Abstract
Background
Co-evolution of host and aeromonads has diversified their spectrums of diseases and antibiograms, while a paucity of data was concerning about this diversity in China. To fill this gap, this study was aimed to investigate and compare antimicrobial resistance (AMR) patterns of clinically important Aeromonas spp. from various clinical sources.
Methods
A multicenter retrospective surveillance study was conducted in Chongqing from 2011 to 2017. Data of strains were retrieved from the database of China Antimicrobial Resistance Surveillance System (CARSS). Whonet 5.6 and Graphpad Prism 6 Software were adopted to determine and compare distribution and AMR patterns.
Results
Among 1135 Aeromonas strains, Aeromonas hydrophila complex (65.6%, 745/1135) was the most predominant species, followed by Aeromonas veronii complex (16.7%, 190/1135) and Aeromonas caviae complex (15.3%, 174/1135). Sputum was the most frequent source of strains (27.7%), followed by wound (20.8%), bloodstream (10.8%) and urine (8.8%). Urinary strains demonstrated the highest resistance rates to ceftriaxone (65.6%), ceftazidime (52.1%), cefepime (38.3%), ciprofloxacin (47.7%) and trimethoprim-sulfamethoxazole (56.6%). Similar AMR pattern was observed in intestinal strains, with corresponding resistance rates of 29.4%, 28.9%, 22.2%, 27.3% and 45%, respectively. However, respiratory, bloodstream and skin strains exhibited resistance rates of less than 20% to most of the antimicrobials tested. In terms of species, approximately 30% of Aeromonas hydrophila complex and Aeromonas caviae complex strains were resistant to ceftriaxone and trimethoprim-sulfamethoxazole, while Aeromonas veronii complex strains harbored resistance rates of less than 20% to all tested antimicrobials. Although antibiograms of these species were distinct, they remained constant from 2011 to 2017.
Conclusions
Distinct AMR patterns between species and sources highlighted the predominance of Aeromonas hydrophila complex and high resistance of strains in urine and intestine to extended-spectrum cephalosporins, ciprofloxacin and trimethoprim-sulfamethoxazole in Southwest China. Temporally constant AMR patterns should not relax the vigilance of antimicrobial resistance in clinically important Aeromonas species.
Keywords: Aeromonas, human, antimicrobial resistance, distribution, time trend
Introduction
The genus of Aeromonas spp., an emerging cluster of opportunistic pathogens, is frequently implicated in a number of human intestinal and extra-intestinal infections.1–5 Since a majority of intestinal infections caused by Aeromonas spp. are self-limiting, Clinical and Laboratory Standard Institution (CLSI) recommends that antimicrobial susceptibility testing is usually applicable to extra-intestinal isolates. 3rd and 4th generation cephalosporins, fluoroquinolones and trimethoprim-sulfamethoxazole are recommended for primary testing.6 However, the abuse of antimicrobial agents, the expression and transmission of mobile resistant elements accelerate the evolution of these opportunistic pathogens in antimicrobial resistance.7,8
In contrast to previous studies of Aeromonas infections in other regions,4,9–11 Asia harbored a relatively high proportion of resistant isolates to ciprofloxacin and ceftriaxone. Southern India found a high resistance rate of 31.0% to ceftriaxone in intestinal strains.12 Korea and Southern Taiwan reported resistance rates of 5%−15% to ciprofloxacin and ceftriaxone in bloodstream strains,13,14 while a very recent study from Northern China further illustrated that resistance rates of extra-intestinal isolates to ciprofloxacin and ceftriaxone were 35.3% and 70.6%,15 respectively. However, a dearth of data was regarding this problem in Southwest China. Moreover, aforementioned studies failed to correlate AMR patterns with possible sources of infections and the heterogeneity of AMR profiling of Aeromonas spp. in distinct infections was unknown.
More importantly, since the interpretive criteria for cefepime, imipenem and meropenem to Aeromonas had been refined by CLSI M45-A3 in 20156 and the epidemiology and AMR patterns of clinically important Aeromonas isolates varies greatly over time by region; therefore, it is necessary to revisit their antibiograms to present concurrent microbial evidence for clinical decision-making and to reflect on the local situation, compared to international data.16,17 However, data concerning about the alteration of antibiogram in clinical Aeromonas species are lacking in mainland China.
