Abstract
Background
Acute pancreatitis (AP) has a high mortality rate and often has serious complications. The Hippo-YAP signaling pathway is mainly involved in cell proliferation and stem cell self-renewal. Recent studies have reported that YAP1 plays a crucial role in pancreatic cancer initiation and acute and chronic pancreatitis (CP). However, the role of YAP1 in AP still needs to be clarified.
Material/Methods
To assess the role of YAP1 in the progression of AP, we established a cell model of AP in AR42J cells. AR42J, a rat pancreatic acinar cell line, was stimulated with caerulein to mimic AP-like acinar cell injury. Levels of interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) were measured by ELISA to investigate the role of YAP1 in the progression of AP.
Results
The results showed that YAP1 and MALAT1 were the targets of miR-194 and were upregulated in caerulein-treated AR42J cells. Overexpression of MALAT1 or YAP1 can increase the levels of IL-6 and TNF-α secreted by AR42J cells, while miR-194 dramatically counteracts this enhancement effect.
Conclusions
Our results demonstrated a regulation loop among MATAL1, miR-194, and YAP1, which dynamically regulates the progression of AP, providing a new therapeutic target for treatment of this disease.
MeSH Keywords: Pancreatitis, Acute Necrotizing; RNA, Long Noncoding; MicroRNAs
Background
Acute pancreatitis (AP) is an acute inflammatory process with a high morbidity and a mortality rate of about 30% [1]. About a one-quarter of severe acute pancreatitis (SAP) patients develop concomitant multiple organ dysfunction and pulmonary microvascular endothelial cell (PMVECs) injury. Gallstones, biliary sludge, hypertriglyceridemia, drug-induced causes, infectious causes, autoimmune pancreatitis, and excessive alcohol use are the common risk factors for AP [2–5]. It was reported that the abnormal activation of trypsinogen in pancreatic cells is the main pathogenesis of AP [6], but the mechanisms regulating the severity and development of AP still need to be clarified.
The Yes-associated protein 1 (YAP1) transcriptional coactivator is a prime mediator of the Hippo signaling pathway, and accumulating evidence shows that the Hippo-YAP signaling pathway plays an important role in regulating organ size and tissue growth, and is also involved in cell proliferation, migration, stem cell self-renewal, and tissue regeneration [7–12]. YAP1 shuttles between the cytoplasm and the nucleus and activates gene transcription during shuttling [13]. The transcriptional activities of YAP and its homolog TAZ are negatively regulated by a series of phosphorylation modifications, catalyzed by Mst1/2 and LATS1/2, leading to cytoplasmic retention and degradation, which block the transfer of YAP from cytosol to the nucleus [14–16]. YAP1 is highly expressed in pluripotent progenitor cells of mouse embryonic 12.5 (E12.5) pancreas [17], and overexpression of YAP1 inhibits the differentiation of endocrine and exocrine compartments during the secondary transformation of pancreas development [18]. In addition, in mice with high expression of YAP1 induced by doxycycline, there was increased pancreas size and more acinar cells [19]. Moreover, the activated YAP1 and TAZ in acinar cells upregulates the signal intensity of JAK-STAT3, thus contributing to the progression of pancreatic ductal adenocarcinoma (PDAC) in mice [20]. Nevertheless, the other mechanisms by which YAP1 regulates the pancreas inflammatory process are poorly defined. The mechanism by which YAP1 affects AP also needs to be further clarified.
Noncoding RNAs, including microRNAs (miRNAs 18–22 nucleotides in length) and long noncoding RNAs (lncRNAs more than 200 nucleotides in length), have been reported to be involved in diverse pathophysiological processes [21,22]. Accumulating evidence has shown that many miRNAs, including miR-21-3p, miR-148a, miR-216a, miR-122, miR-214, and miR-138, are aberrantly expressed in AP [23]. Moreover, some miRNAs have also been found to affect AP-related signaling pathways by suppressing target genes to regulate the progression of AP [24]. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), a highly conserved lncRNA, has roles in regulating various physiological processes, including gene expression and alternative splicing [25,26]. MALAT1 was highly transcribed in non-small cell lung tumors and prostate cancer cells, and increased proliferation and metastasis of these tumor cells [27,28]. Furthermore, high levels of MALAT1 were detected in pancreatic cancer, and the decrease of MALAT1 led to a decline of YAP1 [29]. YAP1 can also act as an inducer to upregulate the transcription of MALAT1 in colorectal cancer [30]. Although MALAT1 has been reported to regulate the progression of tumors, its function in AP is still unclear.
Here, we describe the underlying effect of YAP1 on the progression of AP. Furthermore, we found that levels of YAP1 and MALAT1 were dramatically increased with the reduction of miR-194 in the AP cell model. In summary, we revealed the dynamic regulation of MALAT1/miR-194/YAP1 in the development of pancreatitis, and found that the regulation loop might be a putative therapeutic target for AP.
