Abstract
Background:
Nationwide efforts seek to address the opioid epidemic by increasing access to medications for opioid use disorder (OUD), particularly with buprenorphine. A poorly understood challenge is that among individuals with OUD who do receive buprenorphine, many do not adhere to the pharmacotherapy long enough to achieve sustained benefits. We aimed to identify factors associated with buprenorphine treatment utilization over time.
Methods:
We used random-intercept modeling to identify factors associated with buprenorphine treatment utilization over 2 years after first follow-up by 789 individuals with OUD who had participated in a multi-site randomized clinical trial of buprenorphine compared to methadone. Key predictors were participants’ reports of buprenorphine treatment accessibility and acceptability (assessed at first follow-up) and their interaction effects, controlling for baseline randomization status, sociodemographics, and other covariates.
Results:
Approximately 9.3 – 11.2% of participants utilized buprenorphine treatment over the 2 years of follow-up. Interaction effects indicated that individuals who perceived buprenorphine to be both accessible and acceptable were most likely to use buprenorphine during follow-up, controlling for other factors. In contrast, individuals who perceived buprenorphine to be unacceptable were least likely to use buprenorphine, regardless the level of perceived access to the medication. Buprenorphine treatment utilization was also negatively associated with Hispanic ethnicity, West coast context, and cumulative months receiving methadone treatment and incarceration during follow-up.
Conclusions:
To engage more individuals with OUD in long-term treatment with buprenorphine, interventions should target buprenorphine treatment acceptability, in addition to increasing buprenorphine access, and tailor efforts to meet the needs of vulnerable populations.
Trial registration:
The START Follow-up Study on ClinicalTrials.gov ().
Keywords: buprenorphine; treatment acceptability, access, and utilization; opioid use disorder; pharmacotherapy; longitudinal
1. Introduction
The U.S. opioid epidemic has resulted in extraordinary numbers of accidental injuries, infectious diseases, and premature deaths (Hedegaard et al., 2017), contributing to a historically unprecedented shortening of American life expectancy (Hedegaard et al., 2017; Kochanek et al., 2017). Opioid use disorder (OUD) is typically a chronic condition (Evans & Hser, 2019; Hser et al., 2017, 2007; Nosyk et al., 2014) that requires long-term, or even life-long treatment with medications (e.g., methadone, buprenorphine-naloxone, hereafter referred to as buprenorphine; U.S. Department of Health and Human Services [HHS], 2016). Individuals with OUD who adhere to treatment with medications have lower mortality (Degenhardt et al., 2011; Evans et al., 2015), less opioid use (Hser et al., 2016; Thomas et al., 2014), less HIV risk, and other positive outcomes (Evans et al., 2019; Mattick et al., 2014; Woody et al., 2014). When used appropriately, methadone and buprenorphine are both effective medications for the long-term stabilization of individuals with OUD (Bart, 2012; Bell et al., 2009; Connock et al., 2007; Mattick et al., 2008). Methadone is a Schedule II, full opioid agonist that has been used in the U.S. for almost five decades in licensed specialty opioid treatment programs. In contrast, buprenorphine is a Schedule III partial agonist approved for use in the U.S. in 2002 and available in general health care settings. In addition to buprenorphine’s greater ease of access, its abuse potential is lower than methadone, and it has a higher margin of safety (Bonhomme et al., 2012; Gryczynski et al., 2013; Whelan and Remski, 2012). Despite the advantages of buprenorphine, individuals remain engaged in buprenorphine treatment for less time than those who receive methadone (Burns et al., 2015; Hser et al., 2016; Proctor et al., 2014). Few studies have examined factors that influence use of buprenorphine over time; thus, why buprenorphine is underutilized despite its potential advantages is poorly understood. The present study aims to explore factors associated with utilization of buprenorphine for individuals with opioid use disorder.
Studies of buprenorphine utilization have mostly identified individual-level factors associated with use. For example, one study that compared patients treated with buprenorphine or methadone reported that individuals receiving buprenorphine are more likely than those receiving methadone to be male, have health insurance, be employed, abuse prescription opioids, and have HIV (Fingerhood et al., 2014). Furthermore, studies focused on buprenorphine report that retention in buprenorphine treatment is positively associated with female gender, older age, psychiatric diagnosis, and receipt of prescribed psychiatric medications, and it has been negatively associated with unemployment, Hepatitis C, Black and Hispanic race/ethnicity, and cocaine use (Haddad et al., 2013; Weinstein et al., 2017). A few studies have solicited patient-reported reasons for choosing buprenorphine over methadone. Within this literature, Gryczynski and colleagues found that some individuals perceive buprenorphine to have less severe withdrawal than methadone and fewer unpleasant physical effects (Gryczynski et al., 2013). Individuals have attributed discontinuation of buprenorphine to lack of knowledge, opioid craving and withdrawal symptoms, poor social support, and the experience that buprenorphine works “too well” such that euphoric effects of illicit opioid use cannot be felt (Teruya et al., 2014).
Much less is known about contextual forces that influence access to buprenorphine for individuals with OUD. This gap in knowledge is striking given that individuals’ ability to access buprenorphine is thought to be impacted by several external forces. These include the slow adoption of buprenorphine by U.S. office-based treatment settings (Li et al., 2014), the limited number of health care providers who are willing or able to prescribe buprenorphine (DeFlavio et al., 2015; Netherland et al., 2009; Rosenblatt et al., 2015; Stein et al., 2015), negative perceptions of buprenorphine by treatment payers and regulators (Molfenter et al., 2015), gaps in treatment need versus capacity (Jones et al., 2015), and restrictive policies, such as mandatory counseling, that may function as access barriers (Walley et al., 2008).
Findings suggest that, in addition to sociodemographic characteristics, there are ways in which buprenorphine treatment utilization may be influenced by individual knowledge and attitudes and also contextual forces that shape real or perceived opportunity to access buprenorphine. Here we investigate the extent to which individual knowledge and attitudes toward buprenorphine and access barriers to care are associated with use of buprenorphine over time, while controlling for the effect of sociodemographics, medication received at baseline, and other covariates.
2. Materials and Methods
2.1. Study design
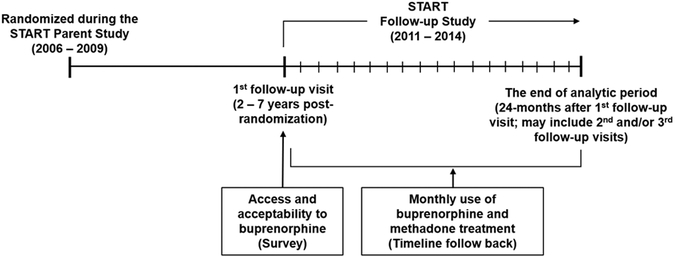
Data were provided by a large multisite prospective study that examined the long-term outcomes of participants who had been randomized to buprenorphine (as buprenorphine/naloxone) (BUP) or methadone (MET). The parent study “Starting Treatment with Agonist Replacement Therapy” (START) was a phase IV, post-marketing study designed to examine the comparative effects of buprenorphine and methadone on indices of liver health in participants with DSM-IV opioid dependence (Saxon et al., 2013). START involved nine federally licensed opioid treatment programs (located in five states: California, Oregon, Washington, Pennsylvania, and Connecticut) that together randomized 1,269 individuals to receive either buprenorphine (n=740) or methadone (n=529) during 2006-2009. Participants were offered medication for 24 weeks and then were tapered off over ≤8 weeks or referred for ongoing clinical treatment. Midway through the study, the randomization scheme was switched from 1:1 buprenorphine:methadone to 2:1 because of higher drop out in the buprenorphine arm. This change accounts for more participants in the follow-up sample having been randomized to buprenorphine.
