Abstract
Salmonella is the most common cause of foodborne illnesses worldwide. Poultry eggs are a major contamination source of Salmonella. The prevalence of Salmonella has been effectively reduced since a series of measures were taken to reduce contamination in egg-laying houses. In the present study, 1,512 environmental samples obtained from layer farms of different production scales were screened in a voluntary Salmonella survey study. Contaminations were detected using a PCR method. Genetic relationships among Salmonella samples were specified using molecular typing by enterobacterial repetitive intergenic consensus (ERIC)-PCR. The survey results showed that two layer farms, located in the Shandong and Hebei provinces, were contaminated with Salmonella. Thirty-one samples from these two farms, including feed, drinking nipples, egg collection belt, air inlets and outlets, air, overshoes, and eggshells, were identified as Salmonella-positive. It was observed that certain samples within the henhouses as well as in the egg collecting areas showed relatively high genetic similarities. The survey conclusively revealed minor Salmonella contamination in northern China. Moreover, various areas within the layer farms were identified as part of the propagation chain of Salmonella. Furthermore, evidence of cross-contamination of Salmonella was found in the laying houses and egg collection areas, even between these two regions. Therefore, it is necessary to establish routine Salmonella detection and subsequent environmental control measures in order to decrease the prevalence of Salmonella.
Keywords: environment, ERIC-PCR, layer farm, PCR, Salmonella
Introduction
With the fast growth of the Chinese economy, the chicken industry in China has been on the rise over the last 30 years, and has achieved great production values. According to the Food and Agriculture Organization of the United Nations (FAO), the total number of chickens was 4,539 billion in 2014, and total egg production reached 24,446 million metric tons in 2013 (FAO, 2016a, b). According to the data published in the annual report of the National Bureau of Statistics of the People's Republic of China, in 2014, the provinces of Shandong, Liaoning, Henan, and Hebei accounted for nearly half of the countrywide egg production capacity (National Bureau of Statistics of China, 2015). There have only been limited reported investigations of Salmonella contamination in poultry flocks and the environment (Li et al., 2013; Gong et al., 2014). However, the worldwide epidemic of infectious diseases known to be caused by various serotypes of Salmonella has brought the public health hazard to the forefront (Morpeth et al., 2009; Arnold et al., 2010; Raufu et al., 2013). Although the egg industry has taken measures, such as cleaning and disinfection, to decrease the prevalence of Salmonella and a large reduction in infections in poultry and humans was subsequently reported, residual contaminations still exist in laying houses (Carrique-Mas et al., 2009; O'Brien, 2013; Gosling et al., 2014).
Salmonella is primarily spread through the food production chain, particularly through contaminated eggs. Salmonella on the surface of eggshells poses a potential threat to the public (De Buck et al., 2004; Schulz et al., 2011). Previous studies have indicated that most Salmonella infections are a consequence of re-invasion, rather than introduction, of pathogens from the farm environment (Van Hoorebeke et al., 2011). Asymptomatic carrier birds may also facilitate the spread of the bacteria (Gast and Holt, 1998; Guard-Petter, 2001). Therefore, it is of utmost importance to detect and monitor Salmonella contaminations in laying house environments. In this study, we conducted a voluntary survey to gain insight into the general Salmonella contamination status of small- and large-scale layer farms in the main egg production areas of China, and potential cross contamination among the confirmed positive eggs and poultry environmental sites was analyzed.
Materials and Methods
Environmental Samples
In this study, environmental samples were collected from eight layer farms located in four provinces (Shandong, Liaoning, Henan, and Hebei) of northern China (Fig. 1). In each province, one large-scale (> 50,000 cages) and one small-scale (< 50,000 cages) farm were selected. Sampling was done between August and September 2014. More detailed information on each of the farms is shown in Table 1.
Fig. 1.
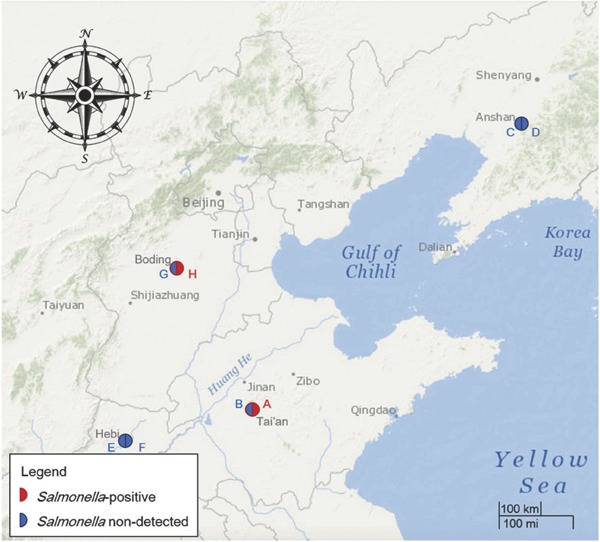
Geographical map showing the locations of the farms in four provinces in northern China where environmental samples were collected. The eight farm locations are marked with red or blue semicircles. Red semicircles represent farms that were contaminated with Salmonella, blue semicircles represent contamination-free farms. Farms are indicated with capital letters, which correspond to those in Table 2.