Material and Methods
Cell culture
The rat pancreatic cell line AR42J was obtained from ATCC (Manassas, VA) and cultured in F-12K medium (Gibco, Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS; HyClone, Logan, UT) in a humidified atmosphere of 5% CO2, at 37°C
Establishment of AP cell model
AR42J cells were cultured for 24 h, followed by incubation with 100nM caerulein (Sigma-Aldrich, St Louis, MO, #C9026). Then, the AR42J cells were harvested at 0, 4,8,12, or 24 h.
Western blot
After caerulein stimulation, cell samples were washed with PBS and lysed with RIPA lysis containing a cocktail of protease inhibitors (Roche) and centrifuged at 15 000 rpm at 4°C for 10 min. The supernatants were collected after centrifugation, and the concentration of precipitated protein was determined using the BCA method. Equal amounts of each sample were separated by SDS-PAGE and then transferred to PVDF membranes (Nanjing KeyGen Biotech Co., Nanjing, China) following the standard steps. Membranes were incubated with 5% (w/v) non-fat milk in Tris-buffered saline-0.1% Tween-20 (TBST) for 1 h at room temperature to block the nonspecific bindings. Then, these pieces of membrane were incubated with primary antibody of YAP1 (Abcam, Hong Kong) overnight at 4°C. After washing 3×10 min in TBST, the membranes were incubated with a second antibody for 1 h at room temperature. Finally, these pieces of membrane were washed with TBST again, and detected by enhanced chemiluminescence plus Western blot detection reagents (Santa Cruz), and exposed to X Film (Eastman Kodak).
Cell transfection
Both the miR-194 mimics and small interfering RNAs (siRNAs) targeting rat YAP1 or MALAT1 were purchased from GenePharma (Shanghai, China). AR21J cells were transfected with miRNA siRNAs, mimics, and plasmids of pcDNA-MALAT1 or pcDNA-YAP1 using Lipofectamine 2000 (Invitrogen). At 24 h after transfections, 100 nM caerulein was added to treat the AR42J cells for another 24 h. After the stimulation, RNAs and/or proteins were extracted for further study.
Luciferase reporter assays
The wild-type sequences of lncRNA MALAT1 and 3′-untranslated regions (3′-UTR) of YAP1 containing predicted miR-194 binding sites (MALAT1-WT and YAP1-WT), as well as the sequences with mutated binding sites (MALAT1-MUT and YAP1-MUT) were subcloned into the pMIR-report vectors (Ambio). To perform the luciferase assays, the constructed luciferase reporter plasmids were co-transfected with miR-194 mimics or NC into the AR42J cells using Lipofectamine 2000. Luciferase activities were measured 48 h after transfection using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Measurement of IL-6 and TNF-α
The culture medium was collected after caerulein treatment for 24 h and we measured the secretion of pro-inflammatory cytokines TNF-α and IL-6 using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Sengxiong Biotechnology) according to the manufacturer’s instruction.
Quantitative real-time PCR
Total RNA was extracted from caerulein-treated AR42J cells using a commercial extraction reagent, RNAiso Plus. RNA was reverse-transcribed using the PrimeScript™ RT Master Mix kit (TaKaRa Biotechnology) and subjected to real-time PCR using gene-specific primers as follows.
Primer for YAP1 forward: 5′-AACCATAAGAACAAGACCACATCC-3′,
reverse: 5′-TATTCCGTATTGCCTGCCGA-3′;
TAZ forward: 5′-TTATCACCGTCTCTAACCACCAG-3′,
reverse: 5′-TTATCACCGTCTCTAACCACCAG-3′;
LATS1 forward: 5′-GAGCATAACCTGAACAAGATGTC-3′,
reverse: 5′-TAACCATATCCTCATCAAAGCC-3′;
TEAD1 forward: 5′-CCGCCATTAAGGTGTCTAGTC-3′,
reverse: 5′-CCGCCATTAAGGTGTCTAGTC-3′;
GAPDH forward: 5′-GAGAAACCTGCCAAGTATGATGAC-4′,
reverse: 5′-GGGAGTTGCTGTTGAAGTCAC-3′.
All miRNA and lncRNA primers were synthesized by RiboBio (Guangzhou, China). The U6 and GAPDH were used as endogenous controls. The qualitative real-time PCR (qRT-PCR) was performed with the 7500 FAST Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Fold changes and subsequent percent gene expression levels were calculated using the comparative CT (2−ΔΔCT) method.
Statistical analysis
The results are presented as means ± standard deviation (SD). Statistical analysis was performed with one-way ANOVA with Dunnett’s multiple comparison tests using Statistical Package for the Social Sciences (SPSS) software. p values of < 0.05 were considered to indicate statistically significant differences.