Using an intent-to-treat design, a follow-up study of all randomized participants was conducted during 2011-2014, consisting of 3 follow-up interviews completed approximately 2 to 8 years (mean 4.5) post-randomization (Hser et al., 2016). The findings reported here utilize data on START participants who completed the first follow-up and one additional follow-up. Of the 1,080 participants who were targeted for follow-up, 89.4% (n=965) were located with 797 interviewed (73.7% of subjects randomized to buprenorphine; 73.6% of subjects randomized to methadone); among the 168 participants located but not interviewed, 49 were deceased, 54 refused, 29 were incarcerated, and 36 were too mentally ill or otherwise unable to be interviewed. Eight of those interviewed were omitted from the present analysis because of missing data, yielding an analytic sample of 789. There were no differences in the demographic characteristics of participants included and omitted from analysis.
2.2. Interview procedures
Research staff at the clinics where participants were originally recruited conducted follow-up interview 1 face-to-face approximately 2-7 years (mean 4.2) post-randomization and obtained a urine sample for drug testing. UCLA staff conducted follow-up interviews 2 and 3 over the telephone approximately 1 (mean 1.3) and 2 (mean 2.4) years, respectively, after follow-up interview 1. Participants were compensated according to local policies, which generally consisted of a $50-$70 gift card for each follow-up interview and $10 for a urine sample. All study procedures were approved by the Institutional Review Board (IRB) at UCLA and by the local site IRBs. A federal Certificate of Confidentiality was obtained to protect against disclosure of sensitive information.
2.3. Primary measures
The dependent variable is self-reported receipt of any prescribed buprenorphine-naloxone over 24 months after follow-up 1 as collected from participants using timeline follow back (TLFB) methodology (Sobell and Sobell, 1992) aided by a calendar and other memory prompts (Hser et al., 1992; Murphy et al., 2010). The TLFB was used to collect buprenorphine treatment use over time from START enrollment to follow-up 1, from follow-up 1 to follow-up 2, and from follow-up 2 to follow-up 3. Data collected at follow-up 1 included utilization of buprenorphine as provided by the original START trial. Therefore, in the present analysis we examined utilization of any buprenorphine during the 24 months after follow-up 1, when participation in the trial had already ended. Finally, given that individuals treated for OUD may have the opportunity to receive either methadone or buprenorphine, we included in analyses individuals who had been randomized by the START trial to methadone in addition to those who had been randomized to buprenorphine.
A key independent variable is buprenorphine accessibility, that is, the extent to which participants reported having experienced barriers to accessing buprenorphine treatment. Participants were asked at follow-up 1 to indicate whether any of 5 factors affect their ability to access buprenorphine treatment when needed (shown in Table 2). Indicators were analyzed separately and then aggregated into one buprenorphine accessibility composite score. To calculate the composite score, a value was assigned to each indicator: 0 for participants who indicated on a single item having no problem accessing buprenorphine treatment when needed and −1 for responses that indicated a constrained or limited ability to access such care. Indicators included: inability to afford buprenorphine because of no health insurance or no pocket money; inability to find free buprenorphine; inability to find a nearby doctor or clinic that prescribed buprenorphine; and inability to receive buprenorphine treatment during the clinic’s usual hours. Instances in which a participant did not indicate a constrained or limited ability to access such care (i.e., those with knowledge of buprenorphine or did not have problem getting it) were coded as 0. A negative value was assigned for each indicator since the questionnaire measures barriers to access. Values were summed such that a higher composite score (possible range was −5 to 0) indicated greater accessibility to buprenorphine.
Table 2.
Accessibility of buprenorphine treatment as reported by participants, by assigned condition in the parent study
| BUP N=460 |
MET N=329 |
Total N=789 |
|
|---|---|---|---|
| Knowledge of buprenorphine treatment*** | N (%) | N (%) | N (%) |
| Yes | 457 (99.4) | 230 (69.9) | 687 (87.1) |
| Problem getting buprenorphine treatment when needed | |||
| Among participants with knowledge of buprenorphine treatment | n=457 | n=230 | n=687 |
| Yes | 237 (51.9) | 113 (49.1) | 350 (51.0) |
| Reason for not getting buprenorphine treatment when needed | |||
| Among participants with problem getting buprenorphine treatment when needed | n=237 | n=113 | n=350 |
| Unaffordable, no health insurance^ | 137 (57.8) | 62 (54.9) | 199 (56.9) |
| Unaffordable, no pocket money^ | 134 (56.5) | 56 (49.6) | 190 (54.3) |
| Can’t find free buprenorphine^ | 117 (49.4) | 61 (54.0) | 178 (50.9) |
| Can’t find nearby physician/clinic prescriber^* | 106 (44.7) | 38 (33.6) | 144 (41.1) |
| Can’t attend clinic hours of operation^ | 13 (5.5) | 3 (2.7) | 16 (4.6) |
| Other reasons* | 40 (16.9) | 30 (26.6) | 70 (20.0) |
| Accessibility of buprenorphine treatment composite score (range: −5 to 0), Mean (SD) | −1.20 (1.44) | −1.06 (1.14) | −1.14 (1.32) |
p<0.05,
p<0.01,
p<0.001; X2 tests for categorical variables and t-tests for continuous variables.
item was used to create the accessibility composite score.
Another key independent variable is buprenorphine acceptability, that is, the extent to which participants express negative attitudes towards buprenorphine medication as self-reported at follow-up 1 in response to 6 items (see Table 3). Indicators were analyzed separately and then aggregated into one composite score. To calculate the composite score, a value of −1 was assigned to each item that indicated participants’ unwillingness to take buprenorphine if it were made available due to reasons that included: dislike of buprenorphine; having heard bad things about buprenorphine; negative physical reactions to buprenorphine; other unwanted side effects; reports that the effect of buprenorphine is not strong enough; and the inability to feel the effects of opioids when also taking buprenorphine. Instances in which a participant did not report such attitudes/experiences (i.e. those without knowledge of buprenorphine or currently taking buprenorphine) were coded as 0. A negative value was assigned for each indicator since the questionnaire measures negative attitudes toward buprenorphine. Values were summed such that a higher composite score (possible range was −6 to 0) indicated stronger acceptability of buprenorphine.
Table 3.
