Background.
Machine perfusion of donor livers is typically performed via the portal vein main stem. Instead, cannulation of a reopened umbilical vein could allow machine perfusion during organ procurement and subsequent implantation in the recipient without interruption of the portal venous circulation. We aimed to assess the feasibility of portal venous machine perfusion via the umbilical vein.
Methods.
During back table inspection of 5 human livers declined for transplantation, the umbilical vein was surgically reopened, dilated, and cannulated. Hypothermic and normothermic oxygenated machine perfusion (NMP) were performed using the umbilical vein for portal inflow. Three livers were perfused with hypothermic machine perfusion, 1 full liver graft underwent NMP for 4 hours, and 1 left lateral split procedure was performed under continuous NMP with portal perfusion via the umbilical vein.
Results.
In all livers, access to the portal venous system via the umbilical vein was successfully achieved with good portal flows and macroscopically homogeneous perfusion. The full liver graft that underwent NMP via the umbilical vein for 4 hours showed good lactate clearance, normalized pH, and achieved good bile production with pH >7.55. During the split procedure under continuous NMP via the umbilical vein, the left lateral segment and extended right lobe remained equally perfused, as demonstrated by Doppler ultrasound.
Conclusions.
Machine perfusion with portal perfusion via the umbilical vein is feasible. Portal venous flows were similar to those obtained after cannulation of the portal vein main stem. This technique enables continuous oxygenated perfusion of liver grafts during procurement, splitting, and implantation.
INTRODUCTION
Liver transplantation is the treatment of choice for patients with end-stage liver disease. However, donor organ shortage remains a limiting factor in the wider application of liver transplantation. This discrepancy between organ availability and demand has led to the increased use of so-called “extended-criteria” donor (ECD) grafts. The use of ECD livers is associated with increased rates of ischemia-reperfusion injury-related complications, such as primary graft nonfunction, early allograft dysfunction, and biliary complications.1 This has led to a large number of livers being declined for transplantation. Ex situ oxygenated machine perfusion is increasingly applied to ECD livers to reduce the risk of graft failure and has made the transition to clinical trials.2
Recently, He et al3 reported the first case of ischemia-free liver transplantation in humans. Their innovative technique of continuous normothermic machine perfusion (NMP) during liver procurement, preservation, and transplantation is a major breakthrough, because for the first time a liver was successfully transplanted without any ischemia or reperfusion. The authors used a donor iliac vein graft that was anastomosed end-to-side to the donor portal vein main stem to enable cannulation and perfusion of the portal venous system without interrupting the blood circulation, even during porto-portal anastomosis in the recipient.3 However, this additional end-to-side anastomosis may predispose to thrombosis, potentially leading to portal venous complications.
Our group recently postulated the use of the surgically reopened umbilical vein as an alternative route to the portal venous system to overcome the need to anastomose an iliac vein graft to the portal vein main stem.4 Access to the portal venous system via a surgically reopened umbilical vein has previously been used for successful retrograde tumor thrombectomy of the portal vein main stem.5 Therefore, we hypothesized that a surgically reopened umbilical vein would provide good access to the portal venous system for machine perfusion (Figure 1A and B). We here present our first experiences of human liver machine perfusion with portal perfusion via the umbilical vein in a preclinical setting.
FIGURE 1.