Results
The YAP signaling pathway is involved in the caerulein-induced AP in AR42J cells
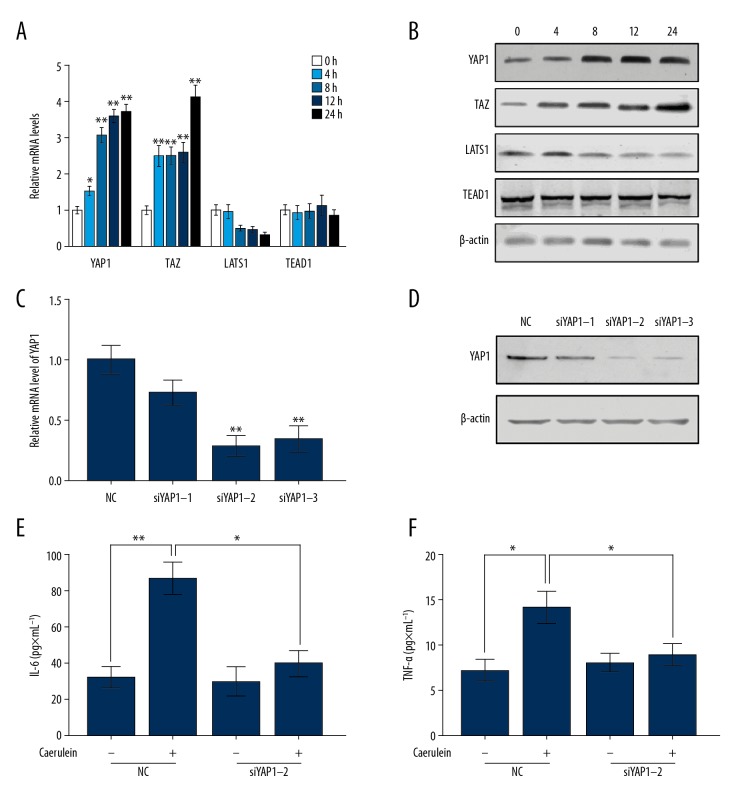
To determine the functional role of the YAP signaling pathway in the AP, we established a caerulein-induced AP model in pancreatic acinar cells AR42J according to the method previously reported [6]. After the induction of AP with caerulein, both the mRNA (Figure 1A) and protein levels (Figure 1B) of YAP1, TAZ, LATS1, and TEAD1 were measured at 0 h, 4 h, 8 h, 12 h, and 24 h. The results showed that caerulein treatment increased the expression of YAP1 and TAZ, and deceased the level of LATS1. However, the level of TEAD1 was not changed by caerulein stimulation. These results suggest that the YAP signaling pathway is involved in caerulein-induced AP. To further investigate the role of the YAP signaling pathway in AP, we transfected 3 siRNAs (siYAP1-1, siYAP1-2, and siYAP1-3) targeting different sites of YAP1 in AR42J cells, and subsequently detected the knockdown efficiency of siRNA at mRNA and protein levels. As shown in Figure 1C and 1D, siYAP1-2 had the highest knockout efficiency, so siYAP1-2 was chosen for the following experiments. Furthermore, the secretion level of pro-inflammatory cytokines, including IL-6 and TNF-α, were detected in AR42J cells to assess the severity of AP. The results in Figure 1E and Figure 1F demonstrate that downregulating YAP1 significantly decreased the secretion levels of IL-6 (Figure 1E) and TNF-α (Figure 1F), which were induced by caerulein stimulation. In summary, YAP1 is upregulated in caerulein-treated AR42J cells and promotes the progression of AP.
Figure 1.
YAP1 was upregulated in caerulein-induced AR42J cells and aggravated the severity of AP. The changes of YAP1, TAZ, LATS1, and TEAD1 at mRNA levels (A) and protein levels (B) were examined in AR42J cells with caerulein stimulation for 0, 4, 8, 12, and 24 h. The AR42J cells were transfected with 3 different siRNAs of YAP1, and the expression level of YAP1 were detected by qRT-PCR (C) and Western blot (D) assays. Measurement of the amount of IL-6 (E) and TNF-α (F) in AR42J cells. (A, C, E, F) The data are shown as means ±SD, * P<0.05, ** P<0.01.
MiRNA-194 is downregulated in AP patients and protects AR42J cells against AP
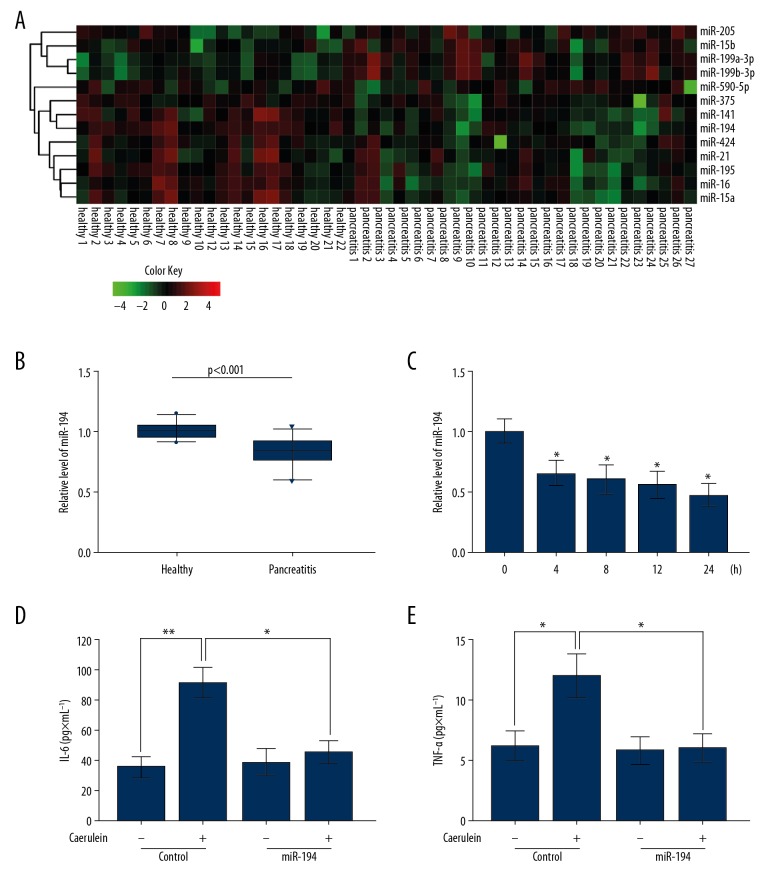