To fill this gap, we launched a seven-year retrospective multicenter study from 2011 to 2017 to elucidate the distributions and antibiogram of clinically important Aeromonas species in Chongqing, Southwest China. AMR patterns were further compared by sources and species. Temporal alterations of AMR profiling were then analyzed between the two periods of 2011–2014 and 2015–2017.
Methods
Study Design And Data Enrollment Criteria
This retrospective study was carried out in the first affiliated hospital of Chongqing Medical University from 2011 to 2017, which was a branch of China Antimicrobial Resistance Surveillance System (CARSS) in Southwest China. All data were collected from the database of CARSS. Only the first isolate characterized with all the following information (patients’ age, unique patient identification number, specimen type and antibiotic susceptibility with minimal inhibitory concentration (MIC) values) was included and a given Aeromonas species with a total number of less than 30 was exempt from AMR pattern analysis according to the recommendation of CLSI M39-A4.18
Bacteria Identification And Antimicrobial Susceptibility
All participated laboratories conformed to standard procedures to perform identification and antimicrobial susceptibility testing by semi or automated microbial system. MICs were interpreted by CLSI M45-A3.6
Definition
Clinically important Aeromonas species included Aeromonas hydrophila complex (A. hydrophila complex), Aeromonas veronii complex (A. veronii complex) and Aeromonas caviae complex (A. caviae complex) on the recommendation of CLSI M45-A3.6
Aeromonas veronii complex was a cluster of pathogens including Aeromonas veronii, Aeromonas sobria, Aeromonas veronii biovar sobria and Aeromonas veronii biovar veronii.19
Strains isolated from stool specimens were defined as intestinal strains, while those isolated from other specimens were defined as extra-intestinal strains.
Statistical Analysis
Raw data were processed by Whonet 5.6 software and then statistically analyzed on Graphpad prism 6 software. Chi-square test or Fisher’s exact test was adopted to examine distribution and changes in AMR patterns. Statistical significance was determined if a two-tailed p value was no more than 0.05.
Results
The Distribution Of Clinical Aeromonas Isolates
During this seven-year study period, a total of 1461 strains was isolated and 1135 strains were included according to the inclusion criteria. A. hydrophila complex (65.6%, 745/1135) was the most predominant species, followed by A. veronii complex (16.7%, 190/1135) and A. caviae complex (15.3%, 174/1135). Interestingly, 21 strains of Aeromonas salmonicida (A. salmonicida) and 5 strains of Aeromonas schubertii (A. schubertii) were firstly reported in our branch (Table 1). The most common specimen source of species was sputum (27.7%, 314/1135), followed by wound (20.8%, 236/1135), bloodstream (10.8%, 123/1135) and urine (8.8%, 100/1135). Intestinal samples contribute to 4.3% (49/1135) of all strains. Significant different distribution of species was observed between nine distinct sources (p = 0.0002).
Table 1.
The Sources And Species Distribution Of Clinical Aeromonas Strains
| Sources | Aeromonas Hydrophila | Aeromonas Veronii | Aeromonas Caviae | Aeromonas Salmonicida | Aeromonas Schubertii | Total |
|---|---|---|---|---|---|---|
| (745)* | (190) | (174) | (21) | (5) | (1135) | |
| Sputum | 185 (24.8%)# | 31 (16.3%) | 79 (45.4%) | 14 (66.7%) | 5 (100%) | 314 (27.7%) |
| Urine | 61 (8.2%) | 14 (7.4%) | 24 (13.8%) | 1 (4.8%) | 100 (8.8%) | |
| Wound secretion | 181 (24.3%) | 28 (14.7%) | 26 (14.9%) | 1 (4.8%) | 236 (20.8%) | |
| Blood | 70 (9.4%) | 40 (21.1%) | 13 (7.5%) | 123 (10.8%) | ||
| Bile | 51 (6.8%) | 11 (5.8%) | 10 (5.7%) | 72 (6.3%) | ||
| Stool | 33 (4.4%) | 12 (6.3%) | 4 (2.3%) | 49 (4.3%) | ||
| Abscess | 61 (8.2%) | 19 (10%) | 6 (3.4%) | 3 (14.3%) | 89 (7.8%) | |
| Ascites | 11 (1.5%) | 13 (6.8%) | 2 (1.1%) | 26 (2.3%) |
Abbreviations: (X)*, X is the total number of strains; Y(Z)#, Z is the proportion of Y in the total strains (Z=Y/X×100%).
