Supplemental Digital Content is Available in the Text.
A ligand-guided, light-activated photosensitizer tool targets TrkA-expressing nociceptors, reversing acute and chronic pain in mice.
Keywords: Nociceptor, TrkA, NGF, Acute pain, Chronic pain, Osteoartritis, CFA-Induced pain, Spared nerve injury, Photoablation, Photosensitizer
Abstract
Nerve growth factor (NGF) and its receptors TrkA and p75 play a key role in the development and function of peripheral nociceptive neurons. Here, we describe novel technology to selectively photoablate TrkA-positive nociceptors through delivery of a phototoxic agent coupled to an engineered NGF ligand and subsequent near-infrared illumination. We demonstrate that this approach allows for on demand and localized reversal of pain behaviors in mouse models of acute, inflammatory, neuropathic, and joint pain. To target peripheral nociceptors, we generated a SNAP-tagged NGF derivative NGFR121W that binds to TrkA/p75 receptors but does not provoke signaling in TrkA-positive cells or elicit pain behaviors in mice. NGFR121W-SNAP was coupled to the photosensitizer IRDye700DX phthalocyanine (IR700) and injected subcutaneously. After near-infrared illumination of the injected area, behavioral responses to nociceptive mechanical and sustained thermal stimuli, but not innocuous stimuli, were substantially reduced. Similarly, in models of inflammatory, osteoarthritic, and neuropathic pain, mechanical hypersensitivity was abolished for 3 weeks after a single treatment regime. We demonstrate that this loss of pain behavior coincides with the retraction of neurons from the skin which then reinnervate the epidermis after 3 weeks corresponding with the return of mechanical hypersensitivity. Thus NGFR121W-SNAP-mediated photoablation is a minimally invasive approach to reversibly silence nociceptor input from the periphery, and control pain and hypersensitivity to mechanical stimuli.
1. Introduction
Current therapeutic strategies for the management of chronic pain have limited efficacy and may be associated with dependence, tolerance, and serious side effects.6,9,29,49,59 To avoid these issues, and develop more efficacious pain therapies, much effort has been made in developing strategies to target the peripheral nervous system (PNS) and molecules that are selectively expressed in nociceptors. Among the most promising of those, nerve growth factor (NGF) and its receptors TrkA/p75 have emerged as frontrunners for the design of next-generation analgesics.13,15,41–43,53
There is a wealth of data supporting the role of NGF in pain signaling. Nerve growth factor and TrkA are required for the development of the PNS; in their absence, nociceptors fail to develop, and animals and humans display severe deficits in pain sensitivity.11,21,27,28,32,50,56 In the adult PNS, TrkA is expressed predominantly on peptidergic nociceptors, and NGF signaling plays an important role in setting pain sensitivity. Thus, administration of NGF leads to hyperalgesia within 1 to 3 hours;20,33,34,47,52 endogenous NGF levels are elevated in chronic pain and promote peripheral and central sensitization.36,40,48 The recognition that NGF has a critical role in the generation and potentiation of pain has created a strong rationale for developing methods that interfere with its signaling. The most successful of these approaches has been the development of anti-NGF antibodies,13 which have advanced to Phase III trials for osteoarthritis (OA) of knee and hip. There are, however, a number of safety issues associated with antagonizing NGF, the most prominent of which is a rapidly progressive OA, leading to joint replacement.24
Rather than inhibiting NGF signaling, here we asked whether NGF may offer a means of accessing nociceptive neurons through their TrkA receptors to deliver a phototoxic agent to silence their activity. To test this hypothesis, we generated a SNAP-tagged NGF that could be conjugated to the photosensitizer IRDye700DX phthalocyanine (IR700). While injection of NGFSNAP IR700 and subsequent near-infrared (NIR) illumination reduced nociceptive behavior in mice, results were confounded by the fact that NGF is in itself proalgesic. We thus turned to “painless” derivatives of NGF which still bind to receptors, but provoke abrogated signaling, to deliver the photosensitizer. Such NGF mutants have been described in patients with Hereditary Sensory and Autonomic Neuropathy-type 5 (HSANV), who display loss of pain perception and other sensory and autonomic abnormalities1 as a result of mutations in NGF.12,21,30,54,57 We selected the NGF p.R121W mutation as a delivery tool because this gave the highest yields in recombinant production when fused to the SNAP-tag. We found that NGFR121W-SNAP binds to TrkA-positive cells but did not elicit TrkA-mediated signaling or sensitization in vivo. Moreover, injection in mice of NGFR121W-SNAP IR700 followed by NIR illumination led to long-term reversal of nociceptive behavior that paralleled retraction of nerve fibers from the epidermis. Thus, through ligand-guided delivery of a small molecule photosensitizer, we are able to achieve selective disruption of nociceptor input and substantially reduce pain.
2. Results
2.1. Generation and characterization of NGFSNAP
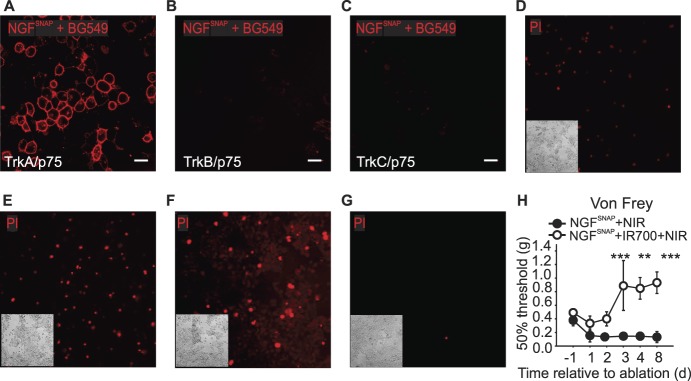
Recombinant NGF was cloned and purified as a SNAP-tag fusion protein (NGFSNAP), which allows for covalent coupling to BG-derived photosensitizers and imaging fluorophores. We first tested the binding selectivity of NGFSNAP by labelling it with a BG-fluorophore and applying it to cells expressing neurotrophin receptors. NGFSNAP was coupled in vitro to BG-Surface549 and applied at a 100-nM concentration to Hek293T cells transiently transfected with TrkA, TrkB. or TrkC and p75. NGFSNAP bound its cognate receptor, decorating the surface of TrkA-/p75-transfected cells (Fig. 1A), while cells transfected with BDNF/NT4 and NT3 receptors with p75 were not stained (Figs. 1B and C), demonstrating that binding occurs faithfully in a receptor-specific fashion.
Figure 1.
Characterization of NGFSNAP. (A–C) Confocal images of Hek293T cells transiently transfected with TrkA, TrkB, or TrkC (A–C, respectively) and p75 stained with 100-nM NGFSNAP conjugated to surface BG-546. NGFSNAP selectively binds only to TrkA-/p75-transfected cells, with virtually no binding to TrkB- or TrkC-transfected cells. Scale bar 20 μm. (D–F) Hek293T cells transiently transfected with TrkA and p75 were incubated with NGFSNAP (0.1, 0.5, and 1 μM, D–F, respectively) conjugated with BG-IR700 and then exposed to NIR illumination for 2 minutes. Twenty-four hours after in vitro photoablation, cells were stained with propidium iodide (PI). One micrometer BG-IR700–conjugated NGFSNAP (G) was applied onto mock transfected cells, and cells exposed to 690-nm light for 2 minutes as a negative control. Insets: images of corresponding brightfields. (H) Von Frey thresholds at baseline and at each of 3 consecutive days of hind paw injection of NGFSNAP conjugated with BG-IR700 (closed circles) or unconjugated (open circles), followed by NIR light exposure. n = 4. Error bars indicate SEM. ***P < 0.001; **P = 0.01 (two-way ANOVA). ANOVA, analysis of variance; NIR, near-infrared.