Table 1. Information on the layer farms of different scale in the main egg production area in northern China evaluated in this study.
| Province | Farm1 | Scale | Layer strain2 | Housing system | Structure |
|---|---|---|---|---|---|
| Shandong | A | 30,000 | Hy-Line Brown | Semi-open | |
| B | 50,000 | Hy-Line Brown | Semi-open | ||
| Liaoning | C | 19,000 | Hy-Line Brown | Semi-open | |
| D | 60,000 | Hy-Line Brown | Battery cage | Semi open | |
| Henan | E | 40,000 | CAU-3 | Semi-open | |
| F | 250,000 | CAU-3 | Closed | ||
| Hebei | G | 4,000 | Hy-Line Sonia | Open | |
| H | 100,000 | Hy-Line Brown | Closed |
Farms were named alphabetically;
Same chicken breed was kept in each laying house
Cotton swabs used for sampling were sterilized by autoclaving prior to use and were then kept in sealed tubes. The sterile cotton swabs were moistened with buffered peptone water (BPW) before sampling. The person collecting the samples wore sterile gloves when handling and moistening the swabs. A 70% ethanol solution was used to decontaminate the top of the BPW-containing tube before opening. Following sampling, each swab was placed in an individual sample tube with 10 mL of BPW. Samples were not pooled. Sampling was carried out under the guidelines of the US Food and Drug Administration (FDA) and the National Institute of Agricultural Research (FDA, 2009; Nys et al., 2011). All samples were collected using sterile cotton swabs, and the same standards were maintained. The sampling process was deemed completed when the surfaces of swabs were fully covered with the targeted samples. The tubes were stored in a cooler box at approximately 4°C and transported back to the laboratory when the sampling process was completed.
Samples obtained from egg collection belts and overshoes were regarded representative environmental samples. The egg collection belts in each henhouse were hand-swabbed at both ends and the central portion of the tire. Three swabs were taken from three adjacent sampling cages, without overlap. This process was continued until all three tires on one side of the bank in the middle of the henhouse had been sampled. Wearing overshoes, a walk was taken along the entire length of each walkway and back. Three random spots were sampled for each overshoe by using sterile cotton swabs. Feed and drinking nipples were sampled as described for the egg collection belt. Samples from the air inlets and outlets were separately taken at nine random locations. Nine air samples were randomly collected by placing moistened swabs on sterile petri dishes close to the ground for 1 h. Thirty eggs were randomly sampled at different positions in the henhouse. Finally, in the egg collection areas, six overshoe, nine air, and 30 egg samples were collected, using the same protocol as that described for henhouse sampling.
Culture Condition and Genomic DNA Extraction
In the laboratory, swab samples in BPW were incubated at 37°C for 18 h with shaking at 170 rpm. Then, 1 mL of bacterial suspension was transferred to 9 mL of selenite cystine broth Salmonella enrichment medium and incubated at 37°C for 18 h with shaking at 170 rpm. Then, the bacterial suspension was stored at 4°C for further analysis.
For genomic DNA extraction, 1 mL of selectively enriched sample was transferred to a microcentrifuge tube and centrifuged at 4°C for 5 min at 12,000 rpm using a Centrifuge 5424R (Eppendorf, Hamburg, Germany). The pellet was washed twice with ddH2O taken from a Millipore Milli-Q® Academic Water Purification System (Merck KGaA, Darmstadt, Germany) and then by suspending it in 1 mL of anhydrous ethanol. The cells were air-dried and then resuspended in 200 µL of ddH2O. Then, the micro-centrifuge tube was boiled for 15 min and immediately chilled on ice for 2 min. The tube was centrifuged at 12,000 rpm at 4°C for 15 min. The supernatant was transferred to a new microcentrifuge tube and used as a DNA template for PCR.
Salmonella Detection Via PCR
The sequences of the primer pair targeting the invA gene used for the identification of Salmonella contamination were as follows: 5′-GTGAAATTATCGCCACGTTCGGGCAA-3′ and 5′-TCATCGCACCGTCAAAGGAACC-3′ (Rahn et al., 1992). For confirmation, all environmental samples were screened using primers targeting hilA: 5′-GTGAAATTATCGCCACGTTCGGGCAA-3′ and 5′-TCATCGCACCGTCAAAGGAACC-3′ (Craciunas et al., 2012). Amplification was carried out in a 20 µL reaction mixture (10 µL 2 × Power Taq PCR MasterMix [BioTeke Corporation, Beijing, China], 1 µL of 10 µM of each of the primers, 2 µL of DNA template, and 6 µL of ddH2O) in an Applied Biosystems Veriti 96-well thermal cycler (Life Technologies, CA, USA). The thermal cycles were: initial denaturation at 95°C for 5 min, 35 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, and final extension at 72°C for 10 min.
