Abstract
Emerging HIV treatment distribution models across sub-Saharan Africa seek to overcome barriers to attaining antiretroviral therapy and to strengthen adherence in people living with HIV. We describe enablers, barriers, and benefits of differentiated treatment distribution models in South Africa, Uganda, and Zimbabwe. Data collection included semistructured interviews and focus group discussions with 163 stakeholders from policy, program, and patient levels. Four types of facility-based and 3 types of community-based models were identified. Enablers included policy, leadership, and guidance; functional information systems; strong care linkages; steady drug supply; patient education; and peer support. Barriers included insufficient drug supply, stigma, discrimination, and poor care linkages. Benefits included perceived improved adherence, peer support, reduced stigma and discrimination, increased time for providers to spend with complex patients, and travel and cost savings for patients. Differentiated treatment distribution models can enhance treatment access for patients who are clinically stable.
Key words: ART, ART distribution, community ART programs, decentralization, differentiation, HIV, treatment
HIV is a chronic disease requiring lifelong access and adherence to antiretroviral therapy (ART). Individuals with access to ART can achieve a near-normal life expectancy (Mills et al., 2011). The Joint United Nations Programme on HIV/AIDS (UNAIDS, 2014) has ambitiously called for 95% of people living with HIV (PLWH) to be diagnosed, 95% of those diagnosed to be started on ART, and 95% of those on ART to be virally suppressed by 2030. The World Health Organization (2015) recommends that PLWH start treatment when diagnosed. In response to the UNAIDS and WHO targets, health systems have begun to develop differentiated treatment distribution models to increase access for the millions of individuals who will require treatment across their lifetimes.
Emerging treatment distribution models across sub-Saharan Africa intend to strengthen adherence by PLWH and streamline services at facilities to allow health providers to focus on patients with more complex clinical needs (Magadzire, Marchal, & Ward, 2015). Differentiated treatment distribution models offer multiple mechanisms through which patients can access ART, thus giving them the ability to pick a mechanism that best meets their needs. Distribution models can be classified into two broad categories: community-based and facility-based models. Facility-based models include decentralization of services from hospitals to primary care centers, which has demonstrated increased patient adherence to ART (Kredo, Ford, Fb, & Garner, 2013). Some facilities allow stable patients to retrieve prepackaged medications only, bypassing other facility services. Community-based models generally focus on distribution and are used most often by clinically stable patients already initiated on ART (Médecins sans Frontières, 2010). Drug pickup is decentralized, and distribution is led and managed by the patient and/or peer groups through a variety of innovative and context-specific approaches.
Models for facility- and community-based approaches have demonstrated favorable outcomes including reductions in workloads (Bedelu, Ford, Hilderbrand, & Reuter, 2007; Bemelmans et al., 2014; Decroo, Damme, Kegels, Remartinez, & Rasschaert, 2012; Decroo, Rasschaert, Telfer, Remartinez, Laga, & Ford, 2013; Médecins sans Frontières, 2010; Rasschaert et al., 2014), staffing needs (Barker, Dutta, & Klein, 2017), and patient costs (Bemelmans et al., 2014; Decroo et al., 2013, 2012; Fatti, Grimwood, & Bock, 2010; Médecins sans Frontières, 2010). Facility-based distribution models have demonstrated that using nonphysician providers can double enrollment (Bemelmans et al., 2010), improve retention (Bemelmans et al., 2010; Kredo et al., 2013), and reduce waiting times (Fatti et al., 2010; Massaquoi et al., 2009; Reidy et al., 2016). Reduced patient loads can also allow more time for clinically complex patients (Dudhia & Kagee, 2015), and community-based distribution models exhibit patient time savings (Decroo et al., 2013; Dudhia & Kagee, 2015; Grimsrud et al., 2016; Magadzire et al., 2015; Prust et al., 2017), reduced patient visit burdens (Mesic et al., 2017), reduced provider workloads (Bemelmans et al., 2014; Decroo et al., 2012; 2013; Médecins sans Frontières, 2010; Mesic et al., 2017; Rasschaert et al., 2014; Wouters, Van Damme, van Rensburg, Masquillier, & Meulemans, 2012), improved treatment access (Grimsrud et al., 2016), retention and adherence (Bemelmans et al., 2014; Decroo et al., 2012; Grimsrud et al., 2016; Médecins sans Frontières, 2010), increased self-efficacy (Bemelmans et al., 2014; Decroo et al., 2012), and peer support (Decroo et al., 2013; Kredo et al., 2013).
The ART scale-up targets established by UNAIDS and WHO give some direction (Grimsrud et al., 2016; Médecins sans Frontières, 2017) but do not identify factors that might facilitate or hinder achievement. We describe differentiated treatment distribution models and identify enablers, barriers, and benefits of the models by synthesizing findings from multistakeholder interviews and focus group discussions (FGDs) with participants in South Africa, Uganda, and Zimbabwe. Understanding the programmatic and systemic barriers and enablers of differentiated treatment distribution from these countries can inform scale-up efforts, which may contribute to reaching 95% viral suppression globally.
Methods
Study Design
We used descriptive research, drawing on a qualitative approach, to collect data via semistructured interviews and FGD with multilevel stakeholders. Convenience sampling was used to recruit key respondents (N = 163) who included policy makers; program managers, designers, and implementers; health service providers; and patients who were purposively selected from 34 organizations across three countries that had varying levels of experience with a range of models.
