Abstract
Pheochromocytoma is a rare, catecholamine secreting tumor arising from chromaffin cells. Presentation of this tumor is highly variable, the most common being hypertension, tachycardia, sweating, and headache. Lactic acidosis and back pain are rare complications of this tumor. We report a 51-year-old gentleman with composite pheochromocytoma, which is rarer than pheochromocytoma, presenting as severe back pain and lactic acidosis.
Keywords: Back pain, composite pheochromocytoma, lactic acidosis
Introduction
Pheochromocytoma is a rare, catecholamine secreting tumor arising from chromaffin cells. The presentation of this tumor is highly variable, the most common being hypertension, tachycardia, sweating, and headache.[1] Lactic acidosis and back pain are rare complications of this tumor.[2] The mechanism of lactic acidosis is due to the effect of catecholamines, epinephrine, and nor-epinephrine on glucose metabolism and the peripheral circulatory changes. We report a 51-year-old gentleman with composite pheochromocytoma (CP) presenting as severe back pain and lactic acidosis.
Case Report
A 51-year-old gentleman, recently diagnosed to have type 2 diabetes mellitus and systemic hypertension, presented to the cardiology outpatient clinic on 28th July 2016 with history of pricking chest pain and shortness of breath for the past 7 years and recurrent attacks of syncope since the previous year. He weighed 49 kg and with a height of 157 cm; body mass index was 19.88 kg/m2. His blood pressure (BP) was 120/80 mmHg and pulse rate was 78/min and regular in rhythm. He was afebrile. Systemic examination was unremarkable.
Laboratory findings revealed hemoglobin of 15.1 g/dL, hematocrit of 47%, white blood cell count of 11,300/mm3 (differential count of neutrophils of 56%, lymphocytes 26%, eosinophils 11%, and monocytes of 7%), and platelet count of 293,000/mm3. Urine on routine examination showed a pH of 6.0, specific gravity of 1015, and was negative for albumin, glucose, red blood cells, and pus cells. Blood glucose fasting was 115 mg/dL and post-prandial was 219 mg/dL with an HbA1C of 7.1%. Blood urea was 27 mg/dL and creatinine was 1.0 mg/dL. Serum electrolytes were as follows: sodium 141 mEq/L, potassium 4.3 mEq/L, chloride 103 mEq/L, and bicarbonate 28 mEq/L. Serum cholesterol was 183 mg/dL, serum triglyceride 121 mg/dL, and serum uric acid 3.9 mg/dl. Two-dimensional (2D) echocardiogram showed normal left ventricular dimensions, no regional wall motion abnormality, and a left ventricular ejection fraction of 69%. Holter monitoring showed occasional supraventricular and ventricular premature complexes. On 2nd August, coronary angiography showed insignificant coronary artery disease with dominant right coronary system and he was discharged with medical advice.
On 3rd August, he attended emergency department with complaints of severe back pain, palpitation, and sweating. His back pain was sudden in onset, gradually progressive, throbbing type, rating 8/10 in pain scale, radiating to the left flank, aggravated by exertion, and partially relieved by rest. He had no other complaints. He was on metformin and calcium channel blocker. He looked anxious, agitated, and restless but was oriented and afebrile. His pulse rate was 112/min, respiratory rate was 28/min, and BP was 210/120 mmHg in the right upper limb and 210/100 mmHg over the left upper limb in supine position. There was no radio-femoral delay. Systemic examination was unremarkable except for a mild tenderness in the lumbar region.
On investigation, electrocardiography, chest X-ray, complete blood counts, liver function tests, 2D echocardiography, urine analysis, and toxicology screen were all within normal limits. Serum amylase and lipase were normal. Blood urea was 58 mg/dL and serum creatinine was 2 mg/dL. Venous blood gas (VBG) analysis showed a pH of 7.21, bicarbonate of 18 mmol/L, base excess of −9.4 mmol/L, and a lactate of 10.81 mmol/L. Serum electrolytes were sodium 138 mmol/L, potassium 3.8 mmol/L, and chloride 108 mmol/L, and anion gap was 12 mmol/L.
The following were considered in the differential diagnosis of this situation: (a) acute mesentric ischemia, (b) aortic dissection (c) hypertensive emergency, and (d) pancreatitis. Contrast-enhanced computed tomography abdomen showed a lesion in the left adrenal gland measuring about 3.5 × 3.2 cm with mild enhancement in the arterial phase, features consistent with pheochromocytoma [Figure 1: Contrast enhanced CT scan of abdomen showing the mildly enhancing left adrenal mass]. No additional extra-adrenal tumors were seen. There was no evidence of mesenteric ischemia, aortic dissection, or pancreatitis. Upon further questioning his family members, it was known that he was unwell in the previous 6 months with on and off headache, palpitation, and anxiousness, which they attributed to the recently diagnosed diabetes mellitus.
