Abstract
Background.
Senegal introduced a 13-valent pneumococcal conjugate vaccine (PCV13) in October 2013, given at 6, 10, and 14 weeks of age. We document trends of meningitis and pneumonia after the PCV13 introduction.
Methods.
From October 2010–October 2016, hospitalization data for clinical meningitis and pneumonia in children aged <5 years were collected from logbooks at a large, tertiary, pediatric hospital in Dakar. We used a set of predetermined keywords to define hospitalizations for extraction from hospital registers. We conducted a time-series analysis and compared hospitalizations before and after the PCV13 introduction, accounting for seasonality. The initial PCV13 uptake period (October 2013–September 2014) was considered to be transitional and was excluded.
Results.
Over the 7-year period, 1836 and 889 hospitalizations with a discharge diagnosis of pneumonia and meningitis, respectively, occurred in children aged <5 years. In children aged <12 months, a small, significant reduction in pneumonia was observed post-PCV13 (−3.8%, 95% confidence interval [CI] −1.5 to −5.9%). No decline was observed among children aged 12–59 months (−0.7%, 95% CI −0.8 to 2.2%). Meningitis hospitalizations remained stable for children aged <12 months (1.8%, 95% CI −0.9 to 4.4%) and 12–59 months (−0.5%, 95% CI −3.6 to 2.6%).
Conclusions.
We used data from 1 hospital to detect a small, significant reduction in all-cause pneumonia hospitalizations 2 years post-PCV13 introduction in infants; the same trend was not measurable in children aged 12–59 months or in meningitis cases. There is a need for continued surveillance to assess the long-term impact of sustained PCV13 use and to monitor how pneumococcus is causing disease in the meningitis belt.
Keywords: meningitis, pneumonia, pneumococcal conjugate vaccine, vaccine impact, hospitalization data
Pneumonia and meningitis, most of which are caused by Streptococcus pneumoniae, are leading infectious causes of morbidity and mortality in children aged <5 years worldwide [1, 2]. Globally, pneumococcus is responsible for 294 000 deaths in human immunodeficiency virus (HlV)-uninfected children aged <5 years annually; nearly 50% of these deaths occur in Africa and 81% occur in children who present with pneumonia [1]. In Africa, over 2.3 million pneumococcal pneumonia cases occur annually in children aged <5 years, resulting in over 137 000 deaths [1]. A previous study conducted at the study hospital—Albert Royer National Children’s Hospital (Centre Hospitalier National d’Enfants Albert Royer [CHNEAR]) in Dakar, Senegal—found that invasive pneumococcal infections accounted for 0.8% of all admissions, with 61% categorized as meningitis and 29% as pneumonia [3].
Pneumococcal conjugate vaccines (PCV) are effective interventions in preventing pneumonia and meningitis caused by S. pneumoniae and are recommended for use in all countries by the World Health Organization [4]. Studies on PCV effectiveness have shown 20–35% reductions in all-cause pneumonia and >90% reductions in invasive pneumococcal disease (including meningitis) after PCV introductions [5, 6]. Furthermore, serotype distribution differs by region, and its effectiveness on serotype-specific diseases may differ by vaccine product. However, the majority of data derive from middle- and high-income settings; data from low-income settings are sparse.