Having confirmed that NGFSNAP specifically labels TrkA-expressing cells, we next asked whether a photosensitizer can be coupled to the ligand to ablate TrkA-positive cells. Hek293T cells transfected with TrkA/p75 were incubated with NGFSNAP conjugated to the NIR photosensitizer BG-IR700 and illuminated with NIR light for 2 minutes. Twenty-four hours later, cell death was analyzed using propidium iodide staining. In cells transfected with TrkA/p75, we observed concentration-dependent increase in cell death upon treatment with NGFSNAP IR700 and NIR illumination (Figs. 1D–F). In the absence of TrkA expression, cell viability was fully preserved after NGFSNAP IR700 application and illumination (Fig. 1G).
We next asked whether NGFSNAP IR700 can be used to photoablate nociceptors in vivo. NGFSNAP conjugated with BG-IR700 (Fig. 1H, filled circles) or unconjugated NGFSNAP (open circles) was injected into the hind paw of C57BL/6J male naive mice, and skin was illuminated for 2 minutes with IR light exposure on 3 consecutive days. Behavioral responses to calibrated von Frey filaments were monitored after each ablation at 24 hours and 5 days after the last treatment. As shown in Figure 1H, mechanical thresholds increased in ablated mice, suggesting that TrkA-expressing nociceptors were targeted by NGFSNAP IR700. However, we also observed a decrease in von Frey thresholds in control NGFSNAP mice (Fig. 1H), indicating that the ligand in itself was having a proalgesic effect and might constitute a confounding factor in the measurement of mechanical thresholds in ablated mice. In further experiments, we thus sought to exploit a “painless” NGF mutant described in HSANV patients, NGFR121W, which has been described to bind to TrkA receptors but not provoke nociceptive signaling.54
2.2. Generation and characterization of NGFR121W-SNAP
A C-terminal fusion of NGFR121W and SNAP (NGFR121W-SNAP) was produced in Chinese hamster ovary cells at yields similar to wildtype NGFSNAP and could be readily labelled with BG549 indicating that the SNAP-tag was fully functional (Fig. 2 A). To characterize the binding and signaling properties of NGFR121W-SNAP, we first assessed its capacity to label Hek293T cells transfected with neurotrophin receptor plasmids. NGFR121W-SNAP was coupled in vitro with BG-Surface549 and applied to cells. Similar to wildtype NGFSNAP, we observed robust membrane labelling of TrkA-/p75-expressing cells (Fig. 2B) but no signal in TrkB/p75- or TrkC/p75-transfected cells (Figs. 2C and D). We further analyzed downstream signaling provoked by wildtype NGFSNAP and NGFR121W-SNAP by assessing phosphorylation of MAPK and AKT upon treatment of PC12 cells with ligands.22 Wildtype NGF treatment produced a substantial increase in phosphorylation of MAPK and AKT, while levels of MAPK and AKT phosphorylation in cells treated with NGFR121W-SNAP were no different from untreated cells (Figs. 2E and F). We next evaluated the neurotrophic activity of wildtype NGF and NGFR121W-SNAP by quantifying the number of differentiated PC12 cells (assessed by neurite outgrowth10) after 6 days of incubation with ligands. A significantly higher proportion of differentiated PC12 cells were present in samples treated with NGFSNAP, while those treated with NGFR121W-SNAP were not significantly different from untreated cells (Figs. 2G–J). Finally, we considered the pronociceptive activity of NGFSNAP and NGFR121W-SNAP by injecting ligands into the hind paw of mice and evaluating mechanical and thermal hyperalgesia with the von Frey and hotplate tests. We found that wildtype NGF induced substantial sensitization to both mechanical and thermal stimuli, while injection of NGFR121W-SNAP did not change thresholds to either of these stimuli (Figs. 2K and L). We conclude that, while binding and receptor specificity of NGFR121W-SNAP are comparable to that of NGFSNAP, it has the distinctive advantage that it allows for uncoupling of receptor engagement from nociceptive activity, making it a valuable tool to selectively target TrkA-expressing peptidergic neurons for the development of an antinociceptive treatment strategy.
Figure 2.
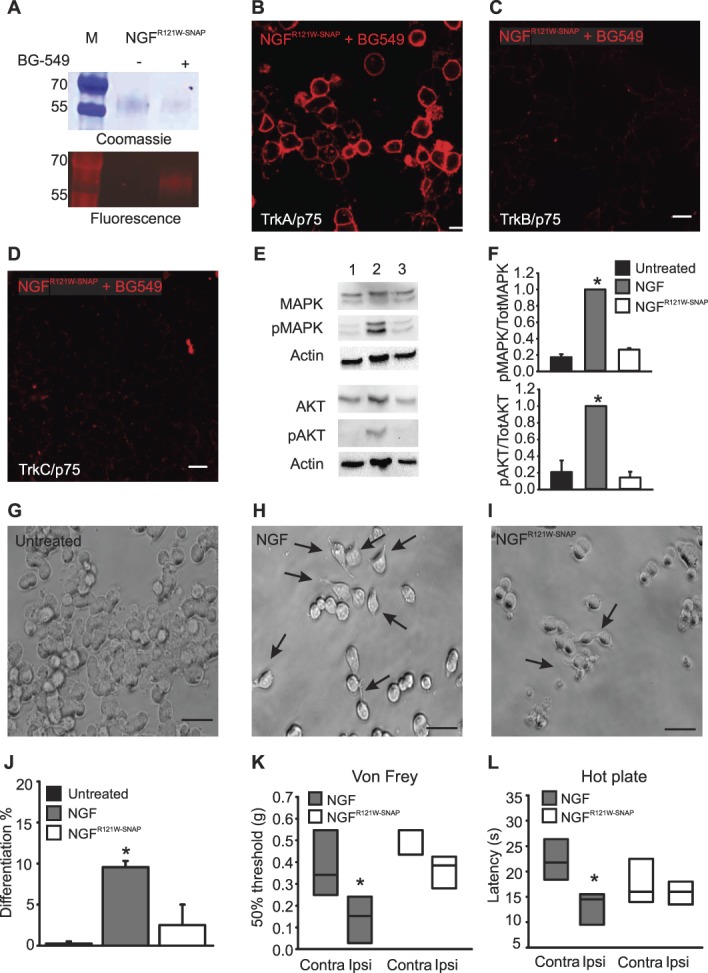
Characterization of NGFR121W-SNAP. (A) NGFR121W-SNAP was coupled with BG549 in vitro, run on an SDS-Page, stained with Coomassie blue, and in-gel fluorescence visualized under a UV light. (B–D) Confocal images of Hek293T cells transiently transfected with TrkA, TrkB, or TrkC (B–D, respectively) and p75 stained with 100-nM NGFR121W-SNAP conjugated to surface BG-546. NGFR121W-SNAP selectively binds only to TrkA-/p75-transfected cells, with virtually no binding to TrkB- or TrkC-transfected cells. Scale bar 20 μm. (E) Representative Western blot of 3 independent experiments, showing the expression level of MAPK, phospho MAPK, AKT, phospho AKT, and actin (loading control) in untreated PC12 cells (lane 1), treated with NGF (lane 2) or NGFR121W-SNAP (lane 3). (F) Levels of each protein were expressed as ratio between the phosphorylated form and the total counterpart and then normalized to the NGF-treated sample. (G–J) Neuron differentiation in untreated (G), NGF-treated (H), and NGFR121W-SNAP-treated (I) PC12 cells, after 6 days of treatment. Arrows indicate differentiated cells. Scale bar 20 µm. (J) Quantitation of neuron-like differentiated PC12 cells, expressed as percentage (%); for the analysis, 241 untreated cells, 246 NGF-treated cells, and 285 NGFR121W-SNAP-treated cells were considered. Error bars indicate SEM. (K) von Frey thresholds after injection of NGF (shaded box) or NGFR121W-SNAP (open box) into the hind paw (ipsi) of mice (n = 4 animals). Box plots represent the force expressed in grams (G) required to trigger a 50% response. *P = 0.05 (two-tailed t test). (L) Hotplate test after injection of NGF (n = 6 animals, red box) and NGFR121W-SNAP (n = 5 animals, gray box) into the hind paw of the mice. Box plots represent the latency expressed in seconds (s) of the paw withdrawal in response to heat (52°C). *P = 0.009 (two-tailed t test). NGF, nerve growth factor.