Figure 1.
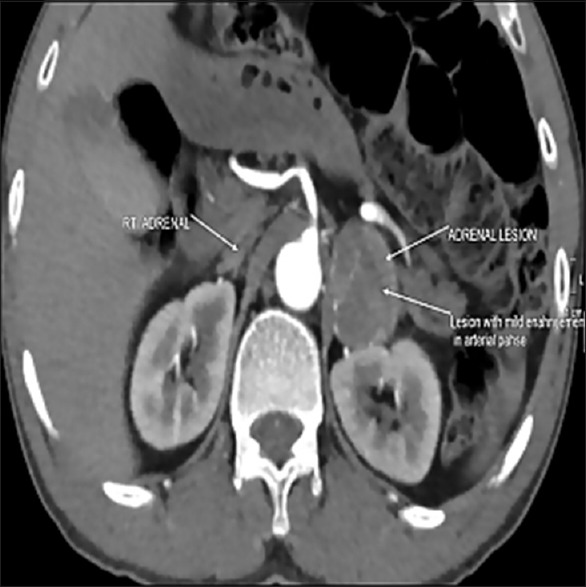
Contrast enhanced CT scan of abdomen showing the mildly enhancing left adrenal mass
His BP was managed with alpha adreno-receptor blocker and calcium channel blocker. Phenoxybenzamine was added at a dose of 10 mg twice a day and later increased to 20 mg three times daily. Persistent tachycardia and BP were stabilized with addition of beta blocker.
On 5th August 2016, the level of 24-h urine metanephrines was 14,536 mcg (reference range: less than 350 mcg/24 h) and nor-metanephrines was 7,607 mcg (reference range: less than 600 mcg/24 h).
Laparoscopic removal of pheochromocytoma was done under general anesthesia on 12th August 2016. After the surgery, there was a brief episode of hypoglycemia and hypotension, which was managed with 25% dextrose and normal saline, respectively. Azotemia resolved and blood gases were normalized. Pathological examination of the resected mass revealed a well-encapsulated tumor of size 5 cm × 4 cm × 3 cm, without hemorrhage. It showed polygonal and round cells with abundant cytoplasm and increased vasculature. There was no capsular or vascular invasion. Tumor cells were seen in the background of spindle cells with ganglion cells and Schwanian stroma. These features were reported as consistent with a CP–ganglioneuroma of the left adrenal gland [Figure 2]. Immunohistochemistry examination showed positivity for chromogranin, synaptophysin, and S-100 stains and negativity for calretinin in pheochromocytoma cells. Calretinin, chromogranin, synaptophysin, and S-100 stains were positive in the ganglioneuromatous component. Vimentin was positive and cytokeratin was negative. These staining characteristics confirmed the composite nature of the tumor.
Figure 2.
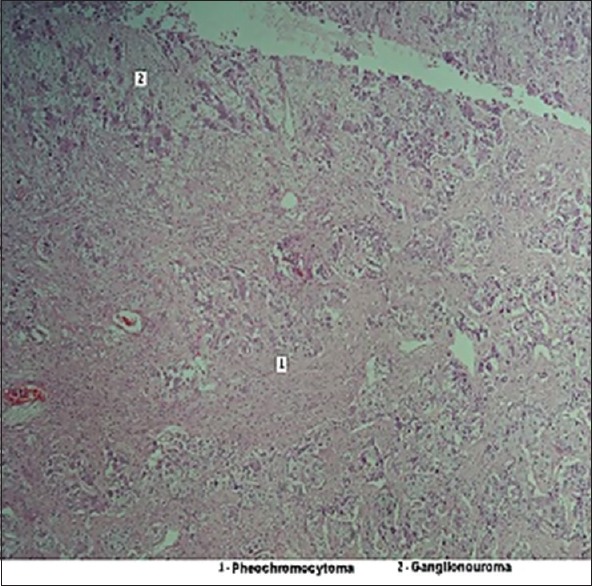
Photomicrograph of the histopathology of tumour
At the time of discharge, his blood pressure and blood glucose were normal. Upon review on 28th February 2017, his BP was 130/80 mmHg without antihypertensive medicine. Blood glucose fasting was 90 mg/dL and post-prandial 66 mg/dL, with an HbA1C of 6.1% without any antidiabetic medicine. The levels of 24-h urinary metanephrines and normetanephrines were 62 and 604, mcg, respectively.