Genomic DNA of Salmonella strains and ddH2O were systematically included as positive and negative controls in each assay, respectively. Five-microliter aliquots of PCR product were analyzed by electrophoresis on 2% (w/v) agarose gel (BIOWEST, Nuaillé, France) using a PowerPac Basic Power Supply (Bio-Rad Laboratories, CA, USA). Gels were stained with GelStain (TransGen Biotech, Beijing, China) and visualized under UV light using an ImageQuant 300 Imager (GE Healthcare, Buckinghamshire, UK).
Enterobacterial Repetitive Intergenic Consensus (ERIC)-PCR Typing
One milliliter of Salmonella-positive bacterial solution in selenite cystine broth was transferred to 9 mL of BPW and incubated overnight for recovery and multiplication. Then, 1 mL of cell solution was pelleted by centrifugation. After the supernatant was discarded, 700 µL of cell lysis buffer containing 20 mM of Tris, 5 mM of EDTA, 400 mM of NaCl and 1% (w/v) of SDS (Sigma-Aldrich, Darmstadt, Germany) was added with 30 µL 2% (w/v) of proteinase K, and 20 µL 1% (w/v) RNase (TransGen Biotech). The mixture was placed in a 55°C water bath and shaken at 220 rpm overnight. Genomic DNA was extracted using a standard phenolchloroform method, air-dried, and dissolved in 200 µL of ddH2O for further utilization. DNA quality was checked by electrophoresis and spectrophotometrically.
The primers used for ERIC-PCR were: ERIC-1R (5′-ATGTAAGCTCCTGG-GGATTCAC-3′) and ERIC-2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′), as previously described (Hulton et al., 1991; Campioni et al., 2014). The PCR mixture (20 µL) consisted of the following: 10 µL of One Taq 2 × Master with Standard Buffer (New England Biolabs, MA, USA), 0.4 µL of 10µM of each primer, 2 µL of DNA template, and 7.2 µL of ddH2O. The PCR amplification program was as follows: initial denaturation at 94°C for 30 s, 30 cycles of 94°C for 30 s, 52°C for 1 min, 68°C for 2 min, and final extension at 68°C for 5 min. PCR products were electrophoresed on 1% (w/v) agarose gel at 30V for 3 h using the equipment mentioned above. To avoid any misinterpretations due to interassay variation, a standard 1-kb ladder (Dongsheng Biotech, Guangdong, China) was added in three lanes on each gel for normalization as well as to facilitate comparison of fingerprints between different gels. All samples were assessed simultaneously and under similar conditions.
Data Analysis
The ERIC-PCR fingerprint patterns, in the form of TIFF images, were analyzed using GelCompar II software version 6.5 (Applied Maths, Sint-Martens-Latem, Belgium). Dice similarity coefficients between samples were calculated with optimization, and the “band matching tolerance” was set as 0.5. A dendrogram was obtained from the resulting similarity matrix by means of an Unweighted Pair Group Method with Arithmetic Averages (UPGMA) clustering algorithm. Default parameters were adopted in accordance with the manufacturer's instruction, except for curve extraction and band definition, where “Averaging thickness” was set as 20 pts, “Min. profiling” was set as 4.00%, and “Shoulder sens.” was set as 3. The discriminatory power of the ERIC-PCR was calculated using Simpson's Index of Diversity by the Hunter-Gaston Diversity Index (Hunter and Gaston, 1988).
Results
Salmonella Environmental Contamination in Layer Farms
In total, 1,512 environmental samples were collected from eight layer farms. Salmonella was detected by PCR using primers targeting invA and primers targeting hilA for confirmation. Samples from six farms were negative for Salmonella. On farms A and H, however, Salmonella was detected. Details on the contamination sources in these two farms are summarized in Table 2.
Table 2. Origins of Salmonella-positive samples in contaminated layer farms.
| Locations | Farm A | Farm H | |||
|---|---|---|---|---|---|
| Contamination rate1 | Key2 | Contamination rate | Key | ||
| Henhouses | Feed | 1/27 (3.7%) | 1 | 0/27 | — |
| Drinking nipples | 1/27 (3.7%) | 2 | 3/27 (11.1%) | 23–25 | |
| Egg belt | 0/27 | — | 1/27 (3.7%) | 26 | |
| Air inlets | 2/9 (22.2%) | 3, 4 | 1/9 (11.1%) | 27 | |
| Air outlets | 3/9 (33.3%) | 5–7 | 0/9 | — | |
| Air | 2/9 (22.2%) | 8, 9 | 1/9 (11.1%) | 28 | |
| Overshoes | 2/6 (33.3%) | 10, 11 | 1/6 (16.7%) | 29 | |
| Eggshells | 5/30 (16.7%) | 12–16 | 0/30 | — | |
| Egg collecting areas | Air | 2/9 (22.2%) | 17, 18 | 0/9 | — |
| Overshoe | 0/6 | — | 0/6 | — | |
| Eggshell | 4/30 (13.3%) | 19–22 | 2/30 (6.7%) | 30, 31 | |
| Total percentage | 11.6% | 4.8% | |||
Number of contaminated samples/number of detected samples for each sample type are shown, with the percentage in parentheses;
Contaminated samples were assigned unique key values.
