Abstract
Background
Differences in proteomic profiles between men and women may provide insights into the biologic pathways that contribute to known sex differences in cardiovascular disease (CVD).
Objectives
To investigate sex differences in circulating biomarkers representative of biologic pathways implicated in the development of CVD among Framingham Heart Study participants.
Methods
We measured 71 circulating CVD protein biomarkers in 7184 participants (54% women, mean age 49). Multivariable models were used to evaluate the associations of sex, menopause and hormone status with biomarkers. Cox models were used to examine whether sex modified the association of biomarkers with incident CVD.
Results
Of 71 biomarkers examined, 61 (86%) differed significantly between men and women, of which 37 were higher in women (including adipokines and inflammatory markers such as leptin and CRP) and 24 were higher in men (including fibrosis and platelet markers such as MMP8 and TIMP1, FDR q<0.05 for all). Sex differences in biomarker profiles were most pronounced between pre-menopausal women vs men, with attenuated sex differences among post-menopausal women not taking hormone replacement therapy. Sex modified the association of specific biomarkers with incident CVD, including CD14 and ApoB (Pinteraction <0.05 for all).
Conclusions
In a predominantly Caucasian population, we identified widespread sex differences in circulating biomarkers that reflect distinct pathways implicated in CVD including inflammation, adiposity, fibrosis, and platelet homeostasis. Menopause and hormone status accounted for some but not all the observed sex differences. Further investigation into factors underlying sex-based differences may provide mechanistic insight into CVD development.
Keywords: biomarkers, female, sex, cardiovascular disease
Condensed Abstract
We sought to investigate sex differences in circulating biomarkers representative of biologic pathways implicated in the development of CVD among Framingham Heart Study participants. Of 71 biomarkers examined, 61 (86%) differed significantly between men and women. Sex differences in biomarker profiles were most pronounced between premenopausal women vs men, with attenuated sex differences among post-menopausal women not taking hormone replacement therapy. We identified widespread sex differences in circulating biomarkers that reflect distinct pathways implicated in CVD. Menopause and hormone status accounted for some but not all of the observed sex differences.
Introduction
Sex differences in cardiovascular disease (CVD) risk are well established but incompletely understood.(1) For example, the incidence of ischemic heart disease is significantly lower in women than men. Moreover, women with coronary artery disease (CAD) are less likely to present with acute coronary syndromes (ACS), and demonstrate a lower prevalence of flow limiting epicardial coronary disease, but significantly increased risk of microvascular dysfunction.(2) Men and women also differ with regard to key features of heart failure (HF). Despite a higher incidence of HF in men, the lifetime risk is similar in men and women. Additionally, women with HF present later and are more likely to have preserved left ventricular ejection fraction and a non-ischemic etiology of HF.(3)
The effects of traditional CVD risk factors also differ between men and women. The relative risks for CVD conferred by diabetes, dyslipidemia, and obesity are greater in women than in men.(4–6) In this context, it has been proposed that inflammation, metabolic dysregulation, and adiposity contribute more significantly to the pathophysiology of CVD in women compared with men.(4,5) Although the exact mechanisms are ill defined, sex hormones have been implicated as prior observational studies reveal an inflection point in CVD incidence after menopause (7).
Circulating proteomic biomarkers of CVD may provide a “biologic snapshot” of potential pathways that may contribute to CVD. Differences in circulating biomarkers between men and women have been described, with a prior focus on biomarkers implicated in myocardial injury and stress. Natriuretic peptide (NP) concentrations differ between men and women(8) and levels are highly influenced by sex hormone status.(9) Cardiac troponins (TnT) are also consistently lower in women, an observation that ultimately led to the adoption of sex-specific cut-offs for the diagnosis of acute myocardial infarction (MI).(10) More recently, studies have demonstrated higher levels of inflammatory markers and adipokines in women in the general population, consistent with previous assertions that inflammation and adiposity pathways are highly relevant to the development of CVD in women.(8,11)
In this context, we sought to examine differences in biochemical profiles of inflammation, metabolic dysfunction, coagulation, fibrosis, and lipid metabolism between men and women free of CVD. Further, we hypothesized that hormone status would in part explain sex differences in biomarkers. Finally, we set out to study whether a given biomarker was differentially associated with incident CVD among men and women. The National Heart, Lung, and Blood Institute established the Systems Approach to Biomarker Research in Cardiovascular Disease (SABRe CVD) Initiative to identify novel proteomic profiles of atherosclerotic CVD and risk factors in participants in the Framingham Heart Study (FHS).(12) Using this platform, we compared levels of CVD biomarkers in men and women in order to ascertain pathophysiologic pathways contributing to sex-based differences in CVD. Identifying these pathways may extend our current understanding of CVD in women and ultimately, improve our targeted strategies for the prevention and treatment of CVD and cardiometabolic disease.
Methods
Study Population
The FHS is a prospective longitudinal community-based observational cohort study.(13) All FHS Offspring cohort participants who attended examination 7 (1998–2001, n=3539) and Third Generation cohort participants who attended examination 1 (2002–2005, n=4095) were included in the SABRe CVD initiative protein assay project. We excluded individuals with prevalent MI (n=173), prevalent HF (n=27), end stage renal disease (n=24), and missing covariates (n=226). The final sample included 7184 individuals. All study protocols were approved by the appropriate Institutional Review Boards and all participants provided informed consent.