Country Selection
Country selection occurred in consultation with the U.S. Agency for International Development (USAID) and via a literature review, detailed in a publication that identified differentiated treatment distribution models in sub-Saharan Africa (Davis, Kanagat, Sharer, Eagan, Pearson, & Amanyeiwe, 2018). Prioritization was made to acknowledge countries at various stages of ART differentiation, from pilot stages (Uganda, Zimbabwe) to scale-up stage (South Africa).
Key Respondent and Site Selection
Key respondents included high-level stakeholders, such as policy makers, advocacy groups, program designers, and implementers with knowledge and experience with treatment distribution models. A literature review (Davis et al., 2018) identified key organizations in each country, which were cross-referenced with incountry researchers/coauthors and with incountry USAID representatives. The result was an initial list of a preselected (nonrandom) group of experts deemed most knowledgeable about differentiated treatment mechanisms in South Africa, Uganda, and Zimbabwe. From this initial list of key informants, snowball sampling, a nonprobability sampling technique, allowed the initial participants to recommend other subjects for subsequent interviews. Semistructured interviews with these individuals helped to identify “big picture” insights and health facilities that were implementing differentiated treatment distribution models.
Health providers (e.g., physicians, nurses, counselors) and patients from program-supported facilities were also invited to participate in interviews and FGDs to provide granular insights into their experiences with the models. Relevant health providers working with the differentiated treatment distribution models were selected using convenience sampling based on the availability on the day the site was visited. This type of convenience sampling was also used to identify patients to participate in FGDs in Uganda and Zimbabwe. Patient FGDs were excluded in South Africa due to ethical restrictions.
Data Collection and Study Locations
Three separate interview tools were developed for policy makers/high-level stakeholders, providers, programmers, and patients. Patient tools were translated into Luganda (Uganda) and Shona (Zimbabwe). Tool content was based on literature review findings and modified using feedback from the AIDSFree project and USAID staff in each country. Tools were designed to gather information about elements of implementation that facilitated or impeded success (enablers and barriers) and specific gains of the models implemented (benefits). South Africa data collection occurred between October and November 2016 in Gauteng, KwaZulu-Natal, Eastern Cape, and Western Cape Provinces. Uganda data collection took place in Kampala and surrounding areas from June to July 2017. Zimbabwe data collection took place in Harare and surrounding areas from October to November 2017.
In each country, the research team consisted of two to four individuals with qualitative research experience and training. Comprehensive notes were taken during interviews and FGDs. All sessions were audiorecorded except for two where respondents agreed to be interviewed but declined consent for audio recording. Comprehensive notes were taken throughout and after interviews and focus groups; the sessions that were audiorecorded were professionally transcribed (and back-checked to original recordings) by research associates in South Africa, Uganda, and Zimbabwe. Notes and recordings were used to create English transcripts.
Data Analysis
Transcription data were analyzed using NVivo 11 software. Axial and open coding of the transcript text allowed for deconstruction of the text and led to the emergence of common themes. A skeleton coding frame was developed by the research team using the key themes identified. Initial transcripts were coded separately by two members of the study team according to the framework and compared to ensure intercoder reliability for each country. Following completion of each country analysis, data sets were merged, and common themes and findings across the three countries were identified. Categories for treatment distribution models were developed across countries and common themes for enablers, barriers, and benefits to these models, which were further categorized by health system, health provider, and patient levels.
Ethical Considerations
Institutional review board approval was received from John Snow, Inc. for all three countries; approval was received from The AIDS Support Organization and the Uganda National Council for Science and Technology in Uganda; from the Joint Research Ethics Committee for the College of Health Sciences, Parirenyatwa Hospital, the Medical Research Council of Zimbabwe, and the Research Council of Zimbabwe in Zimbabwe; in South Africa, the evaluation was approved by the USAID South African Mission. All research was conducted in accordance with approved protocols, and written informed consent was obtained from each participant.
Results
Characteristics of Study Participants
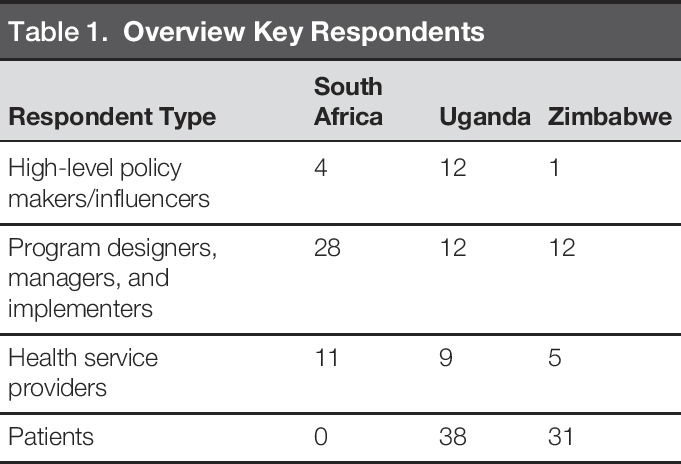
A total of 163 individuals, recruited through convenience sampling, participated in semistructured interviews or FGDs (Table 1). In South Africa, interviews and FGDs took place with 43 individuals from 13 organizations. In Uganda, interviews and FGDs were conducted with 71 individuals, 38 of whom were patients, from 16 organizations. In Zimbabwe, interviews and FGDs were conducted with 49 individuals, 31 of whom were patients, from 5 organizations. In each country, programs selected had received support from the USAID; sample size was determined by the availability of respondents.