To explore the role of miRNA in the caerulein-treated AR42J cells, we analyzed the miRNA microarray data (GSE24279) of AP patients from the public data site Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/gds/?term=). The results showed that the expression of 13 miRNAs in the TargetScan-predicted YAP1-regulated miRNAs were statistically significant, and 9 of them were downregulated (Figure 2A). miR-194 was the miRNA with the greatest fold change, so we focused on miR-194 in further study. The results showed that the level of miR-194 in AP patients was significantly lower than in healthy controls (Figure 2B). qRT-PCR results also indicated that miR-194 expression decreased in AR42J cells with increasing caerulein stimulation time (Figure 2C). ELISA showed that the secretion of IL-6 (Figure 2D) and TNF-α (Figure 2E) by AR42J cells stimulated by caerulein significantly decreased after the transfection of miR-194. Taken together, these results show that endogenous miR-194 protects AR42J cells from caerulein-induced AP.
Figure 2.
miRNA-194 was downregulated in AP patients and protected AR42J cells against AP. (A) Heatmap of differentially expressed miRNAs that potentially target YAP1. (B) Expression of miR-194 was downregulated in AP patients (n=27) compared to healthy controls (n=22). (C) The transcript level of miRNA-194 was detected by qRT-PCR in AR42J cells with caerulein stimulation for 0, 4, 8, 12, and 24 h. (D, E) MiR-194 mimics were transfected in AR42J cells treated with or without caerulein and the IL-6 (D) and TNF-α (E) were measured. (C–E) The data are shown as means ±SD, * P<0.05, ** P<0.01.
MiR-194 directly decreases the level of YAP1 in the caerulein-induced AP in AR42J cells
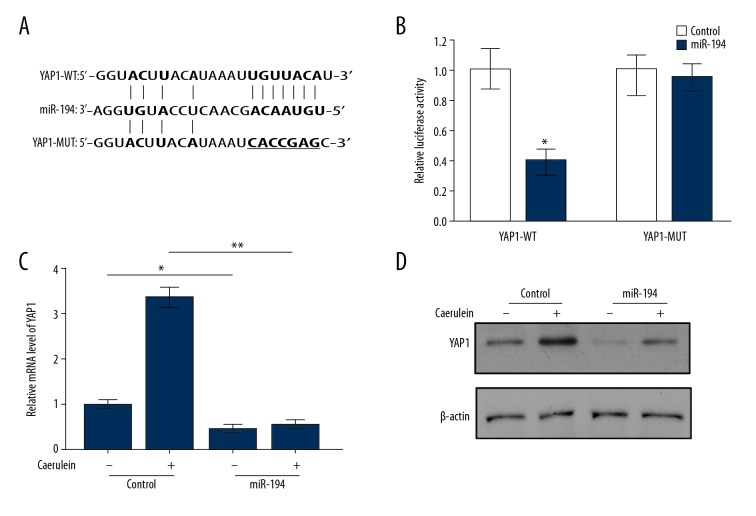
As previously mentioned, YAP1 is a potential target gene of miR-194, and we determined the binding sequence between miR-194 and YAP1 through use of the computational algorithm TargetScan website (Figure 3A). To verify the functional relevance between them, we inserted the sequence of the wild-type YAP1 3′UTR (YAP-WT), containing the potential miR-194 binding site, into the pMIR-Reporter vector and co-transfected it with the miR-194 mimics or negative control (NC). Luciferase reporter assays were performed and the results showed that the luciferase activities in cells that were co-transfected miR-194 and YAP-WT were weaker than in the control group. However, there were no differences in activities between cells co-transfected with miR-194 and those with mutated YAP1 3′UTR (YAP1-MUT) (Figure 3B). Based on the above, we next transfected miR-194 mimics or NC in caerulein -stimulated and control AR42J cells to detect YAP1 expression levels at mRNA and protein levels, respectively. qRT-PCR and Western blot results showed that miR-194 inhibited the expression of YAP1 and significantly attenuated the increase in caerulein-induced AR42J cells (Figure 3C, 3D). These results indicated that miR-194 directly targets YAP1 and inhibits its expression.