Farm A, located in Shandong Province, was a small-scale farm with 30,000 laying hens. Nearly all types of environmental samples from this farm were Salmonella-positive, at levels ranging from 3.7% to 33.3%, with an average of 11.6 %. In the henhouse area, positive samples included: one feed sample taken at the top of the tier close to the air inlet; one water sample from a drinking nipple at the middle of the tier near the air outlet; two samples taken at the bottom of the air inlet; two samples from the middle and one from the bottom of the air outlet; two air samples from locations close to the air inlet and in the middle of the house; two overshoe samples (from both feet); and five eggshells from the top, middle, and bottom of the tier. In the egg collection area, two air samples and four eggshell samples were found to be Salmonella-positive.
Farm H, located in Hebei Province, was a large-scale farm with 100,000 laying hens. Six types of environmental samples were contaminated with Salmonella at levels ranging between 3.7% and 16.7%, with an average of 4.8%. In the henhouse area, positive samples included: three water samples from drinking nipples scattered over three pipelines located at different tiers, away from each other; one sample from the egg collection belt at the bottom of the tier near the air inlet; one sample taken at the top of the air inlet; and one overshoe sample. In the egg collecting area, two eggshells were contaminated with Salmonella.
ERIC-PCR Fingerprinting and Clustering Analysis
Based on a similarity matrix calculated on the basis of ERIC-PCR patterns, a dendrogram was constructed that shows the genetic relationships between Salmonella isolates in positive samples (Fig. 2). The discrimination index of ERIC-PCR typing was calculated to be 99.60% in the present analysis. In each gel lane, 1 to 15 major bands were identified, and amplicons varied in size from 100 bp to more than 10 kb. Based on the ERIC-PCR profiles, three major clusters were distinguished, designated as C1 (keys 02 to 07), C2 (keys 28 to 24), and C3 (keys 13 to 06). Samples from farm A (9/22), which were clustered into C1, showed 25–75% similarity. C2, which covered almost all of the Salmonella-positive samples from farm H (7/9) and six out of 22 samples from farm A, exhibited 30–100% similarity. The samples that were grouped in C3 all originated from farm A (7/22), and displayed 20–60% similarity.
Fig. 2.

Dendrogram produced by cluster analysis of the ERIC-PCR fingerprinting data (UPGMA) of the Salmonella-positive samples, based on a Dice coefficient. Three major clusters (C1 to C3) of related samples were defined. Samples from farm A and farm H are marked with green and red squares, respectively. Each key refers to a unique sample and corresponds to the keys in Table 2. For each ERIC-PCR fingerprint, band sizes, sources, and locations of the samples are shown.
Discussion
Since the late 1990s, the improved biosecurity and hygiene in chicken farms, along with the vaccination of laying hens, have significantly reduced the incidents of Salmonella in poultry and humans (Marcus et al., 2004; Barrow et al., 2012). However, Salmonella infections in laying flocks are still occasionally reported (Gast et al., 2015; Jones et al., 2016; Pande et al., 2016). Environmental contamination is one of the primary problems on layer farms (Wales et al., 2007). Infections of laying hens are difficult to efficiently eliminate by vaccination and other interventions, and are especially challenged by heavy environmental burdens (Barrow, 2007). Salmonella contaminations in laying hens differ depending on the housing systems (Van Hoorebeke et al., 2011). For example, in the United States, with the public awareness of farm animal welfare issues, layers are raised on large ranches where environmental parameters are controlled (Mench, 2008). USDA-graded eggs have been washed and sanitized at processing plants. Eggs packaged for consumption are to be stored and transported under refrigeration, at an ambient air temperature not exceeding 7°C, as per the FDA Egg Safety Rule. In the European Union, the housing of laying hens in conventional battery cages was forbidden in 2012, after which only enriched cages and non-cage housing systems are allowed (Appleby, 2003). The European Union legislation prohibits the washing of class A eggs, partly because chilled, washed shell eggs tend to rot when transported over long distances (Hutchison et al., 2003). In China, egg production has long been led by small-to-medium-scale housing systems with battery cages. A surveillance conducted in 2013 by the National Layer Production Technology System showed that the majority of layer farms (up to 88.28%) penned 2,000 to 50,000 laying hens. Compared with the sporadic reports of poultry Salmonella serovars from South Africa, Egypt, and other developing countries (Barbour et al., 2015), Salmonella infections in China have been hardly investigated. In the present study, 1,512 samples taken from eight layer farms in northern China during the late summer of 2014 were tested for Salmonella using a PCR method. Two farms in different provinces were found to be contaminated with Salmonella. However, the survey revealed the contaminations to be minor, although the samples were collected during an outbreak-prone season. Previous studies have revealed that Salmonella contaminations may fluctuate throughout the year because of seasonal effects, and that contaminations are generally relatively higher during summer (Zdragas et al., 2012; Sivaramalingam et al., 2013).