The Antibiogram Of Clinical Aeromonas Isolates By Specimen Sources
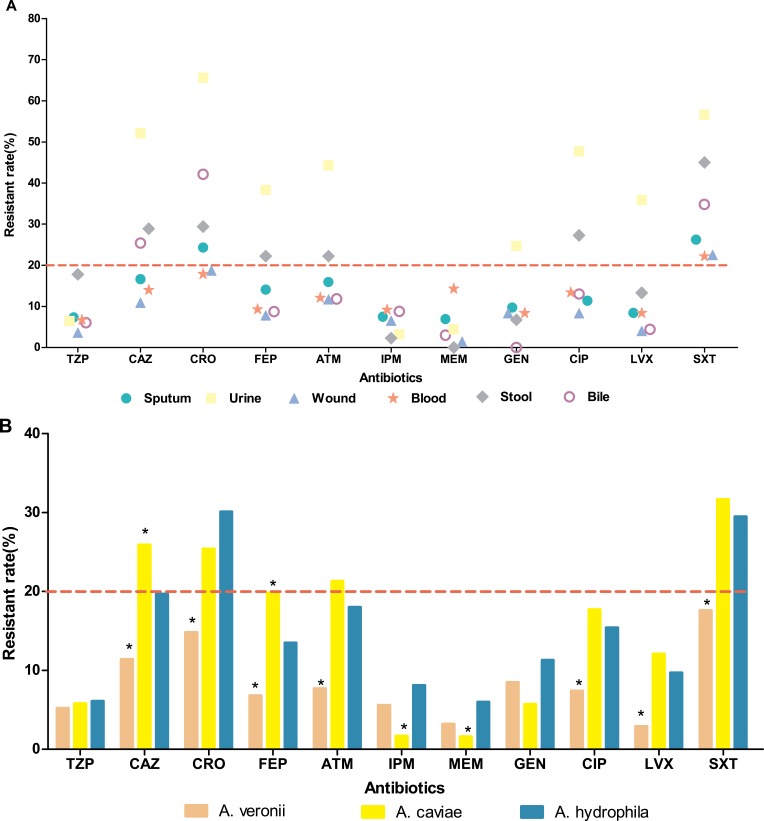
AMR patterns of clinical Aeromonas isolates from the top six specimen sources were illustrated in Figure 1A. Briefly, all isolates demonstrated high resistance to trimethoprim-sulfamethoxazole, with resistance rates ranged from 22.2% to 56.6%, while those to piperacillin/tazobactam, imipenem and meropenem were less than 10%. Interestingly, in comparison to intestinal isolates, urinary isolates exhibited significantly high resistance rates to ceftriaxone (65.6%, X2 = 11.45, df = 1, p = 0.001), ceftazidime (52.1%, X2 = 6.651, df = 1, p = 0.001), aztreonam (44.3%, X2 = 6.247, df = 1, p = 0.012), ciprofloxacin (47.7%, X2 = 5.017, df = 1, p = 0.025), levofloxacin (35.9%, X2 = 7.537, df = 1, p = 0.006), gentamicin (24.7%, X2 = 6.299, df = 1, p = 0.012) and trimethoprim-sulfamethoxazole (56.6%, X2 = 4.409, df = 1, p = 0.035), respectively.
Figure 1.
AMR patterns of clinically important Aeromonas isolates by sources and species. (A) AMR profiling of clinical Aeromonas isolates from top six specimen sources. (B) AMR profiling of three clinically important Aeromonas species. A. hydrophila: Aeromonas hydrophila complex; A. veronii: Aeromonas veronii complex; A. caviae: Aeromonas caviae complex. Asterisks indicate statistical significance as p values were less than 0.05.
Abbreviations: TZP, piperacillin/tazobactam; CAZ, ceftazidime; CRO, ceftriaxone; FEP, cefepime; ATM, aztreonam; IMP, imipenem; MEM, meropenem; GEN, gentamicin; CIP, ciprofloxacin; LEV, levofloxacin; SXT, trimethoprim-sulfamethoxazole.
As observed in urinary strains, intestinal strains found their resistance rates to most of the studied antimicrobials exceeded 20%, except to imipenem, gentamicin and levofloxacin (6.7%, 2.3% and 13.3%, respectively). None of them was resistant to meropenem. Of note, biliary strains presented different AMR profiling and showed high resistance rates particularly to ceftriaxone (42.1%) and ceftazidime (25.4%). It seemed that biliary strains were more resistant to ceftriaxone than intestinal strains, while the difference was not statistically significant (X2 = 1.47, df = 1, p = 0.225). Unlike those from the aforementioned sources, respiratory, bloodstream and skin strains shared similar AMR patterns and illustrated resistance rates of less than 20% to all tested antimicrobials but trimethoprim-sulfamethoxazole. Notably, bloodstream contributed to most of meropenem-resistant strains with a percentage of 14.3% in comparison to other sources (X2 = 17.05, df = 5, p = 0.0009).
