 (Δ = −1.37 ppm). HPLC (B): t = 6.9 min, purity >95%.
4-Dimethylamino-N’-(2-hydroxynaphthylidene)benzohydrazide (5.25c).
Yellow solid (90 % yield); mp >215 °C; 1H NMR (400 MHz DMSO-d6) δ 3.00 (s, 6 H), 6.79 (d, 2 H, J = 8.9 Hz), 7.22 (d, 1 H, J = 9 Hz), 7.39 (t, 1 H, J = 7.5 Hz), 7.59 (t, 1 H, J = 7.5 Hz), 7.84 – 7.91 (m, 4 H), 8.15 (d, 1 H, J = 8.5 Hz), 9.46 (s, 1 H), 11.89 (s, 1 H), 12.99 (s, 1 H); 13C NMR (100 MHz DMSO-d6) δ 108.6, 110.9, 118.5, 119.0, 120.4, 123.4, 127.6, 127.8, 128.95, 129.04, 131.5, 132.2, 145.1, 152.7, 157.7, 162.3; HRMS (TOF) m/z calcd for C20H19N3O2H+: 334.1550, found: 334.1555 (Δ = −1.39 ppm). HPLC (C): t = 4.5 min, purity >95%.
4-Acetamido-N’-(4-dibromo-2-hydroxybenzylidene)benzohydrazide (5.26).
Beige solid (85 % yield); mp >230 °C; 1H NMR (500 MHz DMSO-d6) δ 2.07 (s, 3 H), 7.08 (d, 1 H, J = 9.7 Hz ), 7.13 (d, 1 H, J = 1.3 Hz), 7.52 (d, 1 H, J = 8.3 Hz), 7.71 (d, 2 H, J = 8.7 Hz), 7.88 (d, 2 H, J = 8.6 Hz), 8.59 (s, 1 H), 10.22 (s, 1 H), 11.57 (s, 1 H), 12.03 (s, 1 H); 13C NMR (100 MHz DMSO-d6) δ 24.1, 118.2, 118.5, 119.1, 122.4, 123.7, 126.8, 128.6, 130.5, 142.6, 146.2, 158.0, 162.3, 168.8; HRMS (TOF) m/z calcd for C16H14BrN3O3H+: 376.0291, found: 376.0301 (Δ = −2.51 ppm). HPLC (B): t = 7.5 min, purity >99%.
3-Bromo-N’-(2-hydroxy-5-trifluoromethylbenzylidene)benzohydrazide (5a).
White solid (92 % yield); mp 175–176 °C; 1H NMR (400 MHz DMSO-d6) δ 7.10 (d, 1 H, 88%, J = 8.6 Hz), 7.15 (d, 1 H, 12%, J = 8.5 Hz), 7.51 (t, 1 H, 100%, J = 7.9 Hz), 7.62 (dd, 1 H, 86%, J = 8.6, 1.5 Hz), 7.74 (dd, 1 H, 14%, J = 7.9, 2.4 Hz), 7.81 (d, 1 H, 100%, J = 7.8 Hz), 7.93 (d, 1 H, 100%, J = 7.9 Hz), 8.00 (s, 1 H, 93%), 8.09 (s, 1 H, 7%), 8.12 (s, 1 H, 100%), 8.71 (s, 1 H, 90%), 9.06 (s, 1 H, 10%), 10.69 (s, 1 H, 6%), 11.64 (s, 1 H, 13%), 11.71 (s, 1 H, 87%), 12.27 (s, 1 H, 92%); 13C NMR (100 MHz DMSO-d6) δ 117.2, 119.7, 119.9, 120.2, 120.6, 121.8, 123.1, 125.2, 125.8, 126.9, 128.0, 130.2, 130.8, 134.7, 134.9, 145.7, 160.0, 161.5; 19F NMR (376 MHz DMSO-d6) δ −56.92 (s, 3 F), −60.12 (s, 3 F); HRMS (TOF) m/z calcd for C15H10BrF3N2O2H+:386.9951, found: 386.9957 (Δ = −1.78 ppm). HPLC (B): t = 6.9 min, purity >95%.
2,4-Dibromo-N’-(5-fluoro-2-hydroxybenzylidene)benzohydrazide (5.2f).
White solid (96 % yield); m.p. 182–183 °C; 1H NMR (500 MHz DMSO-d6) δ 6.83 (dd, 1 H, 39%, J = 9, 4.7 Hz), 6.94 (dd, 1 H, 61%, J = 9, 4.7 Hz), 6.98 (dd, 1 H, 39%, J = 9.4, 3.2 Hz), 7.06 (td, 1 H, 39%, J = 8.5, 3.3 Hz), 7.16 (td, 1 H, 61%, J = 8.5, 3.3 Hz), 7.41 (d, 1 H, 39%, J = 8.2 Hz), 7.44 (dd, 1 H, 60%, J = 9.4, 3.2), 7.54 (d, 1 H, 61%, J = 8.2 Hz), 7.70 (dd, 1 H, 40%, J = 8.2, 1.9 Hz), 7.74 (dd, 1 H, 60%, J = 8.2, 1.9 Hz), 8.00 (d, 1 H, 40%, J = 1.9 Hz), 8.02 (d, 1 H, 60%, J = 1.9 Hz), 8.27 (s, 1 H, 40%), 8.47 (s, 1 H, 60%), 9.90 (s, 1 H, 39%), 10.71 (s, 1 H, 61%), 12.15 (s, 1 H, 66%), 12.17 (s, 1 H, 34%); 13C NMR (125 MHz DMSO-d6) δ 112.0, 112.2, 113.2, 113.4, 117.4, 117.5, 117.6, 117.7, 117.7, 118.0, 118.3, 118.4, 119.75, 119.8, 120.18, 120.24, 120.6, 122.7, 123.8, 130.2, 130.7, 130.8, 130.9, 134.1, 134.8, 136.1, 137.2, 141.8, 146.1, 152.8, 153.5, 154.3, 154.4, 156.2, 156.3, 162.5, 168.3; 19F NMR (376 MHz DMSO-d6) δ −124.76 (s, 1 F), −124.91 (s, 1 F); HRMS (TOF) m/z calcd for C14H9Br2FN2O2H+: 414.9088, found: 414.9095 (Δ = −1.7 ppm). HPLC (B): t = 4.6 min, purity >99%.