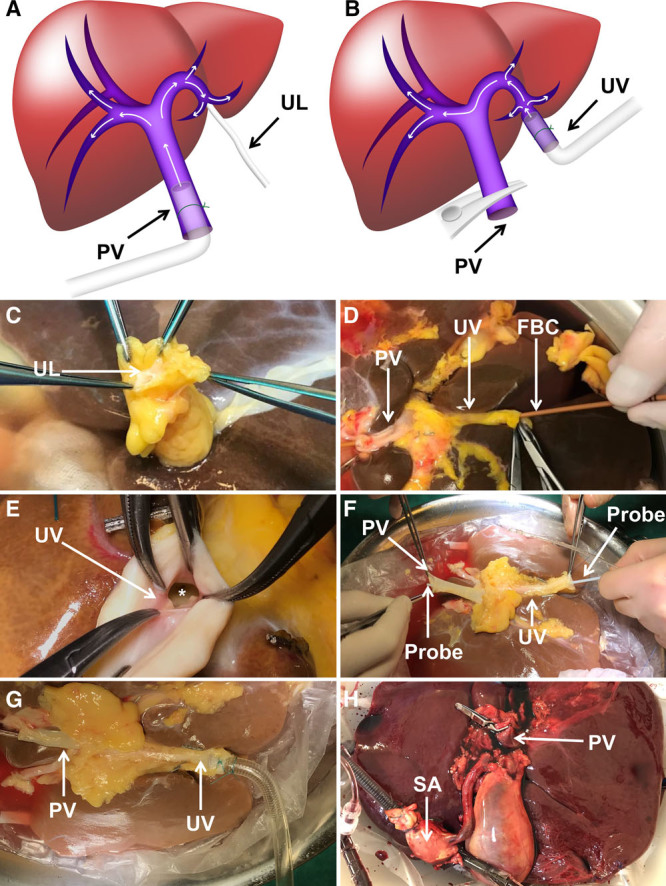
Step-by-step approach for cannulation of the umbilical vein for ex situ machine perfusion. A, Schematic drawing of human portal venous anatomy with usual machine perfusion via the PV main stem. B, Schematic drawing of machine perfusion through the UV with alternative flow pattern. C, The UL of a human donor liver before dissection of the UV. D, Introduction of the FBC to pneumatically dilate the obliterated UV. E, Adequately dilated insertion of the UV into the left intrahepatic PV branch (denoted by the *). F, Connection between UV and PV main stem ensured (demonstrated by a probe). G, Cannulation of the UV with a 24F cannula. H, Normothermic machine perfusion via the UV and the SA. The proximal stump of the PV main stem was closed with a nontraumatic vascular clamp. FBC, Fogarty balloon catheter; PV, portal vein; SA, supratruncal aorta; UL, umbilical ligament; UV, umbilical vein.
MATERIALS AND METHODS
Between January and May 2018, 5 human ECD livers declined for transplantation in the Eurotransplant region were offered to our center for machine perfusion research. This study was approved by the medical ethical committee of the University Medical Center Groningen and the Dutch Transplant Society, the competent authority for organ donation in the Netherlands. Donor relatives provided consent for research before the donation procedure. For all livers, donation after circulatory death combined with donor age exceeding 60 years was the reason for graft discard. All organs were procured using a superrapid procurement technique described previously.6
Organ Inspection and Preparation for Machine Perfusion
All livers were prepared for dual machine perfusion using the umbilical vein for portal perfusion and the supratruncal aorta for arterial perfusion. Opening of the umbilical vein was performed by careful exploration of the round ligament of the liver (Figure 1C). As soon as the umbilical vein was identified and dissected, a Fogarty balloon catheter (8F; Edwards Life Sciences, Nyon, Switzerland) was introduced to dilate the obliterated vessel (Figure 1D) to allow adequate opening of the entrance into the left intrahepatic portal vein branch (Figure 1E). In all 5 livers, a connection between the portal vein and umbilical vein could be obtained easily (Figure 1F). Hereafter, the portal cannula from the Liver Assist device (Organ Assist, Groningen, the Netherlands) was inserted into the umbilical vein with its tip close to the lumen of the left portal vein branch and was secured (Figure 1G). The proximal stump of the portal vein main stem was closed with a nontraumatic vascular clamp (Figure 1H). The first 3 livers were perfused hypothermically (10°C to 12°C) with University of Wisconsin Machine Perfusion solution (Bridge to Life, London, UK). Livers 4 and 5 were perfused normothermically (37°C) with our clinically used NMP solution containing a hemoglobin-based oxygen carrier.6 The hypothermic and oxygenated NMP procedures were performed using the Liver Assist, a pressure-controlled perfusion device, as described previously.6,7
RESULTS
Hypothermic Machine Perfusion via the Umbilical Vein
In the first liver, a smaller cannula (12F) than usual (24F) was introduced in the umbilical vein. However, due to the increased resistance of the smaller cannula, umbilical vein flows were lower (80 to 100 mL/min at a preset pressure of 5 mm Hg) than previously described during hypothermic machine perfusion (ie, 200 to 300 mL/min at 5 mm Hg).5 In livers 2 and 3, the umbilical vein was cannulated with the usual cannula for portal vein cannulation (24F). In these cases, umbilical vein flow rates were similar to values obtained during normal hypothermic perfusion via the portal vein main stem. (ie, >200 mL/min at a pressure of 5 mm Hg).6 Pulsatile arterial flows were comparable (75 to 100 mL/min at a preset pressure of 25 mm Hg) to flows as described previously during dual hypothermic machine perfusion (ie, 50 to 100 mL/min at 25 mm Hg).6
Normothermic Machine Perfusion via the Umbilical Vein
The fourth liver was prepared for NMP through the umbilical vein and supratruncal aorta (Figure 2A). During NMP, this full liver graft cleared lactate (Figure 2B) and the perfusate pH reached normal values within 2 hours (Figure 2C). Doppler ultrasound analysis confirmed accurate position of the tip of the perfusion catheter just before the lumen of the left portal vein branch (Figure 2D), demonstrated adequate blood flow in both the left and the right portal venous branches (Figure 2E), as well as in all the hepatic veins (Figure 2F). To analyze the bile production from the left and right liver lobe separately, both the left and right hepatic bile duct were cannulated and bile was collected from each liver lobe. The amount of bile produced was >10 mL. After 1 hour of NMP, the pH of the bile from both liver lobes was >7.55, and after 2 hours of NMP >7.65.