Acceptability of buprenorphine treatment as reported by participants at follow-up 1, by assigned condition in the parent study
| BUP N=460 |
MET N=329 |
Total N=789 |
|
|---|---|---|---|
| Currently taking buprenorphine | N (%) | N (%) | N (%) |
| Among participants with knowledge of buprenorphine | n=457 | n=230 | n=687 |
| Yes | 80 (17.5) | 39 (17.0) | 119 (17.3) |
| Willingness to take buprenorphine if available* | |||
| Among patients not taking buprenorphine | n=377 | n=191 | n=568 |
| No | 183 (48.5) | 111 (58.1) | 294 (51.8) |
| Reasons not willing to take buprenorphine | |||
| Among patients not taking buprenorphine and unwilling to take buprenorphine | n=183 | n=111 | n=294 |
| Not using opioids now | 99 (54.1) | 52 (46.9) | 151 (51.4) |
| Prefer methadone | 81 (44.3) | 55 (49.6) | 136 (46.3) |
| Do not like buprenorphine^** | 70 (38.3) | 26 (23.4) | 96 (32.7) |
| Cannot find free buprenorphine* | 39 (21.3) | 12 (10.8) | 51 (17.4) |
| Don't know much about buprenorphine | 15 (8.2) | 16 (14.4) | 31 (10.5) |
| Heard bad things about buprenorphine^ | 15 (8.2) | 7 (6.3) | 22 (7.5) |
| Other reasons | 26 (14.2) | 23 (20.7) | 49 (16.7) |
| Reasons for not liking buprenorphine | |||
| Among patients not taking buprenorphine who do not like buprenorphine | n=70 | n=26 | n=96 |
| Had negative physical reactions^* | 51 (72.9) | 13 (50.0) | 64 (66.7) |
| Did not like the side effects^* | 33 (47.1) | 6 (23.1) | 39 (40.6) |
| Medication was not strong enough^ | 21 (30.0) | 13 (50.0) | 34 (35.4) |
| Can’t feel opioids when taking buprenorphine^* | 29 (41.4) | 4 (15.4) | 33 (34.4) |
| Had to come to clinic everyday*** | 27 (38.6) | 0 (0.0) | 27 (28.1) |
| Other reasons | 10 (14.3) | 7 (26.9) | 17 (17.7) |
| Acceptability of buprenorphine treatment composite score (range: −6 to 0), Mean (SD) | −0.72 (1.19) | −0.46 (0.81) | −0.62 (1.05) |
p<0.05,
p<0.01,
p<0.001;
X2 tests for categorical variables and t-tests for continuous variables.
item was used to create the acceptability composite score.
Covariates are conceptualized as person-level predisposing and enabling factors and were selected based on prior findings from this cohort (Hser et al., 2017, 2016). Sociodemographic baseline variables as provided by the parent study include age, gender, race/ethnicity, education level, injection drug use, a cocaine positive urine test, and site (West vs. East coast). Also, included as covariates were experiences after follow-up 1 of methadone treatment and incarceration.
The key measures, sources of data, measurement points, and time horizon of the analysis are outlined in Figure 1.
Figure 1.
Measurement points, sources of data, and time horizon of the analysis
2.4. Statistical analysis
Group differences between participants randomized to buprenorphine and methadone treatments were examined using chi-square test for categorical variables and t-test for continuous variables. Analyses were designed to examine the association between participant utilization of buprenorphine over the 24-month period after follow-up 1 and participant indicators of buprenorphine accessibility and acceptability as measured at follow-up 1, while also considering participants’ baseline randomization status, socio-demographic characteristics, and experiences of incarceration and methadone treatment. First, we computed utilization of buprenorphine treatment at each month over the 24-month follow-up period and plotted the resulting trajectory. Based on the plot, we then applied the generalized linear mixed effects model with a logit function to estimate the trajectory of buprenorphine treatment utilization over the 24-month period. Unstructured covariance was selected, as this model best fit the data with the lowest Akaike (AIC) and Bayesian (BIC) information criteria values (Schwarz, 1978). To examine the association between buprenorphine treatment accessibility and acceptability, participant characteristics, and subsequent buprenorphine treatment utilization over the two-year follow-up period, we built and tested models of greater complexity with stepwise inclusion of covariates. We present two models here. Covariates included randomization condition, and buprenorphine accessibility and acceptability scores. Demographics (e.g., age, gender, race, education), history of cocaine use, drug injection, treatment site, and cumulative methadone treatment utilization over 24 months were included as controlling covariates. Incarceration status was included as a time-varying covariate. Finally, we included a buprenorphine acceptability x buprenorphine accessibility interaction term.
The proportion of missing in each variable was less than 5%, except for incarceration status (27.3%). Missing data were imputed using a multiple imputation method. Ten datasets were generated, and the imputation was informed by the variables in the analysis. Estimates from the 10 datasets were pooled using PROC MI and MIANALYZE (Johnson and Young, 2011; von Hippel, 2009; Yuan, 2010).
Odds ratio (OR) obtained from the models represent change in the likelihood of receiving buprenorphine over time; OR > 1 means an increasing likelihood per month, whereas OR < 1 suggests a decreasing likelihood per month. All models were developed using PROC GLIMMIX in SAS 9.4 (SAS Institute Inc., 2013).
3. Results
3.1. Characteristics of participants
At baseline, mean (sd) age was 37.4 (11.2), 34.4% were female, and most were White (72.8%), followed by Hispanic (11.2%), Black (9.1%), and other race/ethnicity (7.0%) (See Table 1). Most participants had attained a high school degree (45.1%) or at least some college (36.0%), but few were currently employed full- or part-time (18.9%, 11.4%). On average, participants had used heroin for more than a decade (mean [sd] 12.4 [11.4] years) and other opioids for about half as many years (7.2 [8.0] years). Participants also reported a significant number of years using cocaine (7.7 [8.6] years), methamphetamine (7.9 [9.1]), cannabis (12.8 [10.5]), and alcohol (12.8 [10.6]). Participants had received treatment for substance use disorders previously, i.e., drug treatment (mean [sd] 7.1 [7.4] times) or drug detoxification (4.5 [4.8]), alcohol detoxification (3.8 [6.9]), and alcohol treatment (4.2 [6.3]).
Table 1.
