Abstract
SETTING:
Fifty-five public clinics in northern South Africa.
OBJECTIVE:
To estimate patient costs and identify the factors associated with catastrophic costs among individuals treated for tuberculosis (TB).
DESIGN:
We performed cross-sectional interviews of consecutive patients at public clinics from October 2017 to January 2018. ‘Catastrophic costs’ were defined as costs totalling ≥20% of annual household income. For participants with no reported income, we considered scenarios where costs were considered non-catastrophic if 1) costs totaled <US$7.70 (ZAR100) or 2) a multidimensional poverty index was above a certain threshold.
RESULTS:
Among 327 participants, the estimated mean TB episode costs were US$365 (95%CI 233–498): out of-pocket costs comprised 58% of costs, wages lost due to health care-seeking represented 26%, and income reduction accounted for 16% of costs. Ninety (28%) participants experienced catastrophic costs, which were associated with clinic travel times of 60–90 min (adjusted prevalence ratio [aPR] 1.7, 95%CI 0.9–3.1), unemployment (aPR 2.0, 95%CI 1.0–4.0) and having fewer household members (aPR 0.6, 95%CI 0.3–1.0).
CONCLUSIONS:
In rural South Africa, catastrophic costs from TB are common and associated with distance to clinics, unemployment, and household size. These findings can help tailor social protection programs and enhance service delivery to patients at greatest risk of experiencing financial hardship.
Keywords: patient cost, care-seeking, income, socioeconomic, epidemiology
Abstract
CONTEXTE:
Cinquante-cinq centres de santé publics dans le nord de l’Afrique du Sud.
OBJECTIF:
Estimer les coûts pour les patients et identifier les facteurs associés aux coûts catastrophiques pour les patients traités pour la tuberculose (TB).
SCHÉMA:
Nous avons réalisé des entretiens transversaux de patients consécutifs dans des centres de santé publics d’octobre 2017 à janvier 2018. Les coûts catastrophiques ontété définis comme des co ûts atteignant ≥20% du revenu annuel du foyer. Pour les participants n’ayant pas de revenu déclaré, nous avons envisagé des scénarios où les coûts ont été considérés comme non catastrophiques si 1) les coûts totaux ont été inférieurs à 7,70 $US (100 ZAR), ou 2) un index multidimensionnel de pauvreté a été supérieur à uncertain seuil.
RÉSULTATS:
Parmi 327 participants, les coûts moyens estimés parépisode de TB ont été de 365 $US (IC95% 233–498), incluant 58% de coûts directs, 26% de perte de salaire liées à la recherche de soins et 16% de perte de revenus. Quatre-vingt-dix (28%) participants ont subi des coûts catastrophiques, qui ont été associés aux temps de trajets vers le centre de santé de 60–90 min (taux de prévalence ajusté [PRa] 1,7 ; IC95% 0,9–3,1), au chômage (PRa 2,0 ; IC95% 1,0–4,0), et à moins de personnes présentes au foyer (PRa 0.6; IC95% 0.3–1.0).
CONCLUSIONS:
Dans l’Afrique du Sud rurale, les coûts catastrophiques liés à la TB sont fréquents et associés à la distance, au chômage et à la taille du foyer. Ces résultats peuvent contribuer à adapter les programmes de protection sociale et à améliorer la prestation de service aux patients ayant le plus de risqué de subir des difficultés financiéres.
Abstract
MARCO DE REFERENCIA:
Cincuenta y cinco consultorios del sector público en el norte de Suràfrica.
OBJETIVO:
Estimar los costos soportados por los pacientes y reconocer los factores que se asocian con costos catastróficos para las personas que reciben tratamiento antituberculoso.
MÉTODOS:
Se llevaron a cabo entrevistas transversales con pacientes que acudían de manera consecutiva a los consultorios públicos de octubre del 2017 a enero del 2018. Se definieron los costos catastróficos como los costos que correspondían al ≥20% del ingreso familiar anual. En los participantes sin notificaci ón del ingreso, se analizaron hipótesis en las cuales se consideraba que los gastos no eran catastróficos cuando los costos sumaban menos de 7,70 $US (100 ZAR) o cuando el índice multidimensional de pobreza estaba por encima de un determinado umbral.
RESULTADOS:
En los 327 participantes, la estimación del costo medio por episodio de tuberculosis (TB) fue 365 $US (IC95% 233–498), incluidos 58% de costos directos, 26% de salarios perdidos como consecuencia de la búsqueda de atención de salud y 16% de disminución de los ingresos. Noventa participantes (28%) soportaron costos catastróficos, asociados con un tiempo de desplazamiento hasta el consultorio de 60 a 90 min (razón de prevalencias ajustada [PRa] 1,7; IC95% 0,9–3,1), el desempleo (PRa 2,0; IC95% 1,0–4,0) y un menor número de miembros en el hogar (PRa 0,6; IC95% 0,3–1,0).