FIGURE 2.
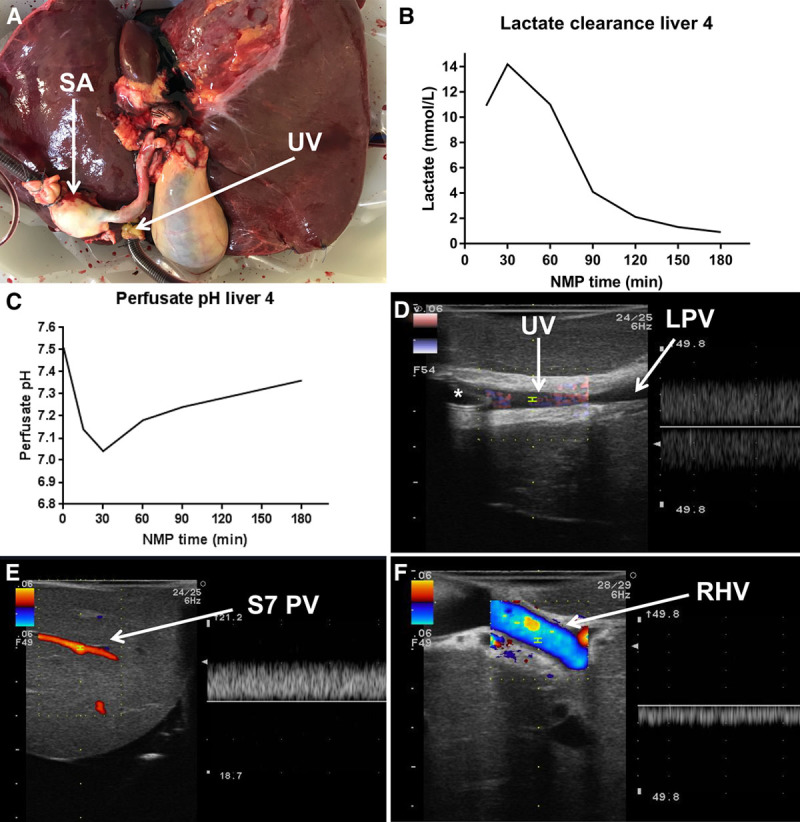
Ex situ evaluation of the liver that underwent NMP via the umbilical vein. A, Macroscopic appearance of the liver during NMP via the umbilical vein. B, Lactate clearance of the full liver graft during NMP. C, Normalization of perfusate pH of the full liver graft during NMP. No sodium bicarbonate was administered. D, Ultrasound image with Doppler acknowledging the position of the 24F perfusion cannula (denoted by the *) in the UV. E, Ultrasound image with Doppler demonstrating the patency of peripheral PV branches in the right liver lobe. F, Ultrasound image with Doppler visualizing adequate venous outflow from the RHV. LPV, left portal vein; NMP, normothermic machine perfusion; PV, portal vein; RHV, right hepatic vein; SA, supratruncal aorta; S7 PV, segment 7 portal vein branch; UV, umbilical vein.
Experimental Ex Situ Left Lateral Segment Split Procedure
The fifth liver was prepared for NMP through the umbilical vein and supratruncal aorta to assess the feasibility of a left lateral segment (LLS) split procedure under continuous NMP with subsequent continued machine perfusion of the LLS graft. The portal vein cannula (24F) was introduced in the umbilical vein and the arterial cannula in the supratruncal aorta after which NMP was initiated (Figure 3A). The organ was perfused at 37°C during transection of the liver parenchyma using the CUSA device (Excel+; Integra LifeSciences, Tullamore, Ireland), followed by sharp division of the hilar plate (Figure 3B and C). Finally, under continuous oxygenated NMP, the right hepatic artery and right portal vein were divided and the left hepatic vein was dissected from the inferior vena cava. The extended right lobe was removed from the reservoir, while the LLS remained under continuous NMP (Figure 3D). The extended right lobe was immediately flushed and preserved in a bowl with University of Wisconsin solution and sterile ice. In the hour following the splitting procedure, the LLS graft cleared lactate and the pH of the perfusion fluid maintained within normal values (between 7.35 and 7.45). The umbilical venous and pulsatile arterial flows of the LLS remained >400 mL/min and 150 to 200 mL/min, respectively.