Participant characteristics and experiences at baseline, by assigned condition in the parent study
| BUP (N=460) |
MET (N=329) | Total (N=789) |
|
|---|---|---|---|
| Female (%) | 33.0 | 36.2 | 34.4 |
| Age (%) | |||
| 18-24 | 13.0 | 14.3 | 13.6 |
| 25-34 | 32.8 | 34.0 | 33.3 |
| 35-44 | 23.3 | 19.2 | 21.6 |
| 45-54 | 23.9 | 25.8 | 24.7 |
| 55-64 | 6.7 | 6.1 | 6.5 |
| 65+ | 0.2 | 0.6 | 0.38 |
| Age (years), Mean (SD) | 37.4 (11.1) | 37.4 (11.3) | 37.4 (11.2) |
| Race/ethnicity (%) | |||
| White | 72.4 | 73.3 | 72.8 |
| Black | 8.7 | 9.7 | 9.1 |
| Hispanic | 13.0 | 8.5 | 11.2 |
| Other | 5.9 | 8.5 | 7.0 |
| Education completed (%) | |||
| Less than high school | 17.2 | 21.3 | 18.9 |
| High school graduate or equivalent (GED) | 45.0 | 45.3 | 45.1 |
| More than high school | 37.8 | 33.4 | 36.0 |
| Education completed (years), Mean (SD) | 12.5 (1.9) | 12.3 (1.9) | 12.4 (1.9) |
| Employment status (%) | |||
| Employed full time | 18.7 | 19.2 | 18.9 |
| Employed part time | 12.0 | 10.6 | 11.4 |
| Unemployed, looking for work | 29.6 | 28.9 | 29.3 |
| Not in the labor force | 39.8 | 41.3 | 40.4 |
| Drug and alcohol use in lifetime | |||
| Heroin (%) | 88.9 | 88.8 | 88.9 |
| Number of years, Mean (SD) | 12.2 (10.9) | 12.7 (12.0) | 12.4 (11.4) |
| Other opioids (%) | 63.3 | 60.2 | 62.0 |
| Number of years, Mean (SD) | 6.9 (7.5) | 7.5 (8.7) | 7.2 (8.0) |
| Cocaine (%) | 66.5 | 71.1 | 68.4 |
| Number of years, Mean (SD) | 7.3 (8.0) | 8.1 (9.3) | 7.7 (8.6) |
| Methamphetamine (%) | 31.3 | 31.9 | 31.6 |
| Number of years, Mean (SD) | 7.9 (8.7) | 7.8 (9.7) | 7.9 (9.1) |
| Amphetamine (%) | 18.3 | 18.2 | 18.3 |
| Number of years, Mean (SD) | 4.0 (4.7) | 4.1 (6.5) | 4.0 (5.5) |
| Cannabis (%) | 80.0 | 79.6 | 79.9 |
| Number of years, Mean (SD) | 13.2 (10.5) | 12.2 (10.4) | 12.8 (10.5) |
| Alcohol (%) | 81.1 | 84.2 | 82.4 |
| Number of years, Mean (SD) | 12.9 (10.9) | 12.8 (10.2) | 12.8 (10.6) |
| Treatment for substance use disorders in lifetime | |||
| Drug treatment (%) | 100.0 | 100.0 | 100.0 |
| Number of times, Mean (SD) | 7.3 (7.1) | 6.9 (7.9) | 7.1 (7.4) |
| Drug detoxification (%) | 68.5 | 65.1 | 67.1 |
| Number of times, Mean (SD) | 4.5 (4.9) | 4.4 (4.8) | 4.5 (4.8) |
| Alcohol treatment (%) | 13.7 | 15.8 | 14.6 |
| Number of times, Mean (SD) | 4.4 (5.5) | 4.0 (7.1) | 4.2 (6.3) |
| Alcohol detoxification (%) | 7.0 | 7.0 | 7.0 |
| Number of times, Mean (SD) | 3.7 (4.7) | 3.9 (9.2) | 3.8 (6.9) |
| State of residence (%) | |||
| California | 29.4 | 28.3 | 28.9 |
| Oregon | 22.0 | 21.0 | 21.6 |
| Washington | 11.7 | 12.2 | 11.9 |
| Pennsylvania | 8.7 | 10.9 | 9.6 |
| Connecticut | 28.3 | 27.7 | 28.0 |
There was no statistically significant difference between groups on any variable.
3.2. Buprenorphine accessibility
Most participants at follow-up 1, 87.1%, reported knowing about buprenorphine treatment (Table 2; see Appendix A for characteristics of the 12.9% participants who reported not knowing about buprenorphine). Of participants who knew about buprenorphine, 51.0% reported problems being able to receive buprenorphine when needed, most commonly because of no health insurance to cover it (56.9%) and/or no pocket money (54.3%), inability to find free buprenorphine (50.9%), and not being able to find a nearby physician or clinic that prescribed it (41.1%). Few subjects reported clinic hours of operation as a reason for not being able to receive buprenorphine (4.6%) or other reasons. The mean (SD) buprenorphine accessibility score was −1.14 (1.32).
3.3. Buprenorphine acceptability
Only 17.3% reported taking buprenorphine at follow-up 1. Of those not taking it, almost half (48.2%) said they would take it if available (Table 3). Of the 51.8% who said they would not take buprenorphine even if it were available, reasons included not using opioids anymore (51.4%), preference for methadone (46.3%), dislike of buprenorphine (32.7%), inability to find free buprenorphine (17.4%), lack of knowledge about buprenorphine (10.5%), and having heard bad things about buprenorphine (7.5%).
Of the 96 participants who said they did not like buprenorphine, reasons for not liking it included negative physical reactions (e.g. allergic reactions, headaches, nausea, stomach pain, and fatigue) (66.7%) or other side effects (e.g. depression, anxiety, anger, suicidal thoughts, sleep difficulties, irritability, paranoia) (40.6%), buprenorphine not being strong enough (35.4%), inability to feel effects of other opioids when taking buprenorphine (34.4%), and the study requirement to come to the clinic every day to receive buprenorphine (28.1%). The mean (SD) buprenorphine acceptability score was −0.62 (1.05).
3.4. Predictors of participant utilization of buprenorphine treatment over time
About 9.3 – 11.2% of participants used buprenorphine treatment and 39.8 – 42.0% of the participants used methadone treatment over 24 months after follow-up 1 (data not shown). Table 4 presents the results of the random-intercept model in which we examined the association between buprenorphine accessibility and acceptability and the trajectory of buprenorphine treatment utilization over 24 months, while also considering participants’ socio-demographic characteristics and other covariates.
Table 4.
Random-intercept model predicting utilization of buprenorphine treatment over 24 months after 1st follow-up (n=789)
| Covariates | OR (95% CI) | |
|---|---|---|
| Model 1 | Model 2 | |
| Intercept | 1.18 (0.25, 5.67) | 1.35 (0.28, 6.48) |
| Slope (months) | 0.98 (0.97, 0.99)** | 0.98 (0.97, 0.99)** |
| Interaction with intercept | ||
| Participant characteristics | ||
| Randomized to BUP (ref: MET) | 1.55 (0.77, 3.15) | 1.65 (0.81, 3.36) |
| Age (years) | 0.97 (0.94, 1.01) | 0.97 (0.94, 1.01) |
| Male (ref: female) | 0.75 (0.36, 1.56) | 0.78 (0.37, 1.65) |
| Race/ethnicity (ref: White) | ||
| Black | 0.35 (0.08, 1.50) | 0.33 (0.08, 1.40) |
| Hispanic | 0.15 (0.04, 0.50)** | 0.14 (0.04, 0.49)** |
| Other race | 0.82 (0.17, 4.07) | 0.78 (0.16, 3.85) |
| Less than high school education (ref: HS or more) | 0.92 (0.37, 2.30) | 0.96 (0.38, 2.43) |
| Cocaine positive at baseline (ref: negative) | 0.70 (0.33, 1.50) | 0.74 (0.35, 1.58) |
| Injection drug use (ref: no injection) | 0.67 (0.33, 1.36) | 0.57 (0.27, 1.19) |
| West coast site (ref: East coast) | 0.29 (0.14, 0.60)*** | 0.29 (0.14, 0.61)*** |
| Total no. of MET treatment received since 1st follow-up | 0.81 (0.76, 0.85)*** | 0.81 (0.76, 0.85)*** |
| Time-varying covariate | ||
| Incarceration since 1st follow-up | 0.29 (0.15, 0.54)*** | 0.28 (0.15, 0.52)*** |
| Participant experiences of buprenorphine treatment | ||
| BUP Accessibility | 0.94 (0.73, 1.22) | 1.06 (0.80, 1.40) |
| BUP Acceptability | 4.57 (2.55, 8.18)*** | 7.11 (3.35, 15.10)*** |
|
Interaction term BUP Accessibility × BUP Acceptability |
-- | 1.51 (1.05, 2.18)* |
BUP=buprenorphine; MET=methadone
p<0.05,
p<0.01,
p<0.001
Initially (i.e., at the initiation of the 24-month follow-up period), higher buprenorphine acceptability score at follow-up 1 was associated with greater likelihood of receiving buprenorphine treatment (OR [CI] = 4.57 [2.55, 8.18], p<0.001) (Table 4, Model 1). Furthermore, Hispanic ethnicity (0.15 [0.04, 0.50], p<.01), West coast context (0.29 [0.14, 0.60], p<0.001), and experiences during follow-up of methadone treatment (0.81 [0.76, 0.85], p<0.001) and incarceration (0.29 [0.15, 0.54], p<0.001) were negatively associated with buprenorphine treatment utilization at the initiation of the 24-month follow-up period.