Table 1.
Overview Key Respondents
Differentiated Treatment Distribution Models
Facility-based models
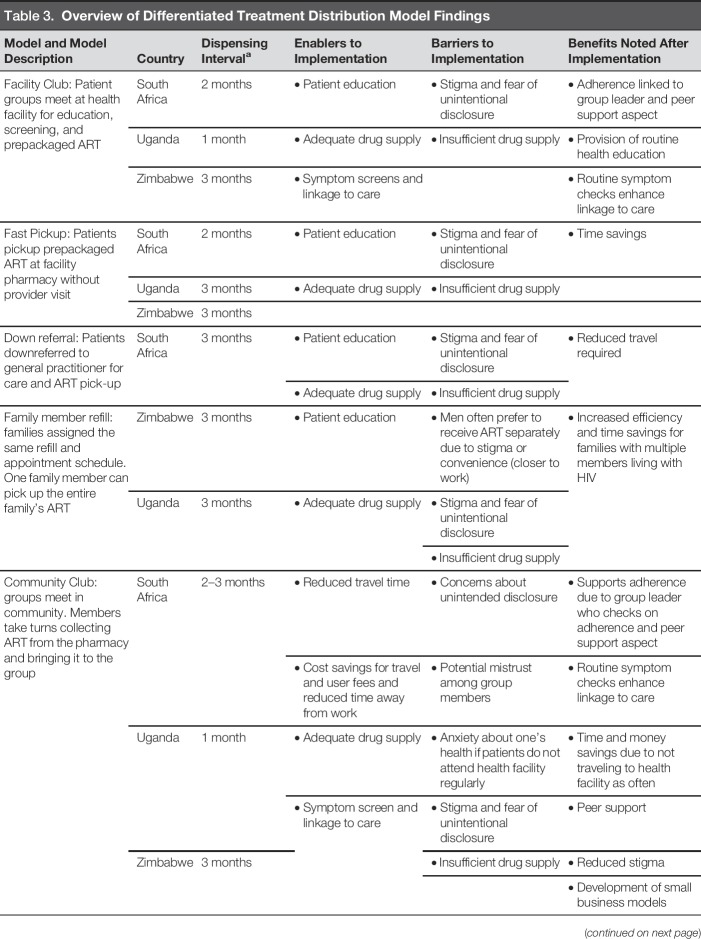
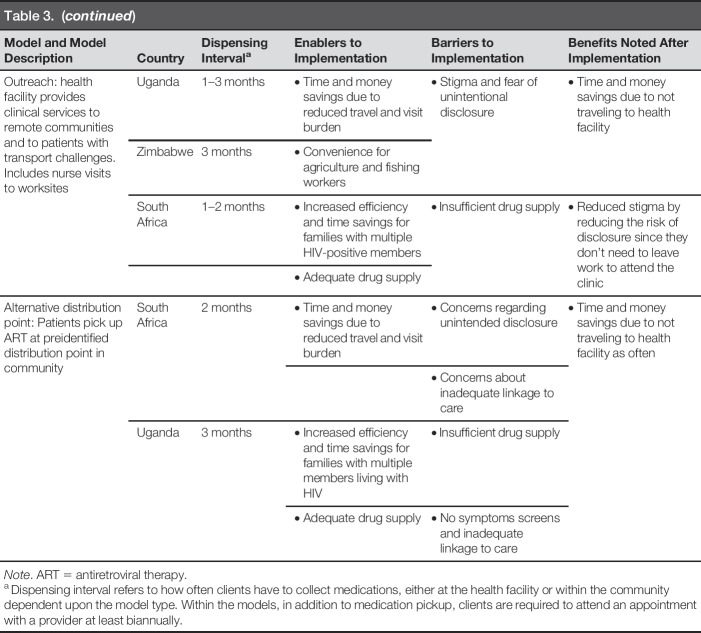
Four facility-based models were identified. The “Facility Club” model existed in all three countries and consisted of patients routinely meeting at the facility to receive health education, health screenings, adherence support, peer support, and collect ART. The amount of ART provided ranged from 1 to 3 months. In South Africa, Facility Clubs included 25–30 participants who met every 2 months. In Uganda, Facility Clubs met monthly or quarterly and had approximately six members. Occasionally, Uganda Facility Clubs were grouped by age, housing proximity, key population type, client schedules, and/or patients with similar interests. In Zimbabwe, Facility Clubs consisted of 10–20 members who met every 3 months.
The “Fast Pickup” model existed in all countries, allowing patients to go directly to the pharmacy to pick up a 2- to 3-month supply of prepackaged ART. Although patients could bypass facility queues and clinician consultation, most were required to undergo a basic screening at the pharmacy window.
The “Down Referral” model in South Africa included only stable patients from high-volume sites. They were contracted to a patient-selected private general practitioner who they visited for routine checkups and annual blood work. ART was dispensed by a central pharmacy to the general practitioner, allowing for quarterly collection by patients.
The “Family Member Refill” model in Uganda and Zimbabwe consolidated trips for families with several members living with HIV. Families were grouped together on the same refill and clinic visit schedule, so that one family member could collect the whole family's ART during their visit.