CONCLUSIONES:
En las zonas rurales de Suràfrica es frecuente que la TB de lugar a costos catastróficos, que se asocian con la distancia del consultorio, el desempleo y el nóumero de miembros del hogar. Estos resultados pueden ayudar a adaptar los programas de protección social a las necesidades y reforzar la prestación de servicios a los pacientes con mayor riesgo de sufrir dificultades económicas.
COSTS INCURRED by individuals with active tuberculosis (TB) are highlighted in the World Health Organization (WHO) End TB Strategy goal of ‘zero TB-affected families facing catastrophic costs due to TB’.1 High patient costs for TB not only degrade the financial wellness of households, but also negatively influence TB treatment outcomes.2 The multifaceted, deleterious nature of such high costs (defined by the WHO as costs exceeding 20% of annual household income) has led to their characterisation as ‘catastrophic’.3–6 Costs accumulate during patients’ pre-diagnostic, diagnostic, and treatment phases, and catastrophic costs are associated with adverse TB treatment outcomes2 and residence in rural areas.3
Rural areas are often epidemiologically and economically distinct from urban environments, and TB-related catastrophic costs may reflect these differences.7–10 People living in rural settings frequently have lower incomes and rely more on remittances, subsistance agriculture, or informal employment.11
We sought to estimate patient costs and to identify demographic, clinical, and socio-economic risk factors for catastrophic costs among people being treated for TB at public primary care clinics in rural South Africa. We also evaluated different methods for estimating catastrophic costs from cross-sectional data and considered different catastrophic cost definitions for those reporting no household income.
STUDY POPULATION AND METHODS
Populations
Limpopo Province, located in northern South Africa, has the highest proportion of households involved in agriculture. It also has the lowest median monthly income of South Africa’s nine provinces, and an estimated 58% of households receive a government social grant.11 In 2016, the annual incidence of TB was estimated to be 301 per 100 000 population (the lowest in South Africa); the prevalence of human immunodeficiency virus (HIV) infection in those aged 15–49 years was estimated to be 8.3% (the third lowest in South Africa).12
The present study was nested within an ongoing pragmatic cluster randomised trial of TB case-finding (the Kharitode TB trial).13 We conducted a cross-sectional study at 55 public clinics in two predominantly rural health districts of Limpopo Province: Vhembe, the northernmost district, and Waterberg, which has large mining and tourism sectors.
Data collection
We developed a patient cost questionnaire based on the WHO tool for assessing TB-related catastrophic costs14 and a national-level socio-economic survey15 (see Supplementary Data). We identified potential participants using data abstraction from presumptive TB case registers at study clinics. Eligible participants were either recently diagnosed with TB or had recently received a negative result on the sputum Xpert® MTB/RIF test (Cepheid, Sunnyvale, CA, USA) (‘Xpert-negative’). Inclusion criteria were enrollment within 2 months of a diagnostic result (Xpert, smear, chest X-ray, or other) at a study clinic; age ≥18 years; and proficiency in English, Sepedi, Xitsonga, or Tshivenda. Patients who transferred from other health care facilities to study clinics for treatment were included. Local study staff interviewed participants in person or by telephone; all individual clinical, socio-economic, and cost-related data were self-reported by participants.
We measured three main components of illness and treatment costs based on WHO guidance.14 These cost components were 1) out-of-pocket costs, 2) wages lost due to time spent in health care-seeking and treatment and 3) decreased monthly income due to illness. Costs were estimated at the household level, and therefore included costs for the participants themselves, as well as their household members and care givers. The three components were summed to obtain the total cost per illness episode for each participant.
We directly ascertained costs experienced before treatment initiation by asking participants about their costs from symptom onset to diagnosis. We used the same methodology to estimate costs for TB patients and those who were Xpert-negative. However, because Xpert-negative participants were not treated for TB, we assumed that they experienced no TB treatment costs. We estimated costs during treatment by estimating the number of health care-seeking visits and applying an estimated per-visit cost. We estimated the number of visits using the median number of clinic visits reported for TB patients by Foster et al. (four visits during the 2-month intensive phase and eight visits during the 4-month continuation phase).3 This number is also consistent with the frequency of visits recommended by the South African Department of Health.16 Because the number of clinic visits varies based on nurse experience and patient adherence,3 we performed a sensitivity analysis using the mean instead of the median number of visits, as reported by Foster et al. Visit costs during treatment cannot be assumed to be equivalent to the cost of the diagnostic visit. We therefore estimated per-visit costs during the intensive phase and continuation phase as respectively 24% and 8% relative to the diagnostic visit; this reflects the total costs divided by the number of visits in each phase of treatment, as measured by Foster et al.3 We used the average currency exchange rate for South African rand (ZAR) during the study period (US$1 = ZAR13).17
Analyses
Our primary analytic objective was to identify potential associations between demographic and socio-economic risk factors and experiencing catastrophic costs. The primary definition of catastrophic costs was illness-related costs exceeding 20% of the annual household income.1,2 However, many participants reported zero annual household income, such that any cost would be deemed ‘catastrophic’. For these individuals, we performed analyses in which costs were reclassified as ‘non-catastrophic’ if total out-of-pocket costs were less than an arbitrary but low ‘minimum cost threshold’ of ZAR100, and total health care-seeking time was <20 h. The amount of ZAR100, equal to US$7.70, is substantially below the catastrophic cost threshold for the participant with the lowest, non-zero household income; 20 h was the mean care-seeking time in our population. We excluded participants from the analysis if their reported pre-symptom income was not known or not reported.