Overall, the likelihood of receiving buprenorphine treatment (i.e., slope) was decreasing across months (0.98 [0.97, 0.99], p<.01). However, the change in the likelihood of buprenorphine treatment receipt over time was not affected by accessibility score, acceptability score, or other predictors.
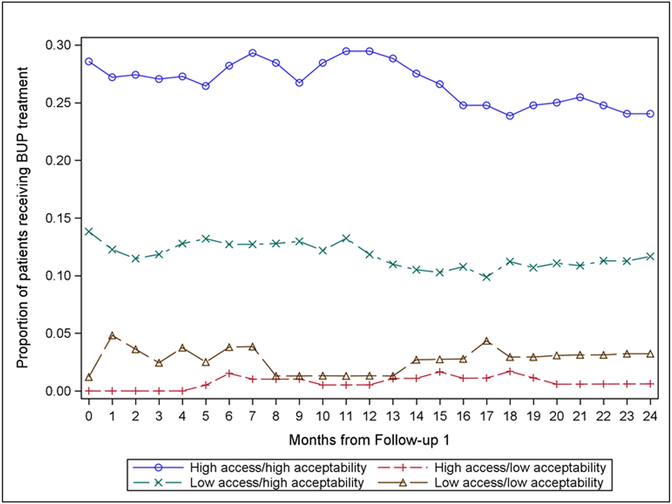
The interaction term of buprenorphine accessibility x buprenorphine acceptability was significant (1.51 [1.05, 2.18], p<.05 on Table 4, Model 2). In other words, individuals who perceived buprenorphine to be both accessible and acceptable were more likely to use buprenorphine treatment at the initiation of the 24-month follow-up period. When the interaction effects were plotted (Figure 2), results indicated that individuals who perceived buprenorphine to be both accessible and also acceptable were most likely to use buprenorphine over time. In contrast, individuals who perceived buprenorphine to be unacceptable were least likely to use buprenorphine, no matter the perceived level of access to the medication.
Figure 2. Buprenorphine (BUP) treatment utilization for opioid use disorder over 24 months after follow-up 1, by BUP accessibility score and BUP acceptability score (n=789).
Note: The median score was used as a cut-off to form low and high groups for access and acceptability scores.
4. Discussion
4.1. Summary of primary findings and implications
In the present paper, we analyze data from an extended long-term follow-up study of participants originally treated with methadone or buprenorphine as part of the START clinical trial. We examine utilization of buprenorphine treatment and its relationship with perceived access barriers and acceptability. Of the study participants, (1) about 9.3 – 11.2% used buprenorphine treatment over a 24-month follow-up period, (2) a significant proportion experienced buprenorphine to be inaccessible or unacceptable, and (3) independent of sociodemographics and other factors, participant likelihood of utilizing buprenorphine over time was associated with buprenorphine treatment accessibility and acceptability. Specifically, individuals who reported buprenorphine to be both accessible and also acceptable were most likely to use buprenorphine during follow-up. In contrast, individuals who reported buprenorphine to be unacceptable were least likely to use buprenorphine, no matter the reported level of access to the medication.
Participant-reported reasons for finding buprenorphine to be unacceptable were consistent with findings from other studies (e.g., Teruya et al., 2014). About half of those who were unwilling to use buprenorphine reported no longer using opioids. Other reasons included negative physical reactions or other adverse side effects, perceived weakness of buprenorphine (“it’s not holding me”), and not being able to feel the effects of other opioids when taking buprenorphine. Results suggest that to increase continued use of buprenorphine over time, we need to address individuals’ experiences that buprenorphine is unacceptable and these engagement strategies should be tailored to the specific reason why there is dislike for the medication. For example, individuals who experience buprenorphine-related negative physical reactions and unwanted side effects, or report that the medication is not strong enough, could benefit from optimization of the medication dosage. In contrast, individuals who report a dislike of their inability to feel the effects of other opioids when taking buprenorphine may be best treated with motivational methods for supporting behavioral changes and strategies to address the underlying causes of opioid misuse. Consideration could also be given to prescribing adjunctive clonidine which has been demonstrated to decrease opioid craving when added to a buprenorphine regimen (Kowalczyk et al., 2015).
We also found that relatively few participants reported utilizing buprenorphine, but about half who were not taking it said they would take it if it were available. Extended duration buprenorphine formulations were developed to improve treatment adherence (Blanco & Volkow, 2019) and may help to improve overall buprenorphine utilization rates, presenting an area for research. We also found that participants’ ability to access buprenorphine when needed was most commonly hindered by the price of buprenorphine, difficulty finding buprenorphine prescribers, and lack of insurance coverage for buprenorphine. In other analyses (data not shown), about two-thirds of participants had health insurance - mostly Medicaid, although significant proportions had private insurance or other forms of insurance – but many did not know if their health insurance covered buprenorphine. Nationwide there have been considerable efforts to expand buprenorphine treatment capacity. This work has focused on needed increases in buprenorphine accessibility, for example by reducing its price, ensuring Medicaid, Medicare, and other types of health insurance cover it, removing barriers such as prior authorization, and training more providers to prescribe it. However, our findings suggest that simply making buprenorphine treatment more available is not enough. To increase buprenorphine utilization, we must also increase knowledge of its benefits and applicable health insurance coverage and develop interventions to improve both accessibility and also acceptability.
About 13% of participants reported not being familiar with buprenorphine. This finding likely reflects, in part, the inability of participants to remember the buprenorphine information that had been provided at baseline. Our findings revealed that compared with participants who did know about buprenorphine, more of those who lacked buprenorphine knowledge were older, living in California, and had less education, more years of alcohol and cocaine use, and less exposure to prior treatment. It is well-known that prior to adopting an innovation it is important to ensure that intended adopters are aware of the innovation, have sufficient information about what it does and how to use it, and are clear about how its use would affect them personally (Hser et al., 2007). When applied to the findings from the present study, it is clear there is a need to implement location-specific strategies for raising individual awareness of buprenorphine as an option for evidence-based OUD treatment. Reducing gaps in knowledge about buprenorphine may also benefit from information dissemination efforts that utilize repeated messaging that is designed to be simple and clear or utilize other techniques that advance understanding. Such an information campaign has already been launched in New York City where advisements about buprenorphine treatment are posted in subway cars and other public venues.