Clinical Assessment
At each examination cycle, participants underwent a comprehensive medical history, examination, anthropometry, and phlebotomy. Reproductive health history was ascertained including menopausal status and use of hormone replacement therapy (HRT). Individuals with missing HRT use (n=1539), unclear menopausal status (n=83), and post-menopausal women age <40 years (n=10) were further excluded in the menopause analyses. The final menopause analysis sample was 5548, of which 529 were pre-menopausal women, 1730 were post-menopausal women, and 3289 men. Among post-menopausal women, 626 (36%) were taking HRT and 1104 (64%) were not taking HRT.
Measurement of Circulating Biomarkers
Fasting blood samples were collected, centrifuged, and stored at −80° C. As part of the SABRe CVD Initiative, 85 circulating protein biomarkers were ascertained from the study population using a discovery proteomic platform. These candidate biomarkers were selected based on association with atherosclerotic CVD, gene expression profiling, published genome-wide associations studies of MI and coronary heart disease (CHD), and discovery proteomics.(14) Of 85 biomarkers, 14 had >25% of samples below the detection limit and were dichotomized.(14) We focused our analysis on the remaining 71 biomarkers. A modified ELISA sandwich approach and Luminex xMAP platform (Sigma-Aldrich, St. Louis, MO) was used to create 17 distinct multiplex panels based on factors including dilution rate, cross-reactivity, the time when the target was added to the assay list, and standard Luminex assays were used.(15,16) Detailed protocols for assay development and measurement have been previously described. Assay characteristics including measures of precision and accuracy are presented in Supplemental Table 1.(14,16) For low-abundance markers, high abundance proteins were depleted using an antibody-based resin designed to deplete 95% of total proteins from plasma called ProteoPrep 20 (Sigma-Aldrich).
Statistical Analysis
Baseline characteristics were summarized separately for men and women. Biomarkers were rank normalized due to skewed distributions. We visually confirmed that distributions of raw biomarker values appeared to have similar shape in men vs women, and we used F values to examine variance ratios and t-statistics (beta estimates and standard errors) from regression models. Relative to raw biomarker values, rank normalization reduced inequality of variances and emphasized mean differences. To assess associations of sex with biomarker concentrations, we performed linear regression analyses in an age-adjusted model and a multivariable model adjusting for age, systolic blood pressure (SBP), hypertension (HTN) treatment, BMI, DM, smoking status, total, and high-density lipoprotein (HDL) cholesterol. In exploratory analyses, we identified biological pathways that were enriched among the SABRe biomarkers using the PANTHER (Protein Analysis Through Evolutionary Relationships) database gene set over-representation and enrichment tools.(17,18)
We investigated the association of menopause status with biomarker levels by comparing pre-menopausal women, post-menopausal women and HRT users, and non-users among post-menopausal women using multivariable models. In secondary analyses, comparator groups (men vs women by hormone status) were matched by age distribution using 5-year age strata.
In exploratory analyses, we examined the association of biomarkers with CVD outcomes (all CVD, HF, CV death, and all cause death) using multivariable Cox models after exclusion of individuals with prevalent disease. We specifically examined sex-biomarker interaction terms to test whether sex modified the association of biomarkers and outcomes. If sex was determined to be an effect modifier, sex-stratified analyses were performed adjusting for age, sex, SBP, HTN treatment, HDL cholesterol, total cholesterol, BMI, DM, and smoking.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc). For primary analyses of cross-sectional biomarker associations with sex and secondary analyses examining menopausal and hormone status, a false discovery rate (FDR) q-value <0.05 was set to account for multiple hypothesis testing. Exploratory analyses relating biomarker*sex interactions with outcomes were deemed suggestive at P<0.05. For Cox regression models, we confirmed that the proportional hazards assumption was met using Schoenfeld residuals.
Results
A total of 7184 individuals were included for analysis, of whom 3895 (54.2%) were women. Baseline characteristics are presented in Table 1. The mean age was 49 years in both men and women. Compared with men, women had lower prevalence of hypertension (25% vs 32%) and diabetes (5% vs 7%), and lower BMI (26.7 ± 6.0 kg/m2 vs 28.2 ±4.7 kg/m2).
Table 1.
Baseline Characteristics in Men and Women
| Women N=3895 | Men N=3289 | p-value | |
|---|---|---|---|
| Clinical Characteristic | |||
| Age, years | 49 ± 14 | 49 ± 14 | 0.28 |
| Systolic BP, mm Hg | 119 ± 18 | 124 ± 15 | <0.0001 |
| Diastolic BP, mm Hg | 71 ± 9 | 75 ± 9 | <0.0001 |
| Hypertension treatment, n (%) | 669 (17) | 671(20) | 0.0005 |
| Body mass index, kg/m2 | 26.7 ± 6.0 | 28.2 ± 4.7 | <0.0001 |
| Diabetes mellitus, n (%) | 207 (5%) | 240 (7%) | 0.0006 |
| Current smoking, n (%) | 582 (15%) | 523 (16%) | 0.26 |
| Total cholesterol, mg/dL | 195 ± 37 | 194 ± 36 | 0.054 |
| HDL cholesterol, mg/dL | 61 ± 17 | 46 ± 13 | <0.0001 |
| eGFR, mL-min−1–1.73m−2 | 81 ± 23 | 83 ± 21 | <0.0001 |
Values are means (standard deviations) or medians (inter-quartile ranges) unless otherwise noted.
