Abstract
The aim of this study was to determine the effects of Toll-like receptor (TLR) ligands on the expression of cytokines in chicken follicular theca and to investigate whether nuclear factor-κB (NFκB) was involved in their expression. The follicular theca was collected from the largest follicle of laying hens. In experiment 1, the expression of TLRs in the theca interna and externa was confirmed using RT-PCR. The theca tissues were then incubated with or without Pam3CSK4 (TLR2 ligand), poly I:C (TLR3 ligand), LPS (TLR4 ligand), flagellin (TLR5 ligand), R837 (TLR7 ligand), and CpG-ODN (TLR21 ligand) for 3 h, after which cytokine expression (IL-1β, IL-6, TNFSF15, CXCLi2, IFN-α, and IFN-β) was analyzed by real-time PCR. In experiment 2, the theca tissues were incubated in a medium containing Pam3CSK4, poly I:C, LPS, or CpG-ODN with or without BAY 11-7085 (an inhibitor of NFκB) for 3 h. The results of experiment 1 revealed that all TLRs, namely TLR1 (type 1 and 2), TLR2 (type 1 and 2), 3–5, 7, 15, and 21, were expressed in the follicular theca, although the PCR products of TLR1 (type 2) and TLR21 were faint. Moreover, Pam3CSK4 and LPS upregulated the expression of all detected cytokines, except for IFN-α, whose expression was not upregulated by LPS. Poly I:C upregulated the expression of IL-6, CXCLi2, and IFN-β, while CpG-ODN upregulated IL-1β. Flagellin and R837 did not significantly affect cytokine expression. In experiment 2, the expression of IL-1β, IL-6, CXCLi2 and IFN-β in tissues incubated with LPS was downregulated by BAY 11-7085. These results suggest that the innate immune system, including pattern recognition by TLRs and cytokine synthesis, occur in the theca; whereas, functions for recognition of bacterial patterns is more developed than that of viral ones.
Keywords: chicken follicular theca, cytokines, NFκB, TLR ligands
Introduction
The hen ovary contains cortical follicles embedded in the ovarian stroma, and numerous white follicles, several hierarchical yellow follicles, and postovulatory follicles protruding from the ovarian surface. The oocyte is surrounded by the granulosa and theca layers (Johnson, 2000; Barua et al., 2001), and microbes in the blood stream may colonize these tissues (Takata et al., 2003). Ovary infections by bacterial and viral pathogens may cause not only ovarian functional disorders, but also the contamination of eggs (Yoshimura, 2015). Therefore, the immune function of the follicular wall is essential for protection from pathogen infection and the production of hygienic eggs.
Toll-like receptors (TLRs) are pattern recognition receptors that recognize pathogen-associated molecular patterns (PAMPs) in the innate immune system. Previous reports have indicated that the hen ovary expresses TLR1-2, 2-1, 3, 4, 5, 7, 15, and 21 (Subedi et al., 2007; Woods et al., 2009; Michailidis et al., 2010). TLR2 forms a heterodimer with TLR1 and recognizes lipoproteins, lipopeptides, and peptidoglycans of Gram-positive bacteria (Keestra et al., 2007). TLR3 and TLR7 can recognize double- and single-strand RNA (dsRNA and ssRNA) viruses, respectively (Alexopoulou et al. 2001; St. Paul et al., 2013). TLR4 recognizes lipopolysaccharides (LPS) of Gram-negative bacteria, and TLR5 can be stimulated by bacterial flagellin (St. Paul et al., 2013). TLR15 is a unique TLR found in birds, which recognizes non-secreted, heat-stable substances and virulence-associated bacterial proteases (Nerren et al., 2010; de Zoete et al., 2011). TLR21 interacts with CpG oligodeoxynucleotides (CpG-ODN) that contain unmethylated CpG motifs of microorganisms (Brownlie and Allan, 2011).