2,4-Dibromo-N’-(5-cyano-2-hydroxybenzylidene)benzohydrazide (5.2g).
Off white solid (99% yield); mp >230 °C; 1H NMR (400 MHz DMSO-d6) δ 6.98 (d, 1 H, J = 5 Hz, 35%), 7.08 (d, 1 H, J = 5 Hz, 65%), 7.41 (d, 1 H, J = 4.6 Hz, 35%), 7.54 (d, 1 H, J = 4.6 Hz, 65%), 7.61 (d, 1 H, 1.2 Hz, 33%), 7.62 – 7.64 (m, 1 H, 35%), 7.70 – 7.71 (m, 1 H, 37%), 7.72 – 7.74 (m, 2 H, 100%, 37%), 8.0 (d, 1 H, J = 1 Hz, 35%), 8.02 (d, 1 H, J = 1 Hz, 65%), 8.08 (d, 1 H, J = 1.2 Hz, 65%), 8.28 (s, 1 H, 35%), 8.48 (s, 1 H, 65%), 11.03 (s, 1 H, 35%), 11.84 (s, 1 H, 65%), 12.26 (s, 1 H, 38%), 12.28 (s, 1 H, 62%); 13C NMR (100 MHz DMSO-d6) δ 48.6, 101.9, 117.5, 117.7, 118.8, 118.9, 119.8, 120.2, 120.6, 122.8, 123.9, 130.2, 130.7, 130.8, 130.9, 131.3, 132.7, 134.2, 134.7, 134.9, 135.0, 135.9, 137.0, 141.0, 145.2, 160.1, 160.7, 162.6, 168.4; HRMS (TOF) m/z calcd for C15H9Br2N3O2H+: 421.9134, found: 421.9130 (Δ = 0.94 ppm). HPLC (B): t = 3.2 min, purity >95%.
2,4-Dibromo-N’-(2-hydroxy-5-trifluoromethoxybenzylidene)benzohydrazide (5.2h).
White solid (99 % yield); mp 170–172 °C; 1H NMR (500 MHz DMSO-d6) δ 6.91 (d, 1 H, 36%, J = 8.8Hz), 7.01 (d, 1 H, 64%, J = 8.9 Hz), 7.16 – 7.19 (m, 2 H, 38 %, 35%), 7.30 (dd, 1 H, 65%, J = 9, 2.8 Hz), 7.40 (d, 1 H, 37%, J = 8.2 Hz), 7.54 (d, 1 H, 63%, J = 8.2 Hz), 7.64 (d, 1 H, 62%, J = 2.7 Hz), 7.69 (dd, 1 H, 37%, J = 8.2, 1.8 Hz), 7.74 (dd, 1 H, 63%, J = 8.2, 1.8 Hz), 7.97 (d, 1 H, 39%, J = 1.8 Hz), 8.02 (d, 1 H, 61%, J = 1.8 Hz), 8.28 (s, 1 H, 37%), 8.50 (s, 1 H, 63%), 10.28 (s, 1 H, 36%), 11.03 (s, 1 H, 64%), 12.18 (s, 1 H, 63%), 12.20 (s, 1 H, 37%); 13C NMR (125 MHz DMSO-d6) δ 117.5, 117.7, 118.5, 119.1, 119.2, 119.86, 119.94, 120.2, 120.61, 120.63, 121.1, 121.2, 122.6, 123.8, 123.9, 124.6, 130.2, 130.6, 130.8, 130.9, 134.0, 134.8, 136.1, 137.2, 140.69, 140.70, 140.9, 145.4, 155.2, 156.0, 162.5, 168.5; 19F NMR (376 MHz DMSO-d6) δ −57.31, −57.40; HRMS (TOF) m/z calcd for C15H9Br2F3N2O3H+: 480.9005, found: 480.9013 (Δ = −1.74 ppm). HPLC (A): t = 10.6 min, purity >98%.
2,5-Dibromo-N’-(5-fluoro-2-hydroxybenzylidene)benzohydrazide (5.3f).
White solid (86% yield), mp >220 °C; 1H NMR (500 MHz DMSO-d6) δ 6.82 – 7.14 (m, 3 H, 100%, 100%, 39%), 7.43 (d, 1 H, 70%, J = 6.3 Hz), 7.59 – 7.68 (m, 3 H, 100%, 100%, 30%), 7.82 (s, 1 H, 61%), 8.27 (s, 1 H, 36%), 8.47 (s, 1 H, 64%), 9.89 (s, 1 H, 37%), 10.69 (s, 1 H, 63%), 12.18 (s, 1 H, 100%); 13C NMR (125 MHz DMSO-d6) δ 112.0, 112.2, 113.2, 113.4, 117.47, 117.53, 117.6, 117.7, 117.8, 117.9, 118.0, 118.3, 118.5, 118.7, 119.77, 119.83, 120.2, 120.3, 120.6, 120.7, 131.0, 131.9, 133.5, 134.1, 134.4, 134.8, 138.7, 139.9, 141.9, 146.2, 152.8, 153.5, 154.3, 154.4, 156.2, 156.3, 161.7, 167.5; 19F NMR (376 MHz DMSO-d6) δ −124.87 (s, 1 F), −124.77 (s, 1 F); HRMS (TOF) m/z calcd for C14H9Br2FN2O2H+: 414.9013, found: 414.9088 (Δ = −3.7 ppm). HPLC (A): t1 = 6.9, t2 = 7.4 min, purity >97%.
4-Bromo-N’-(2-hydroxy-5-trifluoromethoxybenzylidene)benzohydrazide (5.4a).
White solid (93 % yield); mp 213–214 °C; 1H NMR (500 MHz DMSO-d6) δ 7.02 (d, 1 H, J = 9 Hz), 7.30 (dd, 1 H, J = 8.9, 2.6 Hz), 7.64 (d, 1 H, J = 2.5 Hz), 7.77 (d, 2 H, J = 8.5 Hz), 7.89 (d, 2 H, J = 8.5 Hz), 8.67 (s, 1 H), 11.25 (s, 1 H), 12.23 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 117.7, 119.2, 120.2, 120.4, 121.2, 123.3, 124.3, 125.8, 129.8, 131.6, 131.9, 140.7, 145.6, 156.1, 162.1; 19F NMR (376 MHz DMSO-d6) δ −57.30 (s, 3 F); HRMS (TOF) m/z calcd for C15H10BrF3N2O3H+: 402.9900, found: 402.9910 (Δ = −2.47 ppm). HPLC (A): t = 10.0 min, purity >95%.