Although PCV effectiveness data on meningitis and clinical pneumonia endpoints are emerging from Africa concurrent with the increased uptake of PCV into routine immunization programs over the last decade, more data on the impact in West Africa and the meningitis belt (a region in sub-Saharan Africa with high incidences of meningitis) are needed. In this region, pneumococcal meningitis outbreaks due to vaccine serotypes, particularly serotype 1, have occurred despite PCV introductions [7–9]. Senegal, a low-income country in West Africa, introduced the 13-valent pneumococcal conjugate vaccine (PCV13), which protects against disease due to 13 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), in October 2013. The routine immunization schedule includes 3 primary doses (3 + 0) at 6, 10, and 14 weeks of age with no booster dose; there was no catch-up campaign. National coverage for 3 doses of PCV13 was 81% in 2014 and increased to 93% in 2016 [10]. Coverage rates for the Dakar region, where the study hospital is located, ranged between 75% (2015) and 79% (2016; unpublished data). A Haemophilus influenzae type B conjugate vaccine, which prevents meningitis and pneumonia caused by Haemophilus influenzae type B, was introduced into the routine immunization program in 2005, and coverage has remained relatively stable through the study period (range 89–93% from 2014–2016) [11]. A 10-day campaign for MenAfrivac (a vaccine against meningococcal A meningitis) occurred in November 2012, which targeted 1- to 29-year-olds in 35 districts in 8 regions and reached 95% of a targeted cohort of 3.9 million people [12]. The HIV prevalence rate in the general population is 0.5 (95% confidence interval [CI] 0.3–0.6) [13]. Seasonal peaks for respiratory syncytial virus and influenza occur similarly to peaks for pneumonia.
We utilized hospital administrative data, which is readily available and routinely collected for all hospitalizations, from a large pediatric hospital in Dakar to understand the impact of PCV13 on clinical meningitis and pneumonia in children aged <5 years in Senegal.
METHODS
Hospitalization data for clinical meningitis and pneumonia in children aged <5 years were collected at CHNEAR, a large national and subregional tertiary pediatric hospital in Dakar, Senegal. CHNEAR has 120 beds for neonatal, surgical, and pediatric patients and averages 5000 admissions annually.
Trained study staff reviewed the logbooks of 4 pediatric wards to collect the number of admissions for children aged <5 years who were admitted for pneumonia or meningitis from October 2010–October 2016. Admissions for clinical pneumonia and meningitis were based on discharge diagnoses using a set of predetermined keywords (abbreviations were included); no additional laboratory or medical record information were used to determine hospitalizations (Supplementary Appendix). Furthermore, as the data source was admission logbooks, vaccination history was not available and we were unable to determine whether meningitis cases received the PCV13. We excluded hospitalizations with discharge diagnoses of bronchiolitis. Where a discharge diagnosis was missing, an admission diagnosis was used to define the primary clinical syndrome requiring hospitalization. A logbook from March to November 2010 was missing from 1 ward; data from individual medical charts were collected for this period. We stratified data by 2 age groups: 0–11 months and 12–59 months.
To assess PCV13 impact, we conducted an interrupted time-series analysis using Poisson regression to compare hospitalizations before and after the PCV introduction, accounting for seasonality. Data were collected and tallied by age group and month of admission. We calculated the proportions of hospitalizations with a discharge diagnosis of meningitis or pneumonia by age group and year. We defined the pre-PCV period as October 2010–September 2013 and the post-PCV period as October 2014–October 2016. Given that the vaccine was introduced in October 2013, the initial PCV uptake period (October 2013–September 2014) was considered a transitional year and was excluded from analyses. We attempted to use intoxications and ingestions to serve as control conditions, as an indication of the stability of hospitalization trends over time; however, data were too sparse and, therefore, were not used in analyses. Data were analyzed using Microsoft Excel and SAS 9.4 (SAS Institute Inc., Cary, NC).
This protocol was reviewed in accordance with the Centers for Disease Control and Prevention human research protection procedures and was determined to be a nonresearch study.
RESULTS
From October 2010–October 2016, 1836 clinical pneumonia hospitalizations occurred in children aged <5 years; 603 (32.8%) hospitalizations were in children aged 0–11 months and 1233 (67.2%) were in children aged 12–59 months (Table 1). Among children <12 months of age, pneumonia hospitalizations accounted for 7.9% (104 of 1335) of all-cause hospitalizations pre-PCV introduction and 6.5% (93 of 1416) post-PCV introduction. Among children 12–59 months of age, the percentage of pneumonia hospitalizations among all-cause hospitalizations decreased from 17.7% (228 of 1295) before the PCV13 introduction to 14.6% (175 of 1235) after the PCV13 introduction. Figure 1 shows monthly trends of pneumonia and all-cause hospitalizations by age from October 2010–October 2016.
