Abstract
Introduction:
A mandated reduction in the nicotine content of cigarettes may decrease smoking, but also increase demand for other nicotine products. The present study tested the impact of smoking cigarettes with very low nicotine content (VLNC) and concurrent use of a transdermal nicotine patch.
Study design:
A balanced 2 × 2 factorial randomized clinical trial investigating the impact of cigarette nicotine content (double-blind, VLNC versus normal nicotine content) and use of a transdermal nicotine patch (open label, patch versus no patch).
Setting/participants:
Adult daily smokers (N=240) in the Pittsburgh, PA area.
Intervention:
Participants were provided with research cigarettes and transdermal nicotine patches (if assigned to patch condition) for 7 weeks. Cigarettes were Spectrum brand (National Institute on Drug Abuse) and either 15.8 mg nicotine/g tobacco (normal nicotine content) or 0.4 mg nicotine/g tobacco (VLNC). In the 7th week, participants were monetarily incentivized to abstain from smoking.
Main outcome measures:
Participants reported daily cigarette use throughout the trial and the primary outcome was average number of cigarettes smoked per day (study + non-study) during Week 6. Participants were recruited from 2015 to 2017 and data were analyzed between 2017 and 2018.
Results:
Assignment to VLNC cigarettes and assignment to wear a nicotine patch both reduced the number of cigarettes smoked per day during Week 6 (p=0.001 and 0.04, respectively). However, assignment to the patch along with VLNC cigarettes did not significantly reduce cigarette smoking compared to assignment to VLNC cigarettes alone.
Conclusions:
A mandated reduction in the nicotine content of cigarettes is likely to reduce the number of cigarettes smoked per day but the added benefit of concurrent transdermal nicotine is unclear. Future studies should investigate whether alternative sources of non-combusted tobacco, like e-cigarettes, enhance the effects of VLNC cigarettes on smoking.
INTRODUCTION
The U.S. Food and Drug Administration has proposed to mandate a reduction in the nicotine content of cigarettes sold in the U.S. to a minimally addictive or non-addictive level. The goal of such a policy would be to reduce the rate of smoking initiation among youth and help adult smokers quit.1 Clinical trials in which adult smokers are switched to cigarettes with a very low nicotine content (VLNC) have shown that a reduction in cigarette nicotine content can reduce nicotine exposure2–5, smoking rate,2,3,5 toxicant exposure2,5, nicotine dependence,3,5 and increase cessation rates.2,5
Clinical trial data suggest that participants who are switched to VLNC cigarettes seek out additional forms of nicotine, most often commercially available normal nicotine content (NNC) cigarettes, despite instructions not to do so.6 Concurrent use of non-combusted nicotine and tobacco products, including nicotine-replacement therapy (NRT) or e-cigarettes, may facilitate the transition to VLNC cigarettes and reduce non-adherence with VLNC cigarettes. Two existing studies suggest that adjunct NRT may reduce cigarette smoking, reduce withdrawal, and increase adherence with VLNC cigarettes.7,8 However, a third study found that adjunctive NRT did not enhance the effects of VLNC cigarettes on smoking reductions.9
The present study tested the effects of VLNC cigarettes in adult smokers, alone and combined with NRT, across 7 weeks. Participants were randomized to either receive VLNC or NNC cigarettes and either a transdermal nicotine patch or no patch. To explore whether VLNC cigarettes and NRT may impact the ability to abstain from smoking, participants were given a monetary incentive to abstain from smoking during the 7th week.
Participants were asked not to use non-study cigarettes and an adherence incentive system was used. The present paper also includes a secondary analysis to estimate the treatment effects if all participants had been adherent (i.e., causal inference model).10 This analysis is important because following implementation of a tobacco product standard, participants would not have the choice to legally purchase NNC cigarettes.
METHODS
The study was conducted at the University of Pittsburgh, in Pittsburgh, PA, from 2015 to 2017, and data analysis took place between 2017 and 2018. The protocol was approved by the University of Pittsburgh IRB, reviewed by the Food and Drug Administration Center for Tobacco Products, and monitored by an independent data and safety monitoring board. The full protocol can be found in the Appendix.
The present study was a between-subjects balanced 2 × 2 factorial RCT of cigarette nicotine content (NNC, 15.8 mg nicotine/g tobacco; VLNC, 0.4 mg nicotine/g tobacco) and transdermal nicotine patch (patch, no patch). Spectrum research cigarettes were provided by the National Institute on Drug Abuse and have been described in detail elsewhere.3 Participants were assigned a cigarette that matched their menthol preference, an equal number of menthol and non-menthol cigarette smokers were recruited, and randomization was stratified by menthol status.