Environmental samples reportedly provide an accurate and representative risk indicator for Salmonella contamination in layer flocks and eggs (Namata et al., 2008; Denagamage et al., 2015). Certain serotypes of Salmonella spp., in particular for S. enteritidis, are commonly associated with the production environment of laying hens (Murakami et al., 2001). In the present study, Salmonella-positive samples were obtained from both the henhouse and egg collection area on both contaminated farms. These contaminations may not have been newly introduced, but the effects of reinvasions of the pathogen that might have been present in the farm environment long before the investigation (Van Hoorebeke et al., 2010). The contaminated status may persist over successive laying periods (Dewaele et al., 2012b), which poses a potential threat to the farm's entire flocks and eggs, especially during the molting period when the layers' resistance against disease tends to be lower, as contaminated hens are more likely to disseminate pathogens into the environment (Golden et al., 2008). This study revealed that almost all environmental sample types were positive for Salmonella, to varying degrees. These findings may have been strongly associated with the contaminations in layer flocks. The overshoe samples in the present study were positive for Salmonella on both of the contaminated farms, which was consistent with the results reported by Carrique-Mas et al. (2009) and Dewaele et al. (2012a, b), suggesting that samples of overshoes and floors are promising indicators for the presence or absence of Salmonella in layer flocks (O'Brien, 2013; Gosling et al., 2014). In the present study, the Salmonella contamination rate of eggshells was 2.29% (11/480). Other types of pathogens may be present on the surfaces of eggshells that may threaten public health (De Buck et al., 2004). Undercooked or raw eggs are known to be strongly associated with Salmonella infections in humans (Gillespie et al., 2005).
Fewer contaminated spots were detected on farm H (which housed more than 50,000 laying hens) than on farm A. In general, intensive housing systems with effective environmental management can effectively control the prevalence of Salmonella. Meanwhile, environmental cleaning and control processes should cover the entire henhouses and egg collection areas, especially certain blind sites, in order to prevent the spreading of pathogenic bacteria. In farms A and H, some drinking nipples were contaminated with Salmonella. Previous studies have indicated that moist environments promote the multiplication of microflora (Rusin et al., 1998), and bacterial abundance on wet litter was found to more diverse than that in dry conditions (Dumas et al., 2011). Furthermore, the absence of dry cleaning between laying rounds reportedly worsens the Salmonella contamination status in conventional battery cages (Van Hoorebeke et al., 2010). We also detected contamination in air samples. Holt et al. (1998) suggested that Salmonella enteritidis can spread via airborne transmission when the pathogen is contained in aerosols. In addition, eggshells in the egg collection areas as well as in the henhouses were found to be contaminated with Salmonella. Salmonella is capable of growing rapidly in naturally contaminated egg content at room temperature (Humphrey and Whitehead, 1993). A similar proliferation process may occur on the surfaces of the eggshells.
The ERIC-PCR method used in this study has a higher discriminatory power than repetitive sequence-based PCR and classical phenotypic methods, such as serotyping, phage typing, pyocin typing, and biotyping (Wolska and Szweda, 2008; Waturangi et al., 2012). ERIC-PCR-based genotyping method is reproducible and as discriminative as pulsed field gel electrophoresis. It is fast, cheap, and requires no specialized equipment or reagents (Bishi et al., 2008; Kosek et al., 2012). In this study, two Salmonella-positive overshoe samples collected from one person on farm A yielded an identical gel pattern, further demonstrating the reliability and accuracy of the ERIC-PCR. The technique has been successfully applied to the typing of numerous bacterial strains (Dorneles et al., 2014; Silva et al., 2014) and to diversity analyses of bacterial communities, including Salmonella (Pang et al., 2007; Cao et al., 2008). In the present study, ERIC-PCR was used for tracing down the source of the contaminations. As shown in the dendrogram in Fig. 2, the three water samples collected from drinking nipples on farm H dispersed into separate branches. This suggested that the Salmonella contamination at these sites may not have stemmed from the water, but from other sources. Subsequent onsite investigation revealed that the waterlines had been misassembled. Thus, the ERIC-PCR results for the drinking nipples assisted the manager of farm H in adjusting the height of the waterline to solve the problem. However, different samples collected within the henhouse as well as in the egg collecting area held relatively high similarities within the strictly controlled parameter settings, as described in the Methods section, e.g., keys 28, 29, and 30 in Fig. 2. Previous studies have shown that the transfer process of eggs presents a potential risk for cross-contamination (Davies and Wray, 1994). Based on multilocus variable number of tandem repeat analysis-phage typing, Dewaele et al. (2012a) also suggested that cross-contamination may occur between the henhouse and egg collection areas.
In conclusion, based on Salmonella survey results, this study suggested that the Salmonella prevalence in the main egg production areas of China in 2014 was low. Some progress has been achieved in Salmonella control through a public-private partnership. Routine detection and subsequent adoption of effective environmental Salmonella control measures will contribute to the realization of the objective of preventing Salmonella contamination of eggs during production, storage, and transportation.
