Abstract
Mesenchymal stem cells (MSCs) are known for being multi-potent. However, they also possess anticancer properties, which has prompted efforts to adapt MSCs for anticancer therapies. However, MSCs have also been widely implicated in pathways that contribute to tumor growth. Numerous studies have been conducted to adapt MSCs for further clinical use; however, the results have been inconclusive, possibly due to the heterogeneity of MSC populations. Moreover, the conflicting roles of MSCs in tumor inhibition and tumor growth impede their adaptation for anticancer therapies. Antitumorigenic and protumorigenic properties of MSCs in hematologic malignancies are not as well established as they are for solid malignancies, and data comparing them are still limited. Herein the effect of MSCs on hematologic malignancies, such as leukemia and lymphoma, their mechanisms, sources of MSCs, and their effects on different types of cancer, have been discussed. This review describes how MSCs preserve both antitumorigenic and protumorigenic effects, as they tend to not only inhibit tumor growth by suppressing tumor cell proliferation but also promote tumor growth by suppressing tumor cell apoptosis. Thus clinical studies trying to adapt MSCs for anticancer therapies should consider that MSCs could actually promote hematologic cancer progression. It is necessary to take extreme care while developing MSC-based cell therapies in order to boost anticancer properties while eliminating tumor-favoring effects. This review emphasizes that research on the therapeutic applications of MSCs must consider that they exert both antitumorigenic and protumorigenic effects on hematologic malignancies.
Subject terms: Stem-cell research, Stem-cell therapies, Preclinical research, Stem cells, Cell death
Introduction
Since the identification of mesenchymal stem cells (MSCs) from adult bone marrow (BM) [1], numerous studies have been performed globally to understand their characteristics and functions. Therefore, it is widely known that MSCs have multi-lineage potential, differentiating into various types of cells, such as adipocytes, chondroblasts, osteoblasts, and tissue macrophage-like cells [2, 3]. The multi-potent properties of MSCs make them promising therapeutic targets and one of the most indispensable sources of new clinical therapies [3]. In fact, MSCs have been widely used in regenerative medicine for bone and cardiovascular repair [4, 5]. Moreover, they can migrate to damaged tissue, can self-renew, and exert immunomodulatory and antitumor effects [5–7]. Despite extensive research carried out over the past 10 years, it is still unclear whether MSCs have tumor-suppressing or tumor-promoting effects [7].
MSCs have highly heterogeneous features [8]. This may explain why clinical trials involving MSCs have not developed beyond phase 1 and have been inconclusive [9]. Approximately 42 clinical trials investigating the role of MSCs on tumors are registered at www.clinicaltrials.gov, with only 13 targeting hematologic malignancies. These malignancies include myelodysplastic syndrome, leukemia, lymphoma, and multiple myeloma. Among the 13 clinical trials targeting hematologic malignancies, only one focused on anticancer effect of MSCs, while most of the other trials were related to the immunoregulatory effect of MSCs after stem cell transplantation. Further research is required to use MSCs for the treatment of hematologic malignancies. Here the various issues with safety, effectiveness, and the current status regarding the tumor-related effects of MSCs are presented.
Dual role of MSCs in hematologic malignancy progression: MSCs suppress both proliferation and apoptosis
Cancer is difficult to target because it is not a single disease, but a class of diseases in which a group of cells display uncontrolled growth, invasion, and sometimes metastasis. Therefore, it seems impractical to develop one specific method to treat cancer. A variety of promising new therapies, such as cell therapy and immunomodulation, are being developed. Ongoing research suggests that MSCs are excellent targets for cell therapy in a variety of cancers. However, the reported antitumor effects are still controversial. Regardless of the type of cancer, some studies have shown inhibitory effects, while others demonstrate proliferative effects of MSCs on tumors [10]. For example, MSCs have tumoricidal effects on breast and lung cancer cell lines in vitro [11, 12] and on pancreatic tumors in vivo [13]. However, MSCs promote breast and melanoma cancer cell proliferation when co-cultured with tumor cell lines in vitro [14, 15]. They also increase tumor growth when injected into mice with lung or prostate cancer [16, 17]. Interestingly, both inhibitory and proliferative effects of MSCs have been reported in the same study [7, 12]. Several studies suggest that MSCs appear to influence pathways that can suppress both proliferation and apoptosis [18, 19]. The dual role of MSCs can be described as a “double-edged sword.” Therefore, it is important to understand its dual role in tumor cell proliferation.
There is less known about the function of MSCs in hematologic malignancies, such as leukemia, lymphoma, and multiple myeloma, than for solid malignancies, as described above. However, the dual function of MSCs may be applicable to hematologic cancers. According to several studies, it is evident that MSCs possess the ability to inhibit or promote tumor growth by suppressing proliferation or apoptosis of tumor cells, respectively, in hematologic malignancies [7, 10, 12]. Although minor reports have shown that MSCs can directly promote proliferation of hematologic malignant cells or promote apoptosis [20, 21], the primary hypothesis is that MSCs suppress both proliferation and apoptosis. Thus the use of MSCs for the treatment of hematologic malignancies is currently unclear, because inhibitory and promoting effects of MSCs on malignancies are known, both in vitro and in vivo [22, 23].
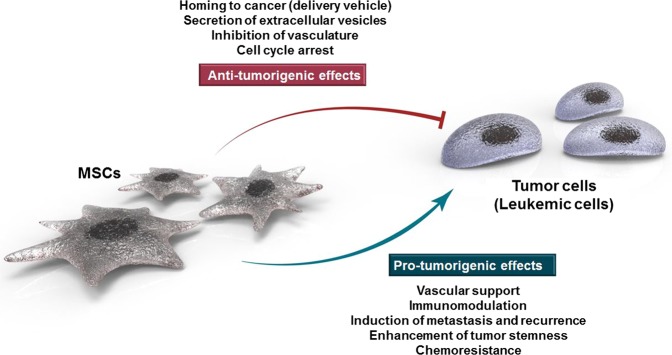
Moreover, mechanisms underlying antitumorigenic or protumorigenic effects remain unclear. Several different mechanisms have been suggested (Tables 1–4), some of which are favorable for the inhibition of hematologic malignancies. These include the possible use of MSCs as a delivery vehicle [24–26] to inhibit vascular growth [27–31] or to decrease cell proliferation by arresting the cell cycle [22, 23, 32–36]. These mechanisms favor the development of MSC-based therapies. However, MSCs are not favorable for clinical use because they have been implicated in supporting tumor vasculature [37–40], exerting immunomodulatory effects in cancer [41–43], and increasing the rate of metastasis and recurrence [44–53]. Moreover, recent studies have focused on how MSCs tend to protect tumor cells from drug-induced apoptosis, leading to chemo-resistance [54–62].
Table 1.
Studies suggesting that MSCs inhibit hematologic malignancy by decreasing tumor cell proliferation in vitro
| Isolated MSC | Tumor cell | Tumor cell no. (cells) | MSC:tumor cell ratio | Proposed mechanism | Reference |
|---|---|---|---|---|---|
| Mouse BM-MSC | Erythroleukemia (FBL3), ALL (P388), and B-lymphoma (A20) | 2 × 104 | 1:0.4, 1:1, 1:4, 1:10 | Induction of cell cycle arrest and apoptosis of tumor cells | Song et al. [22] |
| Human BM-stromal cell line (HFCL) | AML (U937, HL-60, and HL-60/VCR) | 2 × 104 | — | Induction of specific gene expression, leading to cell cycle blockage | Liang et al. [32] |
| Human BM-MSC | CML (BV173 and K562), AML (KG1a), and T-ALL (Jurkat) | 5 × 103 | 1:1, 1:5, 1:10, 1:100 | Transient arrest of tumor cells in G1 phase | Ramasamy et al. [23] |
| Human BM-MSC | CML (K562 and BV173) | 1 × 106 | 1:10 | — | Zhang et al. [54] |
| Human BM-MSC and CML patient’s BM-MSC | CML (K562 and patient’s cells) | — | 1:10 | Regulation of apoptosis-related protein expression and activation of the Wnt signaling pathway | Han et al. [71] |
| Human BM-MSC | CML (BV173) and T-ALL (Jurkat) | 1 × 106 | 1:5, 1:10, 1:50, 1:100 | Induction of cell cycle arrest of leukemic cells | Sarmadi et al. [33] |
| Leukemia patient’s BM-MSC | CML (K562) | 1 × 105 | 1:10 | Induction of cell cycle arrest of leukemic cells | Wei et al. [34] |
| Human UC-MSC | AML (HL-60) and CML (K562) | 1 × 104 | 1:1, 1:5, 1:10 | Activation of p38 MAPK and induction of cell cycle arrest of leukemic cells | Tian et al. [35] |
| Human UC-MSC | CML (K562) | 5 × 103 | MSC secretome used | Paracrine signaling by the secretome | Hendijani et al. [72] |
| Human AT-MSC | AML (HL-60) and CML (K562) | 1 × 106 | 1:10 | Secretion of DKK-1 by NANOG | Zhu et al. [74] |
| Human BM-MSC | CML (patient’s cells) | 1 × 104 | 1:0.1, 1:1, 1:10 | Production of IFN-ɑ | Zhang et al. [75] |
| Human UC-MSC | T-ALL (Jurkat) | 2 × 106 | 1:10 | Activation of Notch signaling pathway | Yuan et al. [76] |
MSC mesenchymal stem cell, BM bone marrow, AML acute myeloid leukemia, CML chronic myeloid leukemia, UM umbilical cord, T-ALL T cell acute lymphoblastic leukemia, MAPK mitogen-activated protein kinase, IFN interferon, AT adipose tissue
Table 4.