Abbreviations: BP = blood pressure, eGFR = estimated glomerular filtration rate
Sex differences in circulating biomarkers
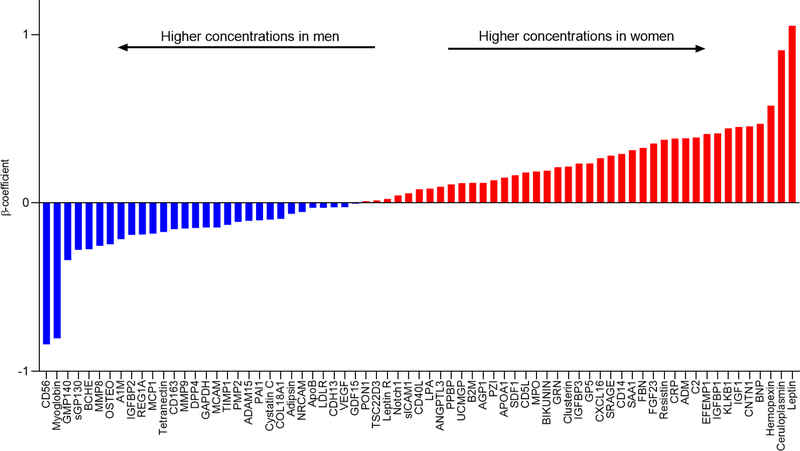
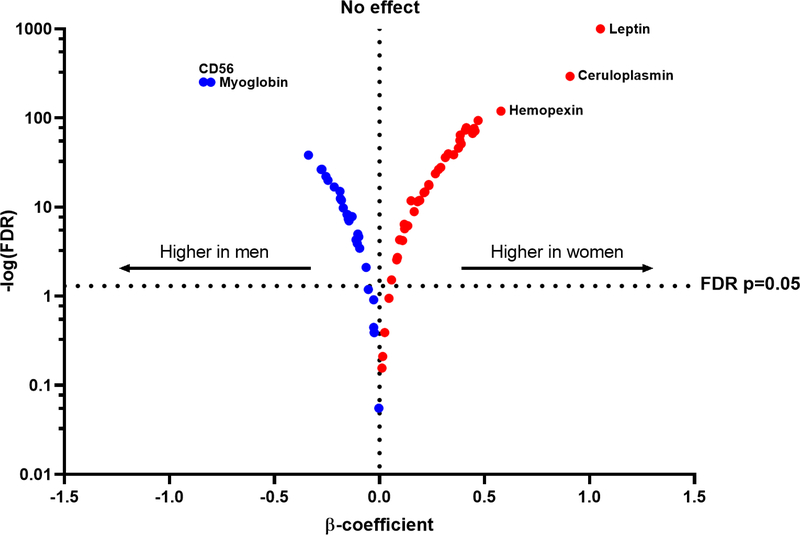
In both age- and multivariable-adjusted models, sex-based differences in circulating biomarkers were observed (Central Illustration, Table 2 with top 20 results, Supplemental Table 2 for full results). Of 71 biomarkers, 61 (86%) were significantly different in men and women (multivariable-adjusted FDR <0.05 for all). Among these 61 biomarkers, 37 were higher in women, with the largest differences observed in circulating levels of leptin, ceruloplasmin, and hemopexin compared with men. Specifically, leptin and ceruloplasmin levels were nearly 1-standard deviation higher in women vs men (multivariable-adjusted ß 1.052, SE 0.018, P<1.00E-1000 and ß=0.908, SE 0.023, P=1.02E-294, respectively). By contrast, 24 of 61 biomarkers were higher in men compared with women, with the largest differences seen in CD56 and myoglobin, both nearly 1-standard deviation higher in men vs women (ß −0.839, SE 0.024, P=5.03E-254 and ß=−0.803, SE 0.023, P=2.00E-252, respectively, see Figure 1). Analyses were largely similar when stratified by smoking status (Supplemental Table 3). Further adjustment for lipid lowering medication use and menopause did not significantly affect analyses for women, but the associations of GMP140, PON1, SRAGE, and Tetranectin with incident HF were attenuated after adjustment for lipid lowering medication use among men (Supplemental Tables 7 & 8). Within the set of biomarkers upregulated in women, pathways associated with inflammation, immune response, and adiposity were overrepresented, while fibrosis and platelet homeostasis pathways were enriched in the set of biomarkers differentially upregulated in men (Supplemental Figure 1).
Central Illustration. Sex Differences in Cardiovascular Biomarkers.
Multivariable-adjusted associations of single biomarkers with sex. Waterfall plot displays ß regression coefficients for individual biomarkers. ß-coefficients represent difference in biomarker concentration between women and men (referent), units expressed in standard deviations of rank-normalized biomarker. Positive ß-coefficient represents higher concentration in women and negative ß-coefficient represents higher biomarker concentration in men. Red bars represent higher concentrations in women, blue bars represent higher concentrations in men. Multivariable model adjusts for age, sex, SBP, HTN treatment, HDL, total cholesterol, BMI, DM, and smoking. Abbreviations: MV = multivariable..
Table 2.
Top 20 protein biomarkers with greatest observed differences between women vs men
| Biomarker | ß-coefficient | SE | p-value |
|---|---|---|---|
| Higher in women | |||
| Leptin | 1.052 | 0.018 | <1.00E-500 |
| Ceruloplasmin | 0.908 | 0.023 | 1.02E-294 |
| Hemopexin | 0.579 | 0.024 | 3.23E-120 |
| BNP | 0.470 | 0.022 | 1.67E-94 |
| CNTN1 | 0.455 | 0.025 | 4.04E-72 |
| IGF1 | 0.451 | 0.024 | 2.84E-77 |
| KLKB1 | 0.443 | 0.025 | 5.94E-68 |
| IGFBP1 | 0.413 | 0.022 | 9.45E-79 |
| EFEMP1 | 0.409 | 0.022 | 6.34E-74 |
| C2 | 0.389 | 0.025 | 5.68E-52 |
| Higher in men | |||
| CD56 | −0.839 | 0.024 | 5.03E-254 |
| Myoglobin | −0.803 | 0.023 | 2.00E-252 |
| GMP140 | −0.338 | 0.025 | 4.72E-39 |
| sGP130 | −0.278 | 0.025 | 3.73E-27 |
| BCHE | −0.274 | 0.025 | 2.31E-27 |
| MMP8 | −0.254 | 0.025 | 7.72E-23 |
| OSTEO | −0.245 | 0.026 | 1.01E-20 |
| A1M | −0.215 | 0.025 | 1.47E-17 |
| IGFBP2 | −0.188 | 0.023 | 1.03E-15 |
| REGIA | −0.187 | 0.025 | 3.52E-13 |
For full list of 71 proteins, please refer to Supplemental Table 1.