4-Bromo-N’-(5-fluoro-2-hydroxybenzylidene)benzohydrazide (5.4b).
White solid (88 % yield); mp >220 °C; 1H NMR (500 MHz DMSO-d6) δ 6.92 – 6.94 (dd, 1 H, J = 9, 4.7 Hz), 7.15 (td, 1 H, J = 8.6, 3.2 Hz), 7.44 (dd, 1 H, J = 9.4 3.1 Hz), 7.76 (d, 2 H, J = 8.5 Hz), 7.89 (d, 2 H, J = 8.5 Hz), 8.63 (s, 1 H), 10.94 (s, 1 H), 12.20 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 113.6, 113. 8, 117.58, 117.64, 118.0, 118.2, 119.7, 119.8, 125.8, 129.7, 131.6, 131.9, 146.3, 153.6, 154.4, 156.3, 162.0; 19F NMR (376 MHz DMSO-d6) δ −125.06 (s, 1 F); HRMS (TOF) m/z calcd for C14H10BrFN2O2H+: 336.9982, found: 336.9996 (Δ = −3.95 ppm). HPLC (B): t = 5.2 min, purity >95%.
2-Methyl-N’-(2-hydroxy-5-trifluoromethylbenzylidene)benzohydrazide (5.27).
White solid (94% yield); mp 194–196 °C; 1H NMR (500 MHz DMSO- d6) δ 2.23 (s, 3 H, 17%), 2.38 (s, 3 H, 83%), 6.96 (d, 1 H, J = 8.6 Hz, 17%), 7.10 (d, 1 H, J = 8.6 Hz, 83%), 7.23 – 7.42 (m, 4 H, 100%, 100%, 15%, 20%), 7.40 (t, 1 H, J = 7.4 Hz, 85%), 7.47 – 7.50 (m, 1 H, 90%, 10%), 7.59 (s, 1 H, 18%), 7.61 (d, 1 H, J = 8.6 Hz), 7.98 (s, 1 H, 82%), 8.32 (s, 1 H, 17%), 8.57 (s, 1 H, 83%), 10.67 (s, 1 H, 17%), 11.72 (s, 1 H, 83%), 12.00 (s, 1 H, 18%), 12.10 (s, 1 H, 82%); 13C NMR (125 MHz, DMSO- d6) δ 19.0, 19.4, 116.9, 117.2, 119.7, 119.9, 120.2, 123.3, 125.3, 125.42, 125.45, 125.50, 125.7, 126.9, 127.6, 127.91, 129.2, 129.8, 130.2, 130.7, 134.2, 134.5, 136.1, 141.1, 145.1, 159.2, 160.0, 165.1; 19F NMR (376 MHz DMSO-d6) δ −60.22 (s, 3 F), −59.94 (s, 3 F); HRMS (TOF) m/z calcd for C16H13F3N2O2H+: 323.1002, found: 323.1001 (Δ = 0.23 ppm). HPLC (A): t = 8.0 min, purity >96%.
2-Methyl-N’-(5-cyano-2-hydroxybenzylidene)benzohydrazide (5.27a).
Beige solid (99% yield); mp 208–209 °C; 1H NMR (700 MHz DMSO-d6) δ 2.23 (s, 3 H, 17%), 2.38 (s, 3 H, 83%), 6.94 (d, 1 H, J = 8.6 Hz, 19%), 7.08 (d, 1 H, J = 8.6 Hz, 81%), 7.26 – 7.32 (m, 3 H, 100%, 100%, 18%), 7.35 – 7.37 (m, 1 H, 19%), 7.40 – 7.42 (m, 1 H, 81%), 7.48 (d, 1 H, J = 7.4 Hz, 82%), 7.60 – 7.64 (m, 2 H, 20%, 20%), 7.71 (dd, 1 H, J = 8.5, 2.1 Hz, 80%), 8.07 (d, 1 H, J = 2.0 Hz, 80%), 8.26 (s, 1 H, 18%), 8.50 (s, 1 H, 82%), 10.93 (s, 1 H, 18%), 12.02 (s, 1 H, 19%), 12.07 (s, 1 H, 82%), 12.16 (s, 1 H, 81%); 13C NMR (175 MHz DMSO-d6) δ 19.0, 19.4, 101.8, 117.5, 117.7, 118.9, 120.2, 120.5, 125.4, 125.7, 126.9, 127.6, 129.3, 129.9, 130.3, 130.8, 131.8, 133.2, 134.2, 134.4, 134.5, 134.8, 135.5, 136.2, 140.7, 145.0, 160.0, 160.8, 165.1, 171.2; HRMS (TOF) m/z calcd for C16H13N3O2H+: 280.1081, found: 280.1086 (Δ = −2.08 ppm). HPLC (A): t = 8.8 min, purity >97%.
4-Fluromethyl-N’-(3,5-dibromo-2-hydroxybenzylidene)benzohydrazide (5.21).
To a solution of tert-butyl 2-(4-(fluoromethyl)benzoyl)hydrazine-1-carboxylate (0.5 mmol) in dichloromethane (5 mL), trifluoroacetic acid (0.5 mL) was added and the reaction mixture was stirred at room temperature for 5 hours. This was followed by the addition of 3,5-dibromosalicylaldedhyde (1.1 ea.), to the reaction mixture and stirred at room temperature overnight. Ice cold water (15 mL) was added to the reaction mixture and filtered to give the product as a white solid (46 % yield); mp >230 °C; 1H NMR (400 MHz DMSO-d6) δ 5.46 (s, 1 H), 5.58 (s, 1 H), 7.57 (d, 2 H, J = 7.6 Hz), 7.80 – 7.82 (m, 2 H), 7.99 (d, 2 H, J = 7.9 Hz), 8.53 (s, 1 H), 12.56 (s, 1 H), 12.71 (s, 1 H); 13C NMR (100 MHz, DMSO-d6) δ 82.7, 84.3, 110.4, 111.2, 121.0, 127.27, 127.33, 128.1, 132.1, 135.6, 140.4, 140.6, 147.2, 153.7, 162.6; 19F NMR (376 MHz DMSO-d6) δ −209.61 (t, 1 F, J = 47.2 Hz); HRMS (TOF) m/z calcd for C15H11Br2FN2O2H+: 428.9244, found: 428.9247 (Δ = −0.69 ppm). HPLC (B): t = 6.2 min, purity >95%.