Studies suggesting that MSCs induce drug resistance of hematologic malignant cells
| Isolated MSC | Tumor cell | Drug | Proposed mechanism | Reference |
|---|---|---|---|---|
| Human BM-MSC | CML (K562 and BV173) | Imatinib | Upregulation of IL-7 | Zhang et al. [54] |
| Human BM-MSC | CML (KBM-5) | Imatinib | Upregulation of CXCR4 | Jin et al. [55] |
| Human BM-MSC | CML (BV173 and patient’s cells) | Imatinib | Upregulation of Bcl-xL expression and CXCL12/CXCR4 interaction | Vianello et al. [62] |
| Human BM-MSC and CML patient’s BM-MSC | CML (K562 and patient’s cells) | Adriamycin | Regulation of apoptosis-related protein expression and activation of the Wnt signaling pathway | Han et al. [71] |
| Human BM-MSC | AML (OCI-AML3) | Cytarabine | Regulation of leukemia–MSC interactions by ARC protein | Carter et al. [56] |
| Human BM-MSC | AML (U937 and KG1a) | Mitoxantrone | Upregulation of c-Myc | Xia et al. [57] |
| Human BM-MSC | AML (HL-60, THP1, U937, and patient’s cells) | Idarubicin | Activation of Notch signaling | Takam Kamga et al. [58] |
| Human BM-stromal cell line (HFCL) | AML (HL-60 and HL-60/VCR) | Topotecan | Upregulation of Bcl-2 expression | Liang et al. [32] |
| Mouse stromal cell line (MS-5) | AML (HL-60 and patient’s cells) | Cytarabine | Increased Bcl-2 and Bcl-xL expression | Konopleva et al. [108] |
| Human stromal cell line (HS-5) | AML (patient’s cells) | Cytarabine and Daunomycin | Direct cell-to-cell interactions | Garrido et al. [107] |
| Human BM-MSC and AML patient’s BM-MSC | AML (OCI-AML3 and patient’s cells) and pre-B ALL (Reh and RS4;11) | Cytarabine, Vincristine, and Doxorubicin | NF-κB activation in MSCs via a VCAM-1/VLA-4 axis | Jacamo et al. [137] |
| Human BM-MSC | T-ALL (Jurkat and patient’s cells) | Cytarabine and Methotrexate | Mitochondrial fission and p21 downregulation by activated ERK/Drp1 | Cai et al. [59] |
| Human BM-MSC | pre-B ALL (Reh) | Genotoxic agents | Downregulation of p21 protein | Zhang et al. [60] |
| Human BM-MSC | T-ALL (Molt-4, Jurkat, CCRF-CEM, and CEM/C1) | Idarubicin | Activation of ERK by direct contact of leukemic cells and MSCs | Wu et al. [61] |
| Human UC-MSC | ALL (Jurkat) | Dexamethasone | Upregulation of Jagged 1 and overexpression of its receptor, Notch 1 | Yuan et al. [76] |
MSC mesenchymal stem cell, BM bone marrow, AML acute myeloid leukemia, CML chronic myeloid leukemia, UM umbilical cord, T-ALL T cell acute lymphoblastic leukemia, CLL chronic lymphocytic leukemia, IL interleukin, ERK extracellular signal–regulated kinase, Drp1 dynamin-related protein 1, CXCR C-X-C chemokine receptor, CXCL C-X-C chemokine ligand, ARC apoptosis repressor with caspase recruitment domain, NF nuclear factor, VCAM-1 vascular cell adhesion molecule-1, VLA-4 very late antigen-4
Antitumorigenic effects of MSCs
Decreased proliferation of tumor cells in vitro
Although MSCs can inhibit and aggravate hematologic malignancies, it can also reduce proliferation of tumor cells in vitro. Studies demonstrating antitumor effects of MSCs and consequently inhibiting tumor growth are shown in Table 1. The mentioned studies utilized MSCs obtained from various sources. These sources include BM, which was the first source discovered for clinical applications, adipose tissue (AT), and umbilical cords (UC) [63, 64]. MSCs originating from these three sources are known to have similar phenotypes, surface antigen expression, and immunosuppressive properties [65, 66]. Our data also show that the antitumor effects of MSCs are not dependent on their origin. Most of the studies in Table 1 were carried out using leukemia cell lines, such as Jurkat, HL-60, and K562, instead of primary cells.
Another important consideration, besides the cell type used, is the concentration of the cells, specifically, the number of MSCs and tumor cells that were co-cultured. Culture conditions, especially the density of MSCs, is known to significantly affect morphology, proliferation rate, and secreted factors [67, 68]. Various types of studies, including gene expression profiles, have demonstrated the multi-functionality of MSCs, including immunoregulation, which can consequently alter the tumor-favoring or -suppressing effects of MSCs [69, 70]. Moreover, it has been suggested that antitumor effects observed in solid cancers are associated with a lower number of MSCs than those with tumor-promoting effects [7]. This association has not yet been suggested for hematologic malignancies but that may be due to lack of data. However, it still seems necessary to standardize the concentration of MSCs and hematologic malignant cells when they are co-cultured in vitro or injected into an in vivo model to safely and effectively use MSCs for further clinical adaptations.
There are many suggested mechanisms explaining the effects of MSCs on tumor cells; however; the most common and widely accepted mechanism is that MSCs induce tumor cell cycle arrest. Song et al. [22] co-cultured C57BL/6 mouse BM-derived MSCs with A20 murine B-lymphoma, FBL3 murine erythroleukemia, and P388 murine acute lymphoblastic leukemia (ALL) cells. They evaluated cell proliferation, apoptosis, cell cycle progression, and cytokine secretion. Consequently, MSCs suppressed the proliferation of lymphoma and leukemia cells in vitro via cell cycle arrest and reduced the levels of interleukin (IL)-10 secretion. Liang et al. [32] also suggested that cell cycle G0/G1 blockage, by transcriptional activation of specific genes, is the underlying mechanism of MSCs’ antitumor effect. In their study, the proliferation of acute myeloid leukemia (AML) cells co-cultured with a human BM fibroblastoid stromal cell line (HFCL) was inhibited. The percentage of G1 phase tumor cells, when co-cultured with HFCL, was significantly higher than that without HFCL and less S phase cells were observed. Similarly, Ramasamy et al. [23] found that MSCs downregulate cyclin D2 levels, leading to a transient cell cycle arrest of tumor cells in the G1 phase. MSCs were found to inhibit the self-renewal ability of cancer cells and their stromal environment could influence malignant diseases [54, 71]. Data presented by Sarmadi et al. [33] and Wei et al. [34] also support this finding, as they found significantly less proliferation of BV173/Jurkat and K562 cell lines when they were co-cultured with MSCs, due to tumor cell cycle arrest in the G0/G1 phase. They showed that proliferation was inhibited in a dose-dependent manner, mainly via cell-to-cell contact. Unlike these five reports mentioned above, Tian et al. [35] used MSCs derived from UC, and not from BM. However, their findings were similar, as proliferation was inhibited by MSCs due to the G0/G1 arrest. Moreover, in this study p38 mitogen-activated protein kinase (MAPK) was suggested as a potent cell proliferation and tumorigenesis suppressor of HL-60 and K562 cells. Gene silencing using small interfering RNA or pharmacological inhibition of p38 MAPK abrogated the inhibitory effect that lead to cell cycle arrest by MSCs. There are other reports suggesting different mechanisms for its antitumor effects besides cell cycle arrest. For example, Hendijani et al. [72] showed antitumor effects of the MSC secretome on leukemia cells. Thus certain secreted substances or paracrine signals may be involved, but the exact mechanism was not demonstrated in their report.