All biomarkers are rank normalized. Original assay units are pg/mL.
ß-coefficient: For women compared to men, 1 standard deviation increase in rank normalized biomarker.
+ ß-coefficient: higher in women
−ß-coefficient: higher in men
MV model adjusts for age, sex, SBP, HTN treatment, HDL, total cholesterol, BMI, DM, and smoking
Figure 1. Multivariable-adjusted associations of single biomarkers with sex.
Volcano plot showing relative biomarker concentrations in men and women. Positive x-values (red) represent biomarkers that are higher in women and negative x-values (blue) represent biomarkers that are higher in men. Abbreviations: FDR = false discovery rate.
The association of menopausal and hormone status on biomarkers
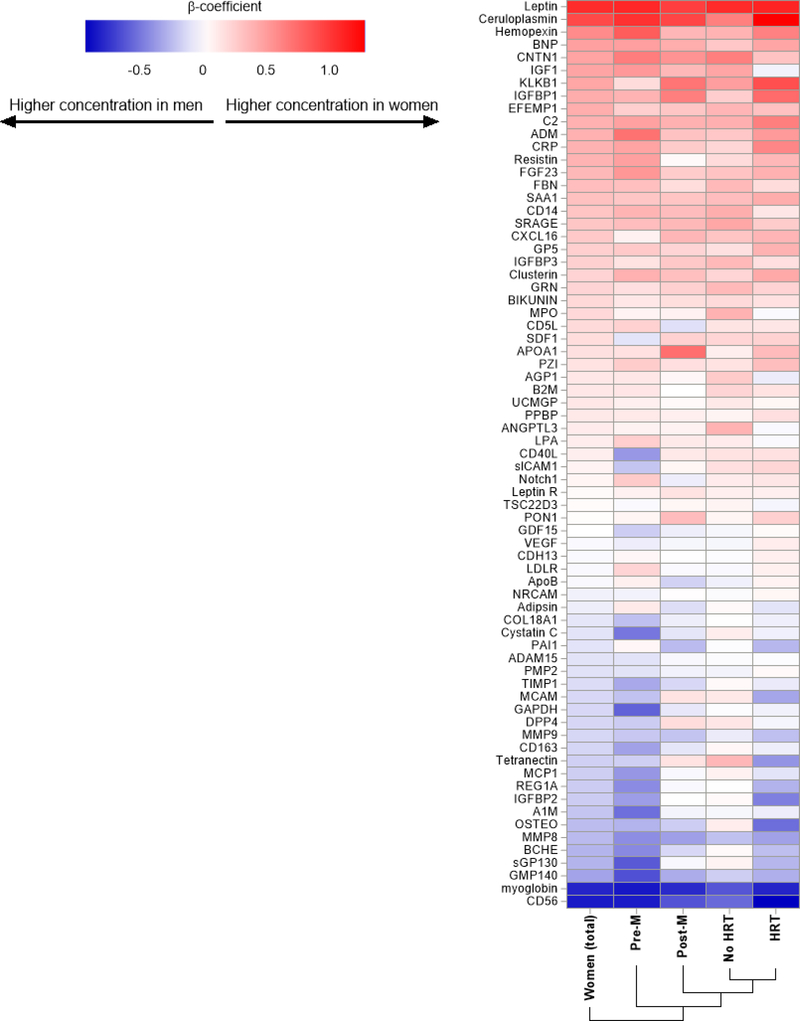
When separately comparing premenopausal and postmenopausal women with men, we made several notable observations. First, the greatest sex differences in biomarker profiles were observed between pre-menopausal women vs men, with some differences preserved among post-menopausal women taking HRT and greatest attenuation of sex differences in post-menopausal women not taking HRT vs men (Figure 2). Second, “attenuation” of sex differences by menopause and hormone status was most apparent among protein biomarkers that were higher in men vs women at baseline (male enriched), while protein biomarkers that were higher in women at baseline (female enriched) were less influenced by hormone or menopausal status.
Figure 2. Heatmap of sex differences in biomarkers by menopausal and hormonal status.
Heatmap displays relative sex differences in biomarker concentrations among (1) all women, (2) pre-menopausal women, (3) post-menopausal women, (4) post-menopausal women not on HRT, and (5) post-menopausal women on HRT compared with men. Positive ß-coefficients (red) represent biomarkers that are higher in women and negative ß (blue) represent biomarkers that are higher in men.
Specifically, among the 24 “male enriched” biomarkers (those higher among men), we found that 18 (75%) of the biomarkers differed significantly between premenopausal women vs men, while only 8 (33%) biomarkers were significantly different among postmenopausal women vs men. When further examining HRT use among post-menopausal women, we found greatest sex differences in “male enriched biomarkers” among women taking HRT vs not taking HRT (71% vs 38% were different in women vs men). By contrast, sex differences in the 37 “female enriched” biomarkers (higher among women) were not attenuated by menopause status: 26 (70%) and 36 (97%) biomarkers differed significantly between pre- and post-menopausal women and men, respectively, while 28 (76%) and 35 (95%) were significantly different in women taking HRT and not taking HRT vs men, respectively (Figure 2). In secondary analyses, we compared women by menopausal and hormone status to age distribution matched men and found similar results (Supplemental Table 4, Supplemental Figure 2).
