Abstract
Purpose
Hyaluronic acid-poly(ethylene glycol)-distearoyl phosphoethanolamine (HA-PEG-DSPE) modified and tocopheryl polyethylene glycol 1000 succinate (TPGS) contained nanostructured lipid carriers (NLCs) were prepared loading ropivacaine and dexmedetomidine to improve the topical anesthetic analgesic anesthesia efficiency.
Methods
NLCs were prepared by the solvent diffusion method. The average particle size, zeta potential, release behavior, and cytotoxicity of the NLCs were tested. Ex vivo skin permeation was studied using a Franz diffusion cell mounted with depilated rat skin. Local anesthesia antinociceptive efficiency was evaluated by rat tail flick latency study in vivo.
Results
NLCs have sizes of about 100 nm, with negative zeta potentials. All the NLCs formulations were found to be significantly less cytotoxic than free drugs at equivalent concentrations. The cumulative amount of drugs penetrated through rat skin from NLCs was 2.0–4.7 folds higher than that of the drugs solution. The in vivo anesthesia antinociception study displayed that NLCs showed stronger and longer anesthesia antinociceptive effect when compared with single drugs loaded NLCs and drugs solution even at a lower dosage of drugs.
Conclusion
The results demonstrated that the HA modified, TPGS contained, dual drugs loaded NLCs could perform a synergistic effect and may reduce the amount of drugs, which can lower the toxicity of the system and at the meanwhile, increase the anesthesia antinociceptive efficiency.
Keywords: topical anesthetic analgesic, skin penetration, hyaluronic acid, long-acting nanostructured lipid carriers, tocopheryl polyethylene glycol 1000 succinate
Introduction
Efficient pain management, especially postoperative pain, is difficult to achieve and remains one of the most common challenges.1 In this field, local anesthesia is one of the most important methods for analgesia either following surgery or for control of other acute and chronic pain through peripheral nerve blocks.2,3 Local anesthetics (LA), such as lidocaine, bupivacaine, ropivacaine (RVC), etc., have small molecule properties like limited acting duration (1–2 hrs for lidocaine; 2–4 hrs for bupivacaine and RVC) and rapid redistribution.4–6 Therefore, there is a compelling need for novel drug delivery formulations of LA to prolong the anesthetic effect and reduce systemic toxicity.
RVC is a local anesthetic frequently indicated for the production of local or regional anesthesia for surgery and for acute pain management.7 Compared with bupivacaine, RVC shows longer duration, and less cardiovascular as well as central nervous system toxicities.8,9 Thus, many anesthesiologists now prefer the use of RVC (Naropin®) in lieu of bupivacaine for peripheral nerve blocks.10 Moreover, to prolong analgesia and reduce local inflammation, research have combined LA with adjuvants, such as dexmedetomidine (DMDT) and dexamethasone recently.11
DMDT, a selective α2-adrenergic agonist, has been approved by the FDA for continuous intravenous sedation in the intensive care setting and procedural sedation in non-intubated patients.12 It acts on both pre- and post-synaptic sympathetic nerve terminal and central nervous system, thereby showing sedative, analgesic, and hemodynamic effects.13,14 Recently, more and more clinical trials have indicated that DMDT has the ability to hasten the onset and prolong the duration blockade when combined with RVC (75% increase in the duration of analgesia).15–18
The drug delivery methods used of penetration enhancement contained chemical penetration enhancers, physical methods, and iontophoresis for various membranes like skin and nail.19 Nanoparticulate drug delivery systems have been applied in pharmaceutical arena like vaccines, etc.20,21 Nanoparticle systems provide promising platforms for combination therapy, sustained release and less systemic side effects in the field of anesthesia and analgesia.2,22 Lipid-based delivery systems, with particular emphasis on nanostructured lipid carriers (NLCs), are among the most attractive strategies for improving penetration of drugs through the skin, especially for encapsulation of the hydrophobic or lipophilic drug.23 Poly(ethylene glycol)-distearoyl phosphoethanolamine (PEG-DSPE) lipids have been widely used in the preparation of lipid carriers and could prolong the circulation time and release drugs at a sustained rate.24 Hyaluronic acid (HA), a linear polysaccharide composed of repeating units of D-glucuronic acid and N-acetyl-D-glucosamine, is proved to be efficient in skin penetration due to the hydrophilic patch domain of HA enables HA to hydrate and diffuse through the skin.25 In this study, HA was conjugated to PEG-DSPE (HA-PEG-DSPE) to produce a long-acting system which could enhance the permeability of RVC and DMDT.
Tocopheryl polyethylene g1000 succinate (TPGS), a water-soluble derivative of natural vitamin E, has been widely used in developing various skin delivery systems to enhance the solubility and percutaneous penetration of drugs to improve their therapeutic effects with minor systemic side effects.26 In the present research, RVC and DMDT co-loaded, HA-PEG-DSPE modified, TPGS contained NLCs (HA-TPGS-RVC/DMDT-NLCs) were designed and the particle size, zeta potential, release behavior, and cytotoxicity of NLCs were characterized. The skin permeation efficiency and in vivo anesthesia antinociceptive effect of NLCs were evaluated on the skin and the tail of rats.
Materials and methods
Materials
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)2000]-NH2 (DSPE-PEG-NH2, molecular weight: 2 kDa) was provided by Pengsheng Biological Co., Ltd. (Shanghai, People's Republic of China). HA (molecular weight: 3 kDa) was obtained from Shandong Freda Biopharm Co., Ltd. (Ji’nan, People's Republic of China). Injectable soya lecithin (ISL) was obtained from Lipoid GmbH (Ludwigshafen, Germany). Compritol® 888 ATO and Glycerol Monostearate (GMS) were obtained as gifts from Gattefossé (Saint-Priest, Lyon, France). RVC hydrochloride, DMDT hydrochloride, N,N-dimethylformamide (DMF), polysorbate 80, DMEM, and MTT were purchased from Sigma Aldrich (St. Louis, MO, USA). All the other chemicals and reagents were of analytical grade or HPLC grade and used without further purification.
Cells
Mouse embryonic fibroblasts (BALB/c 3T3 cells) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Waltham, MA, USA). Cells were grown as suspension cultures and maintained in a humidified atmosphere at 37±2°C and 5% CO2.
Animals
Sprague-Dawley (SD) rats (weighting 350–400 g) were purchased from the Medical Animal Test Center of Shandong University (Jinan, People's Republic of China), fed with standard diet and allowed water and housed in an air conditioned room (22–24°C, 12 hrs light cycle). All the animal experiments were approved by the Animal Ethics Committee of Shandong University and followed the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023).