Table 1.
Average Annual Hospitalizations for Pneumonia and Meningitis Among Children Aged <5 Years
| Age Group |
0 to 59 Months |
0 to 11 Months |
12 to 59 Months |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis Period | Oct 2010– Sept 2011, n = 2434 |
Oct 2011– Sept 2012, n = 2511 |
Oct 2012– Sept 2013, n = 2947 |
Average pre- PCV13, n = 2631 |
Oct 2013– Sept 2014 (transition year), n = 2660 |
Oct 2014–Sept 2015, n = 2665 |
Oct 2015– Sept 2016, n = 2638 |
Average post- PCV13, n = 2652 |
Oct 2010– Sept 2011, n = 1175 |
Oct 2011– Sept 2012, n = 1259 |
Oct 2012– Sept 2013, n = 1572 |
Average pre- PCV13, n = 1335 |
Oct 2013– Sept 2014 (transition year), n = 1447 |
Oct 2014– Sept 2015, n = 1558 |
Oct 2015–Sept 2016, n = 1275 |
Average post- PCV13, n = 1416 |
Oct 2011– Sept 2012, n = 1252 |
Oct 2010– Sept 2011, n = 1259 |
Oct 2012– Sept 2013, n = 1375 |
Average pre- PCV13, n = 1295 |
Oct 2013– Sept 2014 (transition year), n = 1213 |
Oct 2014– Sept 2015, n = 1107 |
Oct 2015– Sept 2016, n = 1363 |
Average post- PCV13, n = 1235 |
| Pneumonia hospitalizations (% total all-cause hospitalizations) | 384 (15.8) | 303 (12.1) | 308 (10.5) | 332 (12.8) | 305 (11.5) | 318 (11.9) | 218 (8.3) | 268 (10.1) | 112 (9.5) | 92 (7.3) | 108 (6.9) | 104 (7.9) | 105 (7.3) | 114 (7.3) | 72 (5.6) | 93 (6.5) | 272 (21.6) | 211 (16.9) | 200 (14.5) | 228 (17.7) | 200 (16.5) | 204 (18.4) | 146 (10.7) | 175 (14.6) |
| Meningitis hospitalizations (% total all-cause hospitalizations) | 131 (5.4) | 249 (9.9) | 154 (5.2) | 178 (6.9) | 108 (4.1) | 133 (5.0) | 114 (4.3) | 124 (4.7) | 76 (6.5) | 132 (10.5) | 77 (4.9) | 95 (7.1) | 51 (3.5) | 88 (5.7) | 56 (4.4) | 72 (5.1) | 55 (4.4) | 117 (9.4) | 77 (5.6) | 83 (6.4) | 57 (4.7) | 45 (4.1) | 58 (4.3) | 52 (4.2) |
Data are from Centre Hospitalier National d’Enfants Albert Royer, October 2010–October 2016.
Abbreviation: PCV13, 13-valent pneumococcal conjugate vaccine.
Figure 1.
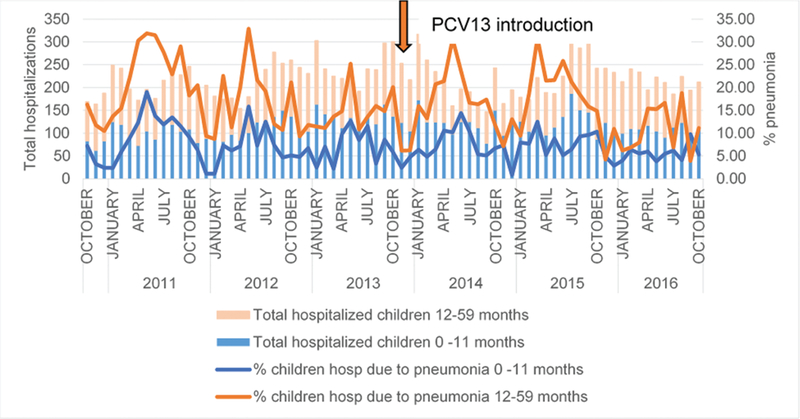
Proportion of pneumonia hospitalizations among all hospitalizations, children 0 to 59 months, by month. Data are from the Centre Hospitalier National d’Enfants Albert Royer, October 2010-Qctober 2016. Abbreviation: PCV13, 13-valent pneumococcal conjugate vaccine.