We used Fisher’s exact test for nominal and binary risk factors and reported statistical associations with P < 0.1 as potential risk factors, although we used a cut-off of P < 0.05 for statistical significance. For univariable and multivariable regressions of risk factors on catastrophic costs, we limited analyses to those participants diagnosed with TB. We employed Poisson regression18 with a robust variance estimator to estimate adjusted prevalence ratios (aPRs) for experiencing catastrophic costs. All analyses were conducted in Stata v14.2 (StataCorp, College Station, TX, USA).
Sensitivity analyses: health care-seeking time estimation
We estimated participants’ lost time due to health care-seeking and treatment using different approaches to gauge the sensitivity of prevalence and risk factor associations to different measures.14 In our primary analysis, we used the median wage of the analytic population (self-reported, excluding social grant incomes), but we also considered four alternative valuations: 1) the median wage in Limpopo Province, 2) mean wage within quintiles of the analytic population based on self-reported pre-symptom household income, 3) the participants’ own estimate of lost wages or lost income-generating opportunities during health care-seeking, and 4) zero cost.
Alternative definitions of catastrophic costs
We also performed sensitivity analyses by developing two thresholds that reclassified costs as non-catastrophic for individuals reporting zero income, based on certain criteria. In the first, we used the minimum cost threshold described above. For the second sensitivity analysis, we defined a socio-economic threshold for catastrophic costs incorporating the South African Multidimensional Poverty Index (SAMPI).15 We reclassified costs as non-catastrophic for those who reported no pre-symptom income if they were not in the lowest socio-economic group, classified using SAMPI. SAMPI dimensions are health, education, living standards, employment, and asset ownership.
Ethical approval
The study protocol was approved by the University of the Witwatersrand’s Human Research Ethics Committee, Johannesburg, and the Limpopo Health Research Committee, Polokwane, South Africa. Informed consent was provided by all study participants.
RESULTS
We interviewed 336 participants being treated for TB at 55 public sector clinics from October 2017 to January 2018. Almost all participants (n= 327, 97%) provided sufficient income data to calculate the catastrophic cost outcome (Table 1). The mean and median total estimated TB episode costs were respectively US$365 (95% confidence interval [CI] 233–498) and US$76 (interquartile range 23–299). Total cost components for each episode were 58% (95%CI 54–62) out-of-pocket costs, 26% (95%CI 21–29%) lost time during health care-seeking, and 16% (95%CI 13–20%) lost wages. The greatest share of out-of-pocket costs was for transportation (Table 2). Costs due to lost household income were right-skewed in the analytic population, with 255 (78%) participants with available income data reporting no change in monthly household income between pre-illness and treatment initiation. Among those who experienced decreases in monthly household income due to illness, income decreases comprised nearly 75% of the mean TB episode cost.
Table 1.
Catastrophic costs among people being treated for TB in the Kharitode study* (n = 327)
| Potential risk factor | Catastrophic costs experienced (n = 90) n (%) | No catastrophic costs experienced (n = 237) n (%) | P value† |
|---|---|---|---|
| Sex | 0.32 | ||
| Male | 49 (54) | 144 (61) | |
| Female | 41 (46) | 93 (39) | |
| Age, years | 0.20 | ||
| 18–24 | 9 (10) | 28 (12) | |
| 25–34 | 13(14) | 61 (26) | |
| 35–44 | 32 (36) | 70 (30) | |
| 45–59 | 28 (31) | 57 (24) | |
| ≥60 | 8 (9) | 21 (9) | |
| Number of household members | 0.37 | ||
| 1–2 | 24 (27) | 45 (19) | |
| 3–4 | 27 (30) | 75 (32) | |
| 5–6 | 18 (20) | 64 (27) | |
| ≥7 | 21 (23) | 53 (22) | |
| Duration of primary symptom, months | 0.41 | ||
| <2 | 52 (58) | 139 (59) | |
| 2–4 | 20 (22) | 56 (24) | |
| >4 | 15 (17) | 26 (11) | |
| Missing | 3 (3) | 16 (7) | |
| Tobacco smoking, pack-years | 0.12 | ||
| Never smoked | 52 (58) | 135 (57) | |
| <5 | 19 (21) | 54 (23) | |
| 5–< 15 | 10 (11) | 29 (12) | |
| ≥15 | 9 (10) | 10 (4) | |