FIGURE 3.
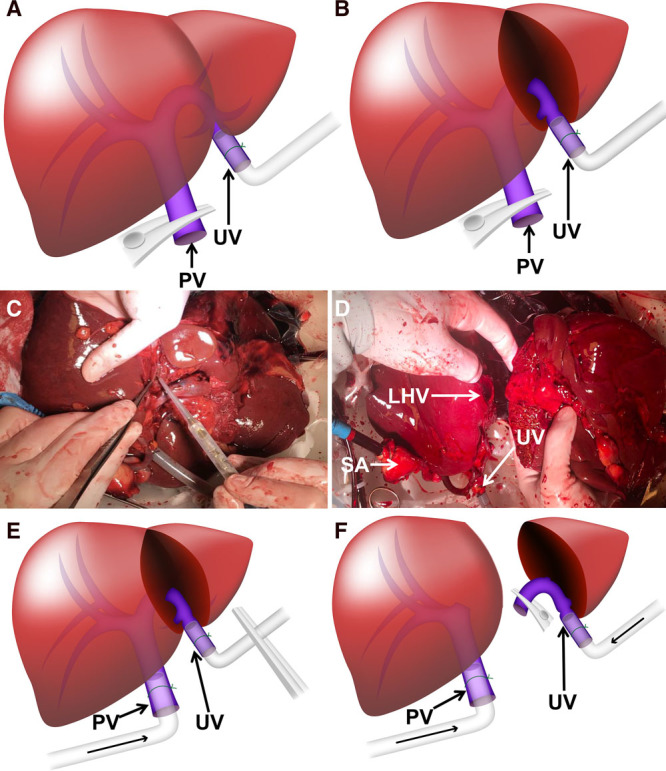
Step-by-step approach to perform an ex situ continuous oxygenated liver split procedure using the umbilical vein. A, Experimental setting for the splitting of liver 5. The PV was clamped and the whole liver graft was perfused normothermically via the UV. B, The LLS was perfused with NMP via the UV and SA after the splitting. The ERL was removed from the perfusion device and preserved on ice. The LLS was perfused with NMP via the UV and SA after the splitting. The ERL was removed from the perfusion device and preserved on ice. C, LLS procedure during continuous NMP. D, NMP of LLS after splitting under NMP via the UV and SA. E, Conceptual clinical ex situ splitting procedure: until PV dissection, continuous oxygenated machine perfusion via the PV main stem is performed. The UV is cannulated but clamped to prevent blackflow. F, Conceptual ex situ split procedure: after PV dissection, hypothermic machine perfusion via both the PV main stem and UV allows continuous oxygenation of both partial liver grafts during and after splitting. ERL, extended right lobe; LHV, left hepatic vein; LLS, left lateral segment; NMP, normothermic machine perfusion; PV, portal vein; SA, supratruncal aorta; UV, umbilical vein.
Proposed Ex Situ Split Procedure Using Continuous Oxygenated Hypothermic Machine Perfusion
The abovementioned concept could also be applied by using single (portal venous only) or dual (portal venous and arterial) hypothermic machine perfusion to allow continuous oxygenated LLS and extended right lobe split, or even full-left and full-right lobe split procedure. To do so, portal vein main stem and umbilical vein should both be cannulated with a portal vein cannula (24F). For dual perfusion, the arterial cannula should be introduced in the supratruncal aorta. For the parenchymal transection phase, hypothermic oxygenated machine perfusion can be initiated via the cannula in the portal vein main stem, while the cannula in the umbilical vein should be clamped to prevent backflow (Figure 3E). After division of the hilar plate, the left portal vein can be divided and clamped. Portal venous perfusion via the umbilical vein can be initiated to perfuse the LLS or full-left lobe, while continuous perfusion of the extended right lobe or full-right lobe is maintained via the portal vein main stem (Figure 3F). After division of the left hepatic artery, perfusion via the supratruncal aorta allows dual hypothermic oxygenated machine perfusion of the extended right lobe or full-right lobe, whereas the LLS or full-left lobe can be perfused via the umbilical vein only.
DISCUSSION
In this proof of concept study, we show for the first time that machine perfusion of human donor livers via the surgically reopened umbilical vein instead of the portal vein main stem is technically feasible. This modification may simplify the previously described technique of ischemia-free liver transplantation as it may allow procurement, preservation, as well as implantation with a standard end-to-end porto-portal anastomosis in the recipient under continuous oxygenated portal venous machine perfusion via the umbilical vein, without the need to anastomose an iliac vein graft to the portal vein main stem. We also demonstrate the technical feasibility of an LLS split procedure under continuous NMP via the surgically reopened umbilical vein as well as the supratruncal aorta.