4-Bromo-2-(5-(2-tolyl)-1,3,4-oxadiazol-2-yl)phenol (6a).
To a solution of 1 (0.23 mmol) in DMF (2 mL) was added bis(trifluoroacetoxy)iodobenzene (1.1 eq.). The reaction mixture was heated at 100 °C for 7 h. After the completion of reaction, the reaction mixture was cooled to room temperature followed by the addition of water (15 mL) which resulted in precipitation of brown solid. The precipitate was filtered, dried and recrystallized in dichloromethane: hexanes (40:60) to give the product as white solid (53% yield); mp 152–153 °C; 1H NMR (500 MHz DMSO-d6) δ 2.67 (s, 3 H), 7.06(d, 1 H, J = 8.8 Hz), 7.40 – 7.45 (m, 2 H), 7.51 (t, 1 H, J = 7.4 Hz), 7.61 (dd, 1 H, J = 8.8, 2.5 Hz), 8.01 (d, 1 H, J = 2.5 Hz), 8.04 (d, 1 H, J = 7.7 Hz), 10.65 (s, 1 H); 13C NMR (125 MHz DMSO-d6) δ 21.5, 110.4, 111.9, 119.4, 122.3, 126.5, 129.0, 130.9, 131.6, 131.8, 135.8, 137.7, 155.7, 161.8, 164.0; HRMS (TOF) m/z calcd for C15H11BrN2O2H+: 331.0076, found: (Δ = −3.6 ppm). HPLC (B): t = 6.0 min, purity >95%.
3-(2-(Benzyloxy)-3,5-dibromophenyl)acrylaldehyde (4f.2).
To a solution of 4f.1 (0.68 mmol) in THF (7 mL), was added (formylmethylene)triphenylposphorane (1.1 eq.). The reaction mixture was refluxed overnight. After completion, the reaction was quenched by the addition of water. The reaction mixture was concentrated under vacuum in a rotary evaporator, followed by the addition of ethyl acetate (40 mL). The organic layer was washed with brine (3×30 mL) and water (3×30 mL). The organic layer was collected, dried with magnesium sulfate, filtered and concentrated under vacuum in a rotary evaporator. The crude product was purified by column chromatography using silica gel and a mixture of hexanes: ethyl acetate (2:1), to give the pure product as beige solid (87% yield); mp 104–105 °C; 1H NMR (500 MHz, CDCl3) δ 5.00 (s, 2 H), 6.49 – 6.53 (m, 1 H), 7.37 – 7.42 (m, 6 H), 7.61 (d, 1 H, J = 2.3 Hz), 7.81 (d, 1 H, J = 2.3 Hz), 9.45 (d, 1 H, J = 7.6 Hz); 13C NMR (125 MHz, CDCl3) δ 118.1, 119.7, 128.9, 129.1, 129.2, 129.5, 130.7, 132.0, 135.4, 138.2, 145.0, 153.9, 193.3; MS (ESI) m/z calcd for C16H12Br2O2H+: 394.9, found: 394.9.
(3-(2-(Benzyloxy)-5-bromophenyl)-1H-pyrazol-1-yl)(3,5-dibromophenyl)methanone (4f.3).
To a solution of 4.2 (1.2 mmol) in acetonitrile was added p-toluenesulfonylhydrazide (1.1 eq.). The reaction mixture was stirred at room temperature for one hour. This was followed by the addition of sodium hydroxide (1.5 eq.), and the reaction mixture was refluxed overnight. The mass peak corresponding to the cyclized product was seen on mass spectrometer. Then, 4-bromobenzoyl chloride (1.5 eq.) was added to the reaction mixture and stirred at room temperature for 3 hours. The reaction mixture was concentrated under vacuum in a rotary evaporator to give the crude product. Recrystallization of the crude product using hexanes: ethyl acetated gave the pure product as white solid (62% yield); mp 140–142 °C; 1H NMR (500 MHz, CDCl3) δ 4.82 (s, 2 H), 7.03 (d, 1 H, J = 2.9 Hz), 7.34 – 7.41 (m, 5 H), 7.65 (d, 2 H, J = 8.8 Hz), 7.78 (d, 1 H, J = 2.4 Hz), 7.96 (d, 1 H, J = 2.4 Hz), 8.10 (d, 2 H, J = 8.7 Hz), 8.43 (d, 1 H, J = 2.9 Hz); 13C NMR (125 MHz, CDCl3) δ 75.7, 111.1, 118.0, 119.9, 128.5, 128.63, 128.64, 128.8, 129.3, 130.0, 131.6, 131.7, 132.0, 132.5, 133.4, 136.0, 136.5, 151.9, 153.3, 165.2; MS (ESI) m/z calcd for C23H15Br3N2O2H+: 588.8, found: 588.8.
(3-(3,5-Dibromo-2-hydroxyphenyl)-1H-pyrazol-1-yl)(3-bromophenyl)methanone (6b).
To a solution of 4f.3 (0.15 mmol) in dichloromethane (3 mL) at 0 °C, BBr3 (1 eq.) was added dropwise. The reaction mixture was stirred at 0 °C for 30 min. This was followed by the addition of dichloromethane (30 mL). The organic layer was washed with brine (3×30 mL) and water (3×30 mL). The organic layer was collected, dried with magnesium sulfate, filtered and concentrated under vacuum in a rotary evaporator. The crude product was purified by column chromatography using silica gel and a mixture of hexanes: ethyl acetate (8:2), to give the pure product as white solid (50 % yield); mp 215–218 °C; 1H NMR (300 MHz, CDCl3) δ 6.95 (d, 1 H, J = 3.0 HZ), 7.69 (s, 2 H), 7.73 (d, 2 H, J = 8.7 Hz), 7.90 (d, 2 H, J = 8.7 Hz), 8.52 (d, 1 H, J = 3.0 Hz), 10.81 (s, 1 H); 13C NMR (125 MHz, CDCl3) δ 107.4, 111.7, 112.5, 117.6, 129.3, 129.4, 129.7, 131.9, 132.1, 132.3, 136.6, 152.4, 154.3, 164.9; HRMS (TOF) m/z calcd for C16H9Br3N2O2H+: 498.8287, found: 498.8286 (Δ = 0.24 ppm). HPLC (B): t1 = 9.0, t2 = 9.3 min, purity >95%.