Community-based models
Three community-based models were identified. The “Community Club” model was identified in all countries and consisted of a group of PLWH who met monthly at predetermined community sites (e.g., community organization, church, mosque) to receive health screenings, provide peer support, and collect ART. Venues were selected to reduce barriers related to time and travel. In South Africa, this model was not yet widely used due to concerns regarding unintended disclosure within one's community (South Africa Department of Health, 2016). Club members met every 2 months. An outside facilitator delivered ART and other chronic care medications during the meetings. In Uganda, Community Club members rotated monthly ART collection at the facility to distribute to the entire group. The collector received a health screening, resulting in each member seeing a provider at least once every 6 months. In Zimbabwe, Community Clubs met every 3 months and Club leaders documented any signs or symptoms experienced by members and shared the information with providers during ART refill collections. Community Clubs were formed by patients and required patients to live in the same geographic areas to facilitate drug distribution.
The “Outreach” model was identified in all countries, allowing ART distribution for patients in remote areas. In Uganda, Outreach provided all facility services, including laboratory workups and ART. In Zimbabwe, HIV care and treatment, including ART distribution, were provided during Outreach. In South Africa, Outreach services were provided at agricultural worksites by nurses/counselors as an extension of a local clinic. Patients were typically able to collect 1 to 2 months of ART during a single visit through Outreach models.
The “Alternative Distribution Point” model was identified in South Africa and Uganda, where patients collected prepackaged ART from trained dispensers at community outreach points, such as churches, private facilities, and community halls. In South Africa, patients could also collect ART from private pharmacies. In both countries, patients typically received 2 to 3 months' worth of ART and were able to quickly queue, receive their ART, and leave. Health education, screenings, and peer support were not provided.
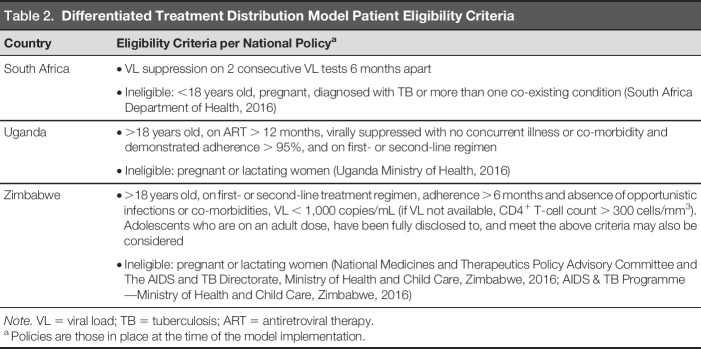
Eligibility Criteria
National policy in each country provided guidance to determine which patients were eligible for differentiated treatment distribution models. In practice, most programs followed national eligibility criteria (Table 2), although some programs were stricter (i.e., only including those who were on a first-line regimen or virally suppressed for longer than required by national policy).
Table 2.
Differentiated Treatment Distribution Model Patient Eligibility Criteria
Enablers and Barriers
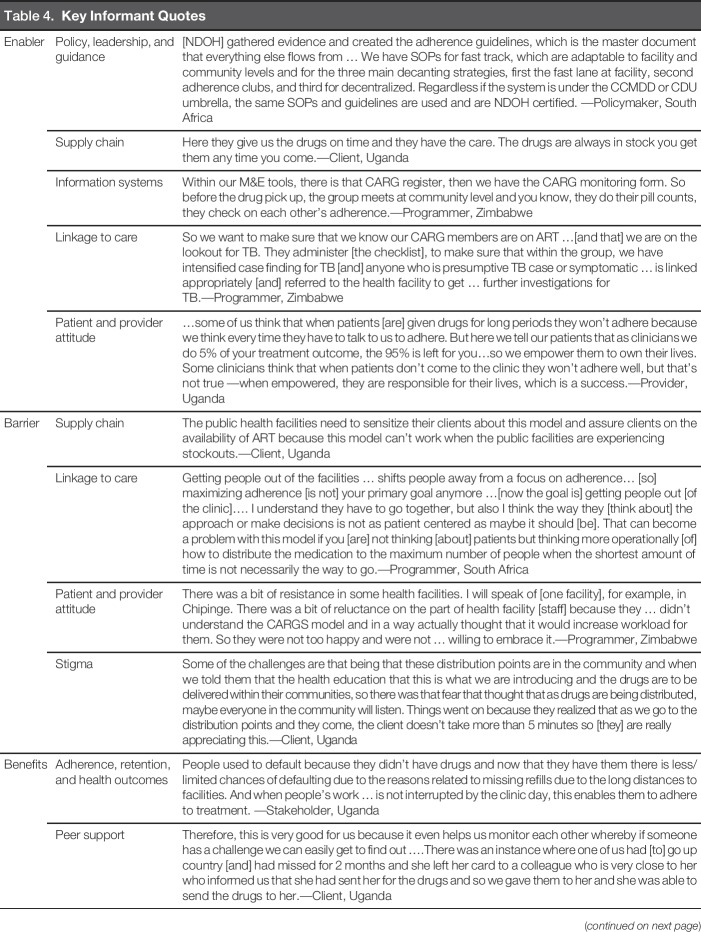
Policy, leadership, and guidance were identified as critical enablers to implementation in all study countries. Leadership from the Ministry of Health provided policy direction, program guidelines, and coordination between donors and implementing organizations, which enabled model development and implementation. Guidelines contributed to standardization and scale-up of services and provided all actors with a common language. In South Africa, training manuals and standard operating procedures facilitated transferring patients to the models and scale-up of existing mechanisms for ART distribution. In Uganda, policy and guideline development standardized practices that had been implemented by various service providers. In Zimbabwe, policies developed for the differentiated treatment distribution models placed pressure on organizations to implement them and were critical to facilitating buy-in, particularly at local levels.