Multiple biomarkers predict CV events differentially in men and women
We investigated the association of biomarkers and CV events in men and women. Over a mean follow-up time of 12.7 years, 294 women and 326 men had incident CV events (details in Supplemental Table 5). In multivariable-adjusted single biomarker analyses, we found that sex modified the associations with incident CV events for the following biomarkers (Figure 3) (Pint value range from 0.008 to 0.05): CV death (PPBP, REG1A, TSC22D3), HF (CD14, ApoB, GMP140, PON1, SRAGE, Tetranectin), all cause death (REG1A, CD56, leptin), and all CVD (GDF15, A1M, CD14, SRAGE) (Table 3). Specifically, ApoB predicted HF in women but not in men (multivariable-adjusted HR 1.55, 95% CI 1.16–2.09, p=0.03 vs HR 0.84, 95% CI 0.63–1.13, p=0.49). After adjusting for interim MI, the association of ApoB with incident HF in women was no longer significant (p=0.11). Similarly, CD14 was associated with HF and CVD among women but not in men (HR for HF:1.55, 95% CI 1.25–1.92, p=0.002 [women]; 1.12, 95% CI 0.91–1.37, p=0.50 [men]). Lastly, PPBP was associated with a lower risk of CV death in women but not in men (HR for CV death: 0.65, 95% CI 0.51–0.84, p=0.031 [women]; 0.96, 95% CI 0.77–1.21, p=0.91 [men]. By contrast, several biomarkers were associated with CV events among men but not women, including GMP140 and SRAGE, both of which predicted HF in men but not women, and PON1 and tetranectin, both of which were associated with lower risk of HF in men but not in women (Table 3).
Figure 3. Sex-specific multivariable-adjusted associations of single biomarkers with outcomes.
Association of biomarkers with CVD outcomes is modified by sex. Biomarkers presented here show sex*biomarker interaction P<0.05. All biomarkers are rank normalized. Original assay units are pg/mL. Abbreviations: HR = hazard ratio Central Illustration. Age-adjusted and multivariable-adjusted associations of single biomarkers with sex. Waterfall plot displays ß regression coefficients for individual biomarkers. ß-coefficients represent difference in biomarker concentration between women and men (referent), units expressed in standard deviations of rank-normalized biomarker. Positive ß-coefficient represents higher concentration in women and negative ß-coefficient represents higher biomarker concentration in men. Red bars represent age-adjusted model, blue bars represent multivariable-adjusted model. Multivariable model adjusts for age, sex, SBP, HTN treatment, HDL, total cholesterol, BMI, DM, and smoking. Abbreviations: MV = multivariable.
Table 3.
Sex-specific multivariable-adjusted associations of single biomarkers with cardiovascular outcomes
| Men (N=3289) | Women (N=3895) | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | pint (sex) | |
| Heart Failure | n = 119 | n = 117 | |||
| CD14 | 1.12 (0.91–1.37) | 0.50 | 1.55 (1.25–1.92) | 0.002 | 0.016 |
| ApoB | 0.84 (0.63–1.13) | 0.49 | 1.55 (1.16–2.09) | 0.030 | 0.01 |
| GMP140 | 1.49 (1.22–1.82) | 0.001 | 1.01 (0.84–1.22) | 0.97 | 0.01 |
| PON1 | 0.66 (0.54–0.81) | 0.001 | 0.98 (0.81–1.20) | 0.96 | 0.008 |
| SRAGE | 1.31 (1.09–1.57) | 0.022 | 0.95 (0.79–1.15) | 0.80 | 0.025 |
| Tetranectin | 0.64 (0.52–0.80) | 0.001 | 0.89 (0.73–1.09) | 0.55 | 0.047 |
| All CVD | n = 326 | n = 294 | |||
| GDF15 | 1.37 (1.13–1.64) | 0.086 | 1.59 (1.30–1.95) | 0.0004 | 0.04 |
| AIM | 0.91 (0.81–1.02) | 0.64 | 1.16 (1.02–1.31) | 0.40 | 0.003 |
| CD14 | 0.99 (0.88–1.12) | 0.92 | 1.27 (1.12–1.45) | 0.011 | 0.0007 |
| SRAGE | 1.08 (0.96–1.21) | 0.80 | 0.90 (0.79–1.01) | 0.42 | 0.027 |
| CV death | n = 91 | n = 65 | |||
| PPBP | 0.96 (0.77–1.21) | 0.91 | 0.65 (0.51–0.84) | 0.031 | 0.024 |
| REG1A | 1.06 (0.85–1.32) | 0.83 | 1.58 (1.17–2.14) | 0.034 | 0.028 |
| TSC22D3 | 0.93 (0.75–1.16) | 0.78 | 1.28 (1.00–1.63) | 0.15 | 0.043 |
| All cause death | n = 430 | n =381 | |||
| REG1A | 1.08 (0.98–1.20) | 0.29 | 1.27 (1.13–1.43) | 0.0009 | 0.036 |
| CD56 | 0.95 (0.86–1.04) | 0.42 | 1.10 (0.97–1.25) | 0.25 | 0.044 |
| Leptin | 1.11 (0.96–1.28) | 0.32 | 0.88 (0.76–1.01) | 0.15 | 0.027 |
HR: hazards ratio per 1-SD increase in rank normalized biomarker Multivariable model adjusts for age, SBP, HTN treatment, HDL cholesterol, total cholesterol, BMI, DM, and smoking. Abbreviations: HR = hazards ratio, CV = cardiovascular, CVD = cardiovascular disease.