4-Bromo-N’-(3,5-dibromo-2-hydroxybenzylidene)-N-methylbenzohydrazide (5.28).
White solid (89% yield); mp 222 – 223 °C; 1H NMR (400 MHz, DMSO-d6) δ 3.47 (s, 3 H), 7.51 (d, 2 H, J = 8.4 Hz), 7.64 (d, 1 H, J = 2.1 Hz), 7.70 (d, 2 H, J = 8.4 Hz), 7.73 (d, 1 H, J = 2.3 Hz), 8.20 (s, 1 H), 10.42 (s, 1 H); 13C NMR (100 MHz, DMSO-d6) δ 28.8, 110.7, 111.4, 121.9, 123.8, 130.1, 131.3, 131.7, 134.1, 135.3, 140.9, 152.2, 168.9; HRMS (TOF) m/z calcd for Cl5HllBr3N2O2H+: 488.8443, found: 488.8449 (Δ = −1.33 ppm). HPLC (A): t = 13.6 min, purity >95%.
For compounds 5.2i, 5.2 j, 5.3g and 5.3h, the same procedure was used as the rest of the acylhydrazones, except for the use of substituted acetophenones instead of salicylaldehydes.
2,4-Dibromo-N’-(1-(3,5-dibromo-2-hydroxyphenyl)ethylidene)benzohydrazide (5.2i).
White solid (88% yield); mp > 220 °C; 1H NMR (400 MHz, DMSO-d6) δ 2.43 (s, 3H), 7.58 (d, J = 8.2 Hz, 1H), 7.75 (dd, J = 1.8 Hz, J = 8.2 Hz, 1H), 7.79 (d, J = 2.3 Hz, 1H), 7.85 (d, J = 2.3 Hz, 1H), 8.04 (d, J = 1.8 Hz, 1H), 11.88 (s, 1H), 14.36 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ14.2, 109.5, 111.9, 120.8, 121.7, 124.0, 130.3, 130.7, 131.2, 134.7, 135.6, 135.9, 154.5, 155.7, 163.6; HRMS (TOF) m/z calcd for C15H10Br4N2O2H+: 566.7549, found 566.7540 (Δ = 1.6 ppm). HPLC (C): t = 4.8 min, purity >98%.
2,4-Dibromo-N’-(1-(5-bromo-2-hydroxyphenyl)ethylidene)benzohydrazide (5.2j).
White solid (83% yield); mp > 220 °C; 1H NMR (700 MHz, DMSO-d6) δ 2.36 (s, 1H, 20%), 2.40 (s, 1H, 80%), 6.70 (d, 1H, 20%, J = 8.8 Hz), 6.90 (d, 1H, 80%, J = 8.8 Hz), 7.34 (dd, 1H, 20%, J = 8.8 Hz, J = 2.4 Hz), 7.43 (d, 1H, 20%, J = 8.2 Hz), 7.46 (dd, 1H, 80%, J = 8.8 Hz, J = 2.4 Hz), 7.57 (d, 1H, 80%, J = 8.2 Hz), 7.59 (d, 1H, 20%, J = 2.4 Hz), 7.71 (dd, 1H, 20%, J = 8.2 Hz, J = 1.7 Hz), 7.74 (m, 2H, 80%), 8.01 (d, 1H, 20%, J = 1.6 Hz), 8.03 (d, 1H, 80%, J = 1.6 Hz), 10.81 (s, 1H, 20%), 11.71 (s, 1H), 13.22 (s, 1H, 80%); 13C NMR (175 MHz, DMSO-d6) δ 14.2, 109.7, 110.0, 119.0, 119.3, 119.6, 120.8, 121.3, 122.1, 123.8, 129.7, 130.7, 130.8, 131.15, 131.20, 133.4, 133.8, 134.5, 134.7, 135.9, 137.0, 152.8, 156.3, 156.4, 157.7, 163.4, 168.8; HRMS (TOF) m/z calcd for C15H11Br3N2O2H+: 488.8443, found 488.8449 (Δ = −1.2 ppm). HPLC (C): t = 3.3 min, purity >97%.
2,5-Dibromo-N’-(1-(3,5-dibromo-2-hydroxyphenyl)ethylidene)benzohydrazide (5.3g).
White solid (68% yield); mp > 220 °C; 1H NMR (700 MHz, DMSO-d6) δ 2.44 (s, 3H), 7.67 (dd, J = 8.6 Hz, J = 2.4 Hz, 1H), 7.70 (d, J = 8.6 Hz, 1H), 7.80(d, J = 2.4 Hz, 1H), 7.85 (d, J = 2.2 Hz, 1H), 7.87 (d, J = 2.2 Hz, 1H), 11.92 (s, 1H), 14.36 (s, 1H); 13C NMR (175 MHz, DMSO-d6) 14.2, 109.6, 111.9, 118.8, 120.6, 121.7, 130.4, 132.1, 134.5, 134.7, 136.0, 138.2, 154.6, 155.8, 162.8; HRMS (TOF) m/z calcd for C15H10Br4N2O2H+: 566.7549, found 566.7553 (Δ = −0.7 ppm). HPLC (B): t1 = 9.0, t2 = 9.3 min, purity >95%.
2,5-Dibromo-N’-(1-(5-bromo-2-hydroxyphenyl)ethylidene)benzohydrazide (5.3h).
White solid (83% yield); mp > 220 °C; 1H NMR (700 MHz, DMSO-d6) δ 2.37 (s, 3H, 20%), 2.41 (s, 3H, 80%), 6.70 (d, 1H, 20%, J = 8.8 Hz), 6.90 (d, 1H, 80%, J = 8.8 Hz), 7.34 (dd, 1H, 20%, J = 8.7 Hz, J = 1.8 Hz), 7.46 (dd, 1H, 80%, J = 8.7 Hz, J = 1.8 Hz), 7.59 (d, 1H, 20%, J = 1.8 Hz), 7.66 (m, 2H), 7.74 (d, 1H, J = 2.1 Hz), 7.86 (d, 1H, 80%, J = 1.8 Hz), 10.77 (s, 1H, 20%), 11.75 (s, 1H), 13.22 (s, 1H, 80%); 13C NMR (175 MHz, DMSO-d6) δ14.2, 109.7, 110.0, 117.3, 118.8, 119.0, 119.6, 120.6, 121.0, 121.3, 122.2, 130.6, 130.7, 130.8, 132.1, 133.4, 133.8, 134.4, 134.5, 134.7, 138.5, 139.7, 152.9, 156.4, 156.5, 157.7, 162.7, 168.0; HRMS (TOF) m/z calcd for C15H11Br3N2O2H+: 488.8443, found 488.8451 (Δ = −1.6 ppm). HPLC (B): t = 6.7 min, purity >97%.