Shen et al. [73] showed that Wnt5a is a major modifier of tumor cell proliferation. When HL-60 leukemia cells were stimulated with the supernatant of adeno-Wnt5a MSCs, proliferation of leukemia cells highly reduced. Zhu et al. [74] have also emphasized the importance of the Wnt signaling pathway in regulating the antitumor effects of AT-derived MSCs, because of the increased secretion of Dickkopf-related protein (DKK)-1, a regulator of the Wnt signaling pathway. Another important underlying mechanism seems to be related to interferon (IFN)-α secretion [75]. Co-culturing MSCs with chronic myeloid leukemia (CML) mononuclear cells greatly inhibited their proliferation and this was associated with higher IFN-α levels in the supernatant of the co-cultured cells. IFN-α secretion increased with the increase in the concentration of MSC and co-culture duration. However, Yuan et al. [76] showed that Jurkat leukemia cell proliferation decreased owing to the UC-derived MSCs. Increased cellular expression of HES-1 transcription factor, which is involved in the Notch signaling pathway, was also observed in this study.
Decreased tumor growth in vivo
Studies demonstrating the antitumor effects of MSCs by inhibiting tumor growth in vivo are shown in Table 2. After intravenous injection of MSCs into BALB/c mice with BALB/c-derived B-lymphoma A20 cells, Song et al. [22] showed a reduction in the incidence of lymphoma and improved survival rates. After co-culturing with MSCs, level of IL-10 in the supernatant of A20 cell cultures significantly decreased in a time-dependent manner. This could contribute to immune evasion. Furthermore, when co-cultured with MSCs, the fraction of A20 cells expressing intracellular IL-10 significantly increased, suggesting that MSCs inhibit the secretion of IL-10 by A20 cells. They concluded that the unexpected tumorigenic effect of MSCs shown in non-obese diabetic/severe combined immunodeficient mice with leukemia was a result of the animal type they used [23]. They mentioned that immunodeficient mice do not reflect the environment of autologous tumor development, and therefore, their in vivo data using BALB/c mice were more reliable. A study by Secchiero et al. [77] supports the tumor-suppressing effect of MSCs. Intraperitoneal injection of MSCs was performed 4 days after lymphoma cell injection. Tumor development was slower and was coupled with a large stromal infiltration and extensive intratumor necrosis. Moreover, when MSCs were directly co-cultured with endothelial cells, they observed a significant induction of endothelial cell apoptosis, suggesting that MSCs, under certain circumstances, may exert antiangiogenic activity. In addition, Zhu et al. [74] demonstrated that MSCs can inhibit K562 proliferation in vivo and that the inhibitory effect of MSCs was achieved through secretion of DKK-1, which suppresses the Wnt signaling pathway and inhibits cell proliferation.
Table 2.
Studies suggesting that MSCs affect hematologic malignancy by decreasing or increasing tumor growth in vivo
| Isolated MSC | Tumor cell | Tumor cell no. (cells) | Animal type | Findings | Proposed mechanism | Reference |
|---|---|---|---|---|---|---|
| Mouse BM-MSC | B-lymphoma (A20) | 1 × 104 | BALB/c mouse | Inhibit lymphoma cell growth | Inhibition of IL-10 secretion to immune evasion of lymphoma cells | Song et al. [22] |
| Human BM-MSC | Lymphoma (BJAB and SKW6.4) | 2 × 106 | SCID mouse | Inhibit lymphoma cell growth | Induction of apoptosis of endothelial cells to form new blood vessels | Secchiero et al. [77] |
| Human AT-MSC | CML (K562) | 2 × 105 | BALB/c-nu mouse | Inhibit leukemic cell proliferation | Induction of cell cycle arrest by secretion of DKK-1 | Zhu et al. [74] |
| Human BM-MSC | CML (BV173) | 1 × 106 | NOD/SCID mouse | Induce leukemic cell growth and reduce apoptosis | Formation of a cancer stem cell niche to preserve the self-renewal ability of leukemic cells | Ramasamy et al. [23] |
| Human AT-MSC | ALL (Reh, CCRF-CEM, SUP-T1, and CCRF-HSB2) | 1 × 105 1 × 107 | NOD/SCID mouse | Induce leukemic cell growth | — | Lee et al. [110] |
MSC mesenchymal stem cell, BM bone marrow, CML chronic myeloid leukemia, ALL acute lymphoblastic leukemia, IL interleukin, AT adipose tissue
Favorable characteristics and mechanisms of MSCs for inhibition of hematologic malignancy
MSCs as delivery vehicles
MSCs are promising delivery vehicles and can be used for cancer therapy [24–26]. They are easily obtainable, hypo-immunogenic, rapidly expanded in vitro, and transplantable [78]. Moreover, MSCs are known to have inherent tumor-tropism capacities and thus can migrate to tumor sites. The cytotoxic effect of MSCs may be beneficial if they could migrate to tumor sites [79]. However, there have been a number of challenges in adapting the homing ability of MSCs for targeted delivery [80, 81]. Pharmacological properties of anticancer drugs improve with the use of drug delivery systems [81, 82]. Although there are limitations, including rapid clearance of nano-carriers from the bloodstream, a combination of the hypo-immunogenic and active targeting abilities of MSCs is promising for anticancer therapies [78]. MSCs can also be adapted as gene therapy carriers in a similar manner. They were first used to deliver IFN-β for treating ovarian cancer [83], reducing tumor growth, and prolonging survival in mouse models. Since then several researchers have used MSCs to deliver genes to certain tumors. Delivery of other factors, such as IFN-γ [84, 85], IL-12 [86, 87], IL-24 [88], and tumor necrosis factor-related apoptosis inducing ligand [89, 90], has also resulted in significant suppression of tumor cell growth.
Moreover, at the cellular and molecular level, MSCs produce most of their effects through paracrine action [91]. Extracellular vesicles (EVs), including exosomes and microvesicles, are lipid membrane-bound vesicles secreted from MSCs. EVs comprise a variety of molecules such as proteins, RNAs, and microRNAs that have originated from MSCs and these molecules are transferred to the other cells, such as cancer cells. Among the many subtypes of EVs, endosome-derived exosomes have emerged as physiologically relevant and powerful components of MSC secretome [92, 93]. Recent report showed that MSC secretome produced an antiproliferative effect on leukemic cells and a cytotoxic effect in combination with doxorubicin [72], indicating anti-leukemic potentials of exosome derived from MSCs. In addition, synthetically personalized exosome mimetics (EMs) could be the alternative vehicles for drug delivery as effective therapeutic agents. EMs from MSCs mixed with paclitaxel by extrusion could be isolated and drug-loaded MSC-EMs have revealed therapeutic efficiency against breast cancer [94]. MSC-EMs may be used as drug delivery vehicles for cancer treatment.
Inhibition of vascular growth
MSCs are known to have proangiogenic characteristics, resulting in tumor growth. However, there is evidence that MSCs can impair angiogenesis or vessel growth under certain conditions. They can migrate to endothelial cell-derived capillaries to produce reactive oxygen species [27, 28]. As a result, MSCs can activate endothelial cell apoptosis in vitro and suppress not only tumor growth but also capillary vessel density in a concentration-dependent manner in mouse melanoma models [28]. Reduced vascular density, leading to tumor growth inhibition, is known in various types of cancers, including breast cancer, glioma, and melanoma [29–31]. The underlying mechanisms appear to be involved in the modulation of the vascular endothelial cadherin/β-catenin signaling pathway [29] and downregulation of platelet-derived growth factor [30], IL-1β [30], and vascular endothelial growth factor (VEGF) [31]. Moreover, a recent study demonstrated that MSCs present in high numbers are potentially cytotoxic. Therefore, local injection of MSCs into tumor tissues may be an effective antiangiogenic treatment [28]. The inhibitory effect on tumor-related vessel growth has not been clearly demonstrated in hematologic cancers, but it may be an important mechanism, as these cancers are still dependent on vascular support [95].