Participants were also randomized to receive either adjunctive transdermal nicotine (Novartis Pharmaceuticals; purchased for the study) or no adjunctive transdermal nicotine. Nicotine patch dose was matched to participants’ baseline number of cigarettes smoked per day (CPD): 21 mg (>20 CPD) or 14 mg (5 to 19 CPD). Participants experiencing discomfort associated with wearing the patch were eligible to be switched to a lower nicotine dose when recommended by the study licensed medical professional (LMP). Research cigarettes and nicotine patches were provided to participants at no cost. Participants, investigators, and study staff were blind to the nicotine content of the cigarettes; patch assignment was not blinded. Procedures for blinding, cigarette management, and randomization were consistent with those reported previously.11
Study Sample
Current daily smokers (N=240) were recruited using standard media outlets. Inclusion criteria were: age ≥18 years and smoking at least five CPD (expired carbon monoxide [CO] ≥10 ppm, or urinary cotinine >2,000 mg/mL). Exclusion criteria were: any intention to quit smoking in the next 30 days or quit attempt resulting in ≥3 days of abstinence in the past 30 days; seeking treatment or using medication for smoking cessation; exclusively using “roll your own” cigarettes; using tobacco products other than cigarettes, including e-cigarettes; binge drinking on >9 days in the past 30 days; testing positive for illicit drugs; having schizophrenia or schizoaffective disorder; a suicide attempt in the past 5 years; being pregnant, trying to become pregnant, or breastfeeding; CO >80 ppm; or having any other psychiatric or medical condition that would put the participant at risk or limit participation as determined by an LMP. There was no racial or gender bias in the recruiting of participants. Participants provided written informed consent. Participants could receive up to $863.50 for study participation.
Measures
Participants attended weekly lab sessions to complete in-person assessments and receive study products. Each week, participants received a 2-week supply of research cigarettes and nicotine patches to ensure that participants did not run out of supplies in the event of an unanticipated missed visit and to allow for any compensatory smoking. Participants reported the number of study and non-study cigarettes they smoked each day via an automated phone system (Intervision Media, IVR).
Participants were instructed to only use the study products and to refrain from using other nicotine or tobacco products. Because of the importance of VLNC adherence for modeling the potential effects of a product standard reducing nicotine to minimally addictive or non-addictive level, a financial incentive system was adopted to increase adherence. The incentive system aimed to increase adherence with exclusively using study cigarettes, honesty about non-study cigarette use, and visit attendance. Briefly, participants earned lottery tickets at each visit for these behaviors that were “validated” as appropriate based on a urinary analysis, which determined compliance. Once per month, lottery tickets were drawn, and participants earned cash prizes if their ticket was drawn and it was valid (Appendix provides details).
During Week 7 of the study, participants were provided with a descending monetary incentive for daily abstinence. Incentive value was high on the first day to encourage the initiation of abstinence, and decreased daily to facilitate the detection of a smoking lapse within this 1-week period.12 Participants assigned to NRT were encouraged to continue using their patches during this week. Participants were instructed to use their assigned study cigarette during the abstinence week if they were not able to abstain from smoking (Appendix provides details).
The primary outcome was the average number of cigarettes smoked (study + non-study) each day during Week 6 of the trial assessed via the daily telephone diaries. Secondary outcomes included breath CO level, collected weekly; the Fagerstrom Test for Nicotine Dependence (FTND) at Weeks 2 and 6; the Brief Wisconsin Inventory of Smoking Dependence Motives at Week 6; and the Minnesota Nicotine Withdrawal Scale, the 10-item Questionnaire of Smoking Urges (QSU), and a modified version of the Cigarette Evaluation Questionnaire (Cigarette Evaluation Scale), collected weekly. Both QSU factors were analyzed (Factor 1: desire and intention to smoke, Factor 2: relief from negative affect or withdrawal).13 Heart rate, blood pressure, and respiratory health symptoms were collected weekly (Appendix Table 2). Participants provided a spot urine at each visit and their first morning urine void at baseline, Week 2, and Week 6. First morning samples were used to quantify the concentrations of total nicotine, total cotinine, total 3-HCOT, and nicotine N-oxide. Total nicotine equivalents (TNEs) were calculated as the molar sum of these four values. Spot urines were analyzed for anatabine levels and used for the adherence incentive program (data not shown). After the trial, an unpublished analysis determined that total anatabine had greater specificity for adherence than anatabine alone, and an analysis of total anatabine at Week 6 was conducted. All urinary analyses were determined by liquid chromatography tandem mass spectrometry analysis using previously described methods, and total refers to the analyte and its glucuronide.14,15 A puff topography assessment from a single cigarette was collected using a pocket CreSS device at baseline (usual brand), Week 2 (study cigarette), and Week 6 (study cigarette); total puff volume is reported here. During Week 7 of the trial when participants were incentivized to remain abstinent from smoking, outcomes included days to first lapse and total days abstinent over the entire week.