The Antibiogram Of Clinical Aeromonas Isolates By Species
In terms of A. hydrophila complex, 30.2% of them was resistant to ceftriaxone. Resistance rates to ceftazidime, cefepime, aztreonam and ciprofloxacin were near to 20%, while those to piperacillin/tazobactam, imipenem, and meropenem were less than 10%. In contrast to A. hydrophila complex, A. caviae complex exhibited notably high resistance rate to ceftazidime (25.9%) and cefepime (19.0%), but low resistance to imipenem (1.8%) and meropenem (1.6%). However, A. veronii complex witnessed a resistance rate of less than 10% to a majority of tested antibiotics and exhibited significantly low resistance rates to ceftriaxone, ceftazidime, cefepime, aztreonam and ciprofloxacin in comparison to A. hydrophila complex (Figure 1B).
Trends Of Antimicrobial Resistance In Extra-Intestinal Isolates From 2012 to 2017
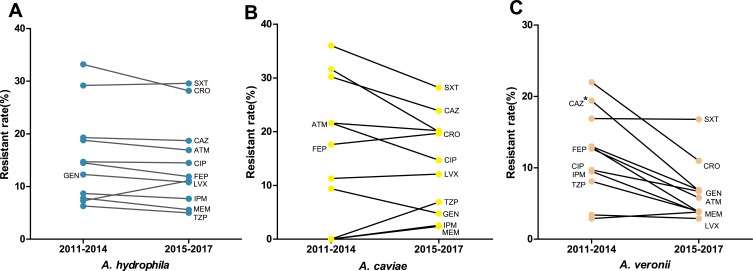
Since AMR patterns of these three predominant species were distinct, we further analyzed changes of their antibiogram after the implementation of CLSI-M45-A3 (2015). Between 2011–2014 and 2015–2017, resistance rates of A. hydrophila complex to all the tested antibiotics remained stable and similar tendency was found in A. caviae complex (Figure 2A and B). Interestingly, A. veronii complex showed a decreasing trend of resistance to a majority of these tested antibiotics and a significant declination to ceftazidime from 19.4% to 6.8% during the same timeframe (X2 = 6.02, df = 1, p = 0.01, Figure 2C).
Figure 2.
Shifting trend in antibiogram of clinically important Aeromonas species from 2011–2014 to 2015–2017. (A) A. hydrophila: Aeromonas hydrophila complex; (B) A. caviae: Aeromonas caviae complex; (C) A. veronii: Aeromonas veronii complex. Asterisks indicate statistical significance as pvalues were less than 0.05.
Abbreviations: TZP, piperacillin/tazobactam; CAZ, ceftazidime; CRO, ceftriaxone; FEP, cefepime; ATM, aztreonam; IMP, imipenem; MEM, meropenem; GEN, gentamicin; CIP, ciprofloxacin; LEV, levofloxacin; SXT, trimethoprim-sulfamethoxazole.
Discussion
This study presented the current available evidence of distributions and AMR patterns of clinical Aeromonas species and illustrated the heterogeneity and evolution of these species in Southwest China. Although sputum was the most common source of clinical Aeromonas species, it is arbitrary to deduce that respiratory tract infection dominates clinical Aeromonas infections during this seven-year investigation period. A nationwide study from France reported that respiratory tract infection contributed to only 6% of the Aeromonas infections.20 Furthermore, clinical study in Taiwan only found 8 pneumonia (9.4%) out of 85 patients with Aeromonas species isolated from respiratory tracts.21 Added that pneumonia tended to be related to aspiration of vomitus in patients with Aeromonas colonizing their gut,22 it is speculated that our high isolating rate may be overestimated by the potential transient colonization of Aeromonas species in patient’s respiratory tract and cross-infections during hospitalization.