N’1,N’4-Bis((E)-5-bromo-2-hydroxybenzylidene)isophthalohydrazide (5.29).
To a solution of isophthalohydrazide (0.170 mmol), 5-bromo-2-hydroxybenzaldehyde (0.374 mmol) in methanol (5 ml) was added 2 drops of glacial acetic acid. The reaction mixture was allowed to stir at room temperature overnight. Addition of water to the reaction mixture resulted in precipitation of the product. The product was filtered, washed with water and dried over vacuum to give the pure product as a white solid in 83% yield; mp > 220 °C; 1H NMR (700 MHz, DMSO-d6) δ 6.92 (d, J = 8.8 Hz, 2H), 7.44 (dd, J = 2.4 Hz, J = 8.7 Hz, 2H), 7.72 (t, J = 7.7 Hz, 1H), 7.83 (d, J = 2.2 Hz, 2H), 8.16 (d, J = 7.6 Hz, 2H), 8.52 (s, 1H), 8.66 (s, 2H), 11.24 (s, 2H), 12.35 (s, 2H). 13C NMR (175 MHz, DMSO-d6) δ 110.5, 118.7, 118.9, 121.4, 127.1, 129.0, 130.3, 131.1, 133.2, 133.7, 145.8, 156.4, 162.4; HRMS (TOF) m/z calcd for C22H16Br2N4O4H+: 558.9611, found: 558.9601 (Δ = 1.79 ppm). This compound was not UV active, for some reason, and thus HPLC analysis for purity assessment was not possible. Nevertheless, we believe that the purity is >95% based on its 1H NMR spectrum (see the Supporting Information).
The same procedure was used for the synthesis of 5.32.
N’1,N’4-Bis((E)-3,5-dibromo-2-hydroxybenzylidene)isophthalohydrazide (5.32).
White solid (83% yield); mp > 220 °C; 1H NMR (700 MHz, DMSO-d6) δ 7.77 (t, J = 7.8 Hz, 1H), 7.85 (d, J = 2.0 Hz, 4H), 8.20 (d, J = 7.7 Hz, 2H), 8.55 (s, 1H), 8.58 (s, 2H), 12.69 (s, 2H), 12.75 (s, 2H). 13C NMR (175 MHz, DMSO-d6) δ 110.5, 111.3, 121.0, 127.3, 129.1, 131.5, 132.2, 132.6, 135.7, 147.5, 153.7, 162.4; HRMS (TOF) m/z calcd for C22H14Br4N4O4H+: 714.7821, Found: 714.7827 (Δ = −0.83 ppm). This compound was not UV active, for some reason, and thus HPLC analysis for purity assessment was not possible. Nevertheless, we believe that the purity is >95% based on its 1H NMR spectrum (see the Supporting Information).
Antifungal activity assay.
MICs were determined following the methods of the Clinical and Laboratory Standards Institutes (CLSI) with modifications. Yeast nitrogen base (YNB) medium without amino acid (pH 7.0, 2% glucose) buffered with (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) was used for MIC studies in C. neoformans. YNB medium without ammonium sulfate, without amino acid and 1% asparagine (pH 7.0, 2% glucose) was used for MIC studies in Candida strains. RPMI medium (pH 7.0, 2% glucose) was used for MIC studies in A. fumigatus. HEPES was used instead of morpholinepropanesulfonic acid (MOPS), because MOPS was found to inhibit the activity of this kind of compounds. The compounds were serially diluted from 32 to 0.03 μg/mL, in a 96-well plate. The inoculum was prepared as described in the CLSI protocol M27A3 guidelines.47 The plates were incubated at 37°C with 5% CO2 for 24 to 72 h and the optical density was measure at 450 nm. The MICs were determined as the lowest concentration of the compound that inhibited 80% of growth compared to the control.
Cytotoxicity assay.
The human cancer cell lines A549 and HepG2 were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% Fetal bovine serum (FBS) and 1% penicillin-streptomycin. At passage 7, 105 cells/well in DMEM containing 10% FBS were transferred into 96-well plates and cultured for 14 h for the cells to adhere to the wells. The compounds were added to the cells at concentrations ranging from 0.03 to 128 μg/mL. The wells without the compound served as controls. The plate was incubated at 37 °C with 5% CO2. After 24 or 48 h, the supernatant was removed, and 50 μl of 5-mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) solution in Phosphate-buffered saline (PBS) was added to each well. The plates were incubated for an additional 4h. The formazan crystal formed inside the cell was dissolved by adding 50 μL dimethyl sulfoxide (DMSO). The optical density was measured at 570 nm.
Time-kill assay.
C. neoformans cells from a culture grown overnight were washed in PBS and resuspended in YNB buffered with HEPES at pH 7.4. The cells were counted, and 2 × 104 cells were incubated with different concentration of the drugs in a final volume of 10 mL with a final concentration of 0.5% DMSO. The tubes were then incubated at 37 °C with 5% CO2 on a rotary shaker at 200 rpm. Aliquots were taken at time points and diluted, and 100-l portions were plated onto yeast extract-peptone-dextrose (YPD) plates. YPD plates were incubated in a 30 °C incubator and after 48 h, the numbers of colony forming units (CFU) were counted and recorded.
Evaluation of synergism in drug combination.