Statistical Analysis
Data were analyzed using R, version 3.4.216 and SAS, version 9.4. A linear mixed-model with a random intercept to account for repeated measures was used. The primary model included terms for cigarette nicotine content (VLNC and NNC), transdermal nicotine patch (patch and no patch), visit, the baseline value of the outcome variable, and menthol status, along with all of the two- and three-way interactions between the two treatment factors and visit. Interactions with visit were dropped from the model if not significant. The secondary model also adjusted for age, gender, and race, along with any other covariates that differed across treatment groups at baseline with p<0.20 (education, natural log of salivary nicotine metabolite ratio). Analyses on the time to lapse in the abstinence incentive week were analyzed using Kaplan–Meier curves and Cox proportional hazards regression. Missing data were imputed using the last observation carried forward, and sensitivity analyses were conducted using complete cases and the baseline observation carried forward. The primary analysis followed the intention-to-treat (ITT) principle and an a value of 0.05 was used to determine statistical significance. Furthermore, the effect of the intervention had all participants been adherent with exclusively using study cigarettes (i.e., the causal inference) was estimated using the inverse probability of compliance weighted estimator.10 The propensity score for adherence included baseline variables that were significantly associated with adherence at Week 6: age, FTND score without CPD, QSU Factor 1, Cigarette Evaluation Scale-craving subscale, Cigarette Evaluation Scale-enjoyment subscale, and treatment assignment. Non-adherent participants were given a weight of 0 and adherent subjects were weighted inversely to their propensity score to estimate the causal effect. The effect of patch was unchanged in all of the causal inference analyses because if cigarette nicotine content is regulated, smokers may still fail to adhere to patch instructions. Adherence with VLNC cigarettes at Week 6 was defined as total anatabine <0.014 nmol/mL and self-reporting zero nonstudy cigarettes smoked. Missed visits were imputed as non-adherence for all analyses.
RESULTS
On average, participants were aged 47.7 years, 50.8% were male, 57.1% were white, and 52.5% had at least some college education. Participants smoked an average of 19.5 CPD, and average FTND was 5.7. There were no significant differences between the groups on any of the demographic or baseline smoking characteristics (Table 1, Appendix Figure 1). There were no significant differences between groups in study completion (Week 6 IVR call). Seventy-one percent of participants in the NNC + NRT group and 68% of participants in the VLNC + NRT group reported using NRT on at least 85% of days in Week 6 (Appendix Table 7).
Table 1.
Demographic and Baseline Smoking Characteristics of Study Participants According to Assigned Group
| Variables | Overall (N=240) | NNC only (n=61) | NNC + NRT (n=59) | VLNC only (n=60) | VLNC + NRT (n=60) | p-valuea |
|---|---|---|---|---|---|---|
| Age, years | 47.7 (12.8) | 47 (12.7) | 47.1 (12.2) | 47.8 (12.4) | 48.9 (13.9) | 0.84 |
| Male | 122 (50.8) | 36 (59.0) | 28 (47.5) | 31 (51.7) | 27 (45.0) | 0.44 |
| Race | ||||||
| White | 137 (57.1) | 34 (55.7) | 31 (52.5) | 34 (56.7) | 38 (63.3) | 0.06 |
| Black | 77 (32.1) | 22 (36.1) | 17 (28.8) | 17 (28.3) | 21 (35.0) | |
| Hispanic | 3 (1.25) | 1 (1.6) | 1 (1.7) | 1 (1.7) | 0 (0.0) | 0.91 |
| Some college or more | 126 (52.5) | 25 (41.0) | 35 (59.3) | 32 (53.3) | 34 (56.7) | 0.19 |
| Menthol | 120 (50.0) | 31 (50.8) | 30 (50.85) | 29 (48.3) | 30 (50.0) | 0.996 |
| CPD | 19.5 (10.4) | 18.2 (8.2) | 19.6 (10.1) | 18.7 (9.4) | 21.5 (13.1) | 0.32 |
| Daily smoking initiation age, years | 19 (6.4) | 19.2 (6.2) | 19.5 (6.2) | 18.7 (6.4) | 18.6 (7) | 0.87 |
| NMRb | 0.296 (0.03, 1.8) | 0.24 (0.05, 0.98) | 0.332 (0.05, 1.46) | 0.293 (0.03, 1.8) | 0.329 (0.03, 1.73) | 0.055 |
| TNE,b nmol/ml | 61.669 (0.6, 327.7) | 63.469 (0.8, 280.8) | 54.667 (0.6, 225.8) | 58.33 (13, 260) | 71.177 (0.6, 327.7) | 0.41 |
| CO, ppm | 24.5 (11.9) | 23.9 (12) | 23.2 (10.6) | 25.4 (13.2) | 25.4 (11.9) | 0.68 |
| FTND | 5.7 (2) | 5.5 (2.1) | 5.8 (2.1) | 5.5 (1.9) | 5.9 (2.1) | 0.54 |
Continuous variables were tested using ANOVA to see if there were any differences between the treatment groups at baseline. Categorical variables were tested using Fisher’s Exact test to see if there were any difference between the treatment groups at baseline.