2.3. NGFR121W-SNAP-mediated photoablation and acute nociception
We first assessed the efficacy of NGFR121W-SNAP IR700-mediated photoablation in vitro in Hek293T cells transfected with TrkA/p75. Similar to NGFSNAP, the mutant ligand induced a robust and concentration-dependent increase in cell death 24 hours after application to cells and NIR illumination (Figs. 3A–C). By contrast, NGFR121W-SNAP IR700 and NIR illumination was without effect in mock-transfected cells not expressing TrkA/p75 (Fig. 3D).
Figure 3.
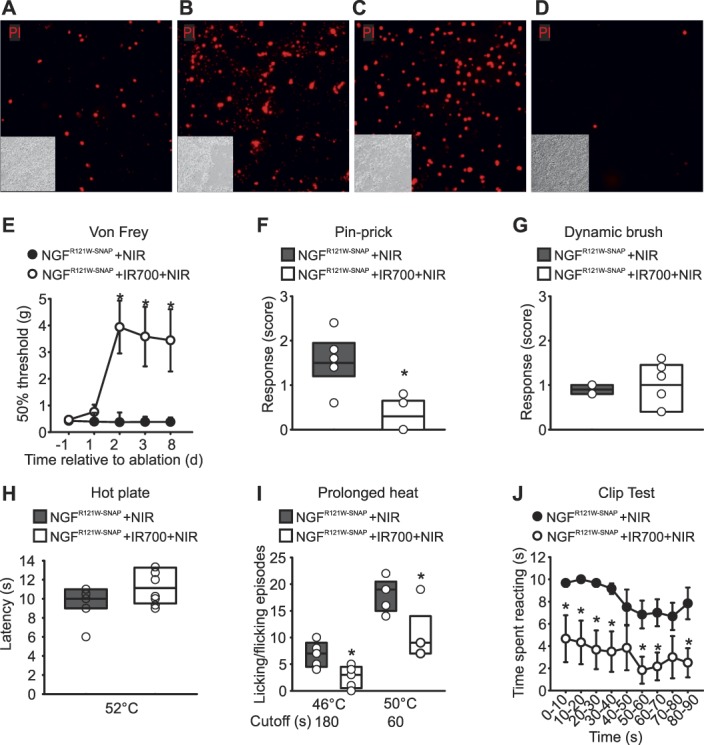
NGFR121W-SNAP-mediated ablation in vitro and in acute nociceptive tests. (A–D) Hek293T cells transiently transfected with TrkA and p75 were incubated with NGFR121W-SNAP (0.1, 0.5, and 1 μM, A–C, respectively) conjugated with BG-IR700 and then illuminated with NIR light for 2 minutes. Twenty-four hours after in vitro photoablation, cells were stained with propidium iodide (PI). One micrometer BG-IR700–conjugated NGFR121W-SNAP (D) was applied onto mock transfected cells, and cells illuminated with 690-nm light for 2 minutes as a negative control. Insets: images of corresponding brightfields. (E) von Frey thresholds after 3 days of injection of NGFR121W-SNAP into the hind paw of the mice, with (open circles) or without (closed circles) IR700, followed by NIR illumination (n = 6 animals). The test was performed at day 1, 2, 3, and 8 after last ablation treatment. The graph shows the force expressed in grams (g) required to trigger a 50% response. Error bars indicate SEM. *P = 0.001 (two-way ANOVA followed by Bonferroni post hoc test). (F and G) Pin-prick and dynamic brush tests after 3 days of injection of NGFR121W-SNAP into the hind paw of the mice with (open box) or without (shaded box) IR700, followed by NIR illumination (n = 6 animals). The test was performed at 7 days after the last ablation treatment. *P = 0.002 (pin-prick, two-tailed t test). The graphs show the response to the stimulus, 0 to 3 score (score = 0 no response; score = 1 paw withdrawal; score = 2 prolonged paw withdrawal; score = 3 paw flicking/licking). (H) Response latency expressing the time needed to observe a nocifensive behavior upon heat stimulation in a hotplate preset at 52°C. Mice were injected for 3 consecutive days with NGFR121W-SNAP into the hind paw, with (open box) or without (shaded box) IR700, followed by NIR illumination (n = 6 animals). The test was performed at 7 days after the last ablation treatment. (I) Paw licking/flicking behavior evoked by a prolonged heat noxious stimuli using a hotplate (46°C, 50°C) in mice injected in both hind paws for 3 days with NGFR121W-SNAP with (open boxes) and without (shaded boxes) IR700, followed by NIR illumination (n = 5 animals). A cutoff time was set at 180 seconds (46°C), and 60 seconds (50°C) *P = 0.018 (two-tailed t test). (J) Clip tail test after 3 days of cream application of NGFR121W-SNAP with (n = 6 animals, white circles) and without (n = animals, black circles) IR700, followed by NIR illumination on the mouse tail. The test was performed at days 1, 5, 7, 10, 14, and 21 after last ablation treatment. The graph shows the latency expressed in seconds (s) to the clip. Error bars indicate SEM. *P = 0.001 (two-way ANOVA followed by Tukey post hoc test). ANOVA, analysis of variance; NIR, near-infrared.
To characterize NGFR121W-SNAP-mediated photoablation in vivo, we evaluated its efficacy in models of acute nociception. Wildtype C57BL/6J male mice were injected for 3 consecutive days in the hind paw with either NGFR121W-SNAP, with or without IR700, or with IR700 alone (some controls shown in Supplementary Fig. 2, available at http://links.lww.com/PAIN/A822) followed by NIR illumination. We first tested mice for sensitivity to punctate mechanical stimuli using von Frey filaments. As shown in Figure 3E, 2 days after ablation, mice treated with NGFR121W-SNAP IR700 displayed significantly reduced sensitivity to these stimuli, requiring weights above 3 g to provoke a behavioral response, and this insensitivity persisted throughout the 8-day monitoring period. Similarly, responses to nociceptive pin-prick stimuli applied to the paw were also substantially reduced in photoablated compared with control mice (Fig. 3F). We further assessed sensitivity to non-nociceptive dynamic brush stimuli and observed no effect of NGFR121W-SNAP IR700 (Fig. 3G) indicating that the photoablation is selective for nociceptors. We also tested thermal responsiveness using the hotplate test. Intriguingly, first-line reflex latencies were not different between photoablated and control animals; however, licking behavior evoked by sustained noxious thermal stimuli was reduced in treated animals at 46°C and 50°C (Figs. 3H and I). Thus, TrkA-positive neurons may mediate pain resulting from tissue damaging thermal stimuli, rather than reflexive defensive responses. Finally, we asked whether NGFR121W-SNAP IR700 can be applied topically to alleviate acute nociception. A microemulsion premixed with either NGFR121W-SNAP with or without IR700, or IR700 alone (Supplementary Fig. 2e, available at http://links.lww.com/PAIN/A822), was applied to the tail skin and illuminated with NIR light. One week later, a calibrated clip was applied to the base of the tail and responses monitored. We observed significantly reduced behavioral responses to the clip in NGFR121W-SNAP IR700 mice throughout the monitoring period, such that mice appeared essentially insensitive to this nociceptive stimulus (Fig. 3J; Supplementary Video 1, available at http://links.lww.com/PAIN/A823).
2.4. NGFR121W-SNAP-mediated photoablation in models of inflammatory, osteoarthritic, and neuropathic pain
We investigated the effects of NGFR121W-SNAP-mediated photoablation on persistent pain using models of inflammatory, osteoarthritic, and neuropathic pain.