Synthesis of HA-PEG-DSPE
DSPE-PEG-NH2 (100 mg) were dissolved in DMF (10 mL) and stirred in an ice bath, followed by adding dicyclohexylcarbodiimide (DCC, 1.2 equivalents) and stirred until completely dissolved (Solution-A). HA (1 equivalent) was dissolved in water (10 mL), added to Solution-A drop by drop, stirred at room temperature overnight and dialyzed (MWCO, 3500 Da) against excess ultrapure water for 24 hrs to get HA-PEG-DSPE. Chemical structure of HA-PEG-DSPE was after dissolved in DMSO-d6 and analyzed by 1H-NMR and fourier transform infrared (FT-IR) spectroscopy . 1H-NMR, δ (ppm) belong to protons of PEG-DSPE: 0.89 (-CH3), 1.22–1.87 (-CH2-), 2.47 (CH2C=O), 2.87 (-OH), 3.45 (-CH2-O-); belong to protons of HA: 3.76 (-CH2-OH), 5.02–5.49 (CH), 8.11 (-NH-), 10.84 (-OH); belong to protons of amide bond: 4.53 (-CH2-C=O-N-), and 7.13 (-NHC=O). FT-IR spectroscopy ν/cm−1: 3442 (-NH-), 2923 (acyl chains on the backbone of HA), 2882 (-CH2-CH2-O- of PEG), 1665 (-HN-CO-), 1416 (-CH2CO-).
Preparation of NLCs
HA-TPGS-RVC/DMDT-NLCs were prepared by solvent diffusion method.27 Lipid dispersion was composed of Compritol® 888 ATO (100 mg), GMS (100 mg), ISL (200 mg), RVC (100 mg), and DMDT (20 mg) were dissolved in DMF (5 mL) and heated at the temperature of 80–85°C to form the lipid phase. Aqueous phase was prepared by dissolving HA-PEG-DSPE (200 mg), TPGS (100 mg), and polysorbate 80 (0.5%, w/v) in water (10 mL), stirred and heated to 30°C. The lipid phase was rapidly injected into the stirred aqueous phase (600 rpm) at 30°C, and the resulting suspension was then dispersed with Milli-Q water and then dialyzed against Milli-Q water for 24 hrs using a centrifuge filter (MW cutoff =30,000 Da) to get the HA-TPGS-RVC/DMDT-NLCs (Figure 1A). Single drug-loaded NLCs were prepared using the same method using only one drug, named HA-TPGS-RVC-NLCs and HA-TPGS-DMDT-NLCs. Non-HA modified NLCs were prepared by the same method using PEG-DSPE instead of HA-PEG-DSPE, named TPGS-RVC/DMDT-NLCs. Non-HA modified, no TPGS contained NLCs were prepared by the same method using PEG-DSPE instead of HA-PEG-DSPE and without the presence of TPGS, named RVC/DMDT-NLCs. Blank NLCs were prepared using the same method without the presence of any drug, named HA-TPGS-NLCs.
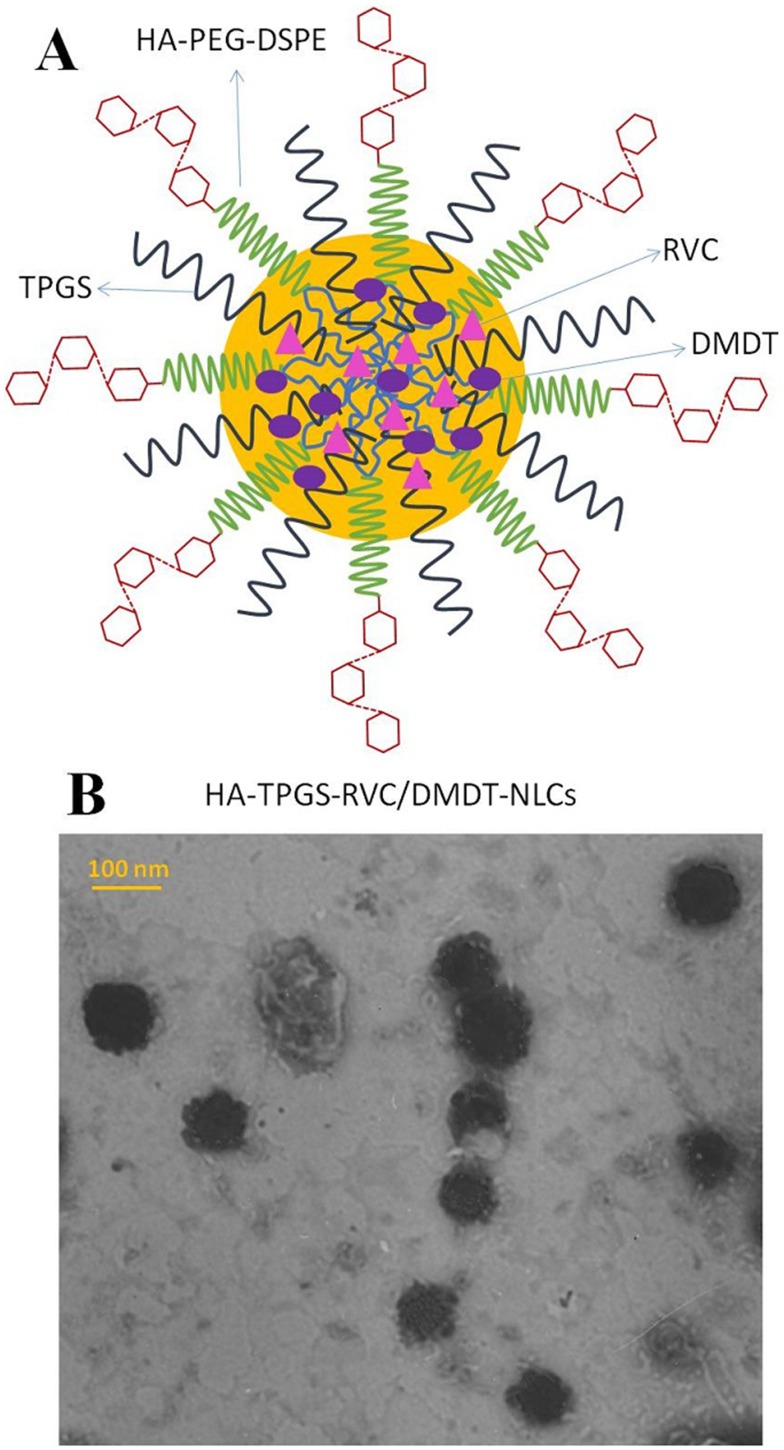
Figure 1.
The sketch diagram (A) and TEM image (B) of HA-TPGS-RVC/DMDT-NLCs. -poly(ethylene glycol)-distearoyl phosphoethanolamine (HA-PEG-DSPE) modified and (TPGS) contained (NLCs) were prepared loading (RPV) and (DMDT).
Abbreviations: TEM, transmission electron microscopy; HA, hyaluronic acid; TPGS, tocopheryl polyethylene glycol 1000 succinate; RVC, ropivacaine; DMDT, dexmedetomidine; NLCs, nanostructured lipid carriers.