Figure 3.
miR-194 directly targeted YAP1. (A) Predicted binding site of miR-194 in the 3′UTR of YAP1. (B) Luciferase assays assessed the activities of wild-type YAP1 3′UTR (YAP1-WT) and mutated YAP1 3′UTR (YAP1-MUT). qRT-PCR (C) and Western blot (D) showed the level of YAP1 in miR-194-transfected AR42J cells with or without caerulein stimulation. (B, C) The data are shown as means ±SD. * P<0.05, ** P<0.01.
MALAT1 competes with YAP1 for miR-194 binding
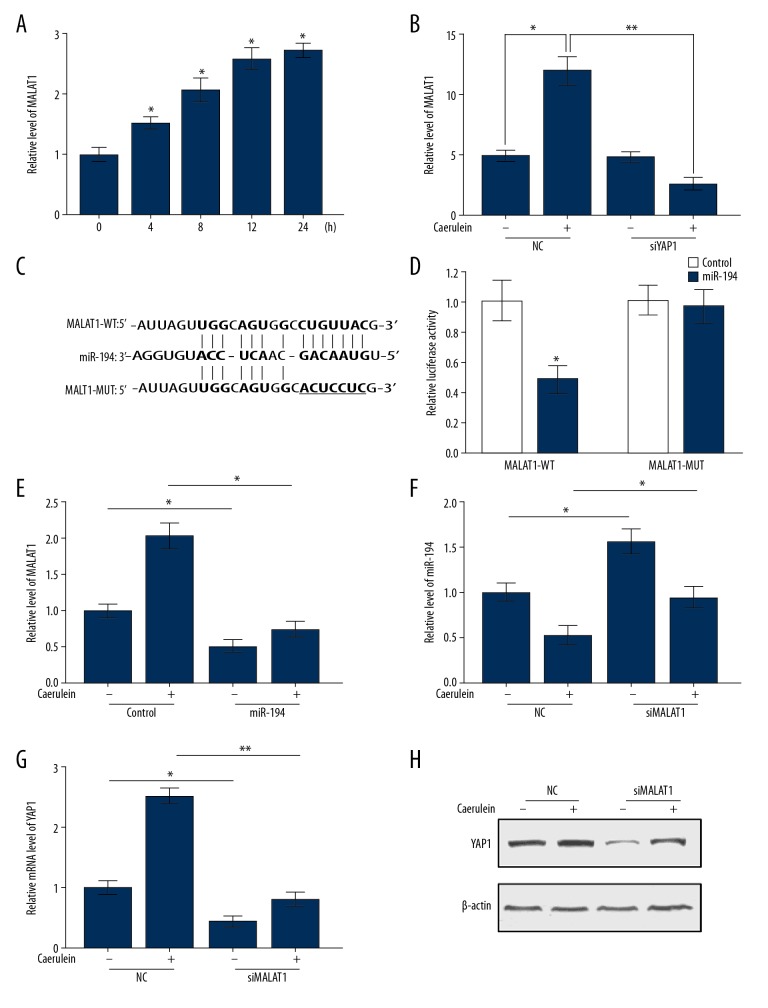
Sun et al. previously reported that YAP1 induced the expression of lncRNA MALAT1 in colorectal cancer [30]. As Figure 4A and 4B show, the level of MALAT1 was increased in AR42J cells after 4 h of caerulein stimulation, and gradually increased with the extension of stimulation time. Bioinformatics results showed that MALAT1 is also a potential target gene of miR-194 and possesses the same miR-194 binding site as YAP1 (Figure 4C). The luciferase report assays showed that MALAT1-dependent luciferase activities are dramatically inhibited by miR-194 mimics when compared to controls (Figure 4D). Meanwhile, the luciferase activities of MALAT1 reporter with the mutation of binding site (MALAT1-MUT) were not affected by miR-194 mimics (Figure 4D).
Figure 4.
MALAT1/miR-194/YAP1 axis implicated in AP. (A) The mRNA level of MALAT1 in AR42J cells with caerulein stimulation for 0, 4, 8, 12, and 24 h. (B) The expression level of MALAT1 was examined in siYAP1-transfected AR42J cells with or without caerulein stimulation. (C) Potential binding sites of MALAT1 and miR-194. (D) Luciferase reporter assays verified the binding relationship between MALAT1 and miR-194. (E) The expression of MALAT1 was inhibited by miR-194 in AR42J cells. (F) The transcription level of miR-194 was upregulated with the downregulation of MALAT1 in AR42J cells. The mRNA (G) and protein (H) level of YAP1 in siMALAT1-transfected AR42J cells with or without caerulein stimulation. (A, B, D–G). Data are shown as means ±SD, * P<0.05, ** P<0.01.
As shown in Figure 4E, the expression level of MALAT1 was decreased in AR42J cells after transfection with miR-194 mimics. Conversely, the silencing of MALAT1 led to a reduction of miR-194 in AR42J cells with or without caerulein treatment (Figure 4F). The normal and caerulein-treated AR42J cells were transfected with miR-194 mimics or NC mimics, and the level of YAP1 was detected by qRT-PCR and Western blot analysis. Overexpression of miR-194 dramatically reduced the level of YAP1 in normal cells and offset caerulein-induced expression (Figure 4G, 4H). In conclusion, our results demonstrate that MALTA1 upregulates the expression level of YAP1 by sponging miR-194 in caerulein-induced AP.