| Missing | 0 | 9 (4) | |
| HIV status | 0.11 | ||
| Not infected | 36 (40) | 119 (50) | |
| Living with HIV | 54 (60) | 118 (50) | |
| Travel time to clinic, min | 0.10 | ||
| <15 | 15 (17) | 44 (19) | |
| 15–60 | 46 (51) | 149 (63) | |
| >60–89 | 17 (19) | 25 (11) | |
| ≥90 | 11 (12) | 16 (7) | |
| Missing | 1 (1) | 3 (1) | |
| Employment | 0.45 | ||
| Temporary/informal | 4 (4) | 15 (6) | |
| Unemployed, cannot work | 9 (10) | 33 (14) | |
| Unemployed, could work | 36 (40) | 62 (26) | |
| Retired | 21 (23) | 56 (24) | |
| Student | 6 (7) | 22 (9) | |
| Self-employed | 4 (4) | 10 (4) | |
| Salaried | 10 (11) | 37 (16) | |
| Missing | 0 | 2 (1) | |
| Highest grade attended by any household member | 0.75 | ||
| ≤4th | 18 (20) | 58 (24) | |
| 5–9th | 25 (28) | 56 (24) | |
| 10–12th | 42 (47) | 106 (45) | |
| Any postgraduate | 5 (6) | 17 (7) | |
| SAMPI,‡ SEP | 0.06 | ||
| Lowest SEP | 33 (37) | 76 (32) | |
| Middle SEP | 42 (47) | 92 (39) | |
| Highest SEP | 15 (17) | 69 (29) | |
The catastrophic cost threshold is set after requiring that costs exceed a minimum of 100 South African rand (US$7.70); health care-seeking time is estimated using the median non-grant income of the study population.
Reflects the difference between people who experienced catastrophic costs vs. those who did not, using Fisher’s exact test.
For 26 (8%) participants, the indicator for years of school attended by adults was assumed to be <5 years if the index case and head of household had attended <5 years, regardless of the number of household members. TB = tuberculosis; HIV = human immunodeficiency virus; SAMPI = South Africa Multidimensional Poverty Index; SEP = socio-economic placement.
Table 2.
Mean patient costs per TB/illness episode, in 2017 currency
| Means of cost components | Mean illness episode cost | Episode cost as proportion of annual household income median % [IQR] | Proportion of participants experiencing catastrophic costs % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Out-of-pocket costs | Indirect costs | |||||||||
| Transport costs | Non-transport: pre-diagnosis | Non-transport: diagnosis and treatment | Total individual care-seeking costs | Care giver costs | Time lost care-seeking | Income lost from decrease in monthly income | ||||
| ТВ-positive (n = 327) | ZAR723 US$56 (15%)* | ZAR530 US$41 (11%)* | ZAR 146 US$11 (3%)* | ZAR 1399 US$108 (29%)* | ZAR 147 US$11 (3%)* | ZAR240 US$18 (5%)* | ZAR2965 US$228 (62%)* | ZAR4751 US$365 (100%)* | 4 [1–18]† | 27.5 |
| Xpert-negative (n = 263) | ZAR40 US$3 (11%)* | ZAR177 US$14 (49%)* | ZAR7 US$1 (2%)* | ZAR224 US$17 (63%)* | ZAR23 US$2 (6%)* | ZAR68 US$5 (19%)* | ZAR43 US$3 (12%)* | ZAR358 US$28 (100%)* | 0 [0–1] | 7.2 |
Proportion of mean episode cost represented by each cost component.
Participants who reported zero pre-illness income (10% of all sampled participants) were excluded from this calculation. Thus, while the 75th percentile of cost as a proportion of annual income for people with TB and non-zero income was 18%, 27.5% of all participants (including those with zero reported income) experienced catastrophic costs, as reported in the text.
TB = tuberculosis; IQR = interquartile range; ZAR = South African rand; US$ = US dollar.
Using the minimum cost threshold method, we found that 28% of patients with TB experienced catastrophic costs, in comparison with 7% of Xpert-negative participants (Table 2). Of the total 327 participants with catastrophic cost data, 30 (9%) were missing data for at least one risk factor; these individuals were excluded from regression analyses. Risk factors associated with catastrophic costs using a threshold of P < 0.1 were clinic travel times of 60–90 min (aPR 1.7, 95%CI 0.9–3.1) and unemployment (aPR 2.0, 95%CI 1.0–4.0) (Table 3). We observed a greater prevalence of catastrophic costs among those aged ≥60 years than the 25–34-year age group (aPR 2.6, 95%CI 1.0–6.8). For those with 5–6 household members, we observed a lower prevalence of catastrophic costs (aPR 0.6, 95%CI 0.3–1.0). In univariable regression, catastrophic costs were associated with smoking >15 pack-years, compared with those who never smoked (unadjusted prevalence ratio, PR1.7, 95%CI 1.0–2.9), although this association did not retain statistical significance in the multivariable model.