Finally, certain participant sociodemographic characteristics were associated with a greater likelihood of participant utilization of buprenorphine during follow-up. Specifically, greater addiction severity (as indicated by injection drug use and cocaine positive urines) was negatively associated with buprenorphine treatment utilization, as was Hispanic ethnicity and living on the West coast relative to the East coast. Results underscore the need to target vulnerable groups who could benefit from buprenorphine and work to curb OUD progression before it worsens.
4.2. Limitations
Study findings must be considered within the context of several limitations. The composite measures of buprenorphine accessibility and buprenorphine acceptability were created specifically for the present study. Key stakeholders from the research team (i.e. physicians and clinical directors with expertise in buprenorphine treatment) assessed the face validity of these independent variables and determined they adequately covered the concepts being measured. Also, participants’ experiences of buprenorphine treatment accessibility and acceptability may have changed over time, particularly in terms of access. However, we lacked repeated measurement of these constructs. Therefore, findings reflect the influence of participants’ experiences as measured at a single time-point. While treatment utilization was tracked during the 2-year period, the access and acceptability scores were measured once at the beginning of the 2-year period. Therefore, those with a high acceptability score who started using buprenorphine at the beginning of the 2-year period and had a negative experience, would have yielded a decreased acceptability score. Similarly, participants who did not seek buprenorphine and had a high accessibility score at the beginning of the 2-year follow-up period, but became aware of barriers once they did seek buprenorphine treatment would have yielded a decreased accessibility score. Investigating the dynamic nature of the relationships between buprenorphine treatment access, acceptability, and utilization is an area for future research. Also, the range of 2-7 years post-randomization is quite wide, which could have implications that we did not account for in relation to recall, persistence of effects of original study arm on acceptability, as well as time trends in the broader diffusion of buprenorphine in the communities. We did not examine predictors of buprenorphine access and acceptability, which are areas for future work. The trial lasted for only 6 months, and across study sites there was variability in post-trial buprenorphine treatment availability and local buprenorphine policies. For these reasons, we have included site as a covariate. Also, to remain in treatment after the trial ended, participants had to make additional arrangements. Therefore, rates of continuing buprenorphine treatment may not reflect what would occur in routine clinical care. Finally, findings are generated from a sample of treated individuals who participated in a clinical trial implemented by a limited number of sites that was designed to evaluate effects of buprenorphine versus methadone on liver function (Saxon et al., 2013). We believe our cohort is reasonably representative of U.S. adults with OUD. Nevertheless, findings may not be representative of the general population of individuals with OUD.
5. Conclusions
To engage more individuals with opioid use disorders in long-term treatment with buprenorphine, interventions should target their experiences regarding buprenorphine treatment acceptability, in addition to increasing buprenorphine access, and tailor efforts to meet the needs of vulnerable populations.
Highlights.
Patients who perceive buprenorphine to be unacceptable are least likely to use it.
Vulnerable populations are least likely to use buprenorphine.
To increase buprenorphine utilization, increase acceptability and target vulnerable populations.
Acknowledgements
Sincere appreciation to our participating networks: the Pacific Northwest Node and Evergreen Treatment Services; the Western States Node and CODA Inc. and Bi-Valley Medical Clinic; the New England Node and Connecticut Counseling Centers and Yale and Hartford Dispensary; the Delaware Valley Node and NET Steps; the Pacific Region Node and Matrix Institute; Emmes Corporation; the CCTN and NIDA.
Role of Funding Source
Main study funding was provided by the National Institute on Drug Abuse (NIDA) through the Clinical Trials Network (CTN) through a series of grants provided to each participating node:
The Pacific Northwest Node (U10 DA01714)
The Western States Node (U10 DA 015815)
The New England Node (U10 DA13038)
The Delaware Valley Node (U10 DA13043)
The Pacific Region Node (U10 DA13045)
The Greater New York Node (UG1 DA013035)
Dr. Evans is also supported by The Greenwall Foundation, the National Institute on Drug Abuse (NIDA) UG3 DA0044830-02S1 and UG1DA050067-01, and the Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Substance Abuse Treatment (CSAT) Grant No. 1H79T1081387-01. Dr. Hser is supported by NIDA R33DA045844 and UG1 DA013035.
Appendix
Appendix A.
Characteristics and experiences of participants by knowledge of buprenorphine treatment
| Do you know about buprenorphine? | |||
|---|---|---|---|
| No (n=102; 13%) |
Yes (n=687; 87%) |
Total (n=789) |
|
| Randomization status, %*** | |||
| Buprenorphine | 3.0 | 66.5 | 58.3 |
| Methadone | 97.1 | 33.5 | 41.7 |
| Female, % | 37.3 | 34.0 | 34.4 |
| Age, %*** | |||
| 22-24 | 0.0 | 2.0 | 1.8 |
| 25-34 | 16.7 | 37.9 | 35.1 |
| 35-44 | 17.7 | 22.1 | 21.6 |
| 45-54 | 42.2 | 23.7 | 26.1 |
| 55-64 | 22.6 | 13.7 | 15.0 |
| 65+ | 1.0 | 0.6 | 0.6 |
| Age, mean (sd)** | 46.6 (10.3) | 40.9 (11.1) | 41.6 (11.2) |
| Race/ethnicity, % | |||
| White | 63.7 | 74.1 | 72.8 |
| Black | 11.8 | 8.7 | 9.1 |
| Hispanic | 17.7 | 10.2 | 11.2 |
| Other | 6.9 | 7.0 | 7.0 |
| Years of education, %* | |||
| Less than high school | 27.5 | 17.6 | 18.9 |
| High school | 46.1 | 45.0 | 45.1 |
| College or more | 26.5 | 37.4 | 36.0 |
| Years of education, mean (sd)** | 11.9 (1.9) | 12.5 (1.9) | 12.4 (1.9) |
| Employment status, %*** | |||
| Employed full time | 12.8 | 19.8 | 18.9 |
| Employed part time | 5.9 | 12.2 | 11.4 |
| Unemployed, looking for work | 22.6 | 30.3 | 29.3 |
| Not in the labor force | 58.8 | 37.7 | 40.4 |
| Months incarcerated in lifetime, mean (sd) | 29.5 (37.4) | 23.9 (33.5) | 24.5 (34.0) |
| ASI severity scores, mean (sd) | |||
| Alcohol | 0.07 (0.16) | 0.07 (0.15) | 0.07 (0.15) |
| Drug | 0.18 (0.13) | 0.18 (0.14) | 0.18 (0.14) |
| Medical | 0.31 (0.35) | 0.31 (0.36) | 0.31 (0.36) |
| Employment | 0.69 (0.30) | 0.64 (0.32) | 0.64 (0.32) |
| Legal | 0.08 (0.15) | 0.14 (0.19) | 0.09 (0.17) |
| Family | 0.12 (0.16) | 0.14 (0.19) | 0.14 (0.18) |
| Psychiatric | 0.24 (0.22) | 0.23 (0.23) | 0.23 (0.23) |
| Age at first use of primary drug, mean (sd) | 21.4 (7.9) | 22.3 (7.8) | 22.2 (7.8) |