Characterization of NLCs and stability
The morphology of NLCs was examined by transmission electron microscopy (TEM). NLCs were placed onto copper grids, one drop of 3% aqueous solution of sodium phosphotungstate was added to each sample and air-dried for TEM analysis. The average particle size (nm), polydispersity index (PDI), and zeta potential (mV) of the NLCs were determined by dynamic light scattering, using a Zetasizer ZS-90 particle analyzer (Malvern Instruments, Malvern, UK).28 Encapsulation efficiency (EE) and drug loading (DL) of the NLCs were determined after ultrafiltration–centrifugation (4000 g for 20 mins) in a Millex 10 kDa regenerated cellulose filtration device (Millipore, Bedford, MA, USA). Samples were diluted (1:9) in 50 mM Hepes buffer (pH 7.4) and the RVC or DMDT present in the filtrate was determined by HPLC. For RVC determination, aliquots were injected onto a Purospher1 STAR RP-18E column (octadecylsilane, 4.6×150 mm; mobile phase: acetonitrile/phosphate buffer =6/4, pH 8.0, flow rate 1 mL/min), the absorbance was measured at 240 nm.29 For DMDT determination, aliquots were injected to a WondaCract ODS-2 column (5 μm, 4.6×150 mm; mobile phase: water/acetonitrile =4/6, flow rate 1 mL/min), the UV absorbance was measured at 254 nm.30 The EE was calculated using the equation: EE (%)=(Trapped drugs in the NLCs)/(Total drugs added)×100. DL (%)=(Trapped drugs in the NLCs)/(Weight of NLCs)×100. The stability of NLCs was evaluated during 3 months of storage at 4°C by assessing the particle size and PDI.
In vitro drug release
In vitro release behaviors of NLCs were performed using Franz diffusion cells.31 NLCs (2 mL, 1 mg/mL) were placed evenly on the surface of a regenerated cellulose membrane (Spectra Por 6, MW cutoff =30,000 Da) mounted between the donor and receiver compartments of Franz diffusion cells. The receiver compartment was filled with pH 7.4 PBS, stirred at 300 rpm and maintained at 32±2 °C (mimicking human skin conditions) using circulating water bath. 0.5 mL of samples was collected at predetermined time points from the receiver compartment and replaced with fresh pH 7.4 PBS. The amount of drugs released was performed by HPLC as described in the “Characterization of NLCs and stability” section.
Cell viability test
In vitro cytotoxicity of drugs encapsulated NLCs and drugs solution (RVC/DMDT SOLU) was measured using MTT assay, in cultures of BALB/c 3T3 cells.32 Cells were seeded in 96-well culture plates at a density of 104 cells/mL and incubated for 24 hrs at 37°C and 5% CO2. The culture medium was then removed and replaced with 100 µL of fresh medium containing different concentrations of NLCs or drugs solution. Untreated cells were used as controls. After the exposure period (2 hrs), the medium was removed and the plate was washed with phosphate-buffered saline (pH 7.4). The medium (100 µL, without serum) with 0.5 mg/mL of MTT reagent was added to each well and incubated for 3 hrs at 37°C. The MTT solution was discarded from each well and 100 µL of ethanol was added to dissolve the formazan crystals. The formazan absorbance was measured at 570 nm.
Ex vivo skin permeation efficiency of NLCs
The abdominal full-thickness skins of SD rats were used for the evaluation of the skin permeation efficiency.26 SD rats were sacrificed and the fur on the abdominal area of the rats were removed. We excised the skin from the abdominal surface and removed the adherent muscle, fat, vasculature, subcutaneous tissue and then equilibrated with PBS buffer. The stripped skin (the epidermal side facing upward) was tied between the donor and receptor compartment of the Franz diffusion cells with an effective permeation area of 3.14 cm2.33 The receptor compartment was filled with PBS (pH 7.4) which was maintained at 37±0.5°C and constantly stirred at 400 rpm. Drugs encapsulated NLCs and drugs solution (0.5 mL, 5 mg/mL) were added to the skin and samples (0.3 mL) were collected from the receptor compartment at predetermined time points and replaced with equivalent amount of PBS. The samples were analyzed for the permeate amount of drugs by HPLC as described in section 2.6 and the cumulative amount of drugs penetrated (Pn) was calculated using equation: 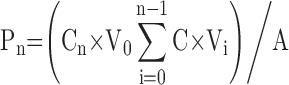 , where Cn and Ci refer to the drug concentration of the receptor medium at each sampling time and the drug concentration of the ith sample. V0 and Vi correspond to the volumes of the receptor compartment and the collected sample. A means the effective diffusion area. The steady-state fluxes (Jss) were obtained from the slope of the linear region of the curve, representing the amount of drug permeated per unit area versus time. Based on the Jss, the permeability coefficient (Kp) was calculated using the equation: Kp = Jss/C0, where C0 corresponds to the drug concentration in the donor compartment.
, where Cn and Ci refer to the drug concentration of the receptor medium at each sampling time and the drug concentration of the ith sample. V0 and Vi correspond to the volumes of the receptor compartment and the collected sample. A means the effective diffusion area. The steady-state fluxes (Jss) were obtained from the slope of the linear region of the curve, representing the amount of drug permeated per unit area versus time. Based on the Jss, the permeability coefficient (Kp) was calculated using the equation: Kp = Jss/C0, where C0 corresponds to the drug concentration in the donor compartment.
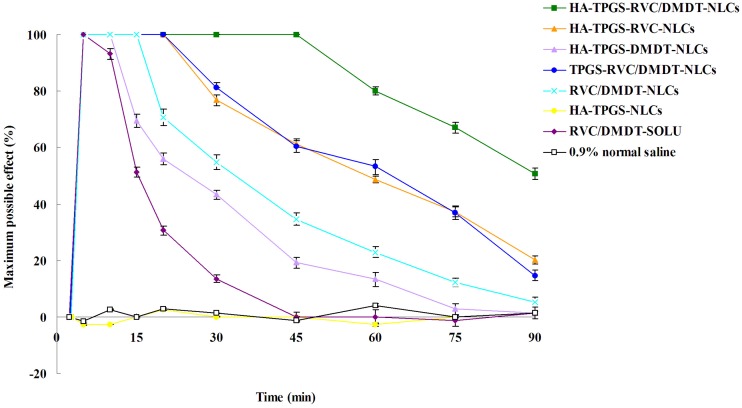
In vivo anesthesia antinociception efficiency of NLCs
In vivo anesthesia antinociceptive effect was assessed by using the tail-flick test.25 The dorsal surface of the tail of SD rats was exposed to a focused, radiant heat light source. HA-TPGS-RVC/DMDT-NLCs (containing 5 mg RVC, 1 mg DMDT), HA-TPGS-RVC-NLCs (containing 10 mg RVC), HA-TPGS-DMDT-NLCs (containing 2 mg DMDT), TPGS-RVC/DMDT-NLCs (containing 5 mg RVC, 1 mg DMDT), RVC/DMDT-NLCs (containing 5 mg RVC, 1 mg DMDT), HA-TPGS-NLCs, RVC/DMDT-SOLU (containing 10 mg RVC, 2 mg DMDT), and 0.9% normal saline were applied locally on the dorsal surface of the tail. Tail-flick test was conducted every 5 mins for a total of 75 mins. Percentage of the maximum possible effect (MPE) was applied to express the anesthesia antinociception effect and calculated using the equation: MPE (%)=(postdrug latency−baseline latency)/(cutoff latency−baseline latency)×100. The baseline latency was calculated as the mean of three different measurements taken at 10-min intervals. A maximum cutoff latency of 10 s was set to avoid tissue damage in analgesic animals.