The first 3 livers were perfused hypothermically, during which it is currently not possible to perform viability testing. We have assessed the flow rates through the umbilical vein and compared this with the usual portal vein flows observed in our clinical hypothermic machine perfusions in humans.6 We noticed that when using a standard 24F cannula for umbilical vein perfusion, flows were comparable to typical hypothermic perfusion via the portal vein main stem (200 to 300 mL/min). Meticulous care should be taken to avoid twisting of the umbilical vein cannula because we observed that this immediately impaired portal venous flow.
The fourth liver perfusion experiment demonstrated that our clinically used viability criteria for donor livers during NMP2 (after 150 min of NMP: perfusate lactate <1.7 mmol/L, perfusate pH between 7.35 and 7.45, bile pH >7.45; Dutch Trial Register No. NTR5972) were reached by using portal venous perfusion via the umbilical vein.
In the fifth liver, we have performed an LLS split procedure under continuous NMP using portal venous perfusion via the surgically reopened umbilical vein. After completion of the split procedure, NMP of the LLS was continued. In the following hour, lactate was cleared and the pH remained within normal values. We propose that by introducing a cannula in the portal vein main stem as well as a cannula in the surgically reopened umbilical vein, machine perfusion of the LLS or full-left lobe as well as the extended right or full-right lobe can be maintained even after complete separation of the 2 liver parts.
We envision that splitting under continuous oxygenated hypothermic machine perfusion could be beneficial, for example, for logistical reasons to allow sequential liver transplantation in the same center (conceptualized in Figure 3E and F). Also, hypothermic oxygenated machine perfusion is less prone to technical failure and is associated with lower flow rates when using a pressure-controlled machine perfusion device. We have, however, not experienced trouble with flow obstruction during the NMP split procedure. When using continuous oxygenated hypothermic machine perfusion, the transfer from one machine to another or to static cold storage before implantation can be performed without additional warm ischemic injury. At normothermic temperatures, we do not consider it beneficial to perfuse both the extended right or full-right lobe and the LLS or full-left lobe simultaneously. For NMP, arterial perfusion is required and arterial cannulation of either the left or the right hepatic artery may risk additional injury to the vascular endothelium. Therefore, we propose that continuous hypothermic oxygenated machine perfusion during splitting will be most beneficial in clinical practice.
In this proof of concept study, we have demonstrated for the first time that machine perfusion through the surgically reopened umbilical vein in discarded human livers is technically feasible, thereby achieving adequate portal venous flows. Future studies should investigate the additional clinical utility of machine perfusion via the umbilical vein, which should include histopathological analysis of the parenchyma and biliary tree.
ACKNOWLEDGMENTS
The authors thank Dr Koert P. de Jong for intellectual contribution and insightful discussion.
Footnotes
The authors declare no funding or conflicts of interest.
O.B.v.L., M.F., R.J.P., and V.E.d.M. participated in study design, writing of the paper, performance of the research, and analyzed the data. R.U., M.J.M.W., and I.M.A.B. contributed to data collection. All authors have critically read and revised the manuscript.
REFERENCES
- 1.Durand F, Renz JF, Alkofer B, et al. Report of the paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl 200814121694–1707 [DOI] [PubMed] [Google Scholar]
- 2.de Meijer VE, Fujiyoshi M, Porte RJ. Ex situ machine perfusion strategies in liver transplantation. J Hepatol 2019701203–205 [DOI] [PubMed] [Google Scholar]
- 3.He X, Guo Z, Zhao Q, et al. The first case of ischemia-free organ transplantation in humans: a proof of concept. Am J Transplant 2018183737–744 [DOI] [PubMed] [Google Scholar]
- 4.van Leeuwen OB, Ubbink R, de Meijer VE, et al. The first case of ischemia-free organ transplantation in humans: a proof of concept. Am J Transplant. 2018;18(8):2091. doi: 10.1111/ajt.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soyama A, Eguchi S, Takatsuki M, et al. Tumor thrombectomy via a surgically reopened umbilical vein combined with right hemihepatectomy in a patient with hepatocellular carcinoma. Dig Surg 2011283222–225 [DOI] [PubMed] [Google Scholar]
- 6.van Rijn R, Karimian N, Matton APM, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg 20171047907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matton APM, Burlage LC, van Rijn R, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl 2018244528–538 [DOI] [PMC free article] [PubMed] [Google Scholar]