Cell cycle arrest
Hematologic malignancies have fewer pathways compared to solid malignancies. The most common underlying process of tumor cell growth inhibition is cell cycle arrest, as listed in Table 1. Although DNA repair processes and cell cycle checkpoints seem to be linked to various cancers, induction mechanism of cancer cell arrest by antitumor agents, is still unknown. Since the precise molecular mechanisms of the cell cycle defects are not well understood, the effects of MSCs on leukemia or lymphoma are not well studied [36]. Several studies showing high level of cells arrested at G0/G1 phase did not reveal the underlying molecular processes. Therefore, further research is needed to study the mechanisms of tumor cell cycle arrest that consequently lead to the antitumor effects of MSCs on hematologic malignancies.
Protumorigenic effects of MSCs
Suppressed apoptosis of tumor cells in vitro
MSCs possess protumorigenic effects and suppress tumor cell apoptosis in vitro, as mentioned above. Studies emphasizing the tumor-favoring effect of MSCs are listed in Table 3. Most of the studies in Table 3 are based on MSCs derived from the BM. More than half of the studies shown in Table 3 used primary cancer cells obtained from leukemia patients instead of the reported cell lines [42–44.96–100], This is due to the difference between primary cancer cells and immortalized cell lines. Immortalized cell lines are known to have significant mutations, which can lead to altered cell traits, which could be a limitation while adapting them for clinical trials [101, 102]. Various factors and signaling pathways have been suggested to be involved in tumor-favoring mechanisms of MSCs. Cell-to-cell contact with MSCs seem to be critical for these factors and signaling pathways to be activated. The specific mechanisms are not fully understood; however, there are several studies emphasizing the importance of cell-to-cell contact. For example, Manabe et al. [103] demonstrated the antiapoptotic activity of MSCs in B-lineage ALL cells. Fifteen of the 18 B-lineage ALL cases showed 50% decrease in viability after 72 h of culture in medium alone, while apoptosis was prevented in 10 of the 12 ALL cases when they were cultured with allogeneic BM stromal cells as feeder layers. They suggested that certain soluble factors play an important role in the interaction between immature B cells and BM stroma cells. Panayiotidis et al. [104] showed that cells in 7 out of 10 cases of chronic lymphocytic leukemia (CLL) remained viable for a longer time when cultured with BM stromal cells. Here adherence of CLL cells to the BM stromal cell layers was also required for MSCs to protect cancer cells from apoptosis. Lagneaux et al. [105] demonstrated the dependence of apoptosis on direct contact between leukemic cells and stromal cells. They showed that adhesion of B-CLL cells to the stromal cells rescued them from apoptosis and extended their life span in vitro. Direct cell-to-cell contact was also found to be critical in a study by Nwabo Kamdje et al. [106]; however, they suggested that the antiapoptotic activity on leukemic cells is mediated by Notch-3 and Notch-4 or Jagged-1/-2 and Delta-like protein 1 in a synergistic manner, while many studies have failed to report specific mechanisms.
Table 3.
Studies suggesting that MSCs aggravate hematologic malignancy by suppressing tumor cell apoptosis in vitro
| Isolated MSC | Tumor cell | Tumor cell no. (cells) | MSC:tumor cell ratio | Proposed mechanism | Reference |
|---|---|---|---|---|---|
| Human BM-MSC | B-ALL (patient’s cells) | 1 × 106 | — | Secretion of soluble factors by MSCs | Manabe et al. [103] |
| Human BM-MSC | CLL (patient’s cells) | 4 × 105, 2 × 106 | — | Cell-to-cell contact of tumor cells with MSCs | Panayiotidis et al. [104] |
| Human BM-MSC | B-CLL (patient’s cells) | 2 × 106 | — | Increased Bcl-2 expression by direct contact between leukemic cells and stromal cells | Lagneaux et al. [105] |
| Human BM-MSC | B-ALL (patient’s cells) | 1 × 105 | 1:10 | Activation of Notch-3 and -4 signaling when tumor cells are in contact with MSCs | Nwabo Kamdje et al. [106] |
| Human stromal cell line (HS-5) | AML (patient’s cells) | 4–6 × 105 | 1:4~1:6 | Direct cell-to-cell interactions regulating antiapoptotic effects, not including Bcl-2 | Garrido et al. [107] |
| Mouse stromal cell line (MS-5) | AML (HL-60 and patient’s cells) | 5 × 105 | — | Increased Bcl-2 expression | Konopleva et al. [108] |
| Human BM-MSC | BCP-ALL (patient’s cells) | — | — | Secretion of PGE2 from MSCs | Naderi et al. [109] |
| Leukemia patient’s BM-MSC | CML (K562) | 1 × 105 | 1:10 | Activation of the PI3K-Akt-Bad pathway | Wei et al. [34] |
| Human UC-MSC | T-ALL (Jurkat) | 2 × 106 | 1:10 | Activation of the Notch signaling pathway | Yuan et al. [76] |
| Human BM-MSC | CML (BV173) | 1 × 106 | 1:10 | Transient cell cycle arrest conferring increased leukemic cell survival by preserving their proliferative ability | Ramasamy et al. [23] |
MSC mesenchymal stem cell, BM bone marrow, AML acute myeloid leukemia, CML chronic myeloid leukemia, UM umbilical cord, T-ALL T cell acute lymphoblastic leukemia, CLL chronic lymphocytic leukemia, PI3K phosphoinositide 3-kinase, PGE2 prostaglandin E2
There are two other studies that used human and mouse stromal cell lines [107, 108] instead of primary MSCs, with similar results to the other studies in Table 3. Garrido et al. [107] cultured leukemic cells of 30 AML patients in direct contact with HS-5 human BM stromal cell monolayers or with HS-5 cells separated by transwell inserts. Leukemic cells were protected from culture- and drug-induced apoptosis when in direct contact. On the other hand, Konopleva et al. [108] used mouse stromal cell lines, which prevented apoptosis of HL-60 cells and primary AML blasts. They also observed increased B cell lymphoma-2 (Bcl-2) expression after co-cultivation of the leukemic cells with MSCs. Moreover, Naderi et al. [109] identified prostaglandin E2(PGE2) as a critical compound for antiapoptotic activity by showing that cell death is reversible upon inhibition of PGE2 synthesis. Primary B cell precursor ALL cells were protected from p53 accumulation and apoptosis through activation of cyclic adenosine monophosphate and protein kinase A signaling. Han et al. [71] explored the effects of MSC on proliferation, apoptosis, and secretion of cytokines during blastic phase-chronic myelogenous leukemia (CML-Bp). CML-Bp MSCs protected K562 CML cells and demonstrated an increased antiapoptotic ability, regulating the expression of apoptosis-related proteins and activating the Wnt pathway.
Some studies have demonstrated tumor-favoring effects as well as tumor-inhibiting effects, which are listed in both Tables 1 and 3 [23, 34, 76]. Apart from tumor cell cycle arrest as an explanation for antitumor effects of MSCs, phosphatidylinositol-3-kinase/protein kinase B (Akt)-Bad signaling [34] and Notch signaling [76] pathways have been implicated as the mechanisms that lead to antiapoptotic processes and tumor growth. Ramasamy et al. [23] showed that MSCs reduce apoptosis of BV173 CML cells. Their in vitro data showed that leukemic cells that had been in contact with MSCs were in a resting state (G0/G1), coupled with downregulation of cyclin D2. Such inhibition is likely to confer improved survival rates for leukemic cells by preserving their proliferative capacity and thus their self-renewal ability. Although the specific underlying processes and their interactions that mediate the effects of MSCs on hematologic malignancies are not yet clear, these reports support the idea of dual functionality of MSCs.
Increased growth of tumors in vivo
Studies showing data regarding the in vivo tumor-favoring effects of MSCs on hematologic cancer are few. As shown in Table 2, Ramasamy et al. [23] suggested that MSCs present different characteristics based on the type of study, i.e., in vitro or in vivo. Specifically, MSCs were found to arrest leukemic cells in vitro, while tumor growth was aggravated when tumor cells were injected into mice. They suggested that MSCs have the ability to form a cancer stem cell niche, in which tumor cells contain the potential to proliferate and maintain malignant processes. Recently, we showed that MSCs facilitate the growth of ALL cells through the detection of viable luminescent ALL cells in an in vivo model [110]. This suggests that MSCs negatively affect hematologic malignancy such as recurrence of ALL cells. This should be considered before developing cell therapy products based on MSCs for the treatment of hematologic malignancy. Therefore, the dual function of MSCs and their effects on hematologic malignancies and solid cancers needs to be further studied before adapting them for clinical uses.