Discussion
Pheochromocytoma is a potentially life-threatening condition occurring in less than 0.2% of patients with hypertension.[3] Although pheochromocytoma may occur at any age, it is most common in the fourth and fifth decades and is equally common in men and women.[4] The classic triad of symptoms include tachycardia, headache, and sweating, and clinical features can be highly variable.[1] Back pain as a presentation in this disease is unusual.[5] Back pain could be due to a large size of the tumor or its rupture or bleeding in to the tumor or due to an extra-adrenal location. None of these findings was seen in this patient. Secretion of both epinephrine and norepinephrine by the tumor and the variable clinical presentations are due to the varying potencies of the catecholamines on alpha (α) and beta (β) adrenergic receptors.[6] Rarely, it does present with lactic acidosis and it has been noted only in a small number of case reports in the past three decades.[2]
Lactate is the final product in the anaerobic pathway of glucose metabolism (pyruvate + NADH ↔ lactate + H+ + NAD+) that develops secondary to tissue hypoxia (classified as type A lactic acidosis as per Cohen and Wood's classification).[7] This typically occurs during shock states, which increase anaerobic glycolysis and the rate of conversion of pyruvate to lactate.
Type B lactic acidosis occurs without tissue hypoxia in a heterogeneous group of disorders (e.g., liver disease, malignancy, thiamine deficiency, mitochondrial enzyme deficiencies) and secondary to alcohol and drug toxicity (e.g., salicylates and nucleoside reverse transcriptase inhibitors).
Pheochromocytomas can cause lactic acidosis by the overproduction of catecholamines, which stimulate glucose production and decrease glucose utilization. Of them, epinephrine stimulates glycogenolysis and lipolysis and increases the concentrations of fuel substrates used for gluconeogenesis. In the Cori cycle [Figure 3], lactate is formed by glycolysis in the muscle and then transported to liver for conversion to glucose by gluconeogenesis and released into circulation. Glycogenolysis and glycolysis are stimulated about a thousand-fold times more than gluconeogenesis by catecholamines resulting in excessive production of lactate.
Figure 3.
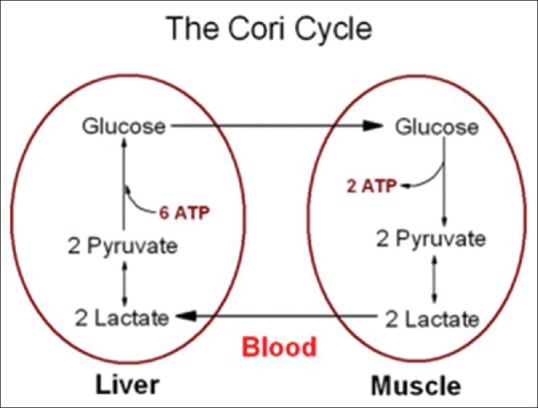
Schematic diagram of Cori Cycle
The other factor resulting in increased lactate levels is the high-energy expenditure of the gluconeogenic limb of Cori cycle, which results in a net loss of four ATP molecules. Clutter et al. have demonstrated that epinephrine increases heart rate, BP, blood glucose, lactate, and glycerol in normal subjects in a dose-dependent manner.[8] This patient's lactic acidosis can be classified as type B lactic acidosis since he had no signs and symptoms of hypoperfusion. Furthermore, catecholamine-induced vasoconstriction results in peripheral tissue ischemia, which causes anaerobic metabolism and lactate production in peripheral tissues. This patient's acute kidney injury is probably related to severe vasoconstriction and a likely left ventricular dysfunction due to catecholamines from the tumor. After the surgical removal of the tumor, renal functions normalized.
Although he presented with acute severe back pain, tachycardia, tachypnoea, anxiousness, hypertension (but no unequal BP on both arms), and VBG showing lactic acidosis, a clinical picture reminiscent of acute mesenteric ischemia or aortic dissection, he did not have nausea, vomiting, diarrhea, passage of bloody stools, constipation, abdominal distention, or obstipation. In addition, an angiogram showed normal coronaries, aorta, and mesenteric vasculature, thus ruling out a mesenteric ischemia or aortic dissection.
Occurrence of lactic acidosis due to metformin is not common, with an incidence of four to nine cases per 100,000 patients a year. According to a recent Cochrane review, there is less evidence to support the association of metformin with lactic acidosis.[9] Our patient had no risk factors such as congestive cardiac failure (CCF) or hypoxia. He was recently (6 months) diagnosed to have diabetes mellitus and he was started only on a low dose of metformin of 250 mg twice a day and also he had no signs of intestinal obstruction. Hence, the cause of lactic acidosis in our patient is less likely due to metformin.