NMR and TNE were summarized using geometric mean, minimum and maximum of the untransformed data. ANOVA was performed to these variables on the log-transformed scale.
NNC, normal nicotine content; NRT, nicotine-replacement therapy; VLNC, very low nicotine content; CPD, cigarettes per day; NMR, nicotine metabolite ratio; TNE, total nicotine equivalents; CO, carbon monoxide; FTND, Fagerstrom Test for Nicotine Dependence.
Figure 1 shows the impact of cigarette nicotine content and NRT on total cigarettes and study CPD from baseline through Week 6 (Appendix Tables 4 and 5). Participants assigned to receive VLNC cigarettes and those assigned to NRT smoked significantly fewer total CPD than participants assigned to NNC cigarettes (−4.58 CPD, 95% CI= −7.18, −1.97) and those not assigned to NRT (−2.74 CPD, 95% CI= −5.36, −0.12), respectively. Those who received VLNC cigarettes also smoked significantly fewer study CPD than participants assigned to NNC cigarettes (−6.35 CPD, 95% CI= −9.27, −3.44), but the effect of NRT on study cigarettes was not significant (−2.58 CPD, 95% CI= −5.51, 0.36). There was no significant interaction between cigarette and NRT assignment on either total or study CPD. When the VLNC + NRT group was compared directly to the VLNC-only group on total CPD and study CPD, the difference was not significant (−0.86 CPD, 95% CI= −3.48, 1.77 and −0.39 CPD, 95% CI= −3.34, 2.55, respectively). There was no significant interaction between menthol status and treatment on total CPD at Week 6 (p=0.0762; Appendix Table 6).
Figure 1.
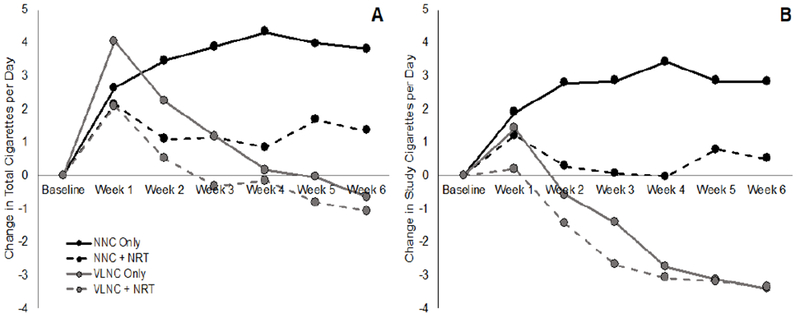
Number of total (A) and study (B) cigarettes smoked per day from baseline through Week 6 of the study.
NNC, normal nicotine content; NRT, nicotine-replacement therapy; VLNC, very low nicotine content.
There was no significant effect of cigarette or NRT on CO or total puff volume, and there was not a significant interaction between cigarette and NRT on CO or total puff volume (Appendix Tables 4 and 5).
Self-reported and biochemically estimated adherence are shown in Figure 2 (Appendix Tables 7 and 8). Participants who received VLNC cigarettes were significantly more likely to self-report use of non-study cigarettes than those who received NNC cigarettes (OR=0.09, 95% CI=0.04, 0.22), but the effect of NRT was not significant, and the cigarette × NRT interaction failed to reach significance (p=0.093). The percentage of participants assigned to VLNC cigarettes who were biochemically determined to be fully adherent was 50% for the VLNC + NRT group and 37% for the VLNC-only group (not significant; p=0.197). Rates of partial adherence, defined as 75% use of study cigarettes, were 66.7% in the VLNC + NRT group and 60% in the VLNC only group. Participants were generally honest about their non-study cigarette use. In both of the VLNC groups, only 5% of participants self-reported adherence but were biochemically determined to be non-adherent.
Figure 2.
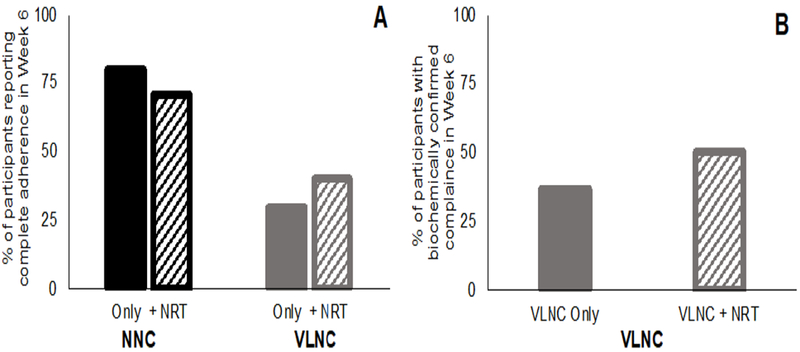
Compliance with use of study cigarettes: (A) percent of subjects in each group who self-report using only study cigarettes in Week 6; (B) percent of participants with urinary total anatabine levels <0.014nmol/ml, the level used to biochemically estimate compliance with study cigarettes.