Discussion
Our study characterizes CVD-related protein biomarker profiles in men and women among a community-based sample of predominantly Caucasian individuals without established CVD. Our principal findings are threefold. First, sex differences in CVD-related protein biomarkers are abundant and reflect important biological pathways implicated in CVD. We observed that biomarkers thought to regulate inflammation and adipokine signaling were preferentially upregulated in women, while biomarkers involving platelet/coagulation homeostasis and fibrosis were enriched in men. Second, sex differences were most pronounced among premenopausal women, followed by postmenopausal women taking HRT compared with non-HRT users. While this supports the potential contribution of sex hormones, it does not explain the totality of the sex differences observed in our study. Finally, we demonstrate that sex may modify the effect of select CVD biomarkers on clinical outcomes, including CVD, HF, and all-cause mortality. Together, these findings highlight important differences in CVD profiles among men and women, including markers of inflammation, metabolic dysfunction, coagulation, and fibrosis, pathways that are integral to the development of CVD. Our results underscore the need for future studies focused on sex-specific risk assessment and therapeutic strategies.
Sexual dimorphism in CVD is well established, although exact mechanisms remain elusive. Previous studies have focused on a small number of established protein biomarkers, and have shown that cardiac troponin concentrations are significantly lower in women than men across the CVD disease spectrum.(19,20) By contrast, NP concentrations are significantly higher in women, both in the general population and in ACS,(21,22) although sex differences in NPs are attenuated among acute and chronic HF samples, potentially explained by the higher prevalence of HFpEF in women (23,24). Beyond single biomarker studies, larger-scale proteomic approaches have the potential to more comprehensively interrogate multiple biological pathways that contribute to CVD development. In a previous study of 30 biomarkers assayed in the Dallas Heart Study, investigators found sex differences in circulating biomarkers including lipids, adipokines (leptin and adiponectin), inflammatory markers (D-dimer and high sensitivity C-reactive protein), and markers of myocyte injury (NT-pro BNP, hs-cTnT, and sST2).(25) We now expand upon these findings and demonstrate that 61 of 71 CVD protein biomarkers examined in our study showed significant differences between men and women that persisted even after accounting for baseline clinical characteristics and comorbidities. These wide-ranging differences highlight potential key pathways including inflammation, adiposity, platelet regulation, and fibrosis, which may provide insight into the biologic basis of sex differences in CVD. It has long been established that female sex is an important modulator of both innate and adaptive immunity.(26) Compared with men, women mount more robust immune responses to infectious stimuli, and relatedly, women possess a stronger autoimmune diathesis. The over-representation of inflammatory biomarkers among women in our study supports a potential link between inflammation and CVD in women preferentially. Consistent with this assertion, recent studies found that coronary microvascular dysfunction and subclinical atherosclerosis associates with systemic inflammation in women with heightened immune states such as HIV(27) and rheumatoid arthritis(28).
In the context of previous studies which have demonstrated a precipitous rise in CVD event rates in women following menopause(29), we specifically examined the effect of menopausal and hormone status on circulating CVD biomarker profiles. Compared with men, pre-menopausal women demonstrated the most pronounced differences in circulating biomarker levels even among age-strata matched samples, while overall sex differences were attenuated in postmenopausal women. Interestingly, the influence of hormone status was most apparent among the biomarkers preferentially enriched in men that represented pathways associated with platelet/coagulation homeostasis, and fibrosis. Here, post-menopausal women taking HRT exhibited a biomarker profile most similar to pre-menopausal women, and postmenopausal women especially those not taking HRT, most resembled men. By contrast, protein biomarkers upregulated in women including adipokines and inflammatory markers were less influenced by menopausal or hormone status. Prior studies have demonstrated similar effects of sex hormones and menopausal status on traditional CVD biomarkers, including NT-proBNP, (9) lipid, and D-dimer concentrations.(25) Similarly, in a study of 2834 post-menopausal women, higher levels of estrogen were associated with lower risk of CHD and higher levels of testosterone were associated with increased risk of CVD and CHD.(30) Our findings now suggest that menopausal and hormone status may disproportionately affect certain biological pathways (platelet/coagulation and fibrosis) whereas less effect is seen on others (inflammation, adipokines).
It is important to recognize that menopausal and hormone status only accounted for part of the sex differences in CVD biomarker profile observed in our study. Despite prior observational data showing lower CVD risk associated with female sex hormones(31,32), it is known that combined hormone therapy (exogenous estrogen and progesterone) administered in the context of a randomized control trial has been shown to increase the risk of CHD. Taken together, these findings underscore that sex differences in CVD are complex and cannot be explained by sex hormones alone (33).