The derangements in carbohydrate metabolism (insulin resistance, impaired fasting glucose, and apparent type 2 diabetes mellitus) are directly related to the increase in catecholamine production from the tumor.[10] These changes normalize after removal of the catecholamine-secreting tumor. Here, we have considered the venous blood lactate as nearly equivalent to arterial blood lactate, which has been accepted generally.[11,12]
CP is a tumor wherein nonpheochromocytoma component is present along with pheochromocytoma, theoretically arising from a common embryonic progenitor, that is, neural crest. Nonpheochromocytoma elements can be ganglioneuroma, ganglioneuroblastoma, neuroblastomatosis-1, and more rarely schwannoma. In our case, like that of Ram et al., the nonpheochromocytoma component was ganglioneuroma.[13] CPs are rare tumors of adrenal gland constituting up to 3% of pheochromocytoma. Kragel and Johnston reported that only 4 of their 13 patients had associated hypertension in composite adrenal medullary tumors.[14]
Some composite adrenal tumors lack endocrine abnormalities and symptoms of pheochromocytoma component. The cause of this phenomenon has not been worked up conclusively. However, our patient presented with labile hypertension and catecholamine excess state similar to that reported by Menon et al.[15]
Conclusion
This case report highlights one of the uncommon presentations of pheochromocytoma, namely, lactic acidosis. It also reiterates the importance of recognizing lactic acidosis in a patient with normal or high BP to suspect pheochromocytoma. In addition, the presentation of this tumor with back pain is itself unusual. Finally, composite tumor of adrenal medulla is a rare occurrence.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors wish to express their thanks to Dr. Ravikrishna of the Delphi anaesthetists' team for excellent patient support during surgery and to pathologists involved in histopathological and immunohistochemical diagnosis and lending photomicrograph of the tumor.
References
- 1.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 2.Madias NE, Goorno WE, Herson S. Severe lactic acidosis as a presenting feature of pheochromocytoma. Am J Kidney Dis. 1987;10:250–3. doi: 10.1016/s0272-6386(87)80182-8. [DOI] [PubMed] [Google Scholar]
- 3.Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med. 2001;134:315. doi: 10.7326/0003-4819-134-4-200102200-00016. [DOI] [PubMed] [Google Scholar]
- 4.Guerrero MA, Schreinemakers JM, Vriens MR, Suh I, Hwang J, Shen WT, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009;209:727. doi: 10.1016/j.jamcollsurg.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Karumanchery R, Nair JR, Hakeem A, Hardy R. An unusual case of back pain: A large pheochromocytoma in an 85 year old woman. Int J Surg. 2012;3:16–8. doi: 10.1016/j.ijscr.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K. Adverse drug reactions in patients with phaeochromocytoma: Incidence, prevention and management. Drug Saf. 2007;30:1031–62. doi: 10.2165/00002018-200730110-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RD, Woods HF. The clinical presentation and classification of lactic acidosis. In: Cohen RD, Woods HF, editors. Clinical and Biochemical Aspects of Lactic Acidosis. Oxford: Blackwell Scientific; 1976. pp. 1–200. [Google Scholar]
- 8.Clutter WE, Bier DM, Shah SD, Cryer PE. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980;66:94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010:CD002967. doi: 10.1002/14651858.CD002967.pub3. [DOI] [PubMed] [Google Scholar]
- 10.La Batide-Alanore A, Chatellier G, Plouin PF. Diabetes as a marker of pheochromocytoma in hypertensive patients. J Hypertens. 2003;21:1703–7. doi: 10.1097/00004872-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 11.TaTheerawit P, Na Petvicharn Cngsujaritvijit V, Sutherasan Y. The correlation between arterial lactate and venous lactate in patients with sepsis and septic shock. J Intensive Care Med. 2016 doi: 10.1177/0885066616663169. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Datta D, Grahamslaw J, Gray AJ, Graham C, Walker CA. Lactate-arterial and venous agreement in sepsis: A prospective observational study. Eur J Emerg Med. 2018;25:85–91. doi: 10.1097/MEJ.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 13.Ram Nawal Rao, Nidhi Singla, and Kamlesh Yadav. Composite pheochromocytoma-ganglioneuroma of the adrenal gland: A case report with immunohistochemical study. Urol Ann. 2013;5(2):115–8. doi: 10.4103/0974-7796.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kragel PJ, Johnston CA. Pheochromocytoma-ganglioneuroma of the adrenal. Arch Pathol Lab Med. 1985;109:470–472. [PubMed] [Google Scholar]
- 15.Menon S, Mahajan P, Desai SB. Composite adrenal medullary tumor: A rare cause of hypertension in a young male. Urol Ann. 2011;3:36–38. doi: 10.4103/0974-7796.75860. [DOI] [PMC free article] [PubMed] [Google Scholar]