Statistical analysis
Results are presented as means±SD. The significance of differences was assessed using two-tailed Student’s t-tests or one-way ANOVA. Differences were considered to be significant at a level of p<0.05.
Results
Characterization of NLCs
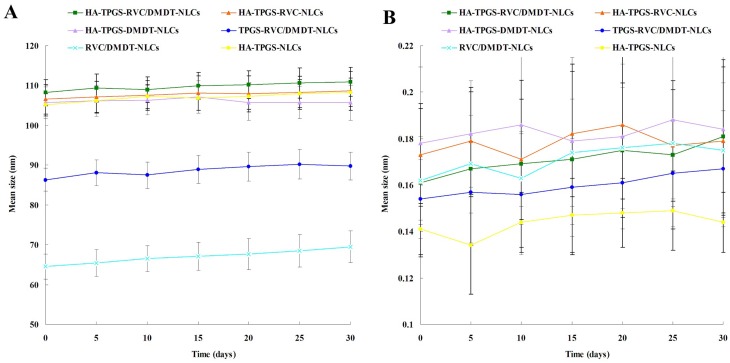
TEM image showed the surface morphology of HA-TPGS-RVC/DMDT-NLCs revealed nano-sized (100 nm), spherical shape (Figure 1B). HA-modified NLCs have sizes of about 100 nm, while sizes of TPGS-RVC/DMDT-NLCs and RVC/DMDT-NLCs were 86.3±2.9 and 64.5±3.1 nm (Table 1). For all formulations the PDIs ranged from 0.1 to 0.2. Zeta potentials of HA-TPGS-RVC/DMDT-NLCs, TPGS-RVC/DMDT-NLCs, and RVC/DMDT-NLCs were −30.7±2.8, −21.2±2.5, and −15.6±2.3 mV, respectively. The EEs of RVC and DMDT were above 80%. The NLCs were stable during 3 months of storage at 4°C for there was no significant change in particle size and PDI (Figure 2).
Table 1.
Characterization of NLCs (mean±SD, n=3)
| NLCs | Particle size (nm) | PDI | Zeta potential (mV) | RVC EE (%) | DMDT EE (%) | RVC DL (%) | DMDT DL (%) |
|---|---|---|---|---|---|---|---|
| HA-TPGS-RVC/DMDT-NLCs | 108.2±3.3 | 0.16±0.03 | −30.7±2.8 | 89.5±2.9 | 88.1±2.1 | 15.9±0.8 | 3.6±0.6 |
| HA-TPGS-RVC-NLCs | 106.5±3.7 | 0.17±0.02 | −28.9±3.2 | 87.6±3.1 | / | 16.1±0.9 | / |
| HA-TPGS-DMDT-NLCs | 105.7±3.9 | 0.18±0.03 | −31.3±2.9 | / | 85.3±3.2 | / | 3.3±0.5 |
| TPGS-RVC/DMDT-NLCs | 86.3±2.9 | 0.15±0.02 | −21.2±2.5 | 90.4±2.7 | 86.5±2.9 | 17.3±0.9 | 3.2±0.6 |
| RVC/DMDT-NLCs | 64.5±3.1 | 0.16±0.02 | −15.6±2.3 | 91.2±2.5 | 89.7±2.6 | 17.1±0.7 | 3.8±0.5 |
| HA-TPGS-NLCs | 105.1±2.8 | 0.14±0.01 | −29.9±2.5 | / | / | / | / |
Abbreviations: HA, hyaluronic acid; TPGS, tocopheryl polyethylene glycol 1000 succinate; RVC, ropivacaine; DMDT, dexmedetomidine; NLCs, nanostructured lipid carriers; PDI, polydispersity index; EE, encapsulation efficiency; DL, drug loading.
Figure 2.
The stability of NLCs evaluated during 3 months of storage at 4°C by assessing the particle size (A) and PDI (B).
Abbreviations: NLCs, nanostructured lipid carriers; PDI, polydispersity index; HA, hyaluronic acid; TPGS, tocopheryl polyethylene glycol 1000 succinate; RVC, ropivacaine; DMDT, dexmedetomidine.
Drug release in vitro
The release of RVC or DMDT from HA-modified NLCs, TPGS contained NLCs and NLCs were different (Figure 3). With the presence of TPGS and HA, release of drugs was in more sustained manners. Release behaviors of RVC and DMDT from HA-TPGS-RVC/DMDT-NLCs, HA-TPGS-RVC-NLCs, and HA-TPGS-DMDT-NLCs were substantially similar with no statistically significant differences. RVC and DMDT release from solution was the fastest; on the contrary, release of drugs from HA-TPGS-RVC/DMDT-NLCs was the slowest.
Figure 3.
The release profiles of RVC (A) and DMDT (B). (Data represent the mean±SD, n=3).
Abbreviations: RVC, ropivacaine; DMDT, dexmedetomidine; TPGS, tocopheryl polyethylene glycol 1000 succinate; DMDT, dexmedetomidine; HA, hyaluronic acid; NLCs, nanostructured lipid carriers.
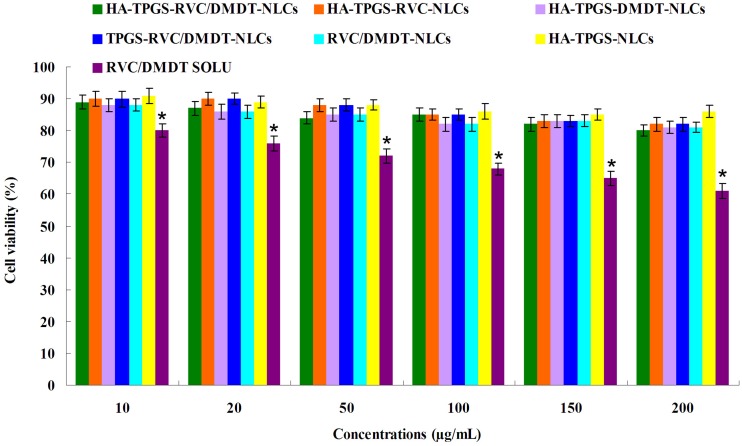
Cell viability
The cell viability of 3T3 cells treated with NLCs and drugs solution using the MTT assay. Figure 4 shows that all kinds of NLCs do not significantly reduce cell viability even at high nanoparticles concentration. However, RVC/DMDT-SOLU presents the dose-dependent cytotoxic effect, led to a decrease in cell viability down to 61.2 ± 1.9%. All the NLCs formulations were found to be significantly less cytotoxic than free drugs at equivalent concentrations (P<0.05).
Figure 4.