Another enabler was a strong supply chain with buffer stocks, supporting ART distribution and reducing shortages; perceived or actual weakness in supply chain was a barrier. Service providers with regular drug supplies had strong reputations for the ability to dispense multimonth quantities, making patients likely to come back. In Uganda, many organizations had drug supply systems in place to maintain ART reserves. However, at times, patients reported seeking a month or multimonth refill but only receiving 2 weeks of ART. Patients preferred nongovernmental organization-supported sites or private providers who were more likely to have sufficient stock to administer a multimonth supply. In Zimbabwe, respondents described concerns about stockouts at lower-level health facilities, where staff were insufficiently trained to quantify and report stock needs. In South Africa, while stockouts were not a significant concern, some districts restricted multimonth dispensing due to concerns that a stock-out could occur.
Respondents in all countries reported that functional information systems increased efficiencies, allowing ART to be prepackaged and set aside in advance of appointments. In Uganda, where many patients accessed multiple facilities and/or needed to be transferred to other facilities, respondents noted the need for a national-level information system to track patients who had been lost to follow-up, identify eligible patients for the differentiated treatment distribution models, and adequately track ART distribution between facility- and community-based distribution models. South Africa respondents stated that the national information system was imperative to tracking patients, although not all facilities had access to this system. Zimbabwe respondents reported that many facilities and organizations had stand-alone tracking systems to monitor patients; a national-level tracking system had not yet been created.
Linkage to care emerged as a theme in all three countries. Linkage experiences were largely dependent on the type of model used. In Community Club models, respondents in all countries noted that even though patients were not always physically at the facility, they were still linked to the facility via the Club facilitator who regularly conducted symptom screenings and weight checks, and made facility referrals as needed. Respondents in South Africa and Uganda voiced concerns that patients accessing Alternative Community Distribution Points might have insufficient linkages to care because these models did not include interactions with a health provider. In Uganda, because more patients received treatment through community-based distribution programs, there was concern that the public sector might not have the capacity and/or resources to maintain linkages, which could result in delays identifying clinical complications.
Patient and provider attitudes were identified in Uganda and Zimbabwe for the potential impact on model acceptability. In facility-based models in Uganda and Zimbabwe, providers were enthusiastic about reduced health facility congestion and patient loads. However, they expressed concern about their perceived inability to provide adequate care (e.g., adherence counseling, chronic disease management) due to reduced visits. Providers also expressed concern that they could feel disconnected from their patients and could miss “silent issues” without regular appointments. Some also doubted patient abilities to adhere. These concerns were often assuaged once the models completed the pilot phase, and providers experienced reduced workloads without worsened adherence and retention. Patients in Uganda and Zimbabwe expressed appreciation that providers trusted them to adhere and check in biannually.
Stigma and fear of unintended disclosure emerged as a barrier; concerns were expressed at three points: (a) the health facility, particularly in Zimbabwe where patients were required to carry registers that could identify them as living with HIV; (b) the community, if patients were participating in a Community Club or an Alternative Distribution Point; and (c) related to ART packaging. Notably, a number of patients in Zimbabwe and Uganda did not feel comfortable collecting ART at distribution points in their own communities due to the risk of unintended disclosure. Although Community Club Models did not require disclosure, many patients perceived HIV disclosure as necessary to joining a club. Conversely, it was suggested in all three countries that Community Club Models might also contribute to normalization of HIV and reduction of stigma. In South Africa, stigma was addressed by integrating many of the model services for patients who had noncommunicable diseases (e.g., hypertension, diabetes) to reduce concerns that individuals accessing medication would be identified as PLWH.
Differentiated Treatment Distribution Model Benefits
Throughout the interviews, respondents reported that benefits of the models included high levels of adherence and retention. In all countries, patients experienced increased motivation for self-care and improved resilience as responsibility for individual health was transitioned from providers to themselves. Patients enrolled in a differentiated model were required to maintain adherence or they would no longer be permitted to participate, effectively increasing the motivation to adhere. When supply chains were secure, and patients were confident that ART was available, they were more likely to enroll in a differentiated model and continue to adhere to treatment.
Peer support emerged as a strong benefit, particularly through the Facility and Community Club Models. Within Clubs, peer support developed organically, allowing members to overcome challenges, including collecting ART, reporting complications, and providing adherence support and appointment reminders. Some Clubs in Uganda and Zimbabwe created savings clubs or businesses to pay transport costs for ART pickup. The money was also allocated to individuals throughout the year and used to start small businesses, including raising chickens, purchasing goats to sell milk, and craftwork.
While stigma was a barrier that contributed to decreased enrollment in differentiated treatment distribution models, some noted that a benefit of community-based distribution models was that PLWH became more open about their status due to the support aspect. Less-frequent facility visits also reduced perceived stigma because patients did not have to make excuses to leave work to attend appointments as often.