Acknowledgments
This work was supported by Special Fund for Agroscientific Research in the Public Interest (201303084), the National Natural Science Foundation of China (31672408), the China Agriculture Research Systems (CARS-41), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R62), and the National Science & Technology Pillar Program during the 12th Five-year Plan Period (2012BAD39B0401).
References
- Appleby MC. The European Union ban on conventional cages for laying hens: history and prospects. Journal of Applied Animal Welfare Science, 6: 103-121. 2003. [DOI] [PubMed] [Google Scholar]
- Arnold ME, Carrique-Mas JJ and Davies RH. Sensitivity of environmental sampling methods for detecting Salmonella Enteritidis in commercial laying flocks relative to the within-flock prevalence. Epidemiology and Infection, 138: 330-339. 2010. [DOI] [PubMed] [Google Scholar]
- Barbour EK, Ayyash DB, Alturkistni W, Alyahiby A, Yaghmoor S, Iyer A, Yousef J, Kumosani T and Harakeh S. Impact of sporadic reporting of poultry Salmonella serovars from selected developing countries. Journal of Infection in Developing Countries, 9: 1-7. 2015. [DOI] [PubMed] [Google Scholar]
- Barrow PA. Salmonella infections: immune and non-immune protection with vaccines. Avian Pathology, 36: 1-13. 2007. [DOI] [PubMed] [Google Scholar]
- Barrow PA, Jones MA, Smith AL and Wigley P. The long view: Salmonella-the last forty years. Avian Pathology, 41: 413-420. 2012. [DOI] [PubMed] [Google Scholar]
- Bishi DK, Verghese S and Verma RS. Molecular typing of colonizing Streptococcus agalactiae strains by enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) in a Chennai based hospital. Indian Journal of Microbiology, 48: 291-296. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni F, Zoldan MM and Falcao JP. Characterization of Salmonella Enteritidis strains isolated from poultry and farm environments in Brazil. Epidemiology and Infection, 142: 1403-1410. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SY, Wang MS, Cheng AC, Qi XF, Yang XY, Deng SX, Yin NC, Zhang ZH, Zhou DC, Zhu DK, Luo QH and Chen XY. Comparative analysis of intestinal microbial community diversity between healthy and orally infected ducklings with Salmonella enteritidis by ERIC-PCR. World Journal of Gastroenterology, 14: 1120-1125. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrique-Mas JJ, Marin C, Breslin M, McLaren I and Davies R. A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian Pathology, 38: 419-424. 2009. [DOI] [PubMed] [Google Scholar]
- Craciunas C, Keul AL, Flonta M and Cristea M. DNA-based diagnostic tests for Salmonella strains targeting hilA, agfA, spvC and sef genes. Journal of Environmental Management, 95 Supplement: S15-S18. 2012. [DOI] [PubMed] [Google Scholar]
- Davies RH and Wray C. An approach to reduction of Salmonella infection in broiler chicken flocks through intensive sampling and identification of cross-contamination hazards in commercial hatcheries. International Journal of Food Microbiology, 24: 147-160. 1994. [DOI] [PubMed] [Google Scholar]
- De Buck J, Van Immerseel F, Haesebrouck F and Ducatelle R. Colonization of the chicken reproductive tract and egg contamination by Salmonella. Journal of Applied Microbiology, 97: 233-245. 2004. [DOI] [PubMed] [Google Scholar]
- Denagamage T, Jayarao B, Patterson P, Wallner-Pendleton E and Kariyawasam S. Risk factors associated with Salmonella in laying hen farms: systematic review of observational studies. Avian Diseases, 59: 291-302. 2015. [DOI] [PubMed] [Google Scholar]
- Dewaele I, Rasschaert G, Wildemauwe C, Van Meirhaeghe H, Vanrobaeys M, De Graef E, Herman L, Ducatelle R, Heyndrickx M and De Reu K. Polyphasic characterization of Salmonella Enteritidis isolates on persistently contaminated layer farms during the implementation of a national control program with obligatory vaccination: a longitudinal study. Poultry Science, 91: 2727-2735. 2012a. [DOI] [PubMed] [Google Scholar]
- Dewaele I, Van Meirhaeghe H, Rasschaert G, Vanrobaeys M, De Graef E, Herman L, Ducatelle R, Heyndrickx M and De Reu K. Persistent Salmonella Enteritidis environmental contamination on layer farms in the context of an implemented national control program with obligatory vaccination. Poultry Science, 91: 282-291. 2012b. [DOI] [PubMed] [Google Scholar]
- Dorneles EM, Santana JA, Ribeiro D, Dorella FA, Guimaraes AS, Moawad MS, Selim SA, Garaldi AL, Miyoshi A, Ribeiro MG, Gouveia AM, Azevedo V, Heinemann MB and Lage AP. Evaluation of ERIC-PCR as genotyping method for Corynebacterium pseudotuberculosis isolates. PloS One, 9: e98758. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas MD, Polson SW, Ritter D, Ravel J, Gelb J, Jr., Morgan R and Wommack KE. Impacts of poultry house environment on poultry litter bacterial community composition. PloS One, 6: e24785. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOWeb. | http://faostat3.fao.org/browse/Q/QA/E. Accessed on January 6, 2016a. [Google Scholar]