Favorable characteristics and mechanisms of MSCs for aggravation of hematologic malignancy
Tumor vasculature support
Both hematologic malignancies and solid tumors require vascular support, which is promoted by MSCs [37–40.111–117]. MSCs are likely to support tumor vasculature directly by differentiating into pericytes or endothelial cells and indirectly by assisting the secretion of proangiogenic factors [15, 37–40]. Transplanted MSCs are engrafted into the perivascular niche when directly interacting with endothelial cells [118]. A population of MSC-like cells have been found in the perivasculature of mouse and human organs [119, 120]. Pericytes play an important role in vascular stabilization, but MSCs can also differentiate into endothelial cells, which may increase the density of vascularity and neovascularization [111, 112]. To support tumor vasculature, certain soluble factors must be secreted by MSCs. Vascular endothelial growth factor (VEGF) is well known as one of the proangiogenic factors involved in tumor angiogenesis [113, 114]. However, other proangiogenic cytokines are required for the angiogenic activity of VEGF. Recombinant VEGF alone does not show the same vascular support [115]. Other soluble factors, such as fibroblast growth factor-1, angiopoietin-1, and IL-6, are known to be secreted from MSCs [116, 117]. Several studies indicate that the vascular-supporting effect of MSCs is much more prominent than the inhibition of tumor capillary growth. Thus, if the angiogenic pathway can be blocked, developing MSC-based cell therapies that focus on the proangiogenic effects of MSCs may be promising.
Immunomodulatory effects of MSCs in cancer
There have been several studies emphasizing the immunoregulatory functions of MSCs, which can be adapted for clinical use. Although the main regulatory pathway remains unclear, MSCs have immunosuppressive properties, which may result in tumor growth in both solid cancers and hematologic malignancies. MSCs affect immunity via interactions with innate cellular components, like natural killer (NK) cells and adaptive cellular components, such as dendritic cells (DCs), B-lymphocytes, and T-lymphocytes [41–43]. MSCs can reduce the proliferative and cytotoxic ability of NK cells and can also inhibit maturation of DCs, which lead to the activation of T-lymphocytes. The involvement of various immunomodulatory factors is also known. These include transforming growth factor (TGF)-β [96, 121, 122], hepatocyte growth factor [96], indoleamine 2,3-dioxygenase with IFN-γ [97, 98], cyclooxygenase (COX)-1/-2 [99], PGE2 [99], inducible nitric oxide synthase [100], and A20 [123]. Some factors with immunomodulatory effects are known to aid MSC-induced tumor growth. TGF-β released by MSCs enhanced the epithelial–mesenchymal transition (EMT) of carcinoma, which is essential for tumor progression [121, 122]. Knockdown of A20 resulted in an antitumorigenic effect both in vitro and in vivo [123]. Moreover, multiple myeloma (MM)-MSC and CML-MSC-educated granulocytic-myeloid-derived suppressor cells showed an increase in immunomodulatory factors, such as arginase 1, tumor necrosis factor-ɑ, IL-1β, COX-2, and IL-6 [124, 125]. This supports an emerging concept regarding the contribution of MM-MSC and CML-MSC to tumor development and progression. However, function of other immunomodulatory factors remains unknown. Further research is needed to elucidate the role of immunomodulatory factors in tumor growth.
Metastasis and recurrence of malignancy
The most important process that contributes to the prometastatic effect of MSCs is the stimulation of EMT, a source of cancer-associated fibroblasts (CAFs) [44]. The EMT process develops more invasive phenotypes, resulting in local invasions and distant metastases [45–47]. It also affects the progression of various types of tumors, such as prostate cancer, pancreatic cancer, and breast cancer [48–50]. Administration of genetically labeled MSCs into mice with tumors significantly induced lung metastases [47].
There are other studies that identify specific molecules or pathways involved in these metastatic events. For example, chemokine (C-X-C motif) ligand (CXCL) type 16 secreted from prostate cancer and the subsequent CXCL16/C-X-C motif receptor (CXCR) type 6 signaling induces the conversion of MSCs into CAFs [48]. Moreover, promoting EMT through the Notch signaling pathway, by co-culturing with MSCs, also induces tumorigenesis [49]. Secretion of chemokine (C-C motif) ligand type 5 by MSCs was shown to be critical for metastasis in breast cancer [14], and MSCs support the entry of breast cancer into the BM through Tac1 regulation [50]. Entry into the BM suggest that the effect of MSCs on metastasis of solid cancers may be applicable to hematologic malignancies, as defects originate in the BM [50, 51].
Owing to an increase in cancer recurrence rate, the development of MSC-based anticancer therapies is considered. The failure of MSCs to be adapted into anticancer therapies is usually due to recurrence or relapse after the therapy, rather than a lack of primary response or initial remission [80]. Moreover, both metastasis and recurrence of malignancies are significantly related to tumor vasculature. MSCs migrate to the tumor parenchyma and differentiate into pericytes, inducing tumor vasculogenesis and promoting tumor recurrence [52]. Higher recurrence rates were also shown in patients with hematologic malignancies in a pilot clinical study [53]. There were two groups in the randomized clinical trial. Patients in one group received hematopoietic stem cells (HSCs) from a human leukocyte antigen-identical sibling donor, while the other group were co-transplanted with MSCs. Graft-versus-host disease was prevented when MSCs were co-transplanted with HSCs, but the relapse of hematologic malignancy was higher compared to the control group. Therefore, further research is required to adapt MSCs for clinical uses.
Enhancement of tumor cell stemness
MSCs are known to provide a favorable tumor-promoting microenvironment and increase tumor cell stemness [126–128]. MSCs are essential to the tumor-promoting microenvironment owing to their multilineage potential. They can differentiate into various types of tumor-related cells such as CAFs [128, 129]. Several studies have shown enhanced stemness of tumor-associated MSCs, which are integral components of the tumor microenvironment in various tumor cell lines [130]. Chosa et al. [131] introduced two novel mechanisms of enhanced stemness in MSCs: the scrapie responsive gene 1/BM stromal cell antigen-1 ligand–receptor combination and cell–cell adhesion through N-cadherin. On the other hand, MSCs have been shown to promote mammosphere formation partially via the epidermal growth factor (EGF)/EGF receptor/Akt pathway to regulate self-renewal through cytokine networks in breast cancer cells [132, 133]. Moreover, they regulate cancer stem cells via bone morphogenic protein signaling in ovarian cancer [134] and provide favorable tumor-promoting microenvironments through WNT/TGF-β signaling pathways in gastric carcinoma [135]. Besides their role in solid cancers, MSCs increase the stemness of cells in hematologic malignancies such as multiple myeloma via an activation of the Bruton tyrosine kinase signal pathway [136]. Thus MSCs play a critical role in tumor cell stemness in various types of tumors; however, the precise underlying mechanisms are still unclear. Therefore, to adapt MSCs for therapeutic use, further study is required to understand the enhancement of tumor cell stemness.
Drug resistance
Several studies demonstrating drug resistance of hematologic malignancies induced by MSCs are listed in Table 4, four of which showed CML cells becoming more resistant to chemotherapy. One of these studies used co-cultured primary CML cells to study the effect of the BM microenvironment in CML drug resistance [54]. They also found higher levels of IL-7 in the BM of CML patients in the blast crisis phase than healthy donors. IL-7 protects leukemic cells from imatinib-induced apoptosis via the Janus kinase 1/signal transducer and activator of transcription 5 pathway. KBM-5 CML cells were also protected from imatinib-induced cell death when they were co-cultured with MSCs [55]. Vianello et al. [62] showed that upregulation of CXCL12 and CXCR4 contribute to the drug-resistant ability of MSCs. In MSCs, differential expression of apoptosis-related proteins and activation of the Wnt pathway boost the antiapoptotic and drug-resistant activity [71].
Seven groups showed that MSCs induce chemo-resistance in AML. Carter et al. [56] emphasized the importance of an apoptosis repressor with caspase recruitment domain (ARC) protein. ARC induces the expression of IL-1β in AML cells. ARC mediates a complex regulatory circuit via nuclear factor (NF)-κB/IL-1β signaling in both AML cells and MSCs. This may be a novel target for AML, because this leads to the activation of numerous chemokine ligand/receptor axes that are closely associated with leukemic cell chemo-resistance. The gene c-Myc, which is involved in the regulation of various apoptotic molecules, may also play an important role, because their levels are upregulated in AML cells co-cultured with stroma [57]. Moreover, Notch inhibition abrogates stroma-induced chemo-resistance in AML, suggesting a potential therapeutic target for leukemia [58]. Expression of Bcl-2 may play an important role in resistance to topotecan [32] and cytarabine [108]. Irrespective of the pathways involved, direct cell-to-cell interaction with MSCs is required to make AML cells resistant to drugs [107]. Drug resistance of AML and ALL cells was due to NF-κB activation in MSCs via a vascular cell adhesion molecule-1/very late antigen-4 axis [137].