NRT, nicotine-replacement therapy; NNC, normal nicotine content; VLNC, very low nicotine content.
A secondary analysis was used to estimate the causal inference of VLNC cigarettes (i.e., the effect assuming all participants had been adherent with the study cigarettes after accounting for observed differences at baseline related to adherence). In this analysis, the impact of VLNC cigarettes on total CPD was even larger than in the ITT analysis (−6.00 CPD, 95% CI= −10.22, −1.22), but there was no significant interaction with NRT (p=0.57). In this analysis, CO was significantly lower for participants assigned to VLNC cigarettes compared with those assigned to NNC cigarettes (−9.27 ppm, 95% CI= −17.5, −1.98), but there was no significant interaction between the cigarette and NRT conditions (Appendix Table 9 shows all causal inference results).
There was a significant interaction between the cigarette and NRT conditions at Week 6 (p=0.03) such that the VLNC-only group had significantly lower TNEs than the NNC-only group, but the VLNC + NRT group did not have significantly different TNEs than the NNC-only group (ratio of geometric means relative to NNC-only condition: VLNC only=0.29, 95% CI=0.19, 0.43; VLNC + patch=0.78, 95% CI=0.52, 1.16; Appendix Tables 10 and 11). TNEs for the NNC + NRT group were not significantly higher than TNEs for the NNC-only group (ratio of geometric means relative to NNC only condition: NNC + NRT=1.47, 95% CI=0.98, 2.19).
There was no reduction in FTND, Brief Wisconsin Inventory of Smoking Dependence Motives, total QSU, QSU Factor 1, or Minnesota Nicotine Withdrawal Scale score as a function of cigarette or NRT assignment, and there was no significant interaction between cigarette and NRT condition on any of these outcomes (Appendix Tables 12 and 13). Participants in the VLNC condition had significantly reduced QSU Factor 2 scores compared with those in the NNC condition, but assignment to NRT did not significantly affect Factor 2 scores, and there was no interaction.
When a causal model was used to estimate the treatment effects assuming all participants had been adherent, FTND and QSU Factor 1 scores were significantly reduced for participants who received VLNC cigarettes compared with those who received NNC cigarettes (−0.79 points, 95% CI= −1.45, −0.08 and −5.15 points, 95% CI= −9.58, −0.62, respectively). Effects on QSU Factor 2 were not significant in the causal inference model. In this analysis, the impact of VLNC cigarettes on Minnesota Nicotine Withdrawal Scale symptoms was larger than in the ITT model, but still failed to reach significance (1.44 points, 95% CI= −0.6, 3.72).
There were 15 serious adverse events, and none of these were deemed related or possibly related to the study assignment (NNC only: five; NNC + NRT: five; VLNC only: four; VLNC + NRT: one). There were 19 adverse events that were deemed by the LMP to be severe, and seven of these were classified by the LMP as possibly related to treatment. All seven of these events were reported concurrently by the same participant in the NNC + NRT group at the Week 3 visit (restlessness, chest pain, nausea, vomiting, headaches, weakness, and confusion). However, the participant was evaluated in an urgent care clinic after the visit and received a diagnosis of costochondritis unrelated to study participation.
There was a significant interaction between the cigarette and NRT conditions on time to lapse during the abstinence week (p=0.02; Figure 3, Appendix Tables 14 and 15). Pairwise comparisons testing the nature of the interaction revealed that no groups differed significantly from the NNC-only group, and there was a trend for the VLNC-only group to lapse sooner than the NNC-only group (hazard ratio=1.48, 95% CI=0.96, 2.29). There was no such trend for the VLNC + NRT group (hazard ratio=0.87, 95% CI=0.55, 1.36). The same pattern was present for the outcome of total number of days abstinent during Week 7 (interaction between VLNC and NRT: p=0.046). Again, none of the groups differed significantly from the NNC-only group, but the mean difference in the total number of days abstinent for the VLNC-only and VLNC + NRT groups compared with the NNC only group were in opposite directions (VLNC only: −0.92, 95% CI= −2.00, 0.015; VLNC + NRT: 0.5, 95% CI= −0.57, 1.57).
Figure 3.