Among children aged <5 years, 889 hospitalizations for meningitis occurred at CHNEAR from October 2010–October 2016: 480 hospitalizations among 0–11 months and 351 among 12–59 months (Table 1). Among children aged <12 months, meningitis hospitalizations accounted for 7.1% (95 of 1335) of all-cause hospitalizations before the PCV introduction and 5.1% (72 of 1416) after the PCV introduction. In children aged 12–59 months, meningitis hospitalizations represented 6.4% (83 of 1295) of all-cause hospitalizations before the PCV13 introduction and 4.2% (52 of 1235) of all-cause hospitalizations after the PCV13 introduction. Figure 2 shows monthly trends for clinical meningitis and all-cause hospitalizations by age group from October 2010 through October 2016.
Figure 2.
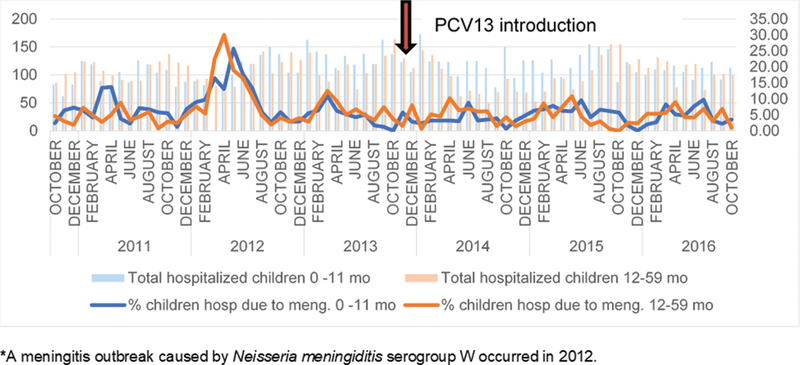
Proportion of meningitis hospitalizations among all hospitalizations, children 0 to 59 months, by month. Data are from the Centre Hospitalier National d’Enfants Albert Royer, October 2010–October 2016. Abbreviation: PCV13, 13-valent pneumococcal conjugate vaccine.
Using a time-series analysis, a statistically significant reduction of 3.8% (95% CI 1.5–5.9%) was observed in children aged <12 months who were hospitalized with clinical pneumonia in the post-PCV13 period, compared to the pre-PCV13 period. In children aged 12–59 months, no significant decline was observed (−0.7%, 95% CI −0.8 to 2.2%). Meningitis hospitalizations remained stable for children aged both <12 months (1.8%, 95% CI −0.9 to 4.4%) and 12–59 months (−0.5%, 95% CI −3.6 to 2.6%) when comparing changes post-PCV versus pre-PCV.