Synergism/cooperativity in the combination of 5 lead compounds with clinical drugs was evaluated by calculating the fractional inhibitory concentration index (FICI). Briefly, in a 96-well plate, drug A (5.2d, 5.2c, 5.6a, 5.8 and 5.8a) was serially diluted from 16 to 0.015 μg/mL (11 dilutions), whereas drug B (either fluconazole, amphotericin B, caspofungin, itraconazole, or voriconazole) was serially diluted from 12 to 0.19 μg/mL for fluconazole, 5 to 0.078 μg/mL for amphotericin B, or 8 to 0.007 μg/mL for caspofungin, itraconazole, or voriconazole (seven dilutions). The FICI is defined as (MICcombined/MICdrugA) + (MICcombined/MICdrugB).31 The level of synergism/cooperativity was determined by using the following scale: strongly synergistic: FIC <0.5; synergistic: FIC <1; autonomous: FIC = 1; additive: FIC = 1~4; antagonistic: FIC >4.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants AI116420, AI125770 and AI134428.
Dr. Maurizio Del Poeta is a Co-Founder and Chief Scientific Officer (CSO) of MicroRid Technologies Inc. Dr. John B. McCarthy is the Chief Executive Officer (CEO) of MicroRid Technologies, Inc. Dr. John Mallamo is the Chief of Research and Development Officer (CRDO) of MicroRid Technologies Inc.
Footnotes
ASSOCIATED CONTENTS
Supporting Information.
1H and 13C spectra of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
All other authors have no conflict of interest.
REFERENCES
- 1.Brown GD; Denning DW; Gow NAR; Levitz SM; Netea MG; White TC, Hidden Killers: Human Fungal Infections. Sci Transl Med 2012, 4, 165rv13. [DOI] [PubMed] [Google Scholar]
- 2.Bongomin F; Gago S; Oladele RO; Denning DW, Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel) 2017, 3, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu N; Tu J; Dong G; Wang Y; Sheng C, Emerging New Targets for the Treatment of Resistant Fungal Infections. J. Med. Chem. 2018, 61, 5484–5511. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong-James D; Meintjes G; Brown GD, A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 22, 120–127. [DOI] [PubMed] [Google Scholar]
- 5.Mousset S; Buchheidt D; Heinz W; Ruhnke M; Cornely OA; Egerer G; Krüger W; Link H; Neumann S; Ostermann H; Panse J; Penack O; Rieger C; Schmidt-Hieber M; Silling G; Südhoff T; Ullmann AJ; Wolf H-H; Maschmeyer G; Böhme A, Treatment of invasive fungal infections in cancer patients—updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 2014, 93, 13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson MD, Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 2005, 56, i5–i11. [DOI] [PubMed] [Google Scholar]
- 7.Singh N; Dromer F; Perfect JR; Lortholary O, Cryptococcosis in Solid Organ Transplant Recipients: Current State-of-the-Science. Clin. Infect. Dis. 2008, 47, 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaoutis TE; Argon J; Chu J; Berlin JA; Walsh TJ; Feudtner C, The Epidemiology and Attributable Outcomes of Candidemia in Adults and Children Hospitalized in the United States: A Propensity Analysis. Clin. Infect. Dis. 2005, 41, 1232–1239. [DOI] [PubMed] [Google Scholar]
- 9.Rajasingham R; Smith RM; Park BJ; Jarvis JN; Govender NP; Chiller TM; Denning DW; Loyse A; Boulware DR, Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017, 17, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roemer T; Krysan DJ, Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb Perspect Med 2014, 4, a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nami S; Aghebati-Maleki A; Morovati H; Aghebati-Maleki L, Current antifungal drugs and immunotherapeutic approaches as promising strategies to treatment of fungal diseases. Biomed. Pharmacother. 2019, 110, 857–868. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA; Diekema DJ, Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller MA; Diekema DJ, Epidemiology of Invasive Mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [DOI] [PubMed] [Google Scholar]
- 14.Hirano R; Sakamoto Y; Kudo K; Ohnishi M, Retrospective analysis of mortality and Candida isolates of 75 patients with candidemia: a single hospital experience. Infect Drug Resist 2015, 8, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepak AJ; Andes DR, Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 5, a019653-a019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brüggemann RJM; Alffenaar J-WC; Blijlevens NMA; Billaud EM; Kosterink JGW; Verweij PE; Burger DM; Saravolatz LD, Clinical Relevance of the Pharmacokinetic Interactions of Azole Antifungal Drugs with Other Coadministered Agents. Clin. Infect. Dis. 2009, 48, 1441–1458. [DOI] [PubMed] [Google Scholar]
- 17.Ghannoum MA; Rice LB, Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanafani ZA; Perfect JR, Resistance to Antifungal Agents: Mechanisms and Clinical Impact. Clin. Infect. Dis. 2008, 46, 120–128. [DOI] [PubMed] [Google Scholar]
- 19.Lyman CA; Walsh TJ, Systemically Administered Antifungal Agents. Drugs 1992, 44, 9–35. [DOI] [PubMed] [Google Scholar]
- 20.Haidar C; Jeha S, Drug interactions in childhood cancer. Lancet Oncol. 2011, 12, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheven M; Schwegler F, Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob. Agents Chemother. 1995, 39, 1779–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazzarini C; Haranahalli K; Rieger R; Ananthula HK; Desai PB; Ashbaugh A; Linke MJ; Cushion MT; Ruzsicska B; Haley J; Ojima I; Del Poeta M, Acylhydrazones as Antifungal Agents Targeting the Synthesis of Fungal Sphingolipids. Antimicrob. Agents Chemother. 