- FAOWeb. | http://faostat3.fao.org/browse/Q/QL/E. Accessed on Januray 6, 2016b. [Google Scholar]
- Food and Drug Administration HHS. Prevention of Salmonella enteritidis in shell eggs during production, storage, and transportation. Final rule. Federal Register, 74: 33029-33101. 2009. [PubMed] [Google Scholar]
- Gast RK and Holt PS. Persistence of Salmonella enteritidis from one day of age until maturity in experimentally infected layer chickens. Poultry Science, 77: 1759-1762. 1998. [DOI] [PubMed] [Google Scholar]
- Gast RK, Guraya R, Jones DR and Anderson KE. Persistence of fecal shedding of Salmonella Enteritidis by experimentally infected laying hens housed in conventional or enriched cages. Poultry Science, 94: 1650-1656. 2015. [DOI] [PubMed] [Google Scholar]
- Gillespie IA, O'Brien SJ, Adak GK, Ward LR and Smith HR. Foodborne general outbreaks of Salmonella Enteritidis phage type 4 infection, England and Wales, 1992–2002: where are the risks? Epidemiology and Infection, 133: 795-801. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden NJ, Marks HH, Coleman ME, Schroeder CM, Bauer NE, Jr. and Schlosser WD. Review of induced molting by feed removal and contamination of eggs with Salmonella enterica serovar Enteritidis. Veterinary Microbiology, 131: 215-228. 2008. [DOI] [PubMed] [Google Scholar]
- Gong J, Zhang J, Xu M, Zhu C, Yu Y, Liu X, Kelly P, Xu B and Wang C. Prevalence and fimbrial genotype distribution of poultry Salmonella isolates in China (2006 to 2012). Applied and Environmental Microbiology, 80: 687-693. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling RJ, Martelli F, Sayers R, Larkin L and Davies RH. A review of the official sampling of flocks of laying hens in the Salmonella National Control Programme in Great Britain. British Poultry Science, 55: 569-575. 2014. [DOI] [PubMed] [Google Scholar]
- Guard-Petter J. The chicken, the egg and Salmonella enteritidis. Environmental Microbiology, 3: 421-430. 2001. [DOI] [PubMed] [Google Scholar]
- Holt PS, Mitchell BW and Gast RK. Airborne horizontal transmission of Salmonella enteritidis in molted laying chickens. Avian Diseases, 42: 45-52. 1998. [PubMed] [Google Scholar]
- Hulton CS, Higgins CF and Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Molecular Microbiology, 5: 825-834. 1991. [DOI] [PubMed] [Google Scholar]
- Humphrey TJ and Whitehead A. Egg age and the growth of Salmonella enteritidis PT4 in egg contents. Epidemiology and Infection, 111: 209-219. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter PR and Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. Journal of Clinical Microbiology, 26: 2465-2466. 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison ML, Gittins J, Walker A, Moore A, Burton C and Sparks N. Washing table eggs: a review of the scientific and engineering issues. World's Poultry Science Journal, 59: 233-248. 2003. [Google Scholar]
- Jones DR, Guard J, Gast RK, Buhr RJ, Fedorka-Cray PJ, Abdo Z, Plumblee JR, Bourassa DV, Cox NA, Rigsby LL, Robison CI, Regmi P and Karcher DM. Influence of commercial laying hen housing systems on the incidence and identification of Salmonella and Campylobacter. Poultry Science, 95: 1116-1124. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M, Yori PP, Gilman RH, Vela H, Olortegui MP, Chavez CB, Calderon M, Bao JP, Hall E, Maves R, Burga R and Sanchez GM. Facilitated molecular typing of Shigella isolates using ERIC-PCR. American Journal of Tropical Medicine and Hygiene, 86: 1018-1025. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Lai J, Wang Y, Liu S, Li Y, Liu K, Shen J and Wu C. Prevalence and characterization of Salmonella species isolated from pigs, ducks and chickens in Sichuan Province, China. International Journal of Food Microbiology, 163: 14-18. 2013. [DOI] [PubMed] [Google Scholar]
- Marcus R, Rabatsky-Ehr T, Mohle-Boetani JC, Farley M, Medus C, Shiferaw B, Carter M, Zansky S, Kennedy M, Van Gilder T, Hadler JL and Emerging Infections Program FoodNet Working Group. Dramatic decrease in the incidence of Salmonella serotype Enteritidis infections in 5 FoodNet sites: 1996–1999. Clinical Infectious Diseases, 38 Supplement 3: S135-S141. 2004. [DOI] [PubMed] [Google Scholar]
- Mench JA. Farm animal welfare in the USA: Farming practices, research, education, regulation, and assurance programs. Applied Animal Behaviour Science, 113: 298-312. 2008. [Google Scholar]