DISCUSSION
Using administrative data collected from 1 large tertiary-care center in Senegal, we showed that pneumonia hospitalizations declined by 4% among children aged <12 months during the 2 years following the PCV13 introduction. No declines in pneumonia or meningitis hospitalizations were observed among children aged 12–59 months. The finding for children aged <12 months is consistent with other studies that observed declines in clinical pneumonia following PCV introductions. A study from Israel found a 7% significant decline in children less than 5 years of age 4 years post-PCV13 introduction, and another study from Italy observed a 5% significant decline in children less than 2 years of age almost 3 years after PCV13 introduction [14, 15]. In Africa, 5 studies found reductions in pneumonia hospitalizations post-PCV introduction, although a statistically significant reduction was observed in only 2 of these. A vaccine effectiveness study in Rwanda documented a significant vaccine effectiveness, of 54%, against severe pneumonia, defined as hospitalization with a clinical diagnosis of severe pneumonia or severe acute lower respiratory infection or 1 or more of the following signs: chest indrawing, respiratory distress, hypoxia, or cyanosis [16]. A time-series analysis in South Africa documented a 39% reduction in pneumonia hospitalizations in HIV-uninfected children aged <5 years post-PCV13 introduction [17]. In Togo, investigators used the indirect cohort method to evaluate the PCV13 impact on severe pneumonia and found a nonsignificant, 80% (95% CI −90 to 100%) reduction; however, the study was conducted 1 year post-PCV13 introduction and included a small sample size [18]. An observational study in Malawi documented a 47% reduction in clinical pneumonia in children aged <5 years; however, this decline was also not significant [19]. Another observational study in Kenya did find a significant 24% reduction in clinical pneumonia in children aged <12 months after PCV10 introduction [20].
We did not observe the same trend in pneumonia hospitalizations among children aged 12–59 months. Instead, in the 3 years prior to the PCV13 introduction, we observed an annual decline in pneumonia hospitalizations, which continued at a slower pace after the PCV13 introduction. However, full indirect effects may not have occurred yet due to the still-increasing uptake in the population targeted for vaccination. We would expect to see further reductions as a result of direct and indirect effects in populations as the PCV coverage improves and there are additional cohorts of newborns vaccinated.
Reductions in the number and percent of hospitalizations for clinical meningitis were not observed in either age group. These findings are unlike other studies in Africa, from Rwanda and the Gambia, evaluating the PCV impact on meningitis, which found significant and large reductions after PCV introductions [16, 21]. However, these studies measured more specific outcomes and were limited to hospitalized probable (eg, cerebrospinal fluid specimen with turbid appearance, elevated white blood cell count) and laboratory-confirmed meningitis cases, and evaluated the PCV impact specifically on pneumococcal meningitis, not on all-cause bacterial meningitis. Our analysis included all hospitalized meningitis cases, defined by predetermined keywords in the ward logbooks and not by laboratory diagnostics; therefore, it is not surprising that the effect of PCV13 was lower in this study, even though pneumococcus is the leading cause of pediatric bacterial meningitis. Furthermore, the number of hospitalizations for meningitis was relatively small and our study was limited to a single hospital, making an assessment of any nationwide trends difficult.
Despite PCV introductions, pneumococcal meningitis outbreaks, especially due to vaccine serotypes, continue to occur throughout countries in the meningitis belt [7–9, 22]. In Senegal, the dominant pneumococcal serotypes causing pediatric meningitis prior to the PCV13 introduction were serotypes 1, 6A, 14, 5, and 23F, all of which are protected against by PCV13 [3]. A recent study conducted at CHNEAR found that post-PCV13 introduction, pneumococcus remained the dominant etiology of confirmed bacterial meningitis cases in children aged <5 years, with 55% of confirmed pneumococcal meningitis cases caused by vaccine serotypes (unpublished data). This finding aligns with what we observed for meningitis hospitalizations and suggests the need for more research to understand factors around the persistence of pneumococcal meningitis in the meningitis belt after PCV introductions.
Data also suggest that a 3 + 0 schedule, without a booster dose, may not provide adequate indirect protection among nonvaccinated children and adults. During a recent pneumococcal meningitis outbreak in Ghana, studies found that the majority of cases occurred in unvaccinated individuals, with serotype 1 causing 50–80% of the pneumococcal meningitis cases [7, 8]. A subsequent study 1 year later found that serotype 1 was still responsible for a large proportion of pneumococcal meningitis cases among older children and adults [22]. Additionally, a study from Burkina Faso evaluated the PCV impact on meningitis and found declines in all PCV13 serotypes except serotype 1 [9]. However, studies from South Africa, the only country in Africa currently using a 2 + 1 schedule (6 weeks, 14 weeks, 9 months), have shown significant declines in vaccine-type invasive disease and, specifically, serotype 1 in all age groups post-PCV introduction [23, 24]. These studies suggest that further evaluations could provide insight on whether a 2 + 1 schedule is a more effective dosing schedule in settings with continued serotype 1 disease post-PCV introduction.