Table 3.
Associations between potential risk factors and catastrophic costs among patients with tuberculosis in Limpopo Province, South Africa* (n = 297)†
| Potential risk factors | Prevalence of catastrophic costs n (%) | Univariable PR (95%CI) | aPR (95%CI) |
|---|---|---|---|
| Sex | |||
| Male | 47 (27) | Reference | Reference |
| Female | 39 (32) | 1.2 (0.8–1.7) | 1.2 (0.7–2.0) |
| Age, years‡ | |||
| 18–24 | 8 (25) | Reference | Reference |
| 25–34 | 13(18) | 0.7 (0.3–1.6) | 0.6 (0.2–1.6) |
| 35–44 | 31 (34) | 1.3 (0.7–2.6) | 1.1 (0.4–2.8) |
| 45–59 | 26 (35) | 1.4 (0.7–2.7) | 1.1 (0.4–2.9) |
| ≥60 | 8 (31) | 1.2 (0.5–2.8) | 1.6 (0.5–5.2) |
| Number of household members | |||
| 1–2 | 23 (38) | Reference | Reference |
| 3–4 | 27 (29) | 0.8 (0.5–1.2) | 0.8 (0.5–1.3) |
| 5–6 | 17(22) | 0.6§ (0.3–1.0) | 0.6§ (0.3–1.0) |
| ≥7 | 19 (28) | 0.8 (0.5–1.2) | 0.8 (0.5–1.3) |
| Duration of primary symptom, months | |||
| <2 | 51 (28) | Reference | Reference |
| 2–4 | 20 (27) | 1.0 (0.6–1.5) | 1.0 (0.6–1.5) |
| >4 | 15 (38) | 1.4 (0.9–2.2) | 1.1(0.7–1.9) |
| Tobacco smoking, pack-years | |||
| Never smoked | 49 (29) | Reference | Reference |
| <5 | 19 (27) | 0.9 (0.6–1.5) | 0.9 (0.5–1.7) |
| 5–< 15 | 9 (24) | 0.8 (0.5–1.6) | 0.8 (0.4–1.6) |
| ≥15 | 9 (50) | 1.7¶(1.0–2.9) | 1.6 (0.8–3.4) |
| HIV status | |||
| Not infected | 34 (26) | Reference | Reference |
| Living with HIV | 52 (32) | 1.2 (0.9–1.8) | 1.0 (0.7–1.6) |
| Distance to clinic, min | |||
| <15 | 14 (25) | Reference | Reference |
| 15–59 | 45 (25) | 1.0 (0.6–1.7) | 1.0 (0.6–1.6) |
| 60–89 | 17 (45) | 1.8§ (1.0–3.1) | 1.7§ (0.9–3.1) |
| ≥90 | 10 (38) | 1.5 (0.8–2.9) | 1.6 (0.8–3.2) |
| Employment category | |||
| Temporary/informal | 9 (22) | Reference | Reference |
| Unemployed, cannot work | 33 (38) | 1.7§ (0.9–3.2) | 2.0§ (1.0–4.0) |
| Unemployed, could work | 20 (28) | 1.3 (0.6–2.5) | 1.8 (0.8–4.1) |
| Retired | 6 (24) | 1.1 (0.4–2.7) | 1.1 (0.3–3.7) |
| Student | 4 (24) | 1.1 (0.4–3.0) | 1.4 (0.4–5.3) |
| Self-employed | 4 (31) | 1.4 (0.5–3.8) | 1.7 (0.6–5.2) |
| Salaried | 10 (24) | 1.1 (0.5–2.4) | 1.4 (0.6–3.3) |
| Highest grade attended by any household member | |||
| ≤4th | 13 (30) | Reference | Reference |
| 5–9th | 25 (29) | 1.3 (0.8–2.2) | 1.1 (0.6–1.9) |
| 10–12th | 42 (28) | 1.1 (0.7–1.8) | 1.2 (0.7–2.0) |
| Any postgraduate | 6 (32) | 1.1 (0.5–2.6) | 1.4 (0.6–3.4) |
| SAMPI,# SEP | |||
| Lowest SEP | 31 (31) | Reference | Reference |
| Middle SEP | 40 (32) | 1 (0.7–1.5) | 1.1 (0.7–1.7) |
| Highest SEP | 15 (21) | 0.7 (0.4–1.2) | 0.7 (0.4–1.4) |
Catastrophic costs assessed using the ‘minimum cost threshold’ method as described in the text.
Thirty observations with missing values of risk factor covariates were excluded from all univariable analyses to obtain the sample size of 297. The missing values are included in separate missing categories in Table 1 under four risk factors: symptom duration, tobacco smoking, employment, and clinic travel time.
Using the 25–34 years age group as reference, we observed a greater prevalence of catastrophic costs among those aged ≥60 years (aPR 2.6, 95%CI 1.0–6.8).
0.10 < P < 0.05.