The cell viability of 3T3 cells treated with NLCs and drugs solution using the MTT assay. (Data represents the mean±SD, n=6). *p<0.05.
Abbreviations: NLCs, nanostructured lipid carriers; RVC, ropivacaine; DMDT, dexmedetomidine; TPGS, tocopheryl polyethylene glycol 1000 succinate; HA, hyaluronic acid.
Skin permeation efficiency
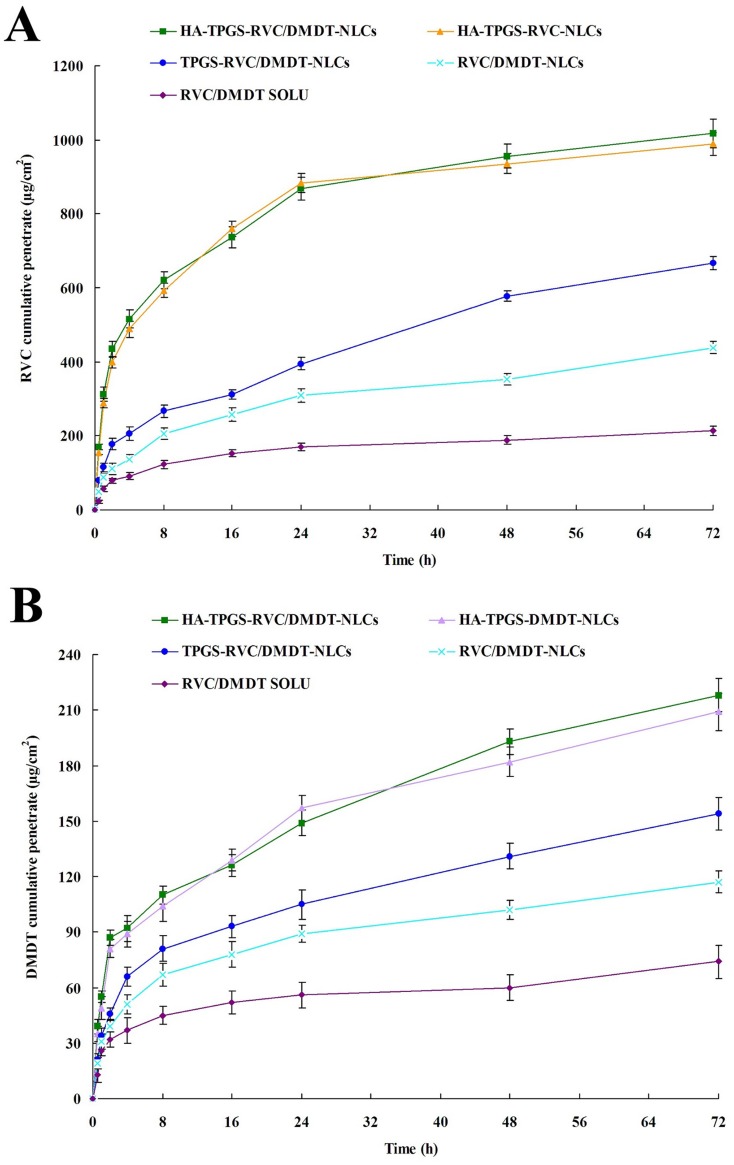
Skin permeation efficiency of drugs from different kinds of NLCs and solution was compared using Franz diffusion cells with the skin of rats. Figure 5 illustrates the accumulative penetration curves of RVC and DMDT during 72 hrs of study. Tables 2 and 3 summarize the P72 (the cumulative amount of drugs penetrated at 72 h), Jss, and Kp-values. Figure 5, Tables 2 and 3 show the RVC/DMDT-NLCs significantly improved the permeation efficiency of drugs when compared with RVC/DMDT-SOLU (P<0.05). TPGS-RVC/DMDT-NLCs exhibited more drugs permeation than RVC/DMDT-NLCs (P<0.05). HA-TPGS-RVC/DMDT-NLCs gain the most remarkable efficiency among all the groups, which is much better than that of TPGS-RVC/DMDT-NLCs (P<0.05). At 72 hrs of study, 1018.3±39.1, 666.8±18.7, 439.2±17.3 and 214.3±13.2 μg cm−2 of RVC was permeated from HA-TPGS-RVC/DMDT-NLCs, TPGS-RVC/DMDT-NLCs, RVC/DMDT-NLCs, and RVC/DMDT-SOLU, respectively. HA-TPGS-RVC/DMDT-NLCs exhibited the most prominent Jss, which is 4.7 folds higher than that of RVC/DMDT-SOLU.
Figure 5.
Accumulative skin penetration curves of RVC (A) and DMDT (B) from NLCs and solution during 72 hrs of study using Franz diffusion cells with the skin of rats. (Data represent the mean±SD, n=6).
Abbreviations: RVC, ropivacaine; DMDT, dexmedetomidine; TPGS, tocopheryl polyethylene glycol 1000 succinate; NLCs, nanostructured lipid carriers.
Table 2.
RVC permeation parameters of NLCs and solution (mean±SD, n=3)
| Formulations | P72 (μg· cm−2) | Kp (cm· h−1) | Jss (μg· cm−2· h−1) |
|---|---|---|---|
| HA-TPGS-RVC/DMDT-NLCs | 1018.3±39.1 | 7.2×10−3 | 14.2 |
| HA-TPGS-RVC-NLCs | 989.4±31.9 | 7.0×10−3 | 13.8 |
| TPGS-RVC/DMDT-NLCs | 666.8±18.7 | 4.7×10−3 | 9.3 |
| RVC/DMDT-NLCs | 439.2±17.3 | 3.1×10−3 | 6.1 |
| RVC/DMDT SOLU | 214.3±13.2 | 1.5×10−3 | 3.0 |
Abbreviations: HA, hyaluronic acid; TPGS, tocopheryl polyethylene glycol 1000 succinate; RVC, ropivacaine; DMDT, dexmedetomidine; NLCs, nanostructured lipid carriers; P72, the cumulative amount of drugs penetrated at 72 hrs; Jss, steady-state fluxes; Kp, permeability coefficient.
Table 3.
DMDT permeation parameters of NLCs and solution (mean±SD, n=3)
| Formulations | P72 (μg· cm−2) | Kp (cm· h−1) | Jss (μg· cm−2· h−1) |
|---|---|---|---|
| HA-TPGS-RVC/DMDT-NLCs | 218.2±12.3 | 1.5×10−3 | 2.9 |
| HA-TPGS-DMDT-NLCs | 209.4±9.7 | 1.5×10−3 | 2.9 |
| TPGS-RVC/DMDT-NLCs | 154.1±9.3 | 1.1×10−3 | 2.1 |
| RVC/DMDT-NLCs | 116.9±6.4 | 8×10−4 | 1.5 |
| RVC/DMDT SOLU | 74.3±8.8 | 5×10−4 | 1.0 |
Abbreviations: HA, hyaluronic acid; TPGS, tocopheryl polyethylene glycol 1000 succinate; RVC, ropivacaine; DMDT, dexmedetomidine; NLCs, nanostructured lipid carriers; P72, the cumulative amount of drugs penetrated at 72 hrs; Jss, steady-state fluxes; Kp, permeability coefficient.