To model inflammatory pain, mice were injected in the hind paw with complete Freund's adjuvant (CFA). After 48 hours, mechanical and thermal hyperalgesia were evident, and mice were treated for 3 days with NGFR121W-SNAP with or without IR700 (Supplementary Fig. 2e, available at http://links.lww.com/PAIN/A822), or with NGFR121W-SNAP IR700, with or without near NIR illumination (Fig. 4A and B). Behavioral thresholds to mechanical and thermal stimuli were monitored for 12 days after CFA injection. As shown in Figure 4A, mice which received NGFR121W-SNAP and NIR inflammation displayed a complete reversal of mechanical hypersensitivity that lasted throughout the 12-day observation period (Fig. 4A). Of note, thermal hyperalgesia induced by CFA was not altered by photoablation (Fig. 4B).
Figure 4.
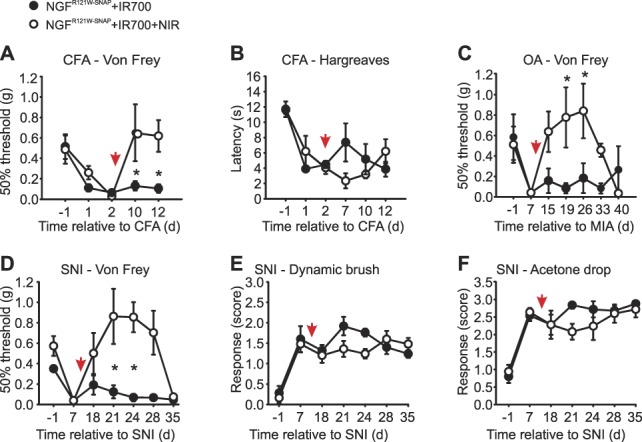
NGFR121W-SNAP-guided photoablation in models of prolonged pain. (A and B) Inflammatory pain was measured 24 and 48 hours after CFA injection into the paw, and at the indicated days, relative to the CFA injection, after a 3-d course of injections with NGFR121W-SNAP + IR700 with (n = 6, open circles) and without (n = 6, closed circles) NIR illumination (red arrow indicates the first day of ablation). Baseline before CFA injection is set as d = −1. Error bars indicate SEM. *P = 0.018 (two-way ANOVA followed by Holm–Sidak post hoc test). (C) von Frey test in osteoarthritis (OA) mouse model induced by a single injection of monosodium iodoacetate (MIA) into the knee. The test was performed at the indicated days, relative to MIA injection, and after a 3-d course of injections of NGFR121W-SNAP + IR700 with (n = 6, open circles) and without (n = 6, closed circles) NIR illumination (red arrow indicates the first day of ablation). Baseline before MIA injection is set as d = −1. Error bars indicate SEM. *P = 0.003 (two-way ANOVA followed by Bonferroni post hoc test). (D–F) von Frey, dynamic brush, and acetone drop tests in spared nerve injury (SNI) mouse model. The tests were performed at the indicated day relative to the SNI procedure, and after a 3-d course of injections with NGFR121W-SNAP + IR700 with (n = 6, open circles) and without (n = 6, closed circles) NIR illumination (red arrow indicates the first day of ablation). Baseline before SNI is set as d = −1. Score = 0, no response; score = 1, paw withdrawal; score = 2, prolonged paw withdrawal; score = 3, paw flicking/licking; and score = 4, prolonged paw licking. *P = 0.005 (two-way ANOVA followed by Bonferroni post hoc test). ANOVA, analysis of variance; NIR, near-infrared.
To model OA-like pain, mice received an intra-articular injection into the knee of monosodium iodoacetate (MIA).45 Seven days after injection, significant fibrosis was evident in the joint (Supplementary Fig. 1a–f, available at http://links.lww.com/PAIN/A822), and mice developed referred mechanical hypersensitivity to von Frey filaments applied to the plantar surface of the paw (Supplementary Fig. 1g, available at http://links.lww.com/PAIN/A822). NGFR121W-SNAP IR700 was injected into the knee, and the area was illuminated with NIR light through the skin for 3 consecutive days. Control mice received NGFR121W-SNAP IR700 but no illumination, or IR700 alone with illumination (Supplementary Fig. 2f, available at http://links.lww.com/PAIN/A822). In treated mice, we observed a complete recovery of mechanical thresholds to preinjury levels, which persisted for 3 weeks before returning to the preablation state (Fig. 4C). Control animals displayed no recovery and remained mechanically hypersensitive throughout the 40-day monitoring period (Fig. 4C; Supplementary Fig. 2f, available at http://links.lww.com/PAIN/A822).
For neuropathic pain, we used the spared nerve injury (SNI) model.17 Seven days after nerve injury when all mice had developed mechanical hypersensitivity, NGFR121W-SNAP IR700 was injected into the paw, and the skin was illuminated with NIR light on 3 consecutive days. Control animals received NGFR121W-SNAP IR700 without illumination (Figs. 4D–F), IR700 with illumination, or NIR illumination alone (Supplementary Fig. 2g, available at http://links.lww.com/PAIN/A822). Mice were then assayed for hypersensitivity to mechanical punctate stimuli, dynamic brush stimuli, and evaporative cooling. In mice treated with NGFR121W-SNAP IR700 and NIR illumination, we again observed that thresholds to punctate mechanical stimuli recovered to preinjury levels for about 3 weeks, before returning to a hypersensitive state (Fig. 4D). Intriguingly, responses to dynamic brushing mechanical stimuli were not altered by photoablation (Fig. 4E) nor were responses to evaporative cooling (Fig. 4F).
2.5. Mechanism of NGFR121W-SNAP-mediated photoablation
To understand how NGFR121W-SNAP-mediated photoablation underlies the loss and then recovery of mechanical sensitivity to nociceptive stimuli in treated mice, we performed a histological analysis of skin at 7 and 28 days after treatment.
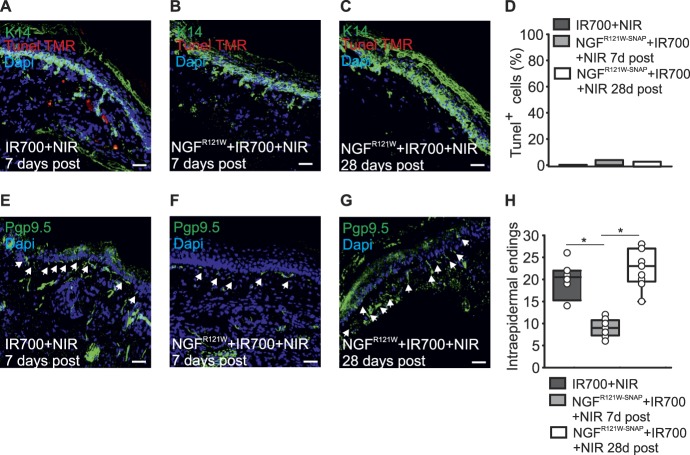
We first investigated the degree of cell death of non-neuronal cells in the skin by performing TUNEL staining to detect apoptotic DNA fragmentation. Skin was harvested at 7 and 28 days after treatment in animals injected with NGFR121W-SNAP IR700 and illuminated with NIR light, or at 7 days after treatment in control animals receiving only IR700 and illumination. Sections were then processed for TUNEL staining and immunohistochemistry with an anti-K14 antibody to mark keratinocytes. As shown in Figures 5A–C, very few apoptotic cells could be detected in the skin in all conditions, and there was no difference in treated vs control samples (Figs. 5A–D). The number of keratinocytes was also constant across conditions and time points. Thus, at the concentrations and light intensities used here, there is no detectable effect of photoablation on non-neuronal cells in the skin.
Figure 5.