Extracellular signal–regulated kinase (ERK)/dynamin-related protein 1 (Drp1)-dependent mitochondrial fission and p21 downregulation are considered crucial for chemo-resistance in ALL. MSCs can alter mitochondrial dynamics induced by Drp1 activation, which can consequently protect leukemic cells from antitumor agents [59]. Downregulation of p21 may also explain the genotoxic agent-induced cell cycle arrest of ALL [60]. Wu et al. [61] used MSCs derived from BM and ALL cells, but the underlying mechanisms seemed different from the studies mentioned above. They have reported that ERK activation is important when ALL cells and MSCs are in direct contact. Yuan et al. [76] used MSCs from UC, instead of the BM, and the result was similar. However, they suggested different underlying mechanisms involving Notch signaling driven by Notch1 receptors, because significant upregulation of Jagged1 and overexpression of Notch1 were observed when Jurkat cells and UC-MSCs were co-cultured.
Conclusions
MSC-based clinical outcomes have shown a wide range of variation likely due to non-standardized experimental methods, lack of specific cell surface markers to identify subsets of MSCs, and heterogeneous characteristics of MSCs that are easily affected by the surrounding environment. Therefore, further research is necessary to develop MSCs for cancer treatment. Moreover, there are many unclear and complicated aspects of cancer, especially hematologic malignancies, such as the tumor-related effects of MSCs. Several studies have been conducted to investigate the effects of MSCs in carcinogenesis or tumor microenvironments, but a single principle cannot explain both the antitumorigenic and protumorigenic functions of MSCs. Even though the underlying process remains unclear, the dual role of MSCs is widely acknowledged.
The antitumor effects of MSCs are mainly a result of suppressed proliferation of malignant cells. More specific mechanisms or molecules involved remain unclear, but arrest at the G0/G1 phase of the cell cycle is an acknowledged mechanism. To utilize this antitumorigenic activity for clinical use in the future, other factors must be considered. MSCs possess certain beneficial characteristics, such as the potential to be used as delivery vehicles and the ability to inhibit vascular growth and arrest the cell cycle (Fig. 1). However, unfavorable characteristics such as favoring tumor growth by suppressing apoptosis, supporting tumor vasculature, involvement in immunomodulation of cancer cells, activation of metastasis/recurrence, and protection of cancer cells from drug-induced apoptosis leading to chemo-resistance are a hindrance to their use as a therapeutic agent. Tumor-associated MSCs, essential components of the tumor microenvironment, are also associated with a protumorigenic effect because they tend to enhance tumor cell stemness (Fig. 1).
Fig. 1.
Scheme for the dual role of mesenchymal stem cells (MSCs) in hematologic malignancy. MSCs have both antitumorigenic and protumorigenic effects, as they tend to not only inhibit tumor growth by suppressing tumor cell proliferation but also promote tumor growth by suppressing tumor cell apoptosis
From the various underlying mechanisms that have been suggested and summarized here, it may be possible to develop MSC-based anticancer therapies by targeting individual pathways. Specifically, the development of molecules that can either increase antitumorigenic effects or decrease protumorigenic effects would be promising for advanced therapies. Detailed studies are required to overcome limitations such as the heterogeneous aspects of MSCs and the lack of standard study methods. Further research regarding the antitumor effects of MSCs should be conducted to develop safe and effective treatments for hematologic malignancies. Development of engineered or genetically modified MSCs may be a promising strategy, as they are safer and more efficient than the unstable and heterogeneous naive MSCs. As numerous researchers continue to overcome limitations and develop MSC-based cell therapies that target hematologic malignancies, there is hope that successful therapies will be developed. Until then, it is imperative that we approach MSC-based cell therapies with caution, considering the unfavorable outcomes described.
Acknowledgments
Funding
This review was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education [Grant No. 2017R1D1A1B03027984] and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea [Grant No. HI15C2963].
Author contributions
MWL and KHY designed the study. All authors drafted and revised the manuscript. SR illustrated the figure. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Myoung Woo Lee, Somi Ryu
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplant. 1968;6:230–7. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 2015;48:62. doi: 10.1186/s40659-015-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji ST, Kim H, Yun J, Chung JS, Kwon SM. Promising therapeutic strategies for mesenchymal stem cell-based cardiovascular regeneration: from cell priming to tissue engineering. Stem Cells Int. 2017;2017:3945403. doi: 10.1155/2017/3945403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–21. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 7.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806–12. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 9.Krampera M. Mesenchymal stromal cells: more than inhibitory cells. Leukemia. 2011;25:565–6. doi: 10.1038/leu.2011.8. [DOI] [PubMed] [Google Scholar]
- 10.Wong RS. Mesenchymal stem cells: angels or demons? J Biomed Biotechnol. 2011;2011:459510. doi: 10.1155/2011/459510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao L, Xu Z, Zhao T, Ye L, Zhang X. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Tian H, Yue W, Zhu F, Li S, Li W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 2011;226:1860–7. doi: 10.1002/jcp.22511. [DOI] [PubMed] [Google Scholar]
- 13.Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, et al. Mesenchymal stromal cells alone or expressing interferon-β suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615–25. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- 14.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, et al. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011;17:579–87. doi: 10.2119/molmed.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463–74. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 17.Lin G, Yang R, Banie L, Wang G, Ning H, Li L, et al. Effects of transplantation of adipose tissue‐derived stem cells on prostate tumor. Prostate. 2010;70:1066–73. doi: 10.1002/pros.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yulyana Y, Ho IA, Sia KC, Newman JP, Toh XY, Endaya BB, et al. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol Ther. 2015;23:746–56. doi: 10.1038/mt.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu YL, Li HY, Zhao XP, Jiao JY, Tang DX, Yan LJ, et al. Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017;108:897–909. doi: 10.1111/cas.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu N, Wang H, Wei J, Wang B, Shan W, Lai X, et al. NR2F2 regulates bone marrow-derived mesenchymal stem cell-promoted proliferation of Reh cells. Mol Med Rep. 2016;14:1351–6. doi: 10.3892/mmr.2016.5389. [DOI] [PubMed] [Google Scholar]
- 21.Lin HD, Fong C, Biswas A, Choolani M, Bongso A. Human umbilical cord Wharton’s jelly stem cell conditioned medium induces tumoricidal effects on lymphoma cells through hydrogen peroxide mediation. J Cell Biochem. 2016;117:2045–55. doi: 10.1002/jcb.25501. [DOI] [PubMed] [Google Scholar]
- 22.Song N, Gao L, Qiu H, Huang C, Cheng H, Zhou H, et al. Mouse bone marrow-derived mesenchymal stem cells inhibit leukemia/lymphoma cell proliferation in vitro and in a mouse model of allogeneic bone marrow transplant. Int J Mol Med. 2015;36:139–49. doi: 10.3892/ijmm.2015.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–10. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 24.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 25.Duchi S, Sotgiu G, Lucarelli E, Ballestri M, Dozza B, Santi S, et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: effective photoinduced in vitro killing of osteosarcoma. J Control Release. 2013;168:225–37. doi: 10.1016/j.jconrel.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Huang B, Tabata Y, Gao J. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J Control Release. 2012;162:464–73. doi: 10.1016/j.jconrel.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Sauer H, Wartenberg M. Reactive oxygen species as signadurg molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423–34. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- 28.Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113:4197–205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menge T, Gerber M, Wataha K, Reid W, Guha S, Cox CS, Jr, et al. Human mesenchymal stem cells inhibit endothelial proliferation and angiogenesis via cell–cell contact through modulation of the VE-cadherin/β-catenin signaling pathway. Stem Cells Dev. 2013;22:148–57. doi: 10.1089/scd.2012.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, et al. Human bone marrow‐derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31:146–55. doi: 10.1002/stem.1247. [DOI] [PubMed] [Google Scholar]