In vivo anesthesia antinociception efficiency
In vivo anesthesia antinociceptive effect was evaluated to determine the efficiency of the two drugs combination in different modified NLCs system. HA-TPGS-NLCs did not show any effect during the test (Figure 6). All the drugs encapsulated NLCs and drugs solution increased the tail-flick test latency significantly compared with the 0.9% normal saline control (P<0.05). RVC/DMDT-SOLU showed a short-lasting antinociceptive effect (reduced rapidly at the first 10 mins), while RVC and/or DMDT-loaded NLCs exhibited a more prolonged antinociceptive effect lasted until 90 mins. To be noticed, HA-TPGS-RVC/DMDT-NLCs showed stronger and longer anesthesia antinociceptive effect when compared with TPGS-RVC/DMDT-NLCs and RVC/DMDT-NLCs (P<0.05). This means the HA modification and TPGS incorporation could enhance and prolong the anesthesia antinociceptive efficiency. Also, single drug-loaded NLCs shower weaker ability than the dual drugs combination system. It is interesting to note that the antinociceptive impact of HA-TPGS-RVC-NLCs and TPGS-RVC/DMDT-NLCs were comparative, which may prove the efficiency of HA modification and drugs combination could lead to the similar efficiency. To be noticed, the uses of both drugs in the combination system were halved but still gain the better results, this may be the evidence that RVC and DMDT co-loaded NLCs could perform a synergistic effect and may reduce the amount of drugs, which can lower the toxicity of the system.
Figure 6.
In vivo anesthesiaantinociceptive effects of NLCs and drugs solution evaluated by tail-flick test on rats. (Data represents the mean±SD, n=8).
Abbreviations: NLCs, nanostructured lipid carriers; RVC, ropivacaine; DMDT, dexmedetomidine; TPGS, tocopheryl polyethylene glycol 1000 succinate.
Discussion
Nanocarrier encapsulation has been reported to increase efficacy and reduce local anesthetic toxicity.34 Studies with solid lipid nanoparticles, silver nanoparticles, and liposomes have been shown to increase the anesthesia duration of drugs formulations, when compared to their respective plain solutions.35–37 As a widely used local anesthesia in clinics, the efficacy of RVC was preferred because of its reduced toxicity.38 Thus, we imagined the treatment of pain with long-acting RVC is exciting. Co-delivery of two adjuvant drugs (DMDT and RVC) was expected to increase the duration of single RVC formulation.6,39 In order to maintain the effectiveness of the drug while minimizing its toxicity, this study considered the development of a nano-sized, controlled release pain management system. Moreover, HA-PEG-DSPE and TPGS were introduced to enhance the percutaneous penetration of drugs, which could improve their therapeutic effects wand produce a long-acting system.
The NLCs in this study were prepared by the solvent diffusion method.40 Drugs loaded NLCs and blank NLCs appeared the similar sizes of about 100 nm. This means the loading of drugs did not enlarge the size of the NLCs. Size and PDI of the carriers are important factors for the nanocarriers, which can influence the distribution of carriers.23 Particle size lower than 200 nm (with a PDI lower than 0.2) could decrease uptake by the liver, prolongs circulation time in the blood, and improves bioavailability.23 In this research, addition of DMDT and/or RVC did not have a significant effect on the size or PDI of the NLCs. The size of HA-modified NLCs is larger than that of TPGS-RVC/DMDT-NLCs and RVC/DMDT-NLCs. These results demonstrated that adding of TPGS and modification of HA enlarged the size of the NLCs. For all formulations, the zeta potentials were negative, an indication of physical stability due to charge repulsion on the surface of the NLCs.41 The maintenance of similar zeta potentials in the presence of DMDT and/or RVC indicates that there are few anesthetic molecules at the surface of the NLCs. Instead, the majority of the anesthetic is in the inner aqueous compartments of the NLCs. Zeta potentials of HA-TPGS-RVC/DMDT-NLCs and TPGS-RVC/DMDT-NLCs were more negative than RVC/DMDT-NLCs, which may be caused by the negatively charged TPGS and HA. The EE values for RVC and DMDT in the NLCs are higher than 80%. A high upload capacity of the NLCs is desirable in order to ensure the encapsulation of drug molecules.
The release profiles of RVC and DMDT could be crucial for achieving long-acting corneal anesthesia. The release profiles illustrated that the drugs released from NLCs in sustained manners. The sustained-release behavior is an important prerequisite for successful long-lasting anesthesia antinociceptive.42 This phenomenon could be explained by the entrapment of the drugs by the lipid matrix which can slowdown the release of the drugs. The release of RVC or DMDT from HA-modified NLCs, TPGS contained NLCs and NLCs were different, release of drugs from HA-TPGS-RVC/DMDT-NLCs was the slowest. These may be explained by the presence of TPGS and HA hindered the release of drugs in more sustained manners.
Cell viability test results of drugs loaded NLCs and drugs solution are different. Drugs loaded and blank NLCs showed no obvious effect on cell viability. In contrast, the cytotoxicity of RVC/DMDT SOLU increased along with the drug concentrations. Lower toxicity of NLCs to the cells may be explained by the protective effects of the carriers to the drugs thus let fewer drugs exposed to the cells.43 The toxic effect of local anesthetics is well known and encapsulation in NLCs has been shown to reduce such intrinsic toxicity.44
The significant permeation difference may depend on the property of the carriers. The skin integrity assessment methods include visual or instrumental. The common methods for screening contain radiotracer method and electrochemical method.45 After application, the NLCs are supposed to form a film covered on the skin surface.46 The oppressed cover makes drug carrier instantly accessible to the skin, which facilitates drug permeation across the stratum corneum. Moreover, the lipids in the formulation may also play a role since it can interact with the skin, joining lipid bilayer and loosening intercellular regions between corneocytes.47 This tendency indicated the potential of NLCs in substantially delivering drugs across skin was considerable, relative to the free solution. The clinically maximum concentration of the RVC was 7.5 mg/mL.48–50 The concentration used in the receptor compartment was 5 mg/mL, which was lower than the maximum concentration of the RVC. HA-TPGS-RVC/DMDT-NLCs exhibited 4.7 folds higher Jss than that of RVC/DMDT-SOLU, higher than TPGS-RVC/DMDT-NLCs (1.5 folds) and RVC/DMDT-NLCs (2.3 folds). These results illustrated the presence of HA and TPGS could enhance the skin permeation ability of NLCs.