NGFR121W-SNAP-guided photoablation induces reversible nerve retraction without affecting keratinocytes in the skin. (A–D) Representative paw skin cryosections stained for keratin 14 (K14, in green) and TUNEL TMR (in red) with quantitation of keratinocytes cell death in mice (n = 3 animals) injected for 3 days with IR700 followed by NIR illumination (A), NGFR121W-SNAP+IR700 followed by NIR illumination (B and C). Skin was collected 7 days after (A and B) and 28 days after (C) the last day of injection. The number of TUNEL+ keratinocytes is expressed as percentage (D). (E–H) Representative paw skin cryosections stained for the neuronal marker Pgp9.5 (in green) of mice injected for 3 days with IR700 followed by NIR illumination (E), NGFR121W-SNAP+IR700 followed by NIR illumination (F and G). The skin was collected 7 days after (E and F) and 28 days after (G) the last day of injection. Arrows indicate free nerve endings. Quantitation of the epidermal free nerve endings for the indicated sample; 8 sections were analyzed (H). *P = 0.001 (two-tailed t test). NIR, near-infrared.
To investigate the effects of NGFR121W-SNAP-mediated photoablation on innervation of the skin, we performed immunohistochemistry using an anti-Pgp9.5 antibody to detect neuronal fibers. Again, skin samples were taken at 7 and 28 days after treatment in treated animals (NGFR121W-SNAP IR700 plus illumination), or at 7 days after treatment in control animals (IR700 and illumination). We observed a significant reduction in the number of the intraepidermal free nerve endings in treated animals compared with control at 7 days after injection (Figs. 5E and F). Importantly, at 28 days after ablation, the number of fibers was restored to a value similar to the one in the control group (Figs. 5G and H). Thus, after photoablation, we observe a retraction of nociceptors from the skin that then reinnervate the epidermis with a similar time course to the loss and return of mechanical sensitivity seen in behavioral experiments.
3. Methods
3.1. Animals
Wildtype C57BL/6J and Balb/c mice were used at age of 8 to 10 weeks for all the experiments. All mice were bred and maintained at the EMBL Neurobiology and Epigenetic Unit, Rome, in accordance with Italian legislation (Art. 9, 27. January 1992, no 116). Experiments were performed under license from the Italian Ministry of Health and in compliance with the ARRIVE guidelines.
3.2. Production of recombinant NGFSNAP and NGFR121W-SNAP
cDNAs encoding for murine NGFSNAP and NGFR121W-SNAP including a C-terminal polyhistidine tag (His6) inserted for purification purposes were cloned into the pMB-PB vector as a fusion protein.37 Protein expression was performed using Chinese hamster ovary cells (ATCC, mycoplasma negative) as described by Balasubramanian.2 Secreted NGFSNAP and NGFR121W-SNAP were purified from cell medium using a Ni-NTA resin (Qiagen, #30210) and eluted with an excess of imidazole. Eluted fractions were then pooled, concentrated, and stored for further analysis.
3.3. Synthesis of BG-IR700
A total of 3 mg of IRDye700DX N-hydroxysuccinimide ester fluorophore (LI-COR Biosciences GmbH, Bad Homburg, Germany) were dissolved in 150 µL of DMSO and treated with 1.5 mg of BG-PEG11-NH2 and 5 µL of diisopropylethylamine. After 1 hour, the product BG-PEG11-IRDye700DX was purified by HPLC using a Waters Sunfire Prep C18 OBD 5 µM; 19 × 150-mm column using 0.1 M of triethylammonium acetate (TEAA) (pH 7.0) and 0.1 M of TEAA in water/acetonitrile 3:7 (pH 7.0) as mobile phases A and B, respectively. A linear gradient from 100% A to 100% B within 30 minutes was used. The fractions containing the product were lyophilized. Stock solutions of BG-IR700 were stored at high concentration in DMSO. Before conjugation with proteins, BG-IR700 was diluted in physiological buffer.
3.4. In vitro experiments
Hek293T cells were transfected with TrkA, TrkB, TrkC, and p75 plasmid using Lipofectamine 2000 (Thermo). For the labelling, 1-µM NGFR121W-SNAP was coupled with 3-µM BG549 surface (NEB # S9112) for 1 hour at 37°C in calcium imaging buffer (NaCl 140 mM; KCl 4 mM; CaCl2 2 mM; MgCl2 1 mM; NaOH; 4.55 mM; glucose 5 mM; and HEPES 10 mM; pH 7.4). The coupling reaction was filtered through a PD MiniTrap G-25 column (GE Healthcare #28-9180-07) to remove the excess of BG. Cells were incubated with the coupling reaction for 15 to 20 minutes at 37°C, then washed 3 times in calcium imaging buffer.
To assess NGF signaling, PC12 cells were cultured on collagen IV–coated 6-well plate in DMEM/F12 medium containing 1% horse serum. Cells were treated for 2 minutes with PBS or 1-µM NGF or 1-µM NGFR121W-SNAP, followed by lysis in Ripa buffer.
For the neuronal differentiation assay, PC12 cells were daily exposed to 100-ng/mL NGF (Alomone labs, #N-100) or NGFR121W-SNAP for 6 days. The number of neuron-like cells was counted at day 6.
All images were visualized with a Leica SP5 confocal microscope.
3.5. SDS-Page and Western blot
To assess the coupling reaction, 1-µM NGFR121W-SNAP was coupled with 1.5-µM BG549 for 1 hour at 37°C. The coupling reactions were analyzed by SDS-Page on a precast acrylamide gel (BioRad #456-9034), along with the same concentrations of NGFR121W-SNAP alone. The bands corresponding to the binding of NGFR121W-SNAP with BG549 were visualized by gel fluorescence. All the samples were visualized by Coomassie staining.
For NGF-mediated signaling, PC12 cells were collected and lysed in Ripa Buffer (Sigma, #R0278) with proteases inhibitor cocktail (Roche #11873580001). Protein lysates were quantified by Bradford assay. For all the experiments, 30-µg total lysate were separated on 10% SDS-Page gel and transferred to a nitrocellulose membrane (Protran #10600007). Membranes were incubated with the following antibodies: anti-MAPK (Cell Signaling #4695), anti-phospho MAPK (Thr202/Tyr204) (Cell Signaling #9106), anti-AKT (Cell Signaling #4691), anti-phospho AKT (Ser473) (Cell Signaling #9271), anti-Actin (Cell signaling #4970). Bands were visualized using the ECL detection system (Amersham #RPN2106); band density was calculated using ImageJ, and the levels of phosphorylated proteins were normalized to the total counterpart.
3.6. Immunofluorescence
Skin from the plantar region of the mice hind paw was dissected 7 days and 28 days after treatment of SNI mice with IR700, with or without NGFR121W-SNAP, followed by near-IR illumination. Skin was fixed with 4% PFA overnight at 4°C, cryoprotected in 30% sucrose for 6 hours and embedded in Tissue-Tek O.C.T. compound. Twenty-five micrometer thick sections were cut using a cryostat (Leica, CM3050S), permeabilized with 0.3% Triton-X, blocked in 5% goat serum + 0.3% Triton-X, and stained for one of the antibodies listed below, prepared in PBS containing 5% goat serum + 0.3% Triton-X. We used K14 (1:200 dilution; Covance # PRB155P) antibody marking for keratinocytes; TUNEL TMR (In situ cell death detection kit—TMR red, Roche # 012156792910) for detecting apoptotic cells; Pgp9.5 (1:200 dilution; Dako # Z5116) marking for nerve fibers. DAPI (1 µg/mL, Invitrogen # D1306) was used to stain nuclei. Slides were mounted with Prolong gold antifade (Invitrogen # P36930), imaged with a Leica SP5 confocal microscope and analyzed with ImageJ. To quantify keratinocytes apoptosis, TUNEL+ K14+ double positive cells were counted in the epidermal basal layer expressed as percentage. For Pgp9.5+ fibers quantitation, the region of dermis/epidermis junction was analyzed and only the intraepidermal free nerve endings per section were considered for counting.38
3.7. Knee histology
After intra-articular injection with saline or MIA, knees were dissected out after 7 and 28 days. The surrounding tissues were trimmed, and knees were postfixed overnight and then placed into a decalcifying solution for 72 hours. Decalcified knees were then washed and embedded in paraffin wax. Eight micrometer thick sections were cut and stained for hematoxylin and eosin and for trichrome staining.