- 31.Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang R, Huang GS, Wang Z, Chen XQ, Bai QX, Zhang YQ, et al. Effects of human bone marrow stromal cell line (HFCL) on the proliferation, differentiation and apoptosis of acute myeloid leukemia cell lines U937, HL-60 and HL-60/VCR. Int J Hematol. 2008;87:152–66. doi: 10.1007/s12185-008-0030-6. [DOI] [PubMed] [Google Scholar]
- 33.Sarmadi V, Tong CK, Vidyadaran S, Abdullah M, Seow HF, Ramasamy R. Mesenchymal stem cells inhibit proliferation of lymphoid origin haematopoietic tumour cells by inducing cell cycle arrest. Med J Malays. 2010;65:209–14. [PubMed] [Google Scholar]
- 34.Wei Z, Chen N, Guo H, Wang X, Xu F, Ren Q, et al. Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res. 2009;28:141. doi: 10.1186/1756-9966-28-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian K, Yang S, Ren Q, Han Z, Lu S, Ma F, et al. p38 MAPK contributes to the growth inhibition of leukemic tumor cells mediated by human umbilical cord mesenchymal stem cells. Cell Physiol Biochem. 2010;26:799–808. doi: 10.1159/000323973. [DOI] [PubMed] [Google Scholar]
- 36.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 37.Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet‐derived growth factor receptors. J Cell Mol Med. 2007;11:1012–30. doi: 10.1111/j.1582-4934.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003;10:621–9. doi: 10.1038/sj.gt.3301934. [DOI] [PubMed] [Google Scholar]
- 39.Roorda BD, ter Elst A, Kamps WA, de Bont ES. Bone marrow-derived cells and tumor growth: contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit Rev Oncol Hematol. 2009;69:187–98. doi: 10.1016/j.critrevonc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem. 2011;286:25007–15. doi: 10.1074/jbc.M110.213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–18. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 44.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–9. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 46.Chang AI, Schwertschkow AH, Nolta JA, Wu J. Involvement of mesenchymal stem cells in cancer progression and metastases. Curr Cancer Drug Targets. 2015;15:88–98. doi: 10.2174/1568009615666150126154151. [DOI] [PubMed] [Google Scholar]
- 47.Meleshina AV, Cherkasova EI, Shirmanova MV, Klementieva NV, Kiseleva EV, Snopova LВ, et al. Influence of mesenchymal stem cells on metastasis development in mice in vivo. Stem Cell Res Ther. 2015;6:15. doi: 10.1186/s13287-015-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kabashima‐Niibe A, Higuchi H, Takaishi H, Masugi Y, Matsuzaki Y, Mabuchi Y, et al. Mesenchymal stem cells regulate epithelial–mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013;104:157–64. doi: 10.1111/cas.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, et al. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE. 2008;3:e2563. doi: 10.1371/journal.pone.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trendowski M. The inherent metastasis of leukaemia and its exploitation by sonodynamic therapy. Crit Rev Oncol Hematol. 2015;94:149–63. doi: 10.1016/j.critrevonc.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Wang HH, Cui YL, Zaorsky NG, Lan J, Deng L, Zeng XL, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016;375:349–59. doi: 10.1016/j.canlet.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 53.Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–9. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Tu H, Yang Y, Wan Q, Fang L, Wu Q, et al. High IL-7 levels in the bone marrow microenvironment mediate imatinib resistance and predict disease progression in chronic myeloid leukemia. Int J Hematol. 2016;104:358–67. doi: 10.1007/s12185-016-2028-9. [DOI] [PubMed] [Google Scholar]
- 55.Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther. 2008;7:48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- 56.Carter BZ, Mak PY, Chen Y, Mak DH, Mu H, Jacamo R, et al. Anti-apoptotic ARC protein confers chemoresistance by controlling leukemia-microenvironment interactions through a NFkappaB/IL1beta signaling network. Oncotarget. 2016;7:20054–67. doi: 10.18632/oncotarget.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia B, Tian C, Guo S, Zhang L, Zhao D, Qu F, et al. c-Myc plays part in drug resistance mediated by bone marrow stromal cells in acute myeloid leukemia. Leuk Res. 2015;39:92–99. doi: 10.1016/j.leukres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Takam Kamga P, Bassi G, Cassaro A, Midolo M, Di Trapani M, Gatti A, et al. Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget. 2016;7:21713–27. doi: 10.18632/oncotarget.7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai J, Wang J, Huang Y, Wu H, Xia T, Xiao J, et al. ERK/Drp1-dependent mitochondrial fission is involved in the MSC-induced drug resistance of T-cell acute lymphoblastic leukemia cells. Cell Death Dis. 2016;7:e2459. doi: 10.1038/cddis.2016.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Hu K, Hu Y, Liu L, Wang B, Huang H. Bone marrow mesenchymal stromal cells affect the cell cycle arrest effect of genotoxic agents on acute lymphocytic leukemia cells via p21 down-regulation. Ann Hematol. 2014;93:1499–508. doi: 10.1007/s00277-014-2069-1. [DOI] [PubMed] [Google Scholar]
- 61.Wu KN, Zhao YM, He Y, Wang BS, Du KL, Fu S, et al. Rapamycin interacts synergistically with idarubicin to induce T-leukemia cell apoptosis in vitro and in a mesenchymal stem cell simulated drug-resistant microenvironment via Akt/mammalian target of rapamycin and extracellular signal-related kinase signaling pathways. Leuk Lymphoma. 2014;55:668–76. doi: 10.3109/10428194.2013.811579. [DOI] [PubMed] [Google Scholar]
- 62.Vianello F, Villanova F, Tisato V, Lymperi S, Ho KK, Gomes AR, et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica. 2010;95:1081–9. doi: 10.3324/haematol.2009.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells - sources and clinical applications. Transfus Med Hemother. 2008;35:272–7. doi: 10.1159/000142333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 65.Russell KA, Chow NH, Dukoff D, Gibson TW, LaMarre J, Betts DH, et al. Characterization and immunomodulatory effects of canine adipose tissue-and bone marrow-derived mesenchymal stromal cells. PLoS ONE. 2016;11:e0167442. doi: 10.1371/journal.pone.0167442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016;7:163. doi: 10.1186/s13287-016-0418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36:1176–85. doi: 10.1016/j.exphem.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho A, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–30. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 69.Wagner W. Senescence is heterogeneous in mesenchymal stromal cells: kaleidoscopes for cellular aging. Cell Cycle. 2010;9:2923–4. doi: 10.4161/cc.9.15.12741. [DOI] [PubMed] [Google Scholar]
- 70.Lee MW, Kim DS, Ryu S, Jang IK, Kim HJ, Yang JM, et al. Effect of ex vivo culture conditions on immunosuppression by human mesenchymal stem cells. Biomed Res Int. 2013;2013:154919. doi: 10.1155/2013/154919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han Y, Wang Y, Xu Z, Li J, Yang J, Li Y, et al. Effect of bone marrow mesenchymal stem cells from blastic phase chronic myelogenous leukemia on the growth and apoptosis of leukemia cells. Oncol Rep. 2013;30:1007–13. doi: 10.3892/or.2013.2518. [DOI] [PubMed] [Google Scholar]
- 72.Hendijani F, Javanmard SH, Sadeghi-aliabadi H. Human Wharton’s jelly mesenchymal stem cell secretome display antiproliferative effect on leukemia cell line and produce additive cytotoxic effect in combination with doxorubicin. Tissue Cell. 2015;47:229–34. doi: 10.1016/j.tice.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Shen YL, Luo Q, Guo YX, Zheng GH, Yu J, Xu YH. Bone marrow mesenchymal stem cell-derived Wnt5a inhibits leukemia cell progression in vitro via activation of the non-canonical Wnt signaling pathway. Oncol Lett. 2014;8:85–90. doi: 10.3892/ol.2014.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23:925–33. doi: 10.1038/leu.2008.384. [DOI] [PubMed] [Google Scholar]
- 75.Zhang HM, Zhang LS. Influence of human bone marrow mesenchymal stem cells on proliferation of chronic myeloid leukemia cells. Chin J Cancer. 2009;28:29–32. [PubMed] [Google Scholar]
- 76.Yuan Y, Chen D, Chen X, Shao H, Huang S. Human umbilical cord-derived mesenchymal stem cells inhibit proliferation but maintain survival of Jurkat leukemia cells in vitro by activating Notch signaling. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:441–7. [PubMed] [Google Scholar]
- 77.Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L, et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin’s lymphoma xenografts. PLoS ONE. 2010;5:e11140. doi: 10.1371/journal.pone.0011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62:1156–66. doi: 10.1016/j.addr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao Z, Zhang L, Hu J, Sun Y. Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug loaded nanoparticles. Nanomedicine. 2013;9:174–84. doi: 10.1016/j.nano.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Ramdasi S, Sarang S, Viswanathan C. Potential of mesenchymal stem cell based application in cancer. Int J Hematol Oncol Stem Cell Res. 2015;9:95–103. [PMC free article] [PubMed] [Google Scholar]