Two of the most widely-used CV biomarkers (cardiac troponin and NPs) have been previously shown to consistently predict CVD events in both men and women.(34,35) However, other biomarkers may have sex-specific effects, such as pro-neurotensin, which was associated with incident DM, CVD, breast cancer, and death in women but not men.(36) In our present analysis, we similarly identified several CVD biomarkers that were differentially associated with outcomes in men vs women. For instance, apolipoprotein B-100 (ApoB) was associated with incident HF in women but not in men. ApoB is a surface protein on atherogenic lipoproteins, primarily LDL, and has previously been associated with CHD in both men and women (37,38) although plasma levels of ApoB are higher in men and post-menopausal women(39). Given the strong association of ApoB to CHD and its role in lipid metabolism, we postulated that ApoB may have mediated HF risk via CHD. Further adjustment for interim MI attenuated the association of ApoB with HF in women, confirming our hypothesis. Why ApoB preferentially predicts HF in women above and beyond total and HDL cholesterol is not clear and deserves further investigation. We also found that CD14 predicted both HF and CVD in women but not men. CD14 is a glycosylphophotidyl (GPI)-anchored membrane glycoprotein expressed on monocytes, macrophages, and neutrophils that is involved in downstream inflammatory pathway signaling.(40) Its gene expression is activated in the liver in response to inflammation, and is therefore, also regarded as an acute phase reactant.(41) In previous studies, soluble CD14 (sCD14) has been associated with traditional CVD risk factors and coronary artery calcification, highlighting the important link between CHD and inflammation among the general population(42) as well as specific disease conditions like HIV(43). Little is known about the association of CD14 and HF nor on the impact of sex on CD14 as a CVD biomarker, but our findings are in keeping with observations that inflammation may preferentially lead to CVD in women. (44)
In contrast to ApoB and CD14, pro-basic platelet protein (PPBP), also known as chemokine (C-X-C motif) ligand 7 (CXCL7), was associated with a lower risk of CV death in women but not in men. Following platelet activation, PPBP is abundantly released from platelets and involved in inflammation and vascular regeneration after injury.(45) PPBP gene expression is increased among patients with hyperlipidemia and CHD compared with controls.(46) Sex differences in platelet aggregation have been previously reported. Compared with men, women consistently demonstrate higher levels of baseline platelet reactivity, although the clinical implications are less clear (47,48). Little else is known about the relationship between PPBP and CVD, but appreciation of sex difference in levels of this protein may provide additional insight into differences in platelet biology between men and women.
Limitations
Our study has several limitations that deserve mention. The majority of biomarkers on the panel were selected based on previously established associations with atherosclerotic CVD, and therefore, pathway analyses comparing pathways enriched in men vs women are not based on an unbiased protein sampling strategy. Due to the observational nature of our study, inferences about causation or mechanism cannot be made. Further, we acknowledge that analyses examining sex*biomarker interactions with respect to outcomes are exploratory and further studies are needed to substantiate our findings. Lastly, participants in our sample are predominantly white, potentially limiting generalization of our findings to other samples.
In conclusion, we demonstrate broad sex differences in circulating CVD protein biomarkers representing pathways of inflammation, adipokine signaling, platelet/coagulation homeostasis, and fibrosis in a predominantly Caucasian sample of individuals free of overt CVD at baseline. While sex differences in key fibrosis and coagulation biomarkers are influenced by menopausal and HRT, inflammatory markers and adipokines appear to be independent of hormone status. Finally, several biomarkers were differentially associated with incident CVD events in women compared with men, including ApoB, CD14, and PPBP. Overall, our findings highlight widespread differences in CVD proteomic biomarkers representing inflammation, metabolism, coagulation, and fibrosis pathways and suggest that sex differences in clinical disease are complex and multifactorial in etiology.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge
Differences between men and women in circulating biomarkers of cardiovascular disease reflect the relationship of sex to inflammation, adiposity, platelet homeostasis, and fibrosis. Menopause and hormone status explain some but not all these differences.
Translational Outlook
Clarification of the biological pathways that differ between men and women could improve targeting of prevention and treatment of cardiovascular disease.
Acknowledgments
This work was supported by grants from the NIH, including N01-HC25195 and HHSN268201500001I (Framingham Heart Study), 5T32HL094301-07 (ESL), R01-HL134893 (JEH), R01-HL140224 (JEH), a Gilead Sciences Research Scholar Award (JEH), and a Hassenfeld Research Scholar award from Massachusetts General Hospital (JEH).
The views expressed in this manuscript are those of the authors and do not necessarily represent the view of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Abbreviations
- ACS
acute coronary syndrome
- BMI
body mass index
- BP
blood pressure
- CAD
coronary artery disease
- CHD
coronary heart disease
- CVD
cardiovascular disease
- DM
diabetes mellitus
- HDL
high-density lipoprotein
- HF
heart failure
- HRT
hormone replacement therapy
- HTN
hypertension
- MI
myocardial infarction
- NP
natriuretic peptides
- OCP
oral contraceptive
- SBP
systolic blood pressure
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merz NC, Shaw LJ, Reis SE et al. Insights From the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study Part II: Gender Differences in Presentation, Diagnosis, and Outcome With Regard to Gender-Based Pathophysiology of Atherosclerosis and Macrovascular and Microvascular Coronary Disease. J Am Coll Cardiol 2006;47. [DOI] [PubMed] [Google Scholar]
- 2.Tamis-Holland JE. Sex and Outcomes After Percutaneous Coronary Intervention: A Cause for Concern for Young Women and Those With ST-Segment Elevation Myocardial Infarction? J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Meara E, Clayton T, McEntegart MB et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;115:3111–20. [DOI] [PubMed] [Google Scholar]