MiR-194 relieves AP by inhibiting expression of MALAT1 and YAP1
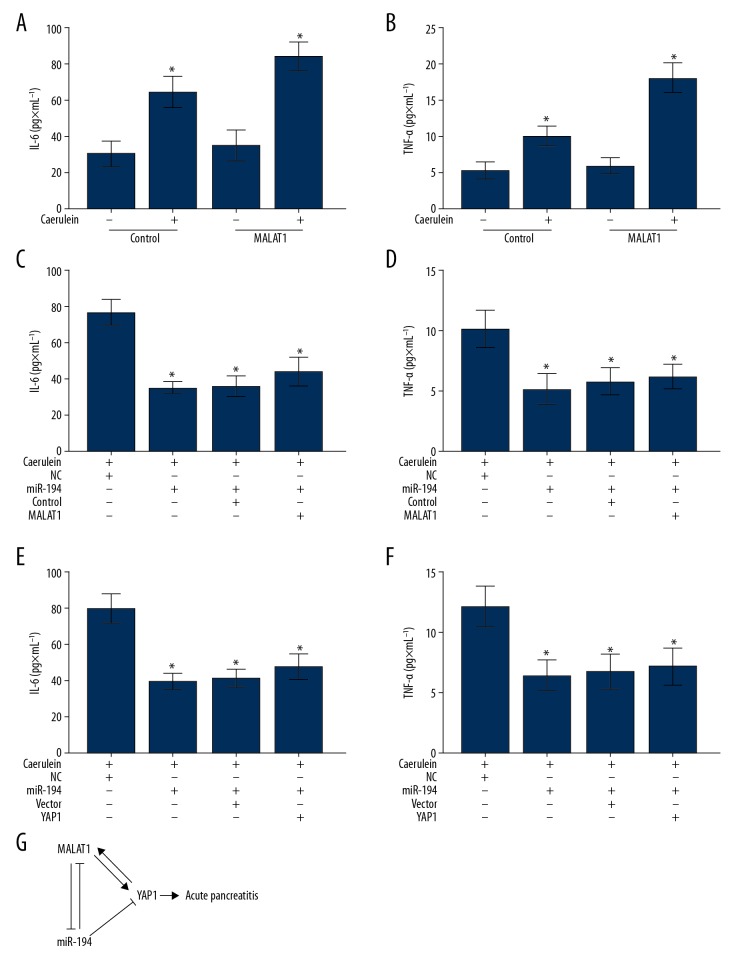
To explore the function of MALAT1 in the progression of AP, MALAT1 was overexpressed in AR42J cells with or without caerulein stimulation. As Figure 5A and 5D showed, overexpression of MALAT1 in caerulein-stimulated AR42J cells promoted the secretion of IL-6 (Figure 5A) and TNF-α (Figure 5B). These results suggest that, similar to YAP1, MALAT1 also acts as a promotor of AP. Since both MALAT1 and YAP1 are target genes of miR-194, we co-transfected miR-194 with MATAL1 or YAP1 in the caerulein-stimulated AR42J cells to examine the level of IL-6 and TNF-α. The ELISA results demonstrated that miR-194 not only inhibited the secretion of IL-6 and TNF-α induced by caerulein, but also counteracted the promotion activity of MALAT1 (Figure 5C, 5D). Similarly, as shown in Figure 5E and 5F, miR-194 also offset the effect of YAP1 in promoting the secretion of IL-6 and TNF-α. In conclusion, the regulation loop (Figure 5G) formed by MALAT1, miR-194, and YAP1 plays roles in regulating the progression of AP, and miR-194 has potential to be used in diagnosis and treatment of acute pancreatitis.
Figure 5.
Enforced expression of miR-194 relieved caerulein-induced AP in AR42J cells. (A, B) Measurement of the concentration levels of IL-6 (A) and TNF-α (B) in the culture supernatant of MALAT1-transfected AR42J cells with or without caerulein stimulation by ELISA. (C, D) The concentration of IL-6 (C) and TNF-α (D) were measured by ELISA in caerulein-induced AP cell model transfected with miR-194 or miR-194 combined with MALAT1. (E, F) The concentration of IL-6 (E) and TNF-α (F) were measured by ELISA in caerulein-induced AP model cells transfected with miR-194 or miR-194 combined with YAP1. (G) Schematic representation of the model, depicting the major molecular mechanisms of the mutual regulatory relationship among MALAT1, miR-194, and YAP1 in AP. (A–F) Data are shown as means ±SD, * P<0.05.
Discussion
As a common manifestation of clinical acute abdomen with many complications, AP has a causal link with high morbidity and mortality [31]. In recent years, it has been pointed out that in the development of AP, pancreatic acinar cells undergo vacuolar accumulation and trypsinogen activation, which are the 2 main pathological reactions, leading to local and systemic inflammatory responses [1,32–34]. However, the molecular mechanisms underlying AP progression remains unclear. It is widely believed that both of these inflammatory cytokines are involved in the progression of pancreatitis [35]. YAP1 is upregulated in the activated stellate cells of CP and PDAC patients. It is worth noting that YAP1 regulates both acinar and ductal cells in mice with AP and CP [36]. However, the details of the role for YAP1 in promoting the development of AP have not been characterized.