Of interest, in this present study, high resistance of urinary strains to primary testing agents (3rd and 4th generation cephalosporins, fluoroquinolones and trimethoprim-sulfamethoxazole recommended by CLSI) was previously unreported in China. This exclusive AMR phenotype resembled that of multidrug-resistant Escherichia coli strains, which we have previously verified co-carried plasmid-encoded extended-spectrum beta-lactamases (ESBLs) genes, aminoglycosides resistance determinants (ARDs) and fluoroquinolones resistance determinants (QRDs).23 Further findings from South India have proved that the blaCTX-M gene originated from ceftriaxone-resistant Aeromonas species were transmissible to recipient (Escherichia coli J53 strain) and resulted in the emergence of ceftriaxone resistant phenotypes.12 Moreover, a recent surveillance study focusing on antibiotic sales of 468 China’s tertiary hospitals from 28 provinces illustrated the preference consumption of cephalosporins and fluoroquinolones.24 More importantly, the consumption of 3rd and 4th generation cephalosporins, as well as fluoroquinolones in China during 2011 and 2015 was higher per capita consumption percentage than that in at least 75% of the 29 European countries.24 This high consumption may accelerate the transmission of mobile resistant elements. Accordingly, it is deduced the high antibiotic selective pressure and the potential transmission of plasmid-encoded ESBLs genes, aminoglycosides and fluoroquinolones resistance determinants between species may contribute to our AMR phenotype.
To date, data of Aeromonas bacteremia in mainland China are lacking, while this present study illustrated A. hydrophila complex was predominant in Aeromonas bacteremia. This is consistent with the results of studies in Korean peninsula and Ethiopia13,25 but contrasts with a 16-year retrospective study in Japan with the predominance of A. caviae.26 Since most of Aeromonas bacteremia were secondary to hepatobiliary tract infections and peritonitis and our present data showed A. hydrophila complex was predominant in bile and ascites, it is supposed that the heterogeneity of primary infections and immune state of patients should be blamed for this discrepancy in Aeromonas bacteremia. Consistent with the aforementioned studies, less than 20% of the bloodstream isolates were resistant to any of the antibiotics tested. However, it was noteworthy that carbapenem resistance presented in 9.6% to 14.3% of the bloodstream isolates. Although Metallo-beta-lactamase CphA has been well recognized to confer carbapenem resistance of Aeromonas, recent studies have alarmed that plasmid-borne blaKPC-2, blaOXA-181, blaVIM-1 and blaVIM-35 genes contribute to carbapenem resistance of clinical Aeromonas species worldwide.27–30 Therefore, the evolution and transmission of carbapenemase genes in clinical Aeromonas species deserved more attention in our branch.
More importantly, our intestinal isolates demonstrated the highest resistance rates of approximately 30% to ceftriaxone, ceftazidime, ciprofloxacin and trimethoprim-sulfamethoxazole in mainland China, hinting the potential clinical empirical therapeutic failure of Aeromonas associated diarrhea and necessitates primary antimicrobial susceptibility testing of other antibiotics among intestinal isolates in our branch. Moreover, in consistent with the results in Tehran31 but on the contrary to two recent studies in mainland China,15,32 intestinal isolates with high resistance to ciprofloxacin was predominant and may be ascribed to the transmission of plasmid-mediated quinolone resistance genes, since qnrS2 gene was found ubiquitous in aquatic environments near Chinese hospitals and Aeromonas spp. might serve as vectors for qnrS2 with the help of IncQ-type plasmids.33 Luckily, gentamicin and carbapenems were still effective to fight against intestinal isolates, less than 7% were resistant to them. Moreover, since resistance rates of isolates from bloodstream, bile and wound were less than 10%, empirical adoption of piperacillin/tazobactam, cefepime, imipenem, gentamicin and levofloxacin may be still effective to fight against these Aeromonas infections. In contrast, only piperacillin/tazobactam, imipenem and meropenem may be still active to fight against urinary infection by Aeromonas spp, less than 7% were resistant to them.