3.8. Behavioral testing
All behavior experiments were performed on adult male mice (8-10 weeks old). Mice were placed on an elevated platform with a mesh floor and habituated for minimum 30 minutes, unless otherwise specified.
For the von Frey test, the plantar side of the hind paw was stimulated with calibrated von Frey filaments (North Coast medical, #NC12775-99). The 50% paw withdrawal threshold was calculated using the Up–Down method.14 After MIA injection, von Frey testing was confined to the posterior-heel region of the hind paw; post-SNI, von Frey testing was confined to the sural nerve innervating region of the paw.
To measure dynamic allodynia, the plantar hind paw was stimulated by stroking using a paint brush (heel-to-toe direction). The responses were scored as previously described19 as 0 = no response; 1 = brief paw withdrawal; 2 = prolonged paw withdrawal; and 3 = paw flicking or licking. Test was performed 5 times, with 1-minute interval between trials.
To measure thermal nociception, mice were placed on a hotplate (Ugo Basile, #35150) preset at 52°C and video recorded. The latency to response (flicking or licking the hind paw) was measured. To avoid tissue damage, a cutoff of 30 seconds was defined.
A hotplate was also used to assess the response to prolonged noxious stimuli. The hotplate was set at 46°C and 50°C, and different cutoffs were set for each temperature: 3 minutes for 46°C, and 1 minute for 50°C, as previously described.25
Thermal nociception was also evaluated using Hargreaves test, in which a thermal heat stimulus (IR 35) was focused onto the plantar region of the hind paw. To avoid tissue damage, a cutoff of 20 seconds was set. The test was repeated 3 times, with 5-minute interval between trials.
To assess evaporative cold allodynia, the acetone drop test was performed.16 Briefly, a single drop of cold acetone was applied onto the plantar side of the hind paw of mice using a syringe without touching the paw of the mice. The responses were scored according to the following scheme: 0 = no response, 1 = paw withdrawal or a single flick, 2 = repeated flicking of the paw, and 3 = licking of the paw. The test was repeated 5 times with 1-minute interval between trials.
For the pin-prick test, the plantar side of the hind paw was gently touched using an insect pin attached to a 1 g von Frey filament, without penetrating the skin, and the yes/no responses were noted for 10 trials, with 1-minute interval between the trials.
For the clip tail test, an alligator clip covered with a rubber casing to reduce the potential tissue damage, was placed on the tail of the mouse, near the base of the tail; then, each mouse was placed on a plexiglass chamber and video recorded. A response was scored when the mice showed a response to the clip by biting, vocalization, grasping of tail, or jumping. A cutoff of 90 seconds was set, and data are plotted as the time to respond (latency) for each mouse.
3.9. Complete Freund's Adjuvant model
Complete Freund's Adjuvant (CFA, 20 µL, 2% in 1:1 saline) (Sigma-Aldrich, F5881) was injected into the hind paw of mice. Behavioral tests were performed at 24- and 48-hour after CFA injection to assess inflammation, followed by photoablation experiments.
3.10. Spared nerve injury model
Peripheral nerve injury was induced by SNI.46 Briefly, mice were anesthetized using 2.5% isoflurane. The sciatic nerve near the thigh region was exposed, and the peroneal and tibial branches were ligated and cut, leaving the sural nerve intact. Behavioral tests were performed starting from 1 week after injury, followed by photoablation experiments.
3.11. Osteoarthritis model
As previously described,45 10-µL monosodium iodoacetate (MIA, 1 mg) (Sigma-Aldrich, I2512) was injected intra-articular, through the intrapatellar ligament, into the knee. Sterile saline was injected in the control mice. Behavioral testing was performed 1 week after the injection.
3.12. In vivo photoablation
The hind paw, the knee, or the tail of the mice was treated for 3 consecutive days with NGFR121W-SNAP (5 µM) coupled to BG-IR700 (15 µM) (20 μL and 10 μL for paw and knee injections, respectively, and 20 μL for microemulsion application). Depending on the experiment, the coupling reaction was delivered by injection, or topically using a microemulsion, as described previously.44 Twenty minutes after delivery, NIR light at 690 nm was applied to the treated skin for 1 minute, at 120 to 150 J/cm2 (PSU-III-FDA diode laser, CNI Laser, China). Behavioral tests were performed starting from 1 day after the last photoablation.
3.13. Statistical analysis
All statistical data are presented as SEM along with the number of samples analyzed (n). Student t test and/or analysis of variance followed by the appropriate post hoc test were used. Statistical significance was assumed at P < 0.05. Sample sizes were determined from the power of the statistical test performed. No animals were excluded, and all experiments were performed blinded with order of testing randomized.
4. Discussion
Here, we describe an approach based on ligand-targeted, light-activated delivery of a phototoxic agent to ablate nociceptors in the skin and reduce pain behavior in mice. To achieve selective nociceptor ablation in vivo, we engineered the “painless” HSANV NGFR121W mutant ligand to specifically deliver a photoactivatable photosensitizer locally to TrkA expressing nociceptors. Upon illumination with NIR light, we show that there is a highly effective reversal in pain behavior in mouse models of inflammatory, neuropathic, and osteoarthritic pain.
The ligand-mediated photoablation technology we describe here has been previously validated in mice using engineered TrkB- and IL31-receptor ligands to control mechanical pain hypersensitivity and inflammatory itch.18,44 Based on extensive evidence supporting a role for TrkA signaling in pain,27,31,34–36,39,56,62 we selected NGF as a delivery agent to target cutaneous nociceptors in vivo. After local illumination of the skin, we observed retraction of fibers innervating the epidermis, corresponding to a long lasting but reversible reduction in nociceptive behavior. When the pain behavior returned 3 weeks after photoablation, we also observed a concomitant regrowth of the cutaneous sensory fibers, suggesting that we were targeting nociceptors, the major transducers of pain. Importantly, no damage to other cellular types was detected under the conditions used, despite the fact that TrkA receptors are expressed on keratinocytes and other cells in the skin.7,36,55 This is in agreement with our previous study using interleukin-31 (IL-31) as a ligand to target pruriceptors in the skin. Like NGF receptors, IL-31 receptors are highly expressed in keratinocytes; however, we found that IL-31-guided photoablation induced keratinocyte cell death only at very high concentrations, and at much higher light intensities than those used here.44 This would suggest that neurons are more sensitive to IR700-induced photoablation than other cells in the skin.
Several recent studies have described naturally occurring mutations in the NGF gene in patients affected by a rare form of congenital insensitivity to pain, HSANV. The variants pro-NGFR221W (corresponding to the mature NGFR100W) and NGFR121W have been characterized at the protein level,10,54,57 and it has been demonstrated that both retain the binding to their receptors TrkA and p75, while downstream signaling (autophosphorylation of TrkA and phosphorylation of MAPK) is impaired, preventing the nociceptive activity typically exerted by NGF. The identification of these “painless” NGF forms opens up the possibility that these mutated ligands may be exploited as drug delivery agents that bind to nociceptors but do not activate them. We explored this possibility here by fusing a SNAP-tag to NGFR121W, and using it to target the small molecule photosensitizer IR700 to nociceptors. Further development of this approach may also allow for the conjugation of other biologically active small molecules to NGFR121W to deliver them into nociceptors in vivo and directly interfere with neuronal activity.