Another benefit emerged related to reduced health system stress. In all three countries, all categories of respondents stated that reduced clinic congestion and patient loads allowed more time for clinically complex patients and documentation, as well as reduced waiting times. In Uganda, patients and providers perceived higher quality of care as clinicians had more time for holistic service provision, including counseling. In Zimbabwe, programmers and providers were better able to plan staffing needs based on more clearly defined patient appointment schedules. Improved triaging of ill patients to physicians and stable patients to nurses was also noted, and patients reported greater self-efficacy related to requesting viral load tests to establish eligibility for a differentiated treatment distribution model.
Conserving resources, including time and money, and increasing work productivity were often mentioned as direct benefits of the differentiated models. Providers overwhelmingly cited increased time available as a benefit and a motivator to improve the quality of care they provided. Financial savings were noted with reduced transport and user fee costs due to reduced visit schedules. Table 3 summarizes each model including enablers, barriers, and benefits. Table 4 provides quotes from informants that support our findings.
Table 3.
Overview of Differentiated Treatment Distribution Model Findings
Table 4.
Key Informant Quotes
Discussion
Differentiated treatment distribution models have a number of benefits including the potential to improve treatment access, improve retention (Bedelu et al., 2007; Brinkhof et al., 2008; Sumbi et al., 2013), conserve time and money, and improve work productivity (Bedelu et al., 2007; Bemelmans et al., 2010; Médecins sans Frontières, 2010), particularly when enablers were potentiated and barriers addressed. These findings add to the growing literature on the acceptability of differentiated distribution models.
Our findings demonstrated that one of the most important facilitators to implementing differentiated treatment distribution models in all countries was buy-in from multiple stakeholders, particularly for community-based models. Sensitizing providers, communities, and networks of PLWH may enable enrollment. Studies have shown that ART decentralization has minimized health system burdens and helped to prioritize health facility resources for the most serious patients (Bedelu et al., 2007; Bemelmans et al., 2014; Decroo et al., 2013; Fatti et al., 2010; Kredo et al., 2013), which may also enable enrollment once these health systems’ benefits have been identified. Results also showed that time and experience with the models were often required for patients and providers to embrace the concept of differentiated treatment distribution models, particularly for providers who were unaware of evidence related to long-term treatment adherence by model participants.
With most facilities offering multiple differentiated treatment distribution models, patient education about how each model would work, what was required for patients to participate in each model, and how to maintain eligibility to stay enrolled in a differentiated treatment distribution model was found to be vital when allowing clients to select a model that would best meet their needs and facilitate easier access to ART. Education was also identified as a key component of self-management with minimal provider oversight, enabling patients to recognize potential health complications and to know when they needed to seek care beyond biannual clinic appointments. These findings were in alignment with the literature on differentiated treatment distribution models, which has also cited patient education as a critical component for effective implementation (Médecins sans Frontières, 2010; Reda & Biadgilign, 2012).
Logistics and information systems were major facilitators of the differentiated treatment distribution models and will require continued attention and adaptation as differentiated services evolve. Robust stock management systems were critical to successful rollout, ensuring that multimonth ART dispensing could be implemented. This was in line with the literature that found that pharmaceutical information management systems resulted in faster ART pickup (International AIDS Society, 2016). Patient information systems were also important to identify eligible patients, track and monitor patient appointments and ART pickup, identify missed appointments, and track patients who transferred to other facilities.
Differentiated treatment distribution models can be complex to implement, and they require contextualization and buy-in. Flexibility within and across models that will increase patient uptake and adapting models to fit patient and health provider needs will optimize treatment access and minimize stigma. Formal approval from district, community, and traditional leadership was instrumental for scale-up in the three countries we studied. As country-specific data on benefits and health outcomes continue to emerge and contribute to the development of formal policy and protocols, this approval stage, essential to successful implementation, may be shortened.
Further research is required to determine the impact of exposure to such models on clinical indicators, including viral load, opportunistic infections, and mortality, as well as the potential for these models to advance treatment goals for specialized populations such as adolescents, pregnant women, key populations, and those struggling with adherence. Differentiated treatment distribution models hold promise to make gains toward attaining the ambitious UNAIDS goals of 95-95-95 when enacted by countries and programs that prepare for implementation challenges and promote differentiated services as part of their commitment to treatment access. Even more notable was the improved agency that this perception of self-care has availed PLWH. The club-styled distribution models, in particular, with their varied affiliated community-based care and support interventions may contribute to improved resiliency in PLWH, to live positively with dignity, and prevent onward transmission with timely viral load monitoring support, thus bringing us even closer to epidemic control.
Our study design had several strengths and weaknesses. While the study captured a range of perspectives from a large number of informants across three countries, our respondents were from a limited convenience sample in mainly urban and semiurban settings, and results may not transfer to rural areas across each country setting. While our results show that the enablers, barriers, and benefits were similar between country settings with a few exceptions, our results are not transferable to all of sub-Saharan Africa. Policy makers and program planners can use data from our study to inform differentiated treatment distribution models within a particular country context and setting while also taking into account the required adaptations that will increase benefits gained at the health system, provider, and patient levels.
Conclusion
Differentiated ART distribution models present exciting opportunities to increase the number of PLWH on treatment, increase service efficiencies, and streamline services without placing increased stress on the health system. To support these opportunities, existing resources must be mobilized to begin meeting the needs of stable PLWH who are ready to move toward greater self-management while maintaining strong linkages to the health facility. Building on and improving existing models, systems, and tools is a critical step toward reaching more PLWH and accelerating progress toward universal HIV treatment.