Interestingly, 21 strains of A. salmonicida were firstly reported in our branch. To the best of our knowledge, no systemic reports were regarding the isolation of A. salmonicida from clinical samples in China. Previous case reports have linked A. salmonicida to postoperative endophthalmitis, bacteremia and diarrhea.32,34,35 Instead, this present study uncovered that A. salmonicida was frequently isolated from sputum, hinting it as a potential causative pathogen to nosocomial pneumonia. Moreover, Vincent AT et al have recently confirmed the pathogenicity and virulence of clinical A. salmonicida isolates in a mouse model and suggested the inclusion of A. salmonicida in clinical diagnosis.36
Distinct AMR patterns were found between these three predominant species. A. caviae complex harbored higher resistance to ceftazidime and cefepime than A. hydrophila complex. Moreover, the phenotype of high resistance to 3rd or 4th generation cephalosporins but low resistance to carbapenems suggested the presence of preponderant mobile resistance elements in A. caviae complex. Case report of A. caviae pneumonia in China firstly uncovered the co-carrier of CTX-M-3, TEM-1 and a new plasmid-mediated MOX-4 AmpC-encoding gene conferred resistance to third or fourth generation cephalosporins but not to carbapenems.37 It is deduced that the emergence and transmission of novel ESBLs and AmpC genes may be blamed for this phenotype. Despite that Study for Monitoring Antimicrobial Resistance Trends (SMART) in the Asia-Pacific region demonstrated the increasing resistance rate of intro-abdominal Aeromonas isolates to ciprofloxacin from 2003 to 2010,38 we did not find statistically significant alteration in the antibiogram of these three predominant species during the timeframe of 2011–2014 and 2015–2017, thus the impact of the revision of breakpoints on antibiogram was insignificant in our setting.
Several limitations should not be neglected in this present study. First, this study was not aimed to study the taxonomy of Aeromonas spp., so the verification of taxonomic affiliation was not fulfilled. Definitely, A. dhakensis, an increasingly recognized human pathogen, previously under the umbrella of A. hydrophila,2 has been isolated from clinical samples in China; however, a total of four clinical isolates underdetermined clinical significance of this species in domestic hospitals.15 Second, due to the inaccessibility of clinical data by CARSS, this study failed to discuss the association of patients’ clinical characteristics with Aeromonas infections, while previous studies have verified that the most common medical conditions among patients with Aeromonas infections were malignancy and liver-transplant related cholecystitis.15 Third, since the strains were not collected from the participated laboratories, this study failed to investigate virulence and resistance mechanisms of Aeromonas isolates.
Conclusions
Distinct distribution and AMR patterns of clinical Aeromonas species in Southwest China highlighted the predominance of A. hydrophila complex and high resistance rates of urine and intestinal isolates against 3rd and 4th generation cephalosporins and ciprofloxacin. Piperacillin/tazobactam and carbapenems are active against these urinary isolates. Routine-use antibiotics may be efficient to fight against Aeromonas bacteremia, while high resistance rate to meropenem may hinder its clinical efficacies. Temporally constant AMR patterns should not unbrace antimicrobial stewardships of Aeromonas infections. The potential role of Aeromonas spp. in nosocomial pneumonia deserves more researches and additional studies are needed.
Acknowledgments
The authors would like to thank all enrolled laboratories for their participation in our branch.
Funding Statement
This work was supported by Chongqing Science and Technology Commission Grant (cstc2016jcyjA0248).
Abbreviations
AMR, antimicrobial resistance; CARSS, China antimicrobial resistance surveillance system; CLSI, Clinical and Laboratory Standards Institute; TZP, piperacillin/tazobactam; CAZ, ceftazidime; CRO, ceftriaxone; FEP, cefepime; ATM, aztreonam; IMP, imipenem; MEM, meropenem; GEN, gentamicin; CIP, ciprofloxacin; LEV, levofloxacin; SXT, trimethoprim-sulfamethoxazole.