As previously described [6,37], we established a cell model of AP in the rat pancreatic acinar cell line AR42J by treatment with caerulein to explore the function of YAP1 in the progression of AP. The results showed that YAP1 is upregulated in AR42J cells with the stimulation of caerulein. ELISA experiments showed that after the knockdown of YAP1 by specific siRNA, the amount of pro-inflammatory cytokines IL-6 and TNF-α secreted by caerulein-induced AR42J cells decreased significantly. These results suggest that inhibition YAP1 expression is a promising therapeutic method for AP.
miRNAs, 18 to 22 nucleotides in length, are noncoding RNAs that regulate post-transcriptional expression of target genes and participate in a variety biological processes [38, 39]. Increasing evidence suggests that multiple miRNAs are dysregulated in AP patients and are involved in the pathogenesis of pancreatitis [40]. To explore whether miRNAs also regulate the level of YAP1 expression in AP models, we used bioinformatics method to analyze the expression profile of miRNAs using the public microarray data of GSE24279. The heatmap showed that miR-194 was significantly decreased in AP patients. miR-194 is involved in the regulation of various tumors, including colon cancer, prostate cancer, breast cancer, and other tumors, but its roles in AP has not been reported [41-43]. Here, we found that miR-194 was downregulated in patients with AP and confirmed that YAP1 is a direct target of miR-194. Enforced expression of miR-194 also significantly reduced the levels of IL-6 and TNF-α in the AP model.
lncRNA, which is more than 200 nucleotides in length, is a type of noncoding RNA without any open reading frame [44]. It has been reported that in colon cancer, YAP1 induces the expression of MALAT1 [30], while the silencing of MALAT1 leads to decreased expression of YAP1 in pancreatic cancer [29]. In this study, we found that MALAT1 and YAP1 contain similar miR-194 binding sites, and both are able to directly bind to miR-194. Overexpression of miR-194 in caerulein-stimulated AR42J cells dramatically inhibited the expression of MALAT1 and YAP1 and decreased the secretion of IL-6 and TNF-α. We also demonstrated that overexpression of MALAT1 or YAP1 in AP model cells increased IL-6 and TNF-α secretion, which was offset by miR-194.
Our study has certain limitations that should be considered. We only used the siRNAs of YAP1 and MALAT1 or miR-194 mimics to investigate their roles in the progression of AP. Further studies of YAP1 and MALAT1 overexpression or miR-194 inhibition should be performed to clarify their functions. Otherwise, all experiments in this study were performed in a cell model of AP, and the animal model should be used to assess the functions of YAP1, MALAT1, and miR-194 in the future.
Conclusions
This present study showed that YAP1 and lncRNA MALAT1 play important roles in AP, and their common suppressor, miR-194, exhibited protective effect against AP. These results suggest that the dynamic balance of the regulatory networks among MALAT1, YAP1, and miR-194 are important in regulating the progression of AP, and enforced expression of miR-194 might be a novel therapeutic strategy.
Footnotes
Source of support: Departmental sources
Conflicts of interests
None.
References
- 1.Gukovsky I, Gukovskaya AS, Blinman TA, et al. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–14. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 2.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142–50. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- 3.Renzulli P, Jakob SM, Tauber M, et al. Severe acute pancreatitis: Case-oriented discussion of interdisciplinary management. Pancreatology. 2005;5:145–56. doi: 10.1159/000085266. [DOI] [PubMed] [Google Scholar]
- 4.Rawla P, Sunkara T, Thandra KC, Gaduputi V. Hypertriglyceridemia-induced pancreatitis: Updated review of current treatment and preventive strategies. Clin J Gastroenterol. 2018;11:441–48. doi: 10.1007/s12328-018-0881-1. [DOI] [PubMed] [Google Scholar]
- 5.Rawla P, Bandaru SS, Vellipuram AR. Review of infectious etiology of acute pancreatitis. Gastroenterology Res. 2017;10:153–58. doi: 10.14740/gr858w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao D, Ge H, Ma B, et al. The interaction between ANXA2 and lncRNA Fendrr promotes cell apoptosis in caerulein-induced acute pancreatitis. J Cell Biochem. 2018 doi: 10.1002/jcb.28097. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Li L, Lu Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang JM, Nagatomo I, Suzuki E, et al. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32:2220–29. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai J, Zhang N, Zheng Y, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–88. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian I, Kim J, Okazawa H, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–72. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Su LL, Ma WX, Yuan JF, et al. Expression of Yes-associated protein in non-small cell lung cancer and its relationship with clinical pathological factors. Chin Med J (Engl) 2012;125:4003–8. [PubMed] [Google Scholar]