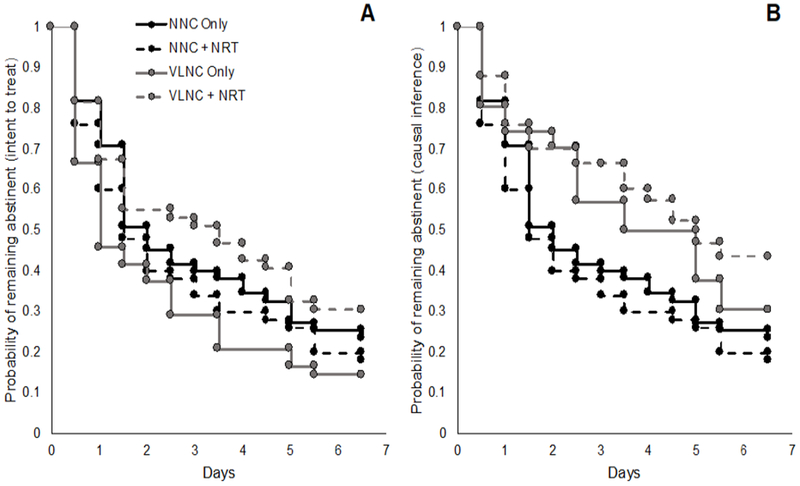
Intent to treat (A) and causal inference model (B) estimates for time to first lapse during the Week 7 abstinence incentive week.
NNC, normal nicotine content; NRT, nicotine-replacement therapy; VLNC, very low nicotine content.
These effects were moderated heavily by study cigarette adherence. When the effect of VLNC cigarettes on abstinence was estimated as if all participants had been adherent with the study cigarettes, the effect of time to lapse and total days abstinent was reversed—assignment to receive VLNC cigarettes increased time to lapse and total days abstinent. However, this effect failed to reach significance (difference in restricted mean survival=0.74, 95% CI= −0.38, 1.92 and 0.98, 95% CI= −0.32, 2.22, respectively).
DISCUSSION
Findings from the present study replicate earlier literature showing that smoking VLNC cigarettes results in a reduction in the total number of CPD.2,3,5 Additionally, a causal inference analysis estimating the treatment effect if all participants had been adherent found a significant reduction in CO in the VLNC condition. These findings, along with previously published trials,2,3,5,17 suggest that if the Food and Drug Administration or a regulatory agency outside of the U.S. were to mandate a reduction in the nicotine content of cigarettes to 0.4 mg nicotine/g tobacco, there would be a reduction in the number of CPD among current smokers.
These data provide information about the likely impact of a tobacco product standard in which nicotine is lowered to a minimally addictive or non-addictive level when smokers are provided with a source of nicotine other than cigarettes, which may include a form of medicinal nicotine or a non-cigarette tobacco product, like e-cigarettes or smokeless tobacco.18 In this study, concurrent use of a nicotine patch and VLNC cigarettes did not further reduce CPD compared to VLNC cigarettes alone. The lack of an interaction between VLNC cigarettes and NRT for CPD is inconsistent with two prior studies7,8 However, a third study also found that the effects of VLNC cigarettes on smoking were not enhanced by NRT.9 Most participants wore their assigned nicotine patch almost every day, suggesting that the lack of the effect was not due to non-adherence with the patch. It is not surprising that NRT would reduce the number of CPD for NNC cigarettes but not VLNC cigarettes, as it may be necessary to reduce smoking of NNC cigarettes when using NRT to keep nicotine levels in a range that is not aversive. However, using VLNC cigarettes would have very little additional impact on nicotine exposure when using NRT. Concurrent use of e-cigarettes, rather than NRT, may be more likely to reduce smoking rates of VLNC cigarettes because e-cigarettes would be more likely to at least partially replace the sensorimotor effects as well as the pharmacologic effects of cigarettes. The nicotine delivery profile of e-cigarettes can also more closely mimic conventional cigarettes than transdermal nicotine.
However, there may be a benefit associated with NRT related to adherence. Although not a significant difference, rates of adherence with VLNC cigarettes were higher among individuals assigned to wear the nicotine patch (biochemically verified adherence: 50% vs 37%). Rates of non-adherence in prior clinical trials have raised concerns that a product standard for cigarettes would create incentives for the development of an illegal cigarette market.19,20 If non-adherence with VLNC cigarettes is predictive of interest in an illicit cigarette market, these data suggest that concurrent use of NRT may reduce seeking of illicit NNC cigarettes.
Adherence with the study cigarettes likely played an important role in this study. Complete adherence was low in this trial, consistent with previous VLNC trials.3,5 VLNC cigarette use would likely have had a larger impact on many of the outcomes if participants had not been able to legally access NNC cigarettes, as would be the case following implementation of a nicotine standard. Indeed, effect sizes were larger for the causal inference analysis than the ITT analysis for many outcomes. Thus, if a nicotine standard is implemented, VLNC use is likely to result in greater reductions in smoking behavior and dependence than reported in the ITT analysis.