Availability Of Data And Materials
All the dataset of this article is available from the corresponding author if reasonably requested.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23(1):35–73. doi: 10.1128/CMR.00039-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen PL, Lamy B, Ko WC. Aeromonas dhakensis, an Increasingly Recognized Human Pathogen. Front Microbiol. 2016;7:793. doi: 10.3389/fmicb.2016.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batra P, Mathur P, Misra MC. Aeromonas spp. an emerging nosocomial pathogen. J Lab Physicians. 2016;8(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghenghesh KS, Rahouma A, Zorgani A, Tawil K, Al Tomi A, Franka E. Aeromonas in Arab countries: 1995–2014. Comp Immunol Microbiol Infect Dis. 2015;42:8–14. doi: 10.1016/j.cimid.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 5.Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. Sci World J. 2012;2012:625023. doi: 10.1100/2012/625023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(CLSI), C.L.S.I. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated of fastidious bacteria. CLSI document M45-A3. Wayne: CLSI; 2015. [Google Scholar]
- 7.Didelot X, Walker AS, Peto TE, Crook DW, Wilson DJ. Within-host evolution of bacterial pathogens. Nat Rev Microbiol. 2016;14(3):150–162. doi: 10.1038/nrmicro.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spengler G, Kincses A, Gajdács M, Amaral L. New roads leading to old destinations: efflux pumps as targets to reverse multidrug resistance in bacteria. Molecules. 2017;22(3):468. doi: 10.3390/molecules22030468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castelo-Branco Dde S, Guedes GM, Brilhante RS, et al. Virulence and antimicrobial susceptibility of clinical and environmental strains of Aeromonas spp. from northeastern Brazil. Can J Microbiol. 2015;61(8):597–601. doi: 10.1139/cjm-2015-0107 [DOI] [PubMed] [Google Scholar]
- 10.McAuliffe GN, Hennessy J, Baird RW. Relative frequency, characteristics, and antimicrobial susceptibility patterns of Vibrio spp., Aeromonas spp., Chromobacterium violaceum, and Shewanella spp. in the northern territory of Australia, 2000–2013. Am J Trop Med Hyg. 2015;92(3):605–610. doi: 10.4269/ajtmh.14-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bargui H, Marzouk M, Benhadj A, Hadj Ali M, Ben Salem Y, Boukadida J. Aeromonas spp. human infection: retrospective study in the region of sousse, 2011–2015. Tunis Med. 2017;5(4):257–261. [PubMed] [Google Scholar]
- 12.Bhaskar M, Dinoop KP, Mandal J. Characterization of ceftriaxone-resistant Aeromonas spp. isolates from stool samples of both children and adults in Southern India. J Health Popul Nutr. 2015;33:26. doi: 10.1186/s41043-015-0036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee JY, Jung DS, Peck KR. Clinical and therapeutic implications of Aeromonas Bacteremia: 14 years nation-wide experiences in Korea. Infect Chemother. 2016;48(4):274–284. doi: 10.3947/ic.2016.48.4.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang HJ, Lai CC, Lin HL, Chao CM. Clinical manifestations of bacteremia caused by Aeromonas species in southern Taiwan. PLoS One. 2014;9(3):e91642. doi: 10.1371/journal.pone.0091642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Yu L, Nan Z, et al. Taxonomy, virulence genes and antimicrobial resistance of Aeromonas isolated from extra-intestinal and intestinal infections. BMC Infect Dis. 2019;19(1):158. doi: 10.1186/s12879-019-3766-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gajdács M, Urbán E. Resistance trends and epidemiology of citrobacter-enterobacter-serratia in urinary tractinfections of inpatients and outpatients (RECESUTI): a 10-year survey. Medicina (Kaunas). 2019;55(6):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gajdács M, Urbán E. Epidemiological trends and resistance associated with Stenotrophomonas maltophilia Bacteremia: a 10-year retrospective cohort study in a tertiary-care hospital in hungary. Diseases. 2019;7(2):41. doi: 10.3390/diseases7020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(CLSI), C.L.S.I. Analysis and presentation of cumulative antimicrobial susceptibility test data: 4th approved guideline. CLSI document M39-A4. Wayne: CLSI; 2014. [Google Scholar]
- 19.Parker JL, Shaw JG. Aeromonas spp. clinical microbiology and disease. J Infect. 2011;62(2):109–118. doi: 10.1016/j.jinf.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Lamy B, Kodjo A, Col BVH Study Group, Laurent F. Prospective nationwide study of Aeromonas infections in France. J Clin Microbiol. 2009;47(4):1234–1237. doi: 10.1128/JCM.00155-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao CM, Lai CC, Tsai HY, et al. Pneumonia caused by Aeromonas species in Taiwan, 2004–2011. Eur J Clin Microbiol Infect Dis. 2013;32(8):1069–1075. doi: 10.1007/s10096-013-1852-6 [DOI] [PubMed] [Google Scholar]