0.05 ≤ P < 0.01.
For 26 (8%) participants, the indicator for years of school attended by adults was assumed to be <5 years if the index case and head of household had attended <5 years, regardless of the number of household members.
PR = prevalence ratio; aPR = adjusted PR; HIV = human immunodeficiency virus; SAMPI = South Africa Multidimensional Poverty Index; SEP = socioeconomic placement.
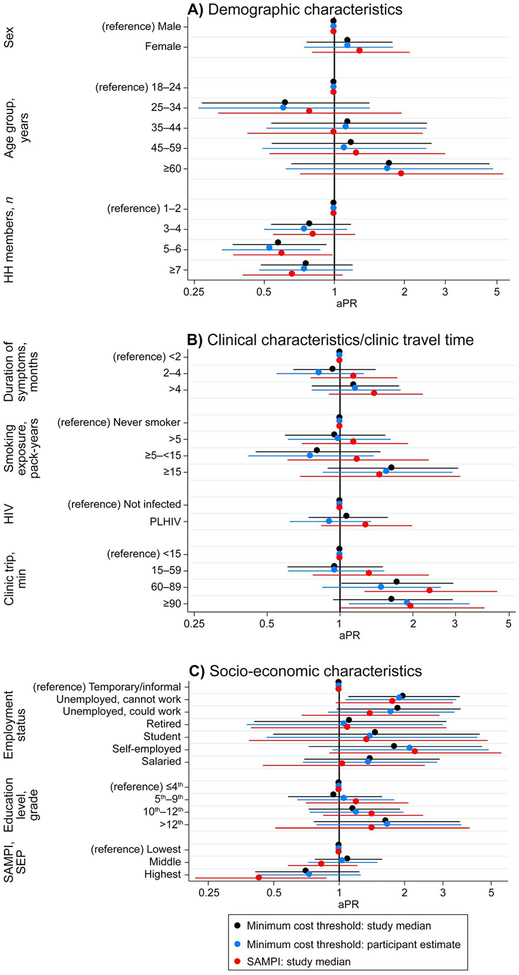
Prevalence estimates of catastrophic costs ranged from 22% to 31%, and varied according to the definition of catastrophic cost and the method for estimating the time lost in seeking health care (Table 4). In a sensitivity analysis using mean instead of median visit numbers, prevalence increased by 6%, to 34% overall. Most associations between risk factors and catastrophic costs were robust to the method for valuing the time lost, but occasionally varied according to the definition of catastrophic cost used (Figure 1). Applying the standard catastrophic threshold definition of ≥20% annual household income, we found that 32 of the 99 (32%) participants who experienced catastrophic costs reported no household income. Of these 32 participants, nine (28%) reported TB episode out-of-pocket costs under ZAR100 (US$7.70) and <20 h spent in health care-seeking. Similarly, 19 (59%) of these 32 participants were grouped in the middle or highest SAMPI socioeconomic categories, whereas only 10 (31%) were both in the lowest socio-economic category and experienced more than ZAR100 in total out-of-pocket costs.
Table 4.
Prevalence of catastrophic costs among patients with tuberculosis in Limpopo Province, South Africa, by catastrophic cost definition and lost time estimation (n = 327)
| Method for estimating lost health care-seeking time* | |||||
|---|---|---|---|---|---|
| Primary analysis | Sensitivity analyses | ||||
| Catastrophic cost definition | Study population, median wage n (%) | Limpopo Province, median wage n (%) | Income quintile, mean wage n (%) | Participant Estimated n (%) | Estimate as zero n (%) |
| 20% of annual income† | 99 (30) | 101 (31) | 101 (31) | 90 (28) | 87 (27) |
| Minimum cost threshold‡ | 90 (28) | 92 (28) | 92 (28) | 88 (27) | 84 (26) |
| SAMPI threshold§ | 80 (24) | 82 (25) | 82 (25) | 76 (23) | 73 (22) |
Methods for estimating lost health care-seeking time were as follows (left to right): 1) median wage of the analytic population (self-reported, excluding income from social grants), 2) median wage in Limpopo Province, 3) mean wage within quintiles of the analytic population, based on self-reported, pre-symptom household income, 4) participants’ own estimate of lost wages or lost income-generating opportunities during health care-seeking based on a summary interview question, and 5) estimation of care-seeking time at zero value (i.e., time spent care-seeking contributing nothing to episode costs).
20% of annual income with no threshold applied to the 10% of participants with no reported income.
20% of annual income with a threshold to reclassify costs as non-catastrophic if participants who reported no household income experienced out-of-pocket costs <ZAR100 (US$7.70), or if total episode health care-seeking time was less than the study mean of 20 h.
20% of annual income with a threshold to reclassify costs as non-catastrophic if participants who reported no household income were not in the lowest socioeconomic placement category of the SAMPI.
SAMPI = South Africa Multidimensional Poverty Index; ZAR = South African rand; US$ = US dollar.