2018, 62, e00156–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mor V; Rella A; Farnoud AM; Singh A; Munshi M; Bryan A; Naseem S; Konopka JB; Ojima I; Bullesbach E; Ashbaugh A; Linke MJ; Cushion M; Collins M; Ananthula HK; Sallans L; Desai PB; Wiederhold NP; Fothergill AW; Kirkpatrick WR; Patterson T; Wong LH; Sinha S; Giaever G; Nislow C; Flaherty P; Pan X; Cesar GV; de Melo Tavares P; Frases S; Miranda K; Rodrigues ML; Luberto C; Nimrichter L; Del Poeta M, Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids. MBio 2015, 6, e00647–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mor V; Rella A; Farnoud AM; Singh A; Munshi M; Bryan A; Naseem S; Konopka JB; Ojima I; Bullesbach E; Ashbaugh A; Linke MJ; Cushion M; Collins M; Ananthula HK; Sallans L; Desai PB; Wiederhold NP; Fothergill AW; Kirkpatrick WR; Patterson T; Wong LH; Sinha S; Giaever G; Nislow C; Flaherty P; Pan X; Cesar GV; de Melo Tavares P; Frases S; Miranda K; Rodrigues ML; Luberto C; Nimrichter L; Del Poeta M, Erratum for Mor et al. , “Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids”. MBio 2018, 9, e00188–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levery SB; Momany M; Lindsey R; Toledo MS; Shayman JA; Fuller M; Brooks K; Doong RL; Straus AH; Takahashi HK, Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett. 2002, 525, 59–64. [DOI] [PubMed] [Google Scholar]
- 26.Noble SM; French S; Kohn LA; Chen V; Johnson AD, Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 2010, 42, 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittershaus PC; Kechichian TB; Allegood JC; Merrill AH; Hennig M; Luberto C; Del Poeta M, Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Invest. 2006, 116, 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baell JB; Ferrins L; Falk H; Nikolakopoulos G, PAINS: Relevance to Tool Compound Discovery and Fragment-Based Screening. Aust. J. Chem. 2013, 66, 1483–1494. [Google Scholar]
- 29.Pouliot M; Jeanmart S, Pan Assay Interference Compounds (PAINS) and Other Promiscuous Compounds in Antifungal Research. J. Med. Chem. 2016, 59, 497–503. [DOI] [PubMed] [Google Scholar]
- 30.Spivak ES; Hanson KE, Candida auris: an Emerging Fungal Pathogen. J. Clin. Microbiol. 2018, 56, e01588–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meletiadis J; Pournaras S; Roilides E; Walsh TJ, Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoang A, Caspofungin acetate: An antifungal agent. Am J Health Syst Pharm 2001, 58, 1206–1214. [DOI] [PubMed] [Google Scholar]
- 33.Feldmesser M; Kress Y; Mednick A; Casadevall A, The Effect of the Echinocandin Analogue Caspofungin on Cell Wall Glucan Synthesis by Cryptococcus neoformans. J. Infect. Dis. 2000, 182, 1791–1795. [DOI] [PubMed] [Google Scholar]
- 34.Pappas PG; Kauffman CA; Andes DR; Clancy CJ; Marr KA; Ostrosky-Zeichner L; Reboli AC; Schuster MG; Vazquez JA; Walsh TJ; Zaoutis TE; Sobel JD, Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dehestani L; Ahangar N; Hashemi SM; Irannejad H; Honarchian Masihi P; Shakiba A; Emami S, Design, synthesis, in vivo and in silico evaluation of phenacyl triazole hydrazones as new anticonvulsant agents. Bioorg. Chem. 2018, 78, 119–129. [DOI] [PubMed] [Google Scholar]
- 36.Liu C; Zhao P; Huang W, New oxadiazole derivatives as promising electron transport materials: synthesis and characterization of thermal, optical and electrochemical properties. In Open Chem2007; Vol. 5, p 303. [Google Scholar]
- 37.Liu Y; Lu B.-w.; Lu J.-r.; Xin C.-w; Li J.-f.; Mu J.-b.; Bao X.-r., Synthesis and antibacterial activities of N-[(1-aryl-3-phenyl-pyrazol-4-yl)methylene]-2-(halo-o-hydroxyphenyl)hydrazide derivatives. Chem Res Chinese U 2013, 29, 449–453. [Google Scholar]
- 38.Monga V; Nayyar A; Vaitilingam B; Palde PB; Singh Jhamb S; Kaur S; Singh PP; Jain R, Ring-substituted quinolines. Part 2: Synthesis and antimycobacterial activities of ring-substituted quinolinecarbohydrazide and ring-substituted quinolinecarboxamide analogues. Bioorg. Med. Chem 2004, 12, 6465–6472. [DOI] [PubMed] [Google Scholar]
- 39.Grimster NP; Connelly S; Baranczak A; Dong J; Krasnova LB; Sharpless KB; Powers ET; Wilson IA; Kelly JW, Aromatic Sulfonyl Fluorides Covalently Kinetically Stabilize Transthyretin to Prevent Amyloidogenesis while Affording a Fluorescent Conjugate. J. Am. Chem. Soc. 2013, 135, 5656–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller CP; Shomali M; Lyttle CR; O’Dea LSL; Herendeen H; Gallacher K; Paquin D; Compton DR; Sahoo B; Kerrigan SA; Burge MS; Nickels M; Green JL; Katzenellenbogen JA; Tchesnokov A; Hattersley G, Design, Synthesis, and Preclinical Characterization of the Selective Androgen Receptor Modulator (SARM) RAD140. ACS Med. Chem. Lett. 2011, 2, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu G; Luo L; Chen S; He F; Xie Y; Luo D; Xue W; Wu J, Synthesis and Insecticidal Activity of Novel Diacylhydrazines Derivatives Containing a N-Pyrazolepyrazole Moiety. ChemistrySelect 2018, 3, 10991–10995. [Google Scholar]
- 42.Husain A; Ahmad A; Alam MM; Ajmal M; Ahuja P, Fenbufen based 3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]-1-(biphenyl-4-yl)propan-1-ones as safer antiinflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 3798–3804. [DOI] [PubMed] [Google Scholar]
- 43.Vallejos S; Muñoz A; Ibeas S; Serna F; García FC; García JM, Forced Solid-State Interactions for the Selective “Turn-On” Fluorescence Sensing of Aluminum Ions in Water Using a Sensory Polymer Substrate. ACS Appl. Mater. Interfaces 2015, 7, 921–928. [DOI] [PubMed] [Google Scholar]
- 44.Guilherme FD; Simonetti JÉ; Folquitto LRS; Reis ACC; Oliver JC; Dias ALT; Dias DF; Carvalho DT; Brandão GC; Souza T. B. d., Synthesis, chemical characterization and antimicrobial activity of new acylhydrazones derived from carbohydrates. J Mol Struct 2019, 1184, 349–356. [Google Scholar]
- 45.Tang X; Wang Z; Zhong X; Wang X; Chen L; He M; Xue W, Synthesis and biological activities of benzothiazole derivatives bearing a 1,3,4-thiadiazole moiety. Phosphorus Sulfur Silicon Relat Elem 2019, 194, 241–248. [Google Scholar]
- 46.Raiford LC; Tanzer LK, PREPARATION OF α,β-UNSATURATED KETONES AND THEIR REACTION WITH PHENYLHYDRAZINE. J. Org. Chem. 1941, 06, 722–731. [Google Scholar]
- 47.Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 4th ed CLSI document M27Ed4. Clinical and Laboratory Standards Institute, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