- 13.Zhou Q, Li L, Zhao B, Guan KL. The hippo pathway in heart development, regeneration, and diseases. Circ Res. 2015;116:1431–47. doi: 10.1161/CIRCRESAHA.116.303311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heallen T, Zhang M, Wang J, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YW, Guo J, Shen H, et al. Phosphorylation of Tyr188 in the WW domain of YAP1 plays an essential role in YAP1-induced cellular transformation. Cell Cycle. 2016;15:2497–505. doi: 10.1080/15384101.2016.1207836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George NM, Day CE, Boerner BP, et al. Hippo signaling regulates pancreas development through inactivation of Yap. Mol Cell Biol. 2012;32:5116–28. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao T, Zhou D, Yang C, et al. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144:1543–53. 1553e1. doi: 10.1053/j.gastro.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Gruber R, Panayiotou R, Nye E, et al. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology. 2016;151:526–39. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eissa S, Safwat M, Matboli M, et al. Measurement of urinary level of a specific competing endogenous RNA network (FOS and RCAN mRNA/miR-324-5p, miR-4738-3p, /lncRNA miR-497-HG) enables diagnosis of bladder cancer. Urol Oncol. 2019;37(4):292.e19–292.e27. doi: 10.1016/j.urolonc.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Meng L, Ma P, Cai R, et al. Long noncoding RNA ZEB1-AS1 promotes the tumorigenesis of glioma cancer cells by modulating the miR-200c/141-ZEB1 axis. Am J Transl Res. 2018;10:3395–412. [PMC free article] [PubMed] [Google Scholar]
- 23.Blenkiron C, Askelund KJ, Shanbhag ST, et al. MicroRNAs in mesenteric lymph and plasma during acute pancreatitis. Ann Surg. 2014;260:341–47. doi: 10.1097/SLA.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 24.Xiang H, Tao X, Xia S, et al. Targeting microRNA function in acute pancreatitis. Front Physiol. 2017;8:726. doi: 10.3389/fphys.2017.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard D, Prasanth KV, Tripathi V, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–93. doi: 10.1038/emboj.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 28.Dai X, Liu L, Liang Z, et al. Silencing of lncRNA MALAT1 inhibits cell cycle progression via androgen receptor signaling in prostate cancer cells. Pathol Res Pract. 2019;215(4):712–21. doi: 10.1016/j.prp.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Shan T, Ding W, et al. Study on mechanism about long noncoding RNA MALAT1 affecting pancreatic cancer by regulating Hippo-YAP signaling. J Cell Physiol. 2018;233:5805–14. doi: 10.1002/jcp.26357. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, Ou C, Liu J, et al. YAP1-induced MALAT1 promotes epithelial-mesenchymal transition and angiogenesis by sponging miR-126-5p in colorectal cancer. Oncogene. 2019;38(14):2627–44. doi: 10.1038/s41388-018-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: Management of acute pancreatitis. Can J Surg. 2016;59:128–40. doi: 10.1503/cjs.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–55. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schepers NJ, Besselink MG, van Santvoort HC, et al. Dutch Pancreatitis Study Group. Early management of acute pancreatitis. Best Pract Res Clin Gastroenterol. 2013;27:727–43. doi: 10.1016/j.bpg.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Wu L, Cai B, Liu X, Cai H. Emodin attenuates calcium overload and endoplasmic reticulum stress in AR42J rat pancreatic acinar cells. Mol Med Rep. 2014;9:267–72. doi: 10.3892/mmr.2013.1773. [DOI] [PubMed] [Google Scholar]
- 35.Steer ML. Relationship between pancreatitis and lung diseases. Respir Physiol. 2001;128:13–16. doi: 10.1016/s0034-5687(01)00259-6. [DOI] [PubMed] [Google Scholar]
- 36.Morvaridi S, Dhall D, Greene MI, et al. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Sci Rep. 2015;5:16759. doi: 10.1038/srep16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai Y, Shen Y, Xu G, et al. TRAM1 protects AR42J cells from caerulein-induced acute pancreatitis through ER stress-apoptosis pathway. In Vitro Cell Dev Biol Anim. 2016;52:530–36. doi: 10.1007/s11626-016-0011-7. [DOI] [PubMed] [Google Scholar]
- 38.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund E, Guttinger S, Calado A, et al. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Zhu ZM, Tu YL, et al. Identfication of key miRNAs in pancreatitis using bioinformatics analysis of microarray data. Mol Med Rep. 2016;14:5451–60. doi: 10.3892/mmr.2016.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao S, Zhao Z, Wu R, et al. MicroRNA-194 regulates cell viability and apoptosis by targeting CDH2 in prostatic cancer. Onco Targets Ther. 2018;11:4837–44. doi: 10.2147/OTT.S169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Wei H, Liu Y, Zheng S. Promotional effect of microRNA-194 on breast cancer cells via targeting F-box/WD repeat-containing protein 7. Oncol Lett. 2018;15:4439–44. doi: 10.3892/ol.2018.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai HK, Chen X, Tang YH, Deng YC. MicroRNA-194 modulates epithelial-mesenchymal transition in human colorectal cancer metastasis. Onco Targets Ther. 2017;10:1269–78. doi: 10.2147/OTT.S125172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brosnan CA, Voinnet O. The long and the short of noncoding RNAs. Curr Opin Cell Biol. 2009;21:416–25. doi: 10.1016/j.ceb.2009.04.001. [DOI] [PubMed] [Google Scholar]