The TFL test is the most frequently used method to measure pain levels and assess the effects of anesthesia.51 It uses radiant heat to measure the latency of the response to thermal noxious stimuli and assess the pain threshold and effect of anesthesia.52 Radiant heat is a type of constant infringement stimulation that does not damage the skin when a proper cutoff time is used. In the present research, all the drugs containing formulations increased the response time of the rats significantly. RVC/DMDT-SOLU showed a short-lasting MPE (10 mins), while NLCs exhibited a more prolonged antinociceptive effect lasted until 90 mins. HA-TPGS-RVC/DMDT-NLCs showed stronger and longer anesthesia antinociceptive effect when compared with TPGS-RVC/DMDT-NLCs and RVC/DMDT-NLCs. This means the HA modification and TPGS incorporation could enhance and prolong the anesthesia antinociceptive efficiency. Also, single drug-loaded NLCs shower weaker ability than the dual drugs combination system. To be noticed, the uses of both drugs in the combination system were halved but still gain the better results, this may be the evidence that RVC and DMDT co-loaded NLCs could perform a synergistic effect and may reduce the amount of drugs, which can lower the toxicity of the system.
Conclusion
In order to improve the anesthesia antinociceptive efficiency, HA-modified long-acting NLCs containing TPGS as a skin penetration enhancer were prepared and characterized as local anesthesia formulation. The cumulative amount of drugs penetrated through rat skin from NLCs was higher than that of the drugs solution. The in vivo anesthesia antinociception study displayed that HA-TPGS-RVC/DMDT-NLCs showed stronger and longer anesthesia antinociceptive effect when compared with other NLCs and drugs solution even at a lower dosage of drugs. HA-TPGS-RVC/DMDT-NLCs constructed in this research could be applied as a promising system for the local anesthetic strategy.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mitra S, Carlyle D, Kodumudi G, Kodumudi V, Vadivelu N. New advances in acute postoperative pain management. Curr Pain Headache Rep. 2018;22(5):35. doi: 10.1007/s11916-018-0690-8 [DOI] [PubMed] [Google Scholar]
- 2.Weiniger CF, Golovanevski M, Sokolsky-Papkov M, Domb AJ. Review of prolonged local anesthetic action. Expert Opin Drug Deliv. 2010;7(6):737–752. doi: 10.1517/17425241003767383 [DOI] [PubMed] [Google Scholar]
- 3.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002;89(3):409–423. [PubMed] [Google Scholar]
- 4.Cohen R, Kanaan H, Grant GJ, Barenholz Y. Prolonged analgesia from Bupisome and Bupigel formulations: from design and fabrication to improved stability. J Control Release. 2012;160(2):346–352. doi: 10.1016/j.jconrel.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 5.Fredrickson MJ, Abeysekera A, White R. Randomized study of the effect of local anesthetic volume and concentration on the duration of peripheral nerve blockade. Reg Anesth Pain Med. 2012;37(5):495–501. doi: 10.1097/AAP.0b013e3182580fd0 [DOI] [PubMed] [Google Scholar]
- 6.Foley PL, Ulery BD, Kan HM, et al. A chitosan thermogel for delivery of ropivacaine in regional musculoskeletal anesthesia. Biomaterials. 2013;34(10):2539–2546. doi: 10.1016/j.biomaterials.2012.12.035 [DOI] [PubMed] [Google Scholar]
- 7.McClure JH. Ropivacaine. Br J Anaesth. 1996;76(2):300–307. Review. doi: 10.1093/bja/76.2.300 [DOI] [PubMed] [Google Scholar]
- 8.Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69(5):563–569. [PubMed] [Google Scholar]
- 9.Chen C, You P. A novel local anesthetic system: transcriptional transactivator peptide-decorated nanocarriers for skin delivery of ropivacaine. Drug Des Devel Ther. 2017;11:1941–1949. doi: 10.2147/DDDT.S135916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chazalon P, Tourtier JP, Villevielle T, et al. Ropivacaine-induced cardiac arrest after peripheral nerve block: successful resuscitation. Anesthesiology. 2003;99(6):1449–1451. doi: 10.1097/00000542-200312000-00030 [DOI] [PubMed] [Google Scholar]
- 11.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109(3):502–511. doi: 10.1097/ALN.0b013e318182c26b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009;111(5):1111–1119. doi: 10.1097/ALN.0b013e3181bbcc26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011;71(11):1481–1501. doi: 10.2165/11207190-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001;17(4):881–897. [DOI] [PubMed] [Google Scholar]
- 15.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115(4):836–843. doi: 10.1097/ALN.0b013e318221fcc9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Boghdadly K, Brull R, Sehmbi H, Abdallah FW. Perineural dexmedetomidine is more effective than clonidine when added to local anesthetic for supraclavicular brachial plexus block: a systematic review and meta-analysis. Anesth Analg. 2017;124(6):2008–2020. doi: 10.1213/ANE.0000000000002014 [DOI] [PubMed] [Google Scholar]
- 17.Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184–196. doi: 10.1097/AAP.0000000000000564 [DOI] [PubMed] [Google Scholar]
- 18.Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. doi: 10.1097/AAP.0000000000000033 [DOI] [PubMed] [Google Scholar]
- 19.Baswan S, Kasting GB, Li SK, et al. Understanding the formidable nail barrier: a review of the nail microstructure, composition and diseases. Mycoses. 2017;60(5):284–295. doi: 10.1111/myc.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik A, Gupta M, Mani R, Gogoi H, Bhatnagar R. Trimethyl chitosan nanoparticles encapsulated protective antigen protects the mice against anthrax. Front Immunol. 2018;9:562. doi: 10.3389/fimmu.2018.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik A, Gupta M, Gupta V, Gogoi H, Bhatnagar R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int J Nanomedicine. 2018;13:7959–7970. doi: 10.2147/IJN.S165876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Paula E, Cereda CM, Fraceto LF, et al. Micro and nanosystems for delivering local anesthetics. Expert Opin Drug Deliv. 2012;9(12):1505–1524. doi: 10.1517/17425247.2012.738664 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wang S, Shi P. Transcriptional transactivator peptide modified lidocaine-loaded nanoparticulate drug delivery system for topical anesthetic therapy. Drug Deliv. 2016;23(9):3193–3199. doi: 10.3109/10717544.2016.1160459 [DOI] [PubMed] [Google Scholar]