- Morpeth SC, Ramadhani HO and Crump JA. Invasive non-Typhi Salmonella disease in Africa. Clinical Infectious Diseases, 49: 606-611. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Horikawa K, Ito T and Otsuki K. Environmental survey of salmonella and comparison of genotypic character with human isolates in Western Japan. Epidemiology and Infection, 126: 159-171. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namata H, Meroc E, Aerts M, Faes C, Abrahantes JC, Imberechts H and Mintiens K. Salmonella in Belgian laying hens: an identification of risk factors. Preventive Veterinary Medicine, 83: 323-336. 2008. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics of China. Web.| http://data.stats.gov.cn/english/easyquery.htm?cn=E0103. Accessed on January 6, 2016. [Google Scholar]
- Nys Y, Bain M and Van Immerseel F. Improving the safety and quality of eggs and egg products. Woodhead Pub., Oxford. 2011. [Google Scholar]
- O'Brien SJ. The “decline and fall” of nontyphoidal salmonella in the United kingdom. Clinical Infectious Diseases, 56: 705-710. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande VV, Devon RL, Sharma P, McWhorter AR and Chousalkar KK. Study of Salmonella Typhimurium Infection in Laying Hens. Frontiers in Microbiology, 7: 203. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Hua X, Yang Q, Ding D, Che C, Cui L, Jia W, Bucheli P and Zhao L. Inter-species transplantation of gut microbiota from human to pigs. ISME Journal, 1: 156-162. 2007. [DOI] [PubMed] [Google Scholar]
- Rahn K, De Grandis SA, Clarke RC, McEwen SA, Galan JE, Ginocchio C, Curtiss R, 3rd and Gyles CL. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Molecular and Cellular Probes, 6: 271-279. 1992. [DOI] [PubMed] [Google Scholar]
- Raufu I, Bortolaia V, Svendsen CA, Ameh JA, Ambali AG, Aarestrup FM and Hendriksen RS. The first attempt of an active integrated laboratory-based Salmonella surveillance programme in the north-eastern region of Nigeria. Journal of Applied Microbiology, 115: 1059-1067. 2013. [DOI] [PubMed] [Google Scholar]
- Rusin P, Orosz-Coughlin P and Gerba C. Reduction of faecal coliform, coliform and heterotrophic plate count bacteria in the household kitchen and bathroom by disinfection with hypochlorite cleaners. Journal of Applied Microbiology, 85: 819-828. 1998. [DOI] [PubMed] [Google Scholar]
- Schulz J, Van Hoorebeke S, Hald B, Hartung J, Van Immerseel F, Radtke I, Kabell S and Dewulf J. The dynamics of Salmonella occurrence in commercial laying hen flocks throughout a laying period. Avian Pathology, 40: 243-248. 2011. [DOI] [PubMed] [Google Scholar]
- Silva GF, Paixao RD, Queiroz CB, Santana MF, Souza A, Sousa NR, Hanada RE and Gasparotto L. Genetic diversity of Mycosphaerella fijiensis in Brazil analyzed using an ERIC-PCR marker. Genetics and Molecular Research, 13: 7698-7707. 2014. [DOI] [PubMed] [Google Scholar]
- Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D and Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Canadian Journal of Veterinary Research, 77: 1-11. 2013. [PMC free article] [PubMed] [Google Scholar]
- Van Hoorebeke S, Van Immerseel F, Schulz J, Hartung J, Harisberger M, Barco L, Ricci A, Theodoropoulos G, Xylouri E, De Vylder J, Ducatelle R, Haesebrouck F, Pasmans F, de Kruif A and Dewulf J. Determination of the within and between flock prevalence and identification of risk factors for Salmonella infections in laying hen flocks housed in conventional and alternative systems. Preventive Veterinary Medicine, 94: 94-100. 2010. [DOI] [PubMed] [Google Scholar]
- Van Hoorebeke S, Van Immerseel F, Haesebrouck F, Ducatelle R and Dewulf J. The influence of the housing system on Salmonella infections in laying hens: a review. Zoonoses Public Health, 58: 304-311. 2011. [DOI] [PubMed] [Google Scholar]
- Wales A, Breslin M, Carter B, Sayers R and Davies R. A longitudinal study of environmental Salmonella contamination in caged and free-range layer flocks. Avian Pathology, 36: 187-197. 2007. [DOI] [PubMed] [Google Scholar]
- Waturangi DE, Joanito I, Yogi Y and Thomas S. Use of REP- and ERIC-PCR to reveal genetic heterogeneity of Vibrio cholerae from edible ice in Jakarta, Indonesia. Gut Pathogens, 4: 2. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska K and Szweda P. A comparative evaluation of PCR ribotyping and ERIC PCR for determining the diversity of clinical Pseudomonas aeruginosa isolates. Polish Journal of Microbiology, 57: 157-163. 2008. [PubMed] [Google Scholar]
- Zdragas A, Mazaraki K, Vafeas G, Giantzi V, Papadopoulos T and Ekateriniadou L. Prevalence, seasonal occurrence and antimicrobial resistance of Salmonella in poultry retail products in Greece. Letters in Applied Microbiology, 55: 308-313. 2012. [DOI] [PubMed] [Google Scholar]