Key Considerations
Programmatic and systemic barriers and enablers of differentiated ART distribution models in South Africa, Uganda, and Zimbabwe can inform global scale-up efforts, which may contribute to reaching 95% viral suppression globally.
Treatment distribution models in sub-Saharan Africa can strengthen adherence by PLWH and streamline services at facilities to allow health providers to focus on patients with more complex clinical needs.
Models for facility- and community-based approaches have favorable outcomes, including reductions in workloads, increased ART enrollment, improved retention in care, and reduced waiting times.
Enrollment in a differentiated treatment distribution model can lead to improved adherence and retention in care and motivate patients to assume self-care and improve resilience as responsibility for personal health is transitioned from providers to themselves.
Peer support can be a strong benefit, particularly through Facility Club and Community Club Models.
Gaining buy-in from multilevel stakeholders is critical for community-based model implementation; sensitizing providers, communities, and networks of PLWH may enable enrollment.
Disclosures
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Acknowledgments
The authors thank the following individuals and organizations: Nida Parks, Sthembile Gombarume, Catherine Brokenshire-Scott, Dr. Seyoum Dejene, Jackie Calnan, Virgininia Mawerewere, Augustine Ndaimani, Dr. Ruth Bulaya-Tembo, AgriAids, ANOVA, BroadReach, Foundation for Professional Development (FPD), Hospice and Palliative Care Association of South Africa, Kheth’Impilo, MatCH, Project Last Mile, Republic of South Africa Department of Health (NDOH), Right to Care, USAID|South Africa, Wikoppen Health and Welfare Centre, Wits Reproductive Health and HIV Institute, AIDS Healthcare Foundation/Uganda Cares (AHF), Infectious Disease Institute (IDI), International Community of Women Living with HIV and AIDS in Eastern Africa-Uganda (ICW-EA), Joint Clinical Research Center (JCRC), Kamwokya Christian Community, Makerere University/Mbarara University AIDS Project (MJAP), Makerere University Walter Reed AIDS Project (MUWRAP), Mildmay Uganda, Ministry of Health (MOH)/District Health Office, Ministry of Health-AIDS Control Program (MOH/ACP), Most at Risk Populations Initiative (MAPI), National Forum of PLHIV Organizations in Uganda (NAFOPHANU), Reach Out Mbuya, The AIDS Support Organization (TASO), Uganda AIDS Commission, Elizabeth Glaser Pediatric AIDS Foundation (EGPAF), FHI 360, Kheth’Impilo, The Organization for Public Health Interventions and Development (OPHID), USAID|Zimbabwe, Zimbabwe Association of Church Related Hospitals (ZACH).
Footnotes
Melissa Sharer contributed equally to this work (shares first authorship).
Sabrina Eagan is co-corresponding author.
This study was funded by the generous support of the American people through the President's Plan for AIDS Relief (PEPFAR) with the U.S. Agency for International Development (USAID) under the terms of the cooperative agreement, Strengthening High Impact Interventions for an AIDS-free Generation (AIDSFree), number AID-OAA-A-14-00046. The contents are the responsibility of AIDSFree and do not necessarily reflect the views of USAID, PEPFAR, or the United States Government. No conflicts of interest declared.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- AIDS and TB Programme—Ministry of Health and Child Care, Zimbabwe. (2017). Operational and service delivery manual for the prevention, care and treatment of HIV in Zimbabwe. [Google Scholar]
- Barker C., Dutta A., Klein K. (2017). Can differentiated care models solve the crisis in HIV treatment financing ? Analysis of prospects for 38 countries in sub-Saharan Africa. Journal of the International AIDS Society, 20(Suppl 4), 68–79. 10.7448/IAS.20.5.21648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedelu M., Ford N., Hilderbrand K., Reuter H. (2007). Implementing antiretroviral therapy in rural communities: The Lusikisiki model of decentralized HIV/AIDS Care. The Journal of Infectious Diseases, 196, S464–S468. 10.1086/521114 [DOI] [PubMed] [Google Scholar]
- Bemelmans M., Baert S., Goemaere E., Wilkinson L., Vandendyck M., van Cutsem G., Ford N. (2014). Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Tropical Medicine and International Health, 19, 968–977. 10.1111/tmi.12332 [DOI] [PubMed] [Google Scholar]
- Bemelmans M., Van Den Akker T., Ford N., Philips M., Zachariah R., Harries A., Massaquoi M. (2010). Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Tropical Medicine & International Health, 15, 1413–1420. 10.1111/j.1365-3156.2010.02649.x [DOI] [PubMed] [Google Scholar]
- Brinkhof M. W. G., Dabis F., Myer L., Bangsberg D. R., Boulle A., Nash D., Anglaret X. (2008). Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bulletin of the World Health Organization, 86, 559–567. 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Kanagat N., Sharer M., Eagan S., Pearson J., Amanyeiwe U. (2018). Review of differentiated approaches to antiretroviral therapy distribution. AIDS Care, 1–7. 10.1080/09540121.2018.1441970. [DOI] [PubMed] [Google Scholar]
- Decroo T., Damme W. Van., Kegels G., Remartinez D., Rasschaert F. (2012). Are expert patients an untapped resource for ART Provision in Sub-Saharan Africa? AIDS Research and Treatment. 10.1155/2012/749718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroo T., Rasschaert F., Telfer B., Remartinez D., Laga M., Ford N. (2013). Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub- Saharan Africa: a systematic review. International Health, 5, 169–179. 10.1093/inthealth/iht016. [DOI] [PubMed] [Google Scholar]