- 22.Nolla-Salas J, Codina-Calero J, Valles-Angulo S, et al. Clinical significance and outcome of Aeromonas spp. infections among 204 adult patients. Eur J Clin Microbiol Infect Dis. 2017;36(8):1393–1403. doi: 10.1007/s10096-017-2945-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Xu X, Pu S, et al. Characterization of carbapenemases, extended spectrum β-lactamases, quinolone resistance and aminoglycoside resistance determinants in carbapenem-non-susceptible Escherichia coli from a teaching hospital in Chongqing, Southwest China. Infect Genet Evol. 2014;27:271–276. doi: 10.1016/j.meegid.2014.07.031 [DOI] [PubMed] [Google Scholar]
- 24.Yang P, Chen Y, Jiang S, Shen P, Lu X, Xiao Y. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob Resist Infect Control. 2018;7:137. doi: 10.1186/s13756-018-0430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arega B, Wolde-Amanuel Y, Adane K, Belay E, Abubeker A, Asrat D. Rare bacterial isolates causing bloodstream infections in Ethiopian patients with cancer. Infect Agent Cancer. 2017;12:40. doi: 10.1186/s13027-017-0150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura M, Araoka H, Yoneyama A. Aeromonas caviae is the most frequent pathogen amongst cases of Aeromonas bacteremia in Japan. Scand J Infect Dis. 2013;45(4):304–309. doi: 10.3109/00365548.2012.737474 [DOI] [PubMed] [Google Scholar]
- 27.Adler A, Assous MV, Paikin S, et al. Emergence of VIM producing Aeromonas caviae in Israeli hospitals. J Antimicrob Chemother. 2014;69(5):1211–1214. doi: 10.1093/jac/dkt505 [DOI] [PubMed] [Google Scholar]
- 28.Anandan S, Gopi R, Devanga Ragupathi NK, et al. First report of blaOXA-181-mediated carbapenem resistance in Aer- omonas caviae in association with pKP3-A: threat for rapid dissemination. J Glob Antimicrob Resist. 2017;10:310–314. doi: 10.1016/j.jgar.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 29.Hughes HY, Conlan SP, Lau AF, et al. Detection and whole-genome sequencing of carbapenemase-producing Aeromonas hydrophila isolates from routine perirectal surveillance culture. J Clin Microbiol. 2016;54(4):1167–1170. doi: 10.1128/JCM.03229-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinclair HA, Heney C, Sidjabat HE, et al. Genotypic and phenotypic identification of Aeromonas species and CphA-mediated carbapenem resistance in Queensland, Australia. Diagn Microbiol Infect Dis. 2016;85(1):98–101. doi: 10.1016/j.diagmicrobio.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 31.Soltan Dallal MM, Mazaheri Nezhad Fard R, Kavan Talkhabi M, Aghaiyan L, Salehipour Z. Prevalence, virulence and antimicrobial resistance patterns of Aeromonas spp. isolated from children with diarrhea. GERMS. 2016;6(3):91–96. doi: 10.11599/germs.2016.1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Wang W, Zhu Z, et al. Distribution, virulence-associated genes and antimicrobial resistance of Aeromonas isolates from diarrheal patients and water, China. J Infect. 2015;70(6):600–608. doi: 10.1016/j.jinf.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 33.Wen Y, Pu X, Zheng W, Hu G. High prevalence of plasmid-mediated quinolone resistance and IncQ plasmids carrying qnrS2 gene in bacteria from rivers near hospitals and aquaculture in China. PLoS One. 2016;11(7):e0159418. doi: 10.1371/journal.pone.0159418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varshney A, Das M, Chaudhary P, Kumari R, Yadav K. Aeromonas Salmonicida as a causative agent for postoperative endophthalmitis. Middle East Afr J Ophthalmol. 2017;24(4):213–215. doi: 10.4103/meajo.MEAJO_238_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore CA, Khalid MF, Patel PD, Goldstein JS. Aeromonas Salmonicida Bacteremia associated with chronic well water consumption in a patient with diabetes. J Glob Infect Dis. 2017;9(2):82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent AT, Fernandez-Bravo A, Sanchis M, Mayayo E, Figueras MJ, Charette SJ. Investigation of the virulence and genomics of Aeromonas salmonicida strains isolated from human patients. Infect Genet Evol. 2019;68:1–9. doi: 10.1016/j.meegid.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 37.Ye Y, Xu XH, Li JB. Emergence of CTX-M-3, TEM-1 and a new plasmid-mediated MOX-4 AmpC in a multiresistant Aeromonas caviae isolate from a patient with pneumonia. J Med Microbiol. 2010;59(Pt 7):843–847. doi: 10.1099/jmm.0.016337-0 [DOI] [PubMed] [Google Scholar]
- 38.Liu YM, Chen YS, Toh HS, et al. In vitro susceptibilities of non Enterobacteriaceae isolates from patients with intra-abdominal infections in the Asia-Pacific region from 2003 to 2010: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int J Antimicrob Agents. 2012;40 Suppl:S11–S17. doi: 10.1016/S0924-8579(12)70004-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the dataset of this article is available from the corresponding author if reasonably requested.