- 24.Li A, Yang F, Xin J, Bai X. An efficient and long-acting local anesthetic: ropivacaine-loaded lipid-polymer hybrid nanoparticles for the control of pain. Int J Nanomedicine. 2019;14:913–920. doi: 10.2147/IJN.S190164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue Y, Zhao D, Yin Q. Hyaluronic acid modified nanostructured lipid carriers for transdermal bupivacaine delivery: in vitro and in vivo anesthesia evaluation. Biomed Pharmacother. 2018;98:813–820. doi: 10.1016/j.biopha.2017.12.103 [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Sun Y, Li Z. Topical anesthesia therapy using lidocaine-loaded nanostructured lipid carriers: tocopheryl polyethylene glycol 1000 succinate-modified transdermal delivery system. Drug Des Devel Ther. 2018;12:4231–4240. doi: 10.2147/DDDT.S187177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu J, Zhang L, Chen Z, et al. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016;23(9):3408–3416. doi: 10.1080/10717544.2016.1189465 [DOI] [PubMed] [Google Scholar]
- 28.Da Silva CMG, Franz-Montan M, Limia CEG, et al. Encapsulation of ropivacaine in a combined (donor-acceptor, ionic-gradient) liposomal system promotes extended anesthesia time. PLoS One. 2017;12(10):e0185828. doi: 10.1371/journal.pone.0185828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Da Silva CM, Fraceto LF, Franz-Montan M, et al. Development of egg PC/cholesterol/α-tocopherol liposomes with ionic gradients to deliver ropivacaine. J Liposome Res. 2016;26(1):1–10. doi: 10.3109/08982104.2015.1022555 [DOI] [PubMed] [Google Scholar]
- 30.Zhan C, Santamaria CM, Wang W, McAlvin JB, Kohane DS. Long-acting liposomal corneal anesthetics. Biomaterials. 2018;181:372–377. doi: 10.1016/j.biomaterials.2018.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You P, Yuan R, Chen C. Design and evaluation of lidocaine- and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: a comparison between solid lipid nanoparticles and nanostructured lipid carriers. Drug Des Devel Ther. 2017;11:2743–2752. doi: 10.2147/DDDT.S141031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couto VM, Prieto MJ, Igartúa DE, et al. Dibucaine in ionic-gradient liposomes: biophysical, toxicological, and activity characterization. J Pharm Sci. 2018;107(9):2411–2419. doi: 10.1016/j.xphs.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 33.Zhai Y, Xu R, Wang Y, Liu J, Wang Z, Zhai G. Ethosomes for skin delivery of ropivacaine: preparation, characterization and ex vivo penetration properties. J Liposome Res. 2015;25(4):316–324. doi: 10.3109/08982104.2014.999686 [DOI] [PubMed] [Google Scholar]
- 34.Moldovan M, Alvarez S, Rothe C, et al. An in vivo mouse model to investigate the effect of local anesthetic nanomedicines on axonal conduction and excitability. Front Neurosci. 2018;12:494. doi: 10.3389/fnins.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Araújo DR, Da Silva DC, Barbosa RM, et al. Strategies for delivering local anesthetics to the skin: focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert Opin Drug Deliv. 2013;10(11):1551–1563. doi: 10.1517/17425247.2013.828031 [DOI] [PubMed] [Google Scholar]
- 36.Jiang Q, Yu S, Li X, Ma C, Li A. Evaluation of local anesthetic effects of Lidocaine-Ibuprofen ionic liquid stabilized silver nanoparticles in Male Swiss mice. J Photochem Photobiol B. 2018;178:367–370. doi: 10.1016/j.jphotobiol.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 37.Cereda CM, Brunetto GB, de Araújo DR, de Paula E. Liposomal formulations of prilocaine, lidocaine and mepivacaine prolong analgesic duration. Can J Anaesth. 2006;53(11):1092–1097. [PubMed] [Google Scholar]
- 38.Wang Z, Huang H, Yang S, et al. Long-term effect of ropivacaine nanoparticles for sciatic nerve block on postoperative pain in rats. Int J Nanomedicine. 2016;11:2081–2090. doi: 10.2147/IJN.S101563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–843. doi: 10.1097/ALN.0b013e318221fcc9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–313. doi: 10.15171/apb.2015.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohanraj VJ, Chen Y. Nanoparticles–A review. Trop J Pharm Res. 2006;5(1):561–573. [Google Scholar]
- 42.Wang J, Zhang L, Chi H, Wang S. An alternative choice of lidocaine-loaded liposomes: lidocaine-loaded lipid-polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016;23(4):1254–1260. doi: 10.3109/10717544.2016.1141259 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Yue Y, Chang M. Local anaesthetic pain relief therapy: in vitro and in vivo evaluation of a nanotechnological formulation co-loaded with ropivacaine and dexamethasone. Biomed Pharmacother. 2017;96:443–449. doi: 10.1016/j.biopha.2017.09.124 [DOI] [PubMed] [Google Scholar]
- 44.Tarba C, Crăcium C. A comparative study of the effects of procaine, lidocaine, tetracaine and dibucaine on the functions and ultrastructure of isolated rat liver mitochondria. Biochim Biophys Acta BBA - Bioenerg. 1990;1019(1):19–28. doi: 10.1016/0005-2728(90)90120-S [DOI] [PubMed] [Google Scholar]
- 45.Baswan SM, Li SK, LaCount TD, Kasting GB. Size and charge dependence of ion transport in human nail plate. J Pharm Sci. 2016;105(3):1201–1208. doi: 10.1016/j.xphs.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai Y, Yang X, Zhao L, Wang Z, Zhai G. Lipid nanocapsules for transdermal delivery of ropivacaine: in vitro and in vivo evaluation. Int J Pharm. 2014;471(1–2):103–111. doi: 10.1016/j.ijpharm.2014.05.035 [DOI] [PubMed] [Google Scholar]
- 47.Rezazadeh M, Emami J, Hassanzadeh F, Sadeghi H, Rostami M, Mohammadkhani H. Targeted nanostructured lipid carriers for delivery of paclitaxel to cancer cells: preparation, characterization, and cell toxicity. Curr Drug Deliv. 2017;14(8):1189–1200. doi: 10.2174/1567201814666170503143646 [DOI] [PubMed] [Google Scholar]
- 48.Pither CE, Emanuelsson BM, Reventlid H, Whitehead E. A comparison of the dynamics and pharmacokinetics of ropivacaine 7.5 mg/mL with and without epinephrine used for epidural anaesthesia in urological surgery. Clin Drug Investig. 2003;23(4):245–253. doi: 10.2165/00044011-200323040-00004 [DOI] [PubMed] [Google Scholar]
- 49.Umbrain VJ, van Gorp VL, Schmedding E, et al. Ropivacaine 3.75 mg/mL, 5 mg/mL, or 7.5 mg/mL for cervical plexus block during carotid endarterectomy. Reg Anesth Pain Med. 2004;29(4):312–316. doi: 10.1097/00115550-200407000-00003 [DOI] [PubMed] [Google Scholar]
- 50.Bjørnestad E, Smedvig JP, Bjerkreim T, Narverud G, Kollerøs D, Bergheim R. Epidural ropivacaine 7.5 mg/mL for elective Caesarean section: a double-blind comparison of efficacy and tolerability with bupivacaine 5 mg/mL. Acta Anaesthesiol Scand. 1999;43(6):603–608. doi: 10.1034/j.1399-6576.1999.430602.x [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Wang J, Chi H, Wang S. Local anesthetic lidocaine delivery system: chitosan and hyaluronic acid-modified layer-by-layer lipid nanoparticles. Drug Deliv. 2016;23(9):3529–3537. doi: 10.1080/10717544.2016.1204569 [DOI] [PubMed] [Google Scholar]
- 52.Kallina CF, Grau JW. Tail-flick test. I: impact of a suprathreshold exposure to radiant heat on pain reactivity in rats. Physiol Behav. 1995;58(1):161–168. doi: 10.1016/0031-9384(95)00046-l [DOI] [PubMed] [Google Scholar]