Our study has several limitations. The definitions for clinical pneumonia and meningitis hospitalizations were based on keywords identified in the ward logbooks. These keywords were defined at the discretion of the clinicians in the hospital and had not been previously validated for accuracy. Second, our use of keywords limited our evaluation to all-cause meningitis hospitalizations: a less-specific outcome than probable/purulent or lab-confirmed meningitis. As a result, we were unable to differentiate meningitis hospitalizations by etiology, likely underestimating the impact from PCV13 on pneumococcal meningitis hospitalizations. Lastly, we evaluated the PCV13 impact just 2 years after the PCV13 introduction; this was perhaps too early to see a significant impact due to vaccination, particularly when monitoring a nonspecific outcome. Though we have reported a small reduction in pneumonia among children aged <12 months, we would expect to see greater reductions in sub-sequent years with increased vaccine coverage.
CONCLUSIONS
We observed a small but significant reduction in clinical pneumonia 2 years after the PCV13 introduction. The lack of impact observed on hospitalizations for pneumonia or meningitis among children aged 12–59 months suggests the need to evaluate the effectiveness of a 3 + 0 schedule in achieving indirect effects or consider evaluating its impact at a time further after the PCV introduction (ie, 3 to 4 years post-introduction). Although PCV impact data on clinical pneumonia and meningitis are limited from Africa, our findings for pneumonia are consistent with currently available data and demonstrate the potential of using this approach, especially in settings with limited or no available surveillance data, to add to the body of evidence for PCV impact. Our study highlights the need for continued surveillance for meningitis and pneumonia to assess the long-term impact of sustained PCV use on African children, as well as to monitor the pneumococcal serotypes causing disease in the meningitis belt.
Supplementary Material
Acknowledgments.
The authors thank all hospital staff and parents of children who used Centre Hospitalier National d’Enfants Albert Royer.
Financial support. This work was supported by funding from Gavi, the Vaccine Alliance, through the CDC Foundation (grant number memo-randum of agreement# 971–17).
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the World Health Organization.
Supplement sponsorship. This supplement was supported with funds from Gavi, the Vaccine Alliance through The World Health Organization and the CDC Foundation, and The Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine.
SUPPLEMENTARY DATA
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wahl B, O’Brien K, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type B disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 2018; 6:e744–e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Estimated Hib and pneumococcal deaths for children under 5 years of age. Available at: http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/ Accessed 31 August 2018.
- 3.Ba I, Ba A, Faye P, et al. Pediatric invasive pneumococcal disease in Senegal. Med Mal Infect 2015; 45:464–469. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Pneumococcal conjugate vaccine for pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Wkly Epidemiol Rec 2019; 94(8):85–104. [Google Scholar]
- 5.Conklin L, Loo JD, Kirk J, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type invasive pneumococcal disease among young children. Pediatr Infect Dis J 2014; 33(Suppl 2):S109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loo JD, Conklin L, Fleming-Dutra KE, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on prevention of pneumonia. Pediatr Infect Dis J 2014; 33(Suppl 2):S140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwambana-Adams B, Asiedu-Bekoe F, Sarkodie B, et al. An outbreak of pneumococcal meningitis among older children (≥5 years) and adults after the implementation of an infant vaccination programme with the 13-valent pneumococcal conjugate vaccine in Ghana. BMC Infect Dis 2016; 16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aku F, Lessa F, Asiedu-Bekoe F, et al. Meningitis outbreak caused by vaccine-preventable bacterial pathogens—Northern Ghana, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kambire D, Soeters HM, Ouedraogo-Traore R, et al. Early impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis-Burkina Faso, 2014–2015. J Infect 2018; 76:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Pneumococcal conjugate (PCV3) immunization coverage estimates by country. Available at: http://apps.who.int/gho/data/node.main.PCV3n?lang=en Accessed 31 August 2018.