- 81.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 82.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 83.Dembinski JL, Wilson SM, Spaeth EL, Studeny M, Zompetta C, Samudio I, et al. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy. 2013;15:20–32. doi: 10.1016/j.jcyt.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang X, Du J, Xu X, Xu C, Song W. IFN-gamma-secreting-mesenchymal stem cells exert an antitumor effect in vivo via the TRAIL pathway. J Immunol Res. 2014;2014:318098. doi: 10.1155/2014/318098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, Lu Y, Huang W, Xu H, Chen X, Geng Q, et al. In vitro effect of adenovirus‐mediated human Gamma Interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematol Oncol. 2006;24:151–8. doi: 10.1002/hon.779. [DOI] [PubMed] [Google Scholar]
- 86.Zhao WH, Cheng JX, Shi PF, Huang JY. Human umbilical cord mesenchymal stem cells with adenovirus-mediated interleukin 12 gene transduction inhibits the growth of ovarian carcinoma cells both in vitro and in vivo. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:903–7. [PubMed] [Google Scholar]
- 87.Chen X, Lin X, Zhao J, Shi W, Zhang H, Wang Y, et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol Ther. 2008;16:749–56. doi: 10.1038/mt.2008.3. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Zhang L, Xu W, Qian H, Ye S, Zhu W, et al. Experimental therapy for lung cancer: umbilical cord-derived mesenchymal stem cell-mediated interleukin-24 delivery. Curr Cancer Drug Targets. 2013;13:92–102. doi: 10.2174/156800913804486665. [DOI] [PubMed] [Google Scholar]
- 89.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–7. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–42. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gangadaran P, Rajendran RL, Lee HW, Kalimuthu S, Hong CM, Jeong SY, et al. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Control Release. 2017;264:112–26. doi: 10.1016/j.jconrel.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 93.Rajendran RL, Gangadaran P, Bak SS, Oh JM, Kalimuthu S, Lee HW, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017;7:15560. doi: 10.1038/s41598-017-15505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rumpel M, Friedrich T, Deininger MW. Imatinib normalizes bone marrow vascularity in patients with chronic myeloid leukemia in first chronic phase. Blood. 2003;101:4641–3. doi: 10.1182/blood-2003-02-0481. [DOI] [PubMed] [Google Scholar]
- 96.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 97.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 98.Chen K, Wang D, Du WT, Han Z, Ren H, Chi Y, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135:448–58. doi: 10.1016/j.clim.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 99.Kang JW, Kang K, Koo HC, Park JR, Choi EW, Park YH. Soluble factors–mediated immunomodulatory effects of canine adipose tissue–derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681–93. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 100.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 101.Marx V. Cell-line authentication demystified. Nat Methods. 2014;11:483–8. doi: 10.1038/nmeth.2932. [DOI] [PubMed] [Google Scholar]
- 102.Maqsood MI, Matin MM, Bahrami AR, Ghasroldasht MM. Immortality of cell lines: challenges and advantages of establishment. Cell Biol Int. 2013;37:1038–45. doi: 10.1002/cbin.10137. [DOI] [PubMed] [Google Scholar]
- 103.Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992;79:2370–7. [PubMed] [Google Scholar]
- 104.Panayiotidis P, Jones D, Ganeshaguru K, Foroni L, Hoffbrand AV. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1996;92:97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 105.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–96. [PubMed] [Google Scholar]
- 106.Nwabo Kamdje AH, Mosna F, Bifari F, Lisi V, Bassi G, Malpeli G, et al. Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood. 2011;118:380–9. doi: 10.1182/blood-2010-12-326694. [DOI] [PubMed] [Google Scholar]
- 107.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5) Exp Hematol. 2001;29:448–57. doi: 10.1016/S0301-472X(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 108.Konopleva M, Konoplev S, Hu W, Zaritskey A, Afanasiev B, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–24. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 109.Naderi EH, Skah S, Ugland H, Myklebost O, Sandnes DL, Torgersen ML, et al. Bone marrow stroma-derived PGE2 protects BCP-ALL cells from DNA damage-induced p53 accumulation and cell death. Mol Cancer. 2015;14:14. doi: 10.1186/s12943-014-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee MW, Park YJ, Kim DS, Park HJ, Jung HL, Lee JW, et al. Human adipose tissue stem cells promote the growth of acute lymphoblastic leuekmia cells in NOD/SCID mice. Stem Cell Rev. 2018;14:451–60. doi: 10.1007/s12015-018-9806-0. [DOI] [PubMed] [Google Scholar]
- 111.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 112.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 113.Li GC, Zhang HW, Zhao QC, Sun LI, Yang JJ, Hong L, et al. Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor β1. Oncol Lett. 2016;11:1089–94. doi: 10.3892/ol.2015.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–31. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 116.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 117.Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 2013;32:4343–54. doi: 10.1038/onc.2012.458. [DOI] [PubMed] [Google Scholar]
- 118.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–6. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kang SG, Shinojima N, Hossain A, Gumin J, Yong RL, Colman H, et al. Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery. 2010;67:711–20. doi: 10.1227/01.NEU.0000377859.06219.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crisan M, Yap S, Casteilla L, Chen C, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Poggi A, Varesano S, Zocchi MR. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front Immunol. 2018;9:262. doi: 10.3389/fimmu.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poggi A, Musso A, Dapino I, Zocchi MR. Mechanisms of tumor escape from immune system: role of mesenchymal stromal cells. Immunol Lett. 2014;159:55–72. doi: 10.1016/j.imlet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Dang R, Yang Y, Zhang L, Cui D, Hong B, Li P, et al. A20 plays a critical role in the immunoregulatory function of mesenchymal stem cells. J Cell Mol Med. 2016;20:1550–60. doi: 10.1111/jcmm.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giallongo C, Tibullo D, Parrinello NL, La Cava P, Di Rosa M, Bramanti V, et al. Granulocyte-like myeloid derived suppressor cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC) Oncotarget. 2016;7:85764–75. doi: 10.18632/oncotarget.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giallongo C, Romano A, Parrinello NL, La Cava P, Brundo MV, Bramanti V, et al. Mesenchymal stem cells (MSC) regulate activation of granulocyte-like myeloid derived suppressor cells (G-MDSC) in chronic myeloid leukemia patients. PLoS ONE. 2016;11:e0158392. doi: 10.1371/journal.pone.0158392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guan J, Chen J. Mesenchymal stem cells in the tumor microenvironment. Biomed Rep. 2013;1:517–21. doi: 10.3892/br.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 2013;1836:321–35. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 128.Nwabo Kamdje AH, Kamga PT, Simo RT, Vecchio L, Seke Etet PF, Muller JM, et al. Mesenchymal stromal cells’ role in tumor microenvironment: involvement of signaling pathways. Cancer Biol Med. 2017;14:129–41. doi: 10.20892/j.issn.2095-3941.2016.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastas- Rev. 2010;29:249–61. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- 130.Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16:35–52. doi: 10.1038/nrd.2016.193. [DOI] [PubMed] [Google Scholar]
- 131.Chosa N, Ishisaki A. Two-novel mechanisms for maintenance of stemness in mesenchymal stem cells: SCRG1/BST1axis and cell-cell adhesion through N-cadherin. Jpn Dent Sci Rev. 2018;54:37–44. doi: 10.1016/j.jdsr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Klopp AH, Lacerda L, Gupta A, Debeb BG, Solley T, Li L, et al. Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PLoS ONE. 2010;5:e12180. doi: 10.1371/journal.pone.0012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q, Yuan HF, et al. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132:153–64. doi: 10.1007/s10549-011-1577-0. [DOI] [PubMed] [Google Scholar]
- 134.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–19. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nishimura K, Semba S, Aoyagi K, Sasaki H, Yokozaki H. Mesenchymal stem cells provide an advantageous tumor microenvironment for the restoration of cancer stem cells. Pathobiology. 2012;79:290–306. doi: 10.1159/000337296. [DOI] [PubMed] [Google Scholar]
- 136.Zhao P, Chen Y, Yue Z, Yuan Y, Wang X. Bone marrow mesenchymal stem cells regulate stemness of multiple myeloma cell lines via BTK signaling pathway. Leuk Res. 2017;57:20–26. doi: 10.1016/j.leukres.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 137.Jacamo R, Chen Y, Wang Z, Ma W, Zhang M, Spaeth EL, et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-κB mediates chemoresistance. Blood. 2014;123:2691–702. doi: 10.1182/blood-2013-06-511527. [DOI] [PMC free article] [PubMed] [Google Scholar]