Adherence appeared to heavily moderate the impact of VLNC cigarettes on ability to remain abstinent from smoking. In the ITT analysis, VLNC use reduced the ability of smokers to remain abstinent from smoking. However, in the causal inference analysis, which estimates the effect of VLNC use under complete adherence, VLNC use increased the ability of smokers to remain abstinent from smoking. If a product standard is implemented, smokers will not be able to legally access NNC cigarettes. Thus, under these conditions, smokers who are motivated to remain abstinent from smoking will be better able to do so. It is unclear why participants in the VLNC condition were less likely to remain abstinent from smoking in the ITT analysis. Participants who failed to be adherent during the trial may have developed learned helplessness, a phenomenon in which repeated failures result in reduced effort in the future.21 Participants may have tried repeatedly to be adherent in the beginning of the trial, but failed, and ultimately exerted less effort toward the end of the trial, including during the abstinence incentive week. Consistent with this theory, time to first lapse was shortest in the VLNC-only group, where non-adherence was highest.
Limitations
The present study had several limitations. First, despite the use of an adherence incentive system, non-adherence was high among those assigned to VLNC cigarettes, and this non-adherence may have affected the dependence, craving, withdrawal, and abstinence outcomes. The effectiveness of the adherence incentive program may have been reduced because it was complex, targeted multiple behaviors (attendance, honesty, adherence), and necessitated delays between the target behaviors and reinforcement. The use of financial incentives for adherence is not a perfect model for a post-regulation marketplace where NNC cigarettes are not legally available. Nonetheless, it is important to understand the impact of VLNC cigarettes in the context of adherence, as nonadherence could affect important outcomes and is unlikely to be widespread following implementation of a policy.22 Second, the causal inference results control for baseline differences between individuals who were adherent and those who were not. However, it is possible that unmeasured baseline differences may exist and would limit the utility of the model to accurately predict the impact of a product standard in a fully adherent sample. Third, the sample size was smaller compared with some prior clinical trials,3,5 which may have limited the power to detect certain effects (e.g., dependence, causal inference model). Fourth, the data may not reflect the impact of a product standard in populations that differ in important ways from this sample (e.g., cigarette smokers who are trying to quit, smokers with schizophrenia).
CONCLUSIONS
This study contributes to a growing body of research on the potential effects of a policy lowering nicotine to a minimally addictive or non-addictive level.3,5 This body of work shows that a reduction in the nicotine content of cigarettes to a very low level is likely to decrease both smoking rates and exposure to harmful constituents among current smokers. This trial showed that NRT failed to produce additional decreases in smoking behavior, although there may be a non-significant trend for NRT to increase adherence with VLNC cigarettes. Trials are underway to investigate the interactive effects of VLNC cigarettes with other non-cigarette tobacco products, like e-cigarettes.23,24 Ongoing or recently completed research has also investigated the impact of a product standard within vulnerable populations, like those with mental illness.25,26 These trials, together with this one and those already published,2–5,7,8,27–30 provide a strong foundation for making regulatory decisions regarding a product standard lowering nicotine to a minimally addictive or non-addictive level in the U.S. and more broadly.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Brian Primack who served as a licensed medical professional on the trial and provided partial review of eligibility and adverse events, and Dr. Michelle Levine who served as a licensed clinical psychologist on the trial. We thank all of the staff involved with data collection, including Cathy Scott, Erin Goldstein, Guy Agostinelli, Lee Bennett, Ravi Choudhuri, Samantha Cwalina, Vanessa Fishel, John Mahalchak, Maura Matvey, Jamie Pearson, Patricia Schademan, Michael Tommarello, and Kristin Yahner. We thank Nicole Thompson for carrying out the anatabine and total nicotine equivalent analyses.
Research reported in this publication was supported by the National Institute on Drug Abuse and U.S. Food and Drug Administration Center for Tobacco Products (U54 DA031659 awarded to ECD and DKH). Research reported in this publication was also supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the NIH Award Number UL1TR000114. The funding source had no role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. The authors declare no other conflicts of interest.
All authors contributed to study design and study protocol. TTS wrote the initial draft of the manuscript. JSK was the lead statistician and analyzed the data with KT. SM analyzed the urinary biomarkers. All authors contributed to the final submitted manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: This trial is registered at ClinicalTrials.gov .
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Food and Drug Administration. FDA announces comprehensive regulatory plan to shift trajectory of tobacco-related disease, death, www.fda.gov/news-events/press-announcements/fda-announces-comprehensive-regulatory-plan-shift-trajectory-tobacco-related-disease-death. Published 2017. Accessed May 21, 2019.