- 11.World Health Organization. Hib (Hib3) immunization coverage estimates by country. Available at: http://apps.who.int/gho/data/node.main.A829 Accessed 21 May 2019.
- 12.USAID Maternal and Child Health Integrated Program. MCHIP end of project report. Washington DC: USAID, 2014. [Google Scholar]
- 13.United States Agency for International Development. Senegal - country quickstats. Available at: https://dhsprogram.com/Where-We-Work/Country-Main.cfm?ctry_id=36&c=Senegal&Country=Senegal&cn=&r=1 Accessed 21 May 2019.
- 14.Baldo V, Cocchio S, Gallo T, et al. Impact of pneumococcal conjugate vaccination: a retrospective study of hospitalization for pneumonia in North-East Italy. J Prev Med Hyg 2016; 57:E61–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Shimol S, Givon-Lavi N, Greenberg D, Dagan R. Cocontribution of rotavirus and pneumococcal conjugate vaccines to the reduction of pediatric hospital visits in young children. J Pediatr 2017; 182:253–9.e2. [DOI] [PubMed] [Google Scholar]
- 16.Gatera M, Uwimana J, Manzi E, et al. Use of administrative records to assess pneumococcal conjugate vaccine impact on pediatric meningitis and pneumonia hospitalizations in Rwanda. Vaccine 2016; 34:5321–8. [DOI] [PubMed] [Google Scholar]
- 17.Izu A, Solomon F, Nzenze SA, et al. Pneumococcal conjugate vaccines and hospitalization of children for pneumonia: a time-series analysis, South Africa, 2006–2014. Bull World Health Organ 2017; 95:618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moïsi J, Alassani I, Tall H, et al. Using the indirect cohort approach to estimate PCV13 effectiveness against meningitis and pneumonia endpoints in Northern Togo In: Program and abstracts of the 10th International Symposium on Pneumonia and Pneumococcal Diseases (Glasgow, Scotland). Geneva, Switzerland: ISPPD, 2016; 181. [Google Scholar]
- 19.McCollum ED, Nambiar B, Deula R, et al. Impact of the 13-valent pneumococcal conjugate vaccine on clinical and hypoxemic childhood pneumonia over three years in Central Malawi: an observational study. PLOS One 2017; 12:e0168209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silaba M, Ooko M, Bottomley C, et al. The impact of 10-valent pneumococcal conjugate vaccine (PCV10) on the incidence of radiologically confirmed pneumonia and on pneumonia hospitalizations among children in Kilifi, Kenya. In: Program and abstracts of the 10th International Symposium on Pneumonia and Pneumococcal Diseases (Glasgow, Scotland). 2016; 22. [Google Scholar]
- 21.Mackenzie GA, Hill PC, Jeffries DJ, et al. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in the Gambia: a population-based surveillance study. Lancet Infect Dis 2016; 16:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozio C, Abdul-Karim A, Abenyeri J, et al. Continued occurrence of serotype 1 pneumococcal meningitis in two regions located in the meningitis belt in Ghana five years after introduction of 13-valent pneumococcal conjugate vaccine. PLOS One 2018; 13(9): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Gottberg A, Kleynhans J, de Gouveia L, et al. Trends in invasive pneumococcal disease among adults aged ≥25 years, South Africa, 2005–2016. In: Program and abstracts of the 11th International Symposium on Pneumonia and Pneumococcal Diseases (Melbourne, Australia). 2018; 18. [Google Scholar]
- 24.von Mollendorf C, Cohen C, Tempia S, et al. Epidemiology of serotype 1 invasive pneumococcal disease, South Africa, 2003–2013. Emerg Infect Dis 2016; 22:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