Previous work has shown that selective antagonism of endogenous NGF is highly effective in animal models of many acute and chronic pain states.23,39,51 Among several agents developed to counteract NGF-mediated sensitization, particular attention has focused on monoclonal antibodies such as tanezumab and fasinumab, which have proven effective in the management of osteoarthritic pain.5,8 Although these anti-NGF antibodies showed greater efficacy in chronic pain compared with the nonsteroidal anti-inflammatory drugs, serious adverse effects, including osteonecrosis, neurogenic arthropathy, and morphologic changes in the sympathetic nervous system were reported,4 leading the US Food and Drug Administration (FDA) to place a hold from 2010 to 2015 on clinical studies involving this class of drugs. The reported adverse effects were similar across anti-NGF monoclonal antibodies, suggesting that they are “class-specific effects”.3 Although new evidence is generated in ongoing phase III clinical trials involving tanezumab and fasinumab in OA, the current evaluation of risk/benefit ratio of anti-NGF therapies remains challenging.5
Similar safety concerns may also be pertinent to NGF-mediated photoablation. However, there are a number of important differences in the photoablation approach compared with monoclonal anti-NGF–based strategies for treating pain. First, photoablation targets cells rather than one specific molecular mediator of pain, enabling the treatment to bypass the enormous molecular complexity that underlies pain, and to circumvent molecular redundancy, a major issue in developing new analgesic drugs. Second, the photoablation is local and can be applied on demand. Thus, dosage, light intensity, and frequency of application may constitute useful checkpoints for patient-personalized modulation. Compared with approaches based on monoclonal antibodies, which are administered systemically, this may reduce off-target effects on systems other than nociception. Third, our data indicate that a single treatment regime of photoablation is sufficient to obtain analgesia that lasts for several weeks, rather than hours or days, potentially reducing toxicity. Indeed, it may also be possible to increase the effect duration further by performing photoablation in the nerve innervating the injured area, or even to selectively ablate nociceptors permanently in the dorsal root ganglion. However, before these approaches are tested, it will be necessary to investigate whether ligand-receptor complexes are endocytosed and transported along the axon to the dorsal root ganglion cell body, and to determine whether the R121W mutation influences NGF trafficking. It will also be important to establish the safety and efficacy of repeated cycles of photoablation.
We found that photoablation using NGFR121W-SNAP was most effective at reducing responses to punctate nociceptive mechanical stimuli. Thus, we observed substantially increased von Frey thresholds under basal, inflammatory, osteoarthritic, and neuropathic states and decreased sensitivity to painful clip and pinprick. Of note, behavioral responses to dynamic mechanical stimuli were not altered by photoablation. In previous work, we have demonstrated that these dynamic stimuli evoke nociceptive reflexes through TrkB-positive mechanoreceptors after nerve injury, and that photoablation using BDNFSNAP is an effective means of reducing these responses.18 As well as demonstrating that NGFR121W-SNAP- and BDNFSNAP-mediated photoablation is selective, these data highlight the complexity of sensory processing under different conditions and indicate that solely targeting nociceptors may not be an optimal strategy in certain pain states.
Given the prominent role of NGF in thermal hyperalgesia, we expected to see a reduction in thermal nociceptive withdrawal latencies in photoablation-treated mice. For example, using a 52°C stimulus, we observed similar latencies between ablated and nonablated mice in acute tests. Similarly, photoablation failed to revert the reduction in latencies induced in a mouse model of inflammatory pain. This apparent discrepancy could be explained by the fact that residual heat-sensitive cutaneous sensory fibers, not expressing TrkA, are preserved by the photoablation and could mediate thermal nociceptive reflexes. Indeed, in a recent study, it has been shown that acute noxious heat sensing in mice depends on a triad of transient receptor potential ion channels: TRPM3, TRPV1, and TRPA1,60 and importantly, none of these channels are expressed exclusively in TrkA-positive neurons.26,58,61 Further work using ligands to target other populations of nociceptors for photoablation may uncover the role of these neurons in mediating thermal nociceptive withdrawal reflexes.
Although we saw no difference in withdrawal reflexes to heat, we did observe a significant reduction in nocifensive behavior upon prolonged thermal stimulation at 46°C and 50°C. It has recently been demonstrated that these 2 behaviors are driven by distinct inputs from the periphery and the spinal cord to the thalamus.25 Indeed, in the periphery, it was shown that nonpeptidergic MRGPRD-positive neurons underlie reflexive responses that limit injury, while TRPV1-positive nociceptors play a role in behavioral responses to prolonged stimuli and the coping response to pain.25 It is thus tempting to speculate that NGFR121W-SNAP-mediated photoablation selectively targets those neurons that input the “suffering” quality of pain, while sparing the first-line defensive neurons. Such qualities would be optimal for analgesic therapy, as the protective aspect of the PNS to noxious heat is preserved, while the perceived pain of the burn is reduced.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgements
The authors acknowledge the assistance of David Hacker, Laurence Durrer, and Soraya Quinche of the Protein Production and Structure Core Facility of the EPFL in generation of NGFSNAP and NGFR121W-SNAP. The authors also thank Violetta Paribeni and Valerio Rossi for the technical support provided with the animals. This work was funded by EMBL.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A822.
Supplemental video content
Video content associated with this article can be found online at http://links.lww.com/PAIN/A823.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
L. Nocchi and C. Portulano contributed equally to this manuscript.
References
- [1].Axelrod FB, Hilz MJ. Inherited autonomic neuropathies. Semin Neurol 2003;23:381–90. [DOI] [PubMed] [Google Scholar]
- [2].Balasubramanian S, Matasci M, Kadlecova Z, Baldi L, Hacker DL, Wurm FM. Rapid recombinant protein production from piggyBac transposon-mediated stable CHO cell pools. J Biotechnol 2015;200:61–9. [DOI] [PubMed] [Google Scholar]
- [3].Bannwarth B, Kostine M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists? Drugs 2014;74:619–26. [DOI] [PubMed] [Google Scholar]
- [4].Belanger P, Butler P, Butt M, Bhatt S, Foote S, Shelton D, Evans M, Arends R, Hurst S, Okerberg C, Cummings T, Potter D, Steidl-Nichols J, Zorbas M. From the cover: evaluation of the effects of tanezumab, a monoclonal antibody against nerve growth factor, on the sympathetic nervous system in adult cynomolgus monkeys (Macaca fascicularis): a stereologic, histomorphologic, and cardiofunctional assessment. Toxicol Sci 2017;158:319–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berenbaum F. Targeting nerve growth factor to relieve pain from osteoarthritis: what can we expect? Joint Bone Spine 2019;86:127–8. [DOI] [PubMed] [Google Scholar]
- [6].Bhangare KP, Kaye AD, Knezevic NN, Candido KD, Urman RD. An analysis of new approaches and drug formulations for treatment of chronic low back pain. Anesthesiol Clin 2017;35:341–50. [DOI] [PubMed] [Google Scholar]
- [7].Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, Raychaudhuri SK, Paus R, Pincelli C. Neurotrophins in skin biology and pathology. J Invest Dermatol 2006;126:1719–27. [DOI] [PubMed] [Google Scholar]
- [8].Bramson C, Herrmann DN, Carey W, Keller D, Brown MT, West CR, Verburg KM, Dyck PJ. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med 2015;16:1163–76. [DOI] [PubMed] [Google Scholar]