- 2.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–769. 10.1158/1055-9965.epi-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatsukami DK, Luo X, Jensen JA, et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. 10.1001/jama.2018.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nardone N, Donny EC, Hatsukami DK, et al. Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction. 2016;111(12):2208–2216. 10.1111/add.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatsukami DK, Hertsgaard LA, Vogel RI, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22(6): 1015–1024. 10.1158/1055-9965.epi-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug Alcohol Depend. 2009;104(1-2):23–33. 10.1016/j.drugalcdep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013;15(1):121–129. 10.1093/ntr/nts098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little RJ, Long Q, Lin X. A comparison of methods for estimating the causal effect of a treatment in randomized clinical trials subject to noncompliance. Biometrics. 2009;65(2):640–649. https://doi.org/10.1111/j.1541-0420.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 11.Kazi A, Fazzi A, Krebs NM, et al. Cigarette Management System: an operating procedures guide to obtaining and managing investigational tobacco products for regulatory science research. Contemp Clin Trials Conmnin. 2018;11:69–74. 10.1016/j.conctc.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweitzer MM, Denlinger RL, Donny EC. Dependence and withdrawal-induced craving predict abstinence in an incentive-based model of smoking relapse. Nicotine Tob Res. 2013;15(1):36–43. 10.1093/ntr/nts080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–1476. 10.1111/j.1360-0443.1991,tb01732.x. [DOI] [PubMed] [Google Scholar]
- 14.Murphy SE, Park SS, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35(11):2526–2533. 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Weymarn LB, Thomson NM, Donny EC, Hatsukami DK, Murphy SE. Quantitation of the minor tobacco alkaloids nornicotine, anatabine, and anabasine in smokers’ urine by high throughput liquid chromatography-mass spectrometry. Chem Res Toxicol. 2016;29(3):390–397. 10.1021/acs.chemrestox.5b00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing. Vienna, Austria; 2018. [Google Scholar]
- 17.Shiffman S, Kurland BF, Scholl SM, Mao JM. Nondaily smokers’ changes in cigarette consumption with very low-nicotine-content cigarettes: a randomized double-blind clinical trial. JAMA Psychiatry. 2018;75(10):995–1002. 10.1001/jamapsychiatry.2018.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith TT, Hatsukami DK, Benowitz NL, et al. Whether to push or pull? Nicotine reduction and non-combusted alternatives - two strategies for reducing smoking and improving public health. Prev Med. 2018;117:8–14. 10.1016/j.ypmed.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates C, Wade C. Reducing nicotine in cigarettes: Challenges and opportunities. R Street. www.rstreet.org/2017/10/24/reducing-nicotine-in-cigarettes-challenges-and-opportunities/ Published 2017. Accessed May 21, 2019. [Google Scholar]
- 20.Britton J Denicotinised cigarettes. Lancet. 2018;392(10142):104–105. 10.1016/s0140-6736(18)31358-8. [DOI] [PubMed] [Google Scholar]
- 21.Maier SF, Seligman ME. Learned helplessness at fifty: insights from neuroscience. Psychol Rev. 2016;123(4):349–367. 10.1037/rev0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths CJ. Illicit trade in tobacco products after implementation of an FDA Product Standard. U.S. Food and Drug Administration Center for Tobacco Products. www.fda.gov/media/112006/download Published 2018. Accessed May 21, 2019. [Google Scholar]
- 23.Donny EC. Project number U54DA031659. Evaluating New Nicotine Standards for Cigarettes. Wake Forest University Health Sciences; https://projectreporter.nih.gov/project_info_description.cfm?aid=9692434&icde=44780645&ddparam=&ddvalue=&ddsub=&cr=4&csb=default&cs=ASC&pball. [Google Scholar]
- 24.Cinciripini PM. Project number R01OD022995. Evaluating concomitant use of very low nicotine content cigarettes and e-cigarettes among daily and non-daily smokers on abuse liability. University of Texas MD Anderson Cancer Center https://projectreporter.nih.gov/project_info_description.cfm?aid=9491784&icde=44780681&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball. [Google Scholar]
- 25.Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10): 1056–1064. 10.1001/jamapsychiatry.2017.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muscat J Project number P50DA036107. Pennsylvania State University Tobacco Center of Regulatory Science (TCORS). Pennsylvania State Univ Hershey Med Center. https://projectreporter.nih.gov/project_info_description.cfm?aid=9334159&icde=44780724&ddparam=&ddvalue=&ddsub=&cr=2&csb=default&cs=ASC&pball. [Google Scholar]
- 27.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P 3rd,. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2479–2485. 10.1158/1055-9965.epi-07-0393. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL, Nardone N, Dains KM, et al. Effect of reducing the nicotine content of cigarettes on cigarette smoking behavior and tobacco smoke toxicant exposure: 2-year followup. Addiction. 2015;110(10):1667–1675. 10.1111/add.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith TT, Cassidy RN, Tidey JW, et al. Impact of smoking reduced nicotine content cigarettes on sensitivity to cigarette price: further results from a multi-site clinical trial. Addiction. 2017;112(2):349–359. 10.1111/add.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