DISCUSSION
In this cross-sectional survey of 327 adults diagnosed with pulmonary TB in rural Limpopo Province, South Africa, we found that one quarter to one third of patients being treated for TB experienced catastrophic costs. Risk factors for experiencing catastrophic costs were longer travel times to clinics, unemployment, fewer household members, and age >60 years. The estimated prevalence of catastrophic costs varied substantially with the definition used and the method for estimating lost health care-seeking time; however, most associations between risk factors and catastrophic costs remained consistent.
Associations of catastrophic TB costs with unemployment and increasing age have been observed in Peru and South Africa,2,3 whereas associations with longer trips to clinics have not been observed previously and may reflect better assessment of non-out-of-pocket costs throughout the TB episode. The 28% prevalence of catastrophic costs was lower than that obtained from recent WHO prevalence surveys in Myanmar (65%) and Viet Nam (63%).19 This difference likely stems from an overall lower burden of out-of-pocket payments under the South African health financing system. However, the high prevalence of catastrophic costs despite publicly available health care in an upper-middle-income country is worrying, and suggests great inequities in wealth and health care access.20 Foster et al., who conducted a patient cost study in urban and rural areas of seven South African provinces, reported a 2.7-fold greater odds of catastrophic costs in rural than in urban communities.3 Those findings suggest that social protection and enhanced service delivery and financing interventions tailored to rural settings will be important considerations in countries such as South Africa if we are to reach the target of zero TB-associated catastrophic costs.
Our data suggest that many of those with no reported income may not experience devastating costs from TB illness. Although consensus has been reached by the WHO on the definition and measurement of catastrophic costs, future studies investigating non-income-based metrics to define catastrophic costs21 and exploring prevalence and risk factors can inform revisions to this guidance, including measurement of catastrophic costs for patients with zero reported income and the challenge of separating lost income due to illness vs. lost wages while seeking care. Such revisions can help generate more accurate and informative prevalence estimates and benchmarks.
While methodology is important, research suggests several potential solutions to reduce catastrophic costs. Interventions which combine poverty reduction and financial assistance may prevent or reverse TB-related costs.22,23 Removing barriers to employment and educational opportunities may also address characteristics that complicate identification of TB cases, heighten transmission, and perpetuate adverse treatment outcomes.2,24
In the CRESIPT (Community Randomized Evaluation of a Socioeconomic Intervention to Prevent TB) study in Lima, Peru,23 a combination intervention utilising conditional cash transfers and psychosocial and educational programs was designed to offset catastrophic costs and demonstrated multiple benefits. Individuals participating in these interventions experienced an 18% increase in isoniazid preventive therapy initiation, an 11% increase in treatment completion and a 14% increase in cure.23 In South Africa, only 5% of people being treated for TB were reported to have accessed short-term cash transfers (‘disability grants’), for which many were presumably eligible.3 Improved TB service delivery and financing, increased access to social protection programs, and mitigation of poverty-related stigma will advance global alleviation of catastrophic costs.25
The limitations of our study included its cross-sectional and exploratory nature. Although we based estimations of TB episode costs on national guidelines and empirical data,3 recall bias of self-reported income and costs may have affected our findings; future work could employ expenditure surveys or other approaches.21 However, our estimated measures of association would only be affected if bias differed across levels of any given risk factor. We may have underestimated patient costs due to the exclusion of patients with multidrug-resistant TB and those who died, although rifampin resistance was uncommon. The cost of HIV/AIDS (human immunodeficiency virus/acquired immune-deficiency syndrome) treatment and illness (outside of TB) was not ascertained here and is an important topic of future research. We did not investigate causality in this cross-sectional study of risk factors, although some of our observed associations have been documented in other settings. The associations reported here should be understood as hypothesis-generating and informative of future research.
CONCLUSION
Over one quarter of all people being treated for TB in this rural South African setting experienced catastrophic costs related to their illness and treatment. Risk factors for catastrophic costs include barriers to accessing the health system, such as long clinic travel times, and sociodemographic factors, such as unemployment, older age and fewer household members. In estimating the prevalence of, and risk factors for, TB-associated catastrophic costs, our results demonstrate the importance of developing and validating definitions of catastrophic costs for people reporting little or no household income. Data from this study and others suggest that increasing access to existing short-term disability grants and other social protection interventions, particularly those tailored to patients with key risk factors in rural settings, would mitigate the economic impact of TB illness on individuals and their households.
Supplementary Material
Figure 1.