- Dudhia R., Kagee A. (2015). Experiences of participating in an antiretroviral treatment adherence club. Psychology, Health and Medicine, 20, 488–494. 10.1080/13548506.2014.953962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatti G., Grimwood A., Bock P. (2010). Better antiretroviral therapy outcomes at primary healthcare facilities: An Evaluation of three tiers of ART Services in Four South African Provinces. PLoS One, 5, e12888. 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud A., Bygrave H., Doherty M., Ehrenkranz P., Ellman T., Ferris R., Bekker L. (2016). Reimagining HIV service delivery: The role of differentiated care from prevention to suppression. Journal of the International AIDS Society, 19, 10–12. 10.7448/IAS.19.1.21484/Viewpoint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredo T., Ford N., Fb A., Garner P. (2013). Decentralising HIV treatment in lower- and middle-income countries (Review ). The Cochrane Database of Systematic Reviews, CD009987. 10.1002/14651858.CD009987.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Médecins sans Frontières. (2010). Closer to home. Retrieved from https://www.msfaccess.org/sites/default/files/MSF_assets/HIV_AIDS/Docs/AIDS_report_ClosertoHome_ENG_2012.pdf
- Médecins sans Frontières. (2017). No time to lose : Retrieved from https://www.msfaccess.org/sites/default/files/HIV_Brief_WCA_NoTimeToLose_ENG_2017.pdf [Google Scholar]
- Magadzire B. P., Marchal B., Ward K. (2015). Improving access to medicines through centralised dispensing in the public sector: a case study of the chronic dispensing unit in the Western Cape Province , South Africa. BMC Health Services Research, 15, 1–8. 10.1186/s12913-015-1164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaquoi M., Zachariah R., Manzi M., Pasulani O., Misindi D., Mwagomba B., Harries A. D. (2009). Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Tropical Medicine and Hygiene, 103, 594–600. 10.1016/j.trstmh.2009.02.012 [DOI] [PubMed] [Google Scholar]
- Mesic A., Fontaine J., Aye T., Greig J., Thwe T. T., Moretó-planas L., Brien D. P. O. (2017). Implications of differentiated care for successful ART scale-up in a concentrated HIV epidemic in Yangon , Myanmar. Journal of the International AIDS Society, 20(Suppl 4), 7–13. 10.7448/IAS.20.5.21644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. J., Bakanda C., Birungi J., Chan K., Ford N., Cooper C. L., Hogg R. S. (2011). Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: A cohort analysis from Uganda. Annals of Internal Medicine, 155, 209–216. 10.7326/0003-4819-155-4-201108160-00358 [DOI] [PubMed] [Google Scholar]
- Prust M. L., Banda C. K., Nyirenda R., Chimbwandira F., Kalua T., Jahn A., Gunda A. (2017). Community ART groups: results from a process evaluation in Malawi on using differentiated models of care to achieve national HIV treatment goals. Journal of the International AIDS Society, 20(Suppl 4), 41–50. 10.7448/IAS.20.5.21650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert F., Decroo T., Remartinez D., Telfer B., Lessitala F., Biot M., Van Damme W. (2014). Adapting a community-based ART delivery model to the patients' needs: A mixed methods research in Tete, Mozambique. BMC Public Health, 14, 364 10.1186/1471-2458-14-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda A. A., Biadgilign S. (2012). Determinants of adherence to antiretroviral therapy among HIV-infected patients in Africa. AIDS Research and Treatment, 2012, 574656. 10.1155/2012/574656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy W. J., Sheriff M., Wang C., Hawken M., Koech E., Elul B.E. (2016). HHS Public Access, 67, 1–17. 10.1097/QAI.0000000000000264.Decentralization [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society, I. A. (2016). Differentiated care for HIV: a decision framework for antiretroviral therapy delivery. Retrieved from http://www.differentiatedcare.org/Portals/0/adam/Content/yS6M-GKB5EWs_uTBHk1C1Q/File/Decision Framework.pdf [Google Scholar]
- South Africa Department of Health (2016). Adherence Guidelines for HIV, TB and NCDs policy and service guidelines for linkage to care, adherence to treatment and retention in care. Department of Health Republic of South Africa, (February), 124. Retrieved from https://www.nacosa.org.za/wp-content/uploads/2016/11/Integrated-Adherence-Guidelines-NDOH.pdf
- Sumbi V., Indongo L., Mabirizi D., Lates J., Mwinga S., Kangudie M., Blom A. (2013). Developing and implementing a Nationwide Electronic Pharmacy Dispensing System in Low-Resource Settings: The Electronic Dispensing Tool in Namibia's ART Program Republic of Namibia Ministry of Health and Social Services. Republic of Namibia Ministry of Health and Social Services, (March), Submitted to the US Agency for International Devel. Retrieved from www.siapsprogram.org
- Uganda Minstry of Health. (2016). Consolidated guidelines for prevention and treatment of HIV in Uganda, (December), 151. Retrieved from https://aidsfree.usaid.gov/sites/default/files/uganda_hiv_gl_2016.pdf [Google Scholar]
- Wouters E., Damme W. Van., Rensburg D. Van., Masquillier C., Meulemans H. (2012). Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Services Research, 12, 194 http://doi.org/http://www.biomedcentral.com/1472-6963/12/194 [DOI] [PMC free article] [PubMed] [Google Scholar]