| Years of alcohol and drug use in lifetime, mean (sd) | |||
| Alcohol* | 13.3 (12.2) | 10.2 (10.5) | 10.6 (10.8) |
| Heroin | 12.7 (11.4) | 10.8 (9.9) | 11.0 (11.4) |
| Opiates | 5.0 (8.1) | 4.4 (7.1) | 4.5 (7.2) |
| Cocaine* | 7.2 (10.1) | 5.0 (9.5) | 5.3 (8.0) |
| Amphetamine* | 0.5 (1.1) | 0.8 (3.0) | 0.7 (2.8) |
| Methamphetamine | 3.4 (7.2) | 2.4 (6.2) | 2.5 (6.3) |
| Cannabis | 10.6 (11.1) | 10.2 (10.6) | 10.2 (10.7) |
| AUDIT-C score, mean (sd) | 2.0 (3.0) | 1.9 (2.6) | 1.9 (2.6) |
| Exposure to treatment in lifetime, mean (sd) | |||
| Number of drug treatments** | 4.9 (3.4) | 7.5 (7.8) | 7.1 (7.5) |
| Number of drug detoxifications** | 1.7 (2.3) | 3.2 (2.9) | 3.0 (4.5) |
| Number of alcohol treatments | 0.5 (0.3) | 0.6 (0.4) | 0.6 (2.8) |
| Number of alcohol detoxifications* | 0.7 (0.9) | 2.1 (5.7) | 1.8 (5.1) |
| Total number of drug and alcohol treatments in lifetime, mean (sd)** | 6.5 (5.3) | 10.7 (12.0) | 10.1 (11.5) |
| Total number of drug and alcohol detoxifications in lifetime, mean (sd) | 0.7 (1.6) | 0.9 (5.0) | 0.9 (4.7) |
| State, %** | |||
| California | 43.1 | 26.8 | 28.9 |
| Connecticut | 13.7 | 30.1 | 28.0 |
| Oregon | 11.8 | 23.0 | 21.6 |
| Pennsylvania | 11.8 | 9.3 | 9.6 |
| Washington | 19.6 | 10.8 | 11.9 |
p<.05
p<.01
p<.001
About 13% of participants reported not knowing about buprenorphine at follow-up 1. Assignment to buprenorphine or methadone in the trial was strongly associated with familiarity with buprenorphine at follow-up 1 (97.1% vs. 33.5%). The group who did not know about buprenorphine was older on average than the group who knew about it (mean age 46.6 vs. 40.9), and more subjects in this group had not attained a high school degree (27.5% vs. 17.6%), and more were not in the labor force (58.8% vs. 37.7%). Participants who did and did not know about buprenorphine began using their primary drug at about the same age on average (at about age 22), and patients in both groups had used heroin and opiates for a similar number of years (11-12 years, 5 on average, respectively), but patients who did not know about buprenorphine had used alcohol (13.3 vs. 10.2 years) and cocaine (7.2 vs. 5.1 years) for more years than participants who did know about buprenorphine. Finally, participants who did not know about buprenorphine had less prior exposure to treatment as indicated by a fewer number of drug treatments (mean [sd] treatments 4.9 [3.4] vs. 7.5 [7.8]) and drug detoxifications (mean [sd] detoxifications 1.7 [2.3] vs. 3.2 [2.9]). Compared with participants who knew about buprenorphine, more of those who did not know about it were living in California (43.1%) than in the other locales [Washington (19.6%), Oregon (11.8%), Pennsylvania (11.8%), or Connecticut (13.7%)].
Footnotes
Declaration of Interest
Authors disclosing relevant financial interests, activities, relationships, and affiliations are:
Andrew J. Saxon: receives royalties as an editor for UpToDate and received an honorarium from Alkermes, Inc. for participation on an advisory board.
All other authors report no financial or other possible conflicts of interest.
References
- Bart G, 2012. Maintenance medication for opiate addiction: the foundation of recovery. J. Addict. Dis. 31, 207–25. 10.1080/10550887.2012.694598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, Trinh L, Butler B, Randall D, Rubin G, 2009. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction 104, 1193–200. 10.1111/j.1360-0443.2009.02627.x [DOI] [PubMed] [Google Scholar]
- Blanco C, Volkow ND, 2019. Management of opioid use disorder in the USA: present status and future directions. Lancet. 27;393(10182):1760–1772. [DOI] [PubMed] [Google Scholar]
- Bonhomme J, Shim RS, Gooden R, Tyus D, Rust G, 2012. Opioid addiction and abuse in primary care practice: a comparison of methadone and buprenorphine as treatment options. J. Natl. Med. Assoc. 104, 342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L, Gisev N, Larney S, Dobbins T, Gibson A, Kimber J, Larance B, Mattick RP, Butler T, Degenhardt L, 2015. A longitudinal comparison of retention in buprenorphine and methadone treatment for opioid dependence in New South Wales, Australia. Addiction 110, 646–55. 10.1111/add.12834 [DOI] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS, 2007. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol. Assess. 11, 1–171, iii-iv. [DOI] [PubMed] [Google Scholar]
- DeFlavio JR, Rolin SA, Nordstrom BR, Kazal LA, 2015. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health 15, 3019. [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J, 2011. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 106, 32–51. 10.1111/j.1360-0443.2010.03140.x [DOI] [PubMed] [Google Scholar]
- Evans E, Hser YI, 2019. The natural history, clinical course, and long-term recovery from opioid use disorders In Kelly and Wakeman (editors), Treating Opioid Addiction. Humana Press, Springer International; DOI: 10.1007/978-3-030-16257-3_9. [DOI] [Google Scholar]
- Evans E, Li L, Min J, Huang D, Urada D, Liu L, Hser Y-I, Nosyk B, 2015. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction 110, 996–1005. 10.1111/add.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans. E, Zhu Y, Yoo C, Huang D, Hser YI, 2019. Criminal justice outcomes over 5 years after randomization to buprenorphine-naloxone or methadone treatment for opioid use disorder. Addiction. 114 (8): 1396–1404. PMCID: PMC6626574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerhood MI, King VL, Brooner RK, Rastegar DA, 2014. A comparison of characteristics and outcomes of opioid-dependent patients initiating office-based buprenorphine or methadone maintenance treatment. Subst. Abus. 35, 122–6. 10.1080/08897077.2013.819828 [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Jaffe JH, Schwartz RP, Dušek KA, Gugsa N, Monroe CL, O’Grady KE, Olsen YK, Mitchell SG, 2013. Patient perspectives on choosing buprenorphine over methadone in an urban, equal-access system. Am. J. Addict. 22, 285–91. 10.1111/j.1521-0391.2012.12004.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad MS, Zelenev A, Altice FL, 2013. Integrating buprenorphine maintenance therapy into federally qualified health centers: Real-world substance abuse treatment outcomes. Drug Alcohol Depend. 131, 127–135. 10.1016/j.drugalcdep.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, Miniño AM, 2017. Drug overdose deaths in the United States, 1999-2016., NCHS Data Brief. [PubMed] [Google Scholar]
- Hser Y-I, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Woody G, Liu D, Wakim P, Matthews AG, Hatch-Maillette M, Jelstrom E, Wiest K, McLaughlin P, Ling W, 2016. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 111, 695–705. 10.1111/add.13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y-I, Huang D, Saxon AJ, Woody G, Moskowitz AL, Matthews AG, Ling W, 2017. Distinctive trajectories of opioid use over an extended follow-up of patients in a multisite trial on buprenorphine + naloxone and methadone. J. Addict. Med. 11, 63–69. 10.1097/ADM.0000000000000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y-I, Longshore D, Anglin MD, 2007. The life course perspective on drug use: a conceptual framework for understanding drug use trajectories. Eval. Rev. 31, 515–47. 10.1177/0193841X07307316 [DOI] [PubMed] [Google Scholar]