- [9].Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, Mulla SM, Lopes LC, Vogel N, Chen E, Kirmayr K, De Oliveira K, Olivieri L, Kaushal A, Chaparro LE, Oyberman I, Agarwal A, Couban R, Tsoi L, Lam T, Vandvik PO, Hsu S, Bala MM, Schandelmaier S, Scheidecker A, Ebrahim S, Ashoorion V, Rehman Y, Hong PJ, Ross S, Johnston BC, Kunz R, Sun X, Buckley N, Sessler DI, Guyatt GH. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA 2018;320:2448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Capsoni S, Covaceuszach S, Marinelli S, Ceci M, Bernardo A, Minghetti L, Ugolini G, Pavone F, Cattaneo A. Taking pain out of NGF: a “painless” NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PLoS One 2011;6:e17321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron 1992;9:779–88. [DOI] [PubMed] [Google Scholar]
- [12].Carvalho OP, Thornton GK, Hertecant J, Houlden H, Nicholas AK, Cox JJ, Rielly M, Al-Gazali L, Woods CG. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet 2011;48:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chang DS, Hsu E, Hottinger DG, Cohen SP. Anti-nerve growth factor in pain management: current evidence. J Pain Res 2016;9:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [15].Chi KR. Outlook for NGF inhibitor painkiller class brightens. Nat Biotechnol 2016;34:679–80. [DOI] [PubMed] [Google Scholar]
- [16].Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. PAIN 1994;59:369–76. [DOI] [PubMed] [Google Scholar]
- [17].Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. PAIN 2000;87:149–58. [DOI] [PubMed] [Google Scholar]
- [18].Dhandapani R, Arokiaraj CM, Taberner FJ, Pacifico P, Raja S, Nocchi L, Portulano C, Franciosa F, Maffei M, Hussain AF, de Castro Reis F, Reymond L, Perlas E, Garcovich S, Barth S, Johnsson K, Lechner SG, Heppenstall PA. Control of mechanical pain hypersensitivity in mice through ligand-targeted photoablation of TrkB-positive sensory neurons. Nat Commun 2018;9:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014;159:1417–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O'Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology 1997;48:501–5. [DOI] [PubMed] [Google Scholar]
- [21].Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet 2004;13:799–805. [DOI] [PubMed] [Google Scholar]
- [22].Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A 1976;73:2424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, Davies AM. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci 2006;27:85–91. [DOI] [PubMed] [Google Scholar]
- [24].Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015;23(suppl 1):S18–21. [DOI] [PubMed] [Google Scholar]
- [25].Huang T, Lin SH, Malewicz NM, Zhang Y, Zhang Y, Goulding M, LaMotte RH, Ma Q. Identifying the pathways required for coping behaviours associated with sustained pain. Nature 2019;565:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ikeda-Miyagawa Y, Kobayashi K, Yamanaka H, Okubo M, Wang S, Dai Y, Yagi H, Hirose M, Noguchi K. Peripherally increased artemin is a key regulator of TRPA1/V1 expression in primary afferent neurons. Mol Pain 2015;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 1996;13:485–8. [DOI] [PubMed] [Google Scholar]
- [28].Johnson EM, Jr, Gorin PD, Brandeis LD, Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science 1980;210:916–18. [DOI] [PubMed] [Google Scholar]
- [29].Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. [DOI] [PubMed] [Google Scholar]
- [30].Larsson E, Kuma R, Norberg A, Minde J, Holmberg M. Nerve growth factor R221W responsible for insensitivity to pain is defectively processed and accumulates as proNGF. Neurobiol Dis 2009;33:221–8. [DOI] [PubMed] [Google Scholar]
- [31].Lewin GR, Lechner SG, Smith ES. Nerve growth factor and nociception: from experimental embryology to new analgesic therapy. Handb Exp Pharmacol 2014;220:251–82. [DOI] [PubMed] [Google Scholar]
- [32].Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci 1993;16:353–9. [DOI] [PubMed] [Google Scholar]
- [33].Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci 1993;13:2136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci 1994;6:1903–12. [DOI] [PubMed] [Google Scholar]
- [35].Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 1989;337:362–4. [DOI] [PubMed] [Google Scholar]
- [36].Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011;115:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Matasci M, Baldi L, Hacker DL, Wurm FM. The PiggyBac transposon enhances the frequency of CHO stable cell line generation and yields recombinant lines with superior productivity and stability. Biotechnol Bioeng 2011;108:2141–50. [DOI] [PubMed] [Google Scholar]
- [38].McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol 1998;55:1513–20. [DOI] [PubMed] [Google Scholar]
- [39].McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci 1996;351:431–40. [DOI] [PubMed] [Google Scholar]
- [40].Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech 1999;45:252–61. [DOI] [PubMed] [Google Scholar]
- [41].Miller RE, Block JA, Malfait AM. Nerve growth factor blockade for the management of osteoarthritis pain: what can we learn from clinical trials and preclinical models? Curr Opin Rheumatol 2017;29:110–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mullard A. Drug developers reboot anti-NGF pain programmes. Nat Rev Drug Discov 2015;14:297–8. [DOI] [PubMed] [Google Scholar]
- [43].Mullard A. Painkilling anti-NGF antibodies stage phase III comeback. Nat Rev Drug Discov 2018;17:697. [DOI] [PubMed] [Google Scholar]
- [44].Nocchi L, Roy N, D'Attilia M, Dhandapani R, Maffei M, Traista A, Castaldi L, Perlas E, Chadick CH, Heppenstall PA. Interleukin-31-mediated photoablation of pruritogenic epidermal neurons reduces itch-associated behaviours in mice. Nat Biomed Eng 2019;3:114–25. [DOI] [PubMed] [Google Scholar]
- [45].Ogbonna AC, Clark AK, Malcangio M. Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice. Age (Dordr) 2015;37:9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pertin M, Gosselin RD, Decosterd I. The spared nerve injury model of neuropathic pain. Methods Mol Biol 2012;851:205–12. [DOI] [PubMed] [Google Scholar]
- [47].Petty BG, Cornblath DR, Adornato BT, Chaudhry V, Flexner C, Wachsman M, Sinicropi D, Burton LE, Peroutka SJ. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol 1994;36:244–6. [DOI] [PubMed] [Google Scholar]
- [48].Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 2006;29:507–38. [DOI] [PubMed] [Google Scholar]
- [49].Riediger C, Schuster T, Barlinn K, Maier S, Weitz J, Siepmann T. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Front Neurol 2017;8:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ritter AM, Lewin GR, Kremer NE, Mendell LM. Requirement for nerve growth factor in the development of myelinated nociceptors in vivo. Nature 1991;350:500–2. [DOI] [PubMed] [Google Scholar]
- [51].Ro LS, Chen ST, Tang LM, Jacobs JM. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. PAIN 1999;79:265–74. [DOI] [PubMed] [Google Scholar]
- [52].Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. PAIN 2010;148:407–13. [DOI] [PubMed] [Google Scholar]
- [53].Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage 2015;23(suppl 1):S8–17. [DOI] [PubMed] [Google Scholar]
- [54].Shaikh SS, Nahorski MS, Woods CG. A third HSAN5 mutation disrupts the nerve growth factor furin cleavage site. Mol Pain 2018;14:1744806918809223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol 1996;148:1807–18. [PMC free article] [PubMed] [Google Scholar]
- [56].Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 1994;368:246–9. [DOI] [PubMed] [Google Scholar]
- [57].Sung K, Ferrari LF, Yang W, Chung C, Zhao X, Gu Y, Lin S, Zhang K, Cui B, Pearn ML, Maloney MT, Mobley WC, Levine JD, Wu C. Swedish nerve growth factor mutation (NGF(R100W)) defines a role for TrkA and p75(NTR) in nociception. J Neurosci 2018;38:3394–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015;18:145–53. [DOI] [PubMed] [Google Scholar]
- [59].Vadivelu N, Gowda AM, Urman RD, Jolly S, Kodumudi V, Maria M, Taylor R, Jr, Pergolizzi JV., Jr Ketorolac tromethamine—routes and clinical implications. Pain Pract 2015;15:175–93. [DOI] [PubMed] [Google Scholar]
- [60].Vandewauw I, De Clercq K, Mulier M, Held K, Pinto S, Van Ranst N, Segal A, Voet T, Vennekens R, Zimmermann K, Vriens J, Voets T. A TRP channel trio mediates acute noxious heat sensing. Nature 2018;555:662–6. [DOI] [PubMed] [Google Scholar]
- [61].Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011;70:482–94. [DOI] [PubMed] [Google Scholar]
- [62].Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994;62:327–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.