- 4.Savji N, Meijers WC, Bartz TM et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam C, Carson PE, Anand IS et al. Sex Differences in Clinical Characteristics and Outcomes in Elderly Patients With Heart Failure and Preserved Ejection Fraction. Circ Heart Fail 2018;5:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton CB, Pettinger M, Rossouw J et al. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ Heart Fail 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex Differences in Stroke Risk Among Older Patients With Recently Diagnosed Atrial Fibrillation. JAMA 2012;307:1952–1958. [DOI] [PubMed] [Google Scholar]
- 8.Motiwala SR, Sarma A, Januzzi JL, O’Donoghue ML. Biomarkers in ACS and Heart Failure: Should Men and Women Be Interpreted Differently? Clin Chem 2014;60:35–43. [DOI] [PubMed] [Google Scholar]
- 9.Lam C, Cheng S, Choong K et al. Influence of Sex and Hormone Status on Circulating Natriuretic Peptides. J Am Coll Cardiol 2011;58:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 11.Khera A, McGuire DK, Murphy SA et al. Race and Gender Differences in C-Reactive Protein Levels. J Am Coll Cardiol 2005;46:464–469. [DOI] [PubMed] [Google Scholar]
- 12.Yin X, Subramanian S, Hwang S-J et al. Protein Biomarkers of New-Onset Cardiovascular Disease. Arterioscler Thromb Vasc Biol 2018;34:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 14.Ho JE, Lyass A, Courchesne P et al. Protein Biomarkers of Cardiovascular Disease and Mortality in the Community. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods 1999;227:41–52. [DOI] [PubMed] [Google Scholar]
- 16.dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol 2005;66:175–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pursnani A, Massaro JM, D’Agostino RB, O’Donnell CJ, Hoffmann U. Guideline-Based Statin Eligibility, Cancer Events, and Noncardiovascular Mortality in the Framingham Heart Study. J Clin Oncol 2017;35:2927–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc 2013;8:1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical Validation of a High-Sensitivity Cardiac Troponin T Assay. Clin Chem 2010;56:254–261. [DOI] [PubMed] [Google Scholar]
- 20.Antman EM, Morrow DA. Biomarker Release After Percutaneous Coronary Intervention. Circ Cardiovasc Interv 2008;1:3–6. [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Larson MG, Levy D et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254–258. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–982. [DOI] [PubMed] [Google Scholar]
- 23.Meyer S, van der Meer P, van Deursen VM et al. Neurohormonal and clinical sex differences in heart failure. Eur Heart J 2013;34:2538–47. [DOI] [PubMed] [Google Scholar]
- 24.Krauser DG, Chen AA, Tung R, Anwaruddin S, Baggish AL, Januzzi JL Jr. Neither Race nor Gender Influences the Usefulness of Amino-Terminal Pro-Brain Natriuretic Peptide Testing in Dyspneic Subjects: A ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Substudy. J Card Fail 2006;12:452–457. [DOI] [PubMed] [Google Scholar]
- 25.Lew J, Sanghavi M, Ayers CR et al. Sex-Based Differences in Cardiometabolic Biomarkers. Circulation 2017;135:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Anastos K, Kaplan RC et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J Infect Dis 2017;215:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amigues I, Russo C, Giles Jon T et al. Myocardial Microvascular Dysfunction in Rheumatoid Arthritis. Circ Cardiovasc Imaging 2019;12:e007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiovasc Dis 1995;38:199–210. [DOI] [PubMed] [Google Scholar]
- 30.Zhao D, Guallar E, Ouyang P et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J Am Coll Cardiol 2018;71:2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000;133:933–941. [DOI] [PubMed] [Google Scholar]
- 32.Bush TL, Barrett-Connor E, Cowan LD et al. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation 2018;75:1102–1109. [DOI] [PubMed] [Google Scholar]
- 33.Manson JE, Hsia J, Johnson KC et al. Estrogen plus Progestin and the Risk of Coronary Heart Disease. N Engl J Med 2003;349:523–534. [DOI] [PubMed] [Google Scholar]
- 34.Everett BM, Berger JS, Manson JE, Ridker PM, Cook NR. B-Type Natriuretic Peptides Improve Cardiovascular Disease Risk Prediction in a Cohort of Women. J Am Coll Cardiol 2014;64:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everett BM, Brooks M, Vlachos H et al. Sex Differences in Cardiac Troponin and the Risk of Death or Major Cardiovascular Events. J Am Coll Cardiol 2016;68:978–980. [DOI] [PubMed] [Google Scholar]
- 36.Melander O, Maisel AS, Almgren P et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 2012;308:1469–75. [DOI] [PubMed] [Google Scholar]
- 37.Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med 2006;259:481–492. [DOI] [PubMed] [Google Scholar]
- 38.Ingelsson E, Schaefer EJ, Contois JH et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA 2007;298:776–785. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, McNamara JR, Fruchart JC et al. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J Lipid Res 1996;37:1886–1896. [PubMed] [Google Scholar]
- 40.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science (New York, NY) 1990;249:1431–3. [DOI] [PubMed] [Google Scholar]
- 41.Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol 2004;172:4470–9. [DOI] [PubMed] [Google Scholar]
- 42.Reiner AP, Lange EM, Jenny NS et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 2013;33:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longenecker CT, Jiang Y, Orringer CE et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014;28:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daubert MA, Douglas PS. Primary Prevention of Heart Failure in Women. JACC Heart Fail 2019;7:181–191. [DOI] [PubMed] [Google Scholar]
- 45.Gleissner CA, von Hundelshausen P, Ley K. Platelet Chemokines in Vascular Disease. Arterioscler Thromb Vasc Biol 2008;28:1920–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maneerat Y, Prasongsukarn K, Benjathummarak S, Dechkhajorn W. PPBP and DEFA1/DEFA3 genes in hyperlipidaemia as feasible synergistic inflammatory biomarkers for coronary heart disease. Lipids Health Dis 2017;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet Function Monitoring in Patients With Coronary Artery Disease. J Am Coll Cardiol 2007;50:1822–1834. [DOI] [PubMed] [Google Scholar]
- 48.Becker DM, Segal J, Vaidya D et al. Sex Differences in Platelet Reactivity and Response to Low-Dose Aspirin Therapy. JAMA 2006;295:1420–1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.