- Hser Y, Anglin MD, Chou C, 1992. Reliability of retrospective self-report by narcotics addicts. Psychol. Assess. 4, 207–213. 10.1037/1040-3590.4.2.207 [DOI] [Google Scholar]
- Johnson DR, Young R, 2011. Toward best practices in analyzing datasets with missing data: Comparisons and recommendations. J. Marriage Fam. 73, 926–945. 10.1111/j.1741-3737.2011.00861.x [DOI] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E, 2015. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am. J. Public Health 105, e55–63. 10.2105/AJPH.2015.302664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek KD, Murphy S, Xu J, Arias E, 2017. Mortality in the United States, 2016., NCHS Data Brief. [PubMed] [Google Scholar]
- Kowalczyk WJ, Phillips KA, Jobes ML, Kennedy AP, Ghitza UE, Agage DA, Schmittner JP, Epstein DH, Preston KL, 2015. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: A randomized controlled trial with ecological momentary assessment. Am. J. Psychiatry 172, 760–767. 10.1176/appi.ajp.2014.14081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shorter D, Kosten TR, 2014. Buprenorphine in the treatment of opioid addiction: opportunities, challenges and strategies. Expert Opin. Pharmacother. 15, 2263–2275. 10.1517/14656566.2014.955469 [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. CD002207 10.1002/14651858.CD002207.pub4 [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M, 2008. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence, in: Mattick RP (Ed.), Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, Chichester, UK, p. CD002207 10.1002/14651858.CD002207.pub3 [DOI] [PubMed] [Google Scholar]
- Molfenter T, Sherbeck C, Zehner M, Quanbeck A, McCarty D, Kim J-S, Starr S, 2015. Implementing buprenorphine in addiction treatment: payer and provider perspectives in Ohio. Subst. Abuse Treat. Prev. Policy 10, 13 10.1186/s13011-015-0009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Hser Y-I, Huang D, Brecht M-L, Herbeck DM, 2010. Self-report of longitudinal substance use: A comparison of the UCLA Natural History Interview and the Addiction Severity Index. J. Drug Issues 40, 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherland J, Botsko M, Egan JE, Saxon AJ, Cunningham CO, Finkelstein R, Gourevitch MN, Renner JA, Sohler N, Sullivan LE, Weiss L, Fiellin DA, BHIVES Collaborative, 2009. Factors affecting willingness to provide buprenorphine treatment. J. Subst. Abuse Treat. 36, 244–51. 10.1016/j.jsat.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Li L, Evans E, Urada D, Huang D, Wood E, Rawson R, Hser Y-I, 2014. Utilization and outcomes of detoxification and maintenance treatment for opioid dependence in publicly-funded facilities in California, USA: 1991-2012. Drug Alcohol Depend. 143, 149–57. 10.1016/j.drugalcdep.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SL, Copeland AL, Kopak AM, Herschman PL, Polukhina N, 2014. A naturalistic comparison of the effectiveness of methadone and two sublingual formulations of buprenorphine on maintenance treatment outcomes: findings from a retrospective multisite study. Exp. Clin. Psychopharmacol. 22, 424–33. 10.1037/a0037550 [DOI] [PubMed] [Google Scholar]
- Rosenblatt RA, Andrilla CHA, Catlin M, Larson EH, 2015. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann. Fam. Med. 13, 23–6. 10.1370/afm.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc., 2013. SAS Enterprise Guide 5.1. SAS Institute Inc., Cary, NC. [Google Scholar]
- Saxon AJ, Ling W, Hillhouse M, Thomas C, Hasson A, Ang A, Doraimani G, Tasissa G, Lokhnygina Y, Leimberger J, Bruce RD, McCarthy J, Wiest K, McLaughlin P, Bilangi R, Cohen A, Woody G, Jacobs P, 2013. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug Alcohol Depend. 128, 71–76. 10.1016/j.drugalcdep.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G, 1978. Estimating the dimension of a model. Ann. Stat. 6, 461–464. 10.1214/aos/1176344136 [DOI] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back: a technique for assessing self-reported alcohol consumption, in: Litten R, Allen J (Eds.), Measuring Alcohol Consumption. Humana Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- Stein BD, Pacula RL, Gordon AJ, Burns RM, Leslie DL, Sorbero MJ, Bauhoff S, Mandell TW, Dick AW, 2015. Where is buprenorphine dispensed to treat opioid use disorders? The role of private offices, opioid treatment programs, and substance abuse treatment facilities in urban and rural counties. Milbank Q. 93, 561–83. 10.1111/1468-0009.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruya C, Schwartz RP, Mitchell SG, Hasson AL, Thomas C, Buoncristiani SH, Hser Y-I, Wiest K, Cohen AJ, Glick N, Jacobs P, McLaughlin P, Ling W, 2014. Patient perspectives on buprenorphine/naloxone: a qualitative study of retention during the starting treatment with agonist replacement therapies (START) study. J. Psychoactive Drugs 46, 412–26. 10.1080/02791072.2014.921743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME, 2014. Medication-assisted treatment with buprenorphine: Assessing the evidence. Psychiatr. Serv. 65, 158–170. 10.1176/appi.ps.201300256 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (HHS), O. of the S.G., 2016. Facing addiction in America: The surgeon general’s report on alcohol, drugs, and health. HHS, Washington, DC. [PubMed] [Google Scholar]
- von Hippel PT, 2009. How to impute interactions, squares, and other transformed variables. Sociol. Methodol. 39, 265–291. 10.1111/j.1467-9531.2009.01215.x [DOI] [Google Scholar]
- Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, Alford DP, 2008. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J. Gen. Intern. Med. 23, 1393–8. 10.1007/s11606-008-0686-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein ZM, Kim HW, Cheng DM, Quinn E, Hui D, Labelle CT, Drainoni M-L, Bachman SS, Samet JH, 2017. Long-term retention in Office Based Opioid Treatment with buprenorphine. J. Subst. Abuse Treat. 74, 65–70. 10.1016/j.jsat.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan PJ, Remski K, 2012. Buprenorphine vs methadone treatment: A review of evidence in both developed and developing worlds. J. Neurosci. Rural Pract. 3, 45–50. 10.4103/0976-3147.91934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Bruce D, Korthuis PT, Chhatre S, Poole S, Hillhouse M, Jacobs P, Sorensen J, Saxon AJ, Metzger D, Ling W, 2014. HIV risk reduction with buprenorphine-naloxone or methadone: findings from a randomized trial. J. Acquir. Immune Defic. Syndr. 66, 288–93. 10.1097/QAI.0000000000000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YC, 2010. Multiple imputation for missing data: Concepts and new development (version 9.0). Rockville, MD. [Google Scholar]