Sensitivity of associations with catastrophic costs to thresholds and care-seeking time associations. aPR = adjusted prevalence ratio; HH = household members; HIV = human immunodeficiency virus; PLHIV = people living with HIV; SAMPI = South Africa Multidimensional Poverty Index; SEP = socio-economic placement
Acknowledgements
The authors thank the National Institutes of Health (Bethesda, MD, USA) and US taxpayers for, directly and indirectly, financially contributing to this research; our partner organization Perinatal HIV Research Unit (Soweto, South Africa) and our amazing Kharitode study staff, who struggled daily against many challenges—logistical, communicative and conceptual—yet succeeded in obtaining these data; the Johns Hopkins University (JHU; Baltimore, MD, USA) Center for Global Health for funding through the Global Health Established Field Placement grant; and our colleagues, mentors and administrators in the Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, in addition to our many colleagues at the JHU Center for TB Research for the many helpful suggestions, outstanding instruction, and expert logistical support.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2016. WHO/HTM/TB/2016.13. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2.Wingfield T, Boccia D, Tovar M, et al. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLOS Med 2014; 11: e1001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster N, Vassall A, Cleary S, Cunnala L, Churchyard G. The economic burden of TB diagnosis and treatment in South Africa. Soc Sci Med 2015; 130: 42–50. [DOI] [PubMed] [Google Scholar]
- 4.Laokri S, Dramaix-Wilmet M, Kassa F, Anagonou S, Dujardin B. Assessing the economic burden of illness for tuberculosis patients in Benin: determinants and consequences of catastrophic health expenditures and inequities. Trop Med Int Health 2014; 19: 1249–1258. [DOI] [PubMed] [Google Scholar]
- 5.Mauch V, Woods N, Kirubi B, Kipruto H, Sitiene J, Klinkenberg E. Assessing access barriers to tuberculosis care with the tool to estimate patients’ costs: pilot results from two districts in Kenya. BMC Public Health 2011; 11: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayé R, Wyss K, Abdualimova H, Saidaliev S. Household costs of illness during different phases of tuberculosis treatment in Central Asia: a patient survey in Tajikistan. BMC Public Health 2010; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ukwaja KN, Alobu I, Igwenyi C, Hopewell PC. The high cost of free tuberculosis services: patient and household costs associated with tuberculosis care in Ebonyi State, Nigeria. PLOS ONE 2013; 8: e73134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedrazzoli D, Siroka A, Boccia D, et al. How affordable is TB care? Findings from a nationwide TB patient cost survey in Ghana. Trop Med Int Health 2018; 23: 870–878. [DOI] [PubMed] [Google Scholar]
- 9.Nhung NV, Hoa NB, Anh NT, et al. Measuring catastrophic costs due to tuberculosis in Viet Nam. Int J Tuberc Lung Dis 2018; 22: 983–990. [DOI] [PubMed] [Google Scholar]
- 10.Fuady A, Houweling TA, Mansyur M, Richardus JH. Catastrophic total costs in tuberculosis-affected households and their determinants since Indonesia’s implementation of universal health coverage. Infect Dis Poverty 2018; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Statistics South Africa. South Africa General Household Survey, 2016. Pretoria, South Africa: STATSA, 2016. [Google Scholar]
- 12.Limpopo Provincial AIDS Council. Annual Progress Report 2015/16: Provincial Strategic Plan 2012–2016. Pietersburg, South Africa: Office of the Premier, 2016. [Google Scholar]
- 13.Kerrigan D, West N, Tudor C, et al. Improving active case finding for tuberculosis in South Africa: Informing innovative implementation approaches in the context of the Kharitode trial through formative research. Health Res Policy Syst 2017; 15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Protocol for survey to determine direct and indirect costs due to TB and to estimate proportion of TB-affected households experiencing catastrophic costs: field testing version. Geneva, Switzerland: WHO, Global TB Programme, 2015. [Google Scholar]
- 15.Statistics South Africa. The South African MPI: creating a multidimensional poverty index using census data. Pretoria, South Africa: STATSA, 2014. [Google Scholar]
- 16.Department of Health, Republic of South Africa. National Tuberculosis Management Guidelines. Pretoria, South Africa: National Department of Health, 2014. [Google Scholar]
- 17.Oanda Corporation. Historical exchange rates. London, UK: OANDA Corporation, 1996–2019; https://www.oanda.com. Accessed March 2019. [Google Scholar]
- 18.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Global tuberculosis report, 2017. WHO/HTM/TB/2017.23. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 20.Ataguba JE, Akazili J, McIntyre D. Socio-economic-related health inequality in South Africa: evidence from General Household Surveys. Int J Equity Health 2011; 10: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney S, Mukora R, Candfield S, Guinness L, Grant AD, Vassall A. Measuring income for catastrophic cost estimates: limitations and policy implications of current approaches. Soc Sci Med 2018; 215: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J 2014; 43: 1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingfield T, Tovar MA, Huff D, et al. Beyond pills and tests: addressing the social determinants of tuberculosis. Clin Med 2016; 16 (Suppl 6): S79–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mesfin MM, Newell JN, Madeley RJ, et al. Cost implications of delays to tuberculosis diagnosis among pulmonary tuberculosis patients in Ethiopia. BMC Public Health 2010; 10: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudgard WE, Evans CA, Sweeney S, et al. Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: an economic modelling study. PLoS Med 2017; 14: e1002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.