Subedi et al. (2007) demonstrated that the follicular theca of laying hens expresses TLR2, 4, 5, and 7. They have also reported that the intravenous injection of LPS in hens upregulated the expression of interleukin (IL)-1β, IL-6, and chemokine CXCLi2 in association with the recruitment of heterophil-like cells in the theca (Abdelsalam et al., 2011). Abdelsalam et al. (2012) also found that IL-1β stimulated the expression of avian β-defensin (AvBD) 12 in the theca. These reports suggest that the TLR-mediated immune system is developed in the follicular theca (Abdelsalam et al., 2012). However, it is unknown whether theca cells recognize TLR ligands other than LPS. Nuclear factor-κB (NF-κB) is known to be the major transcription factor involved downstream of the TLR signaling pathway. NFκB is known to induce the expression of cytokines downstream of TLRs in oviduct cells (Kamimura et al., 2017); however, the transcription factor(s) that induce the expression of cytokines in the theca tissues is unknown.
The aim of this study was to determine whether the expression of proinflammatory cytokines was induced by different TLR ligands, so as to improve our understanding of the innate immunodefense system in the theca. We also examined whether NFκB is the transcription factor responsible for the induction of cytokine expression in the theca. BAY 11-7085, which inhibits the activation of NFκB, was used as the inhibitor of NFκB.
Materials and Methods
Experimental Birds
White Leghorn hens, approximately 350-day-old and laying five or more eggs in a sequence, were used for this study. They were kept in individual cages under a 14 h light:10 h dark regimen, and provided with feed and water ad libitum. The hens were euthanized under anesthesia with sodium pentobarbital (Somnopentyl; Kyoritsu Pharmaceutical Co., Tokyo, Japan), and the largest preovulatory follicles were collected 3 h after oviposition (approximately 22 h prior to predicted time of ovulation). This study was approved by the Hiroshima University Animal Research Committee (No. C15–17).
TLR Ligands and Inhibitors
Pam3CSK4 (TLR2 ligand; a synthetic triacylated lipopeptide), poly I:C (TLR3 ligand; a synthetic analog of doublestranded RNA), flagellin from S. typhimurium (TLR5 ligand), and imiquimod (R837; TLR7 ligand; an imidazoquinoline amine analog to guanosine) were purchased from Novus Biologicals (Littleton, CO, USA). LPS from S. minnesota (TLR4 ligand) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and Class B CpG-ODN (2007) (TLR21 ligand) was purchased from InvivoGen (San Diego, CA, USA). The sequence of CpG-ODN was 5′-TCGTCGTTGTCGTTTTGTCGTT-3′. Stock solutions of Pam3CSK4 and poly I:C were prepared by dissolving them in sterile water (100 µg/mL and 100 mg/mL, respectively). LPS and flagellin were dissolved in phosphate-buffered saline (PBS) (1 mg/mL and 100 µg/mL, respectively), imiquimod was dissolved in dimethyl sulfoxide (DMSO) (100 mg/mL), and CpG-ODN was dissolved in water (10 mg/mL). BAY 11-7085 (NFκB inhibitor) was purchased from Abcam Co. (Cambridge, MA, USA), and was dissolved in DMSO at a concentration of 1 mM and stored at −20°C until use.
Experimental Design
In experiment 1, we first examined the expression of TLRs in the theca before culturing. The effects of TLR ligands on cytokine expression in the cultured theca tissues were then studied. In experiment 2, the effect of the NFκB inhibitor, BAY 11-7085, on the induction of cytokine expression by TLR ligands was examined.
Experiment 1. Effect of TLR Ligands on Cytokine Expression in the Theca
The largest follicles were collected from seven birds for each treatment group and washed with sterile PBS containing 10U/mL penicillin and 10 µg/mL streptomycin (Cosmo Bio Co., Ltd., Tokyo, Japan). The superficial tissue was removed, and an incision was made in the follicular wall to remove the yolk and granulosa layer from the theca. First, a piece of the theca layer was put on a rubber plate with the theca interna on the upper side, and then the theca interna was gently scraped off with a scalpel blade (as evidenced by a change in the tissue color from pink to near white). The theca interna and the theca externa where the theca interna was removed were then used. The absence of the theca interna tissue in the theca externa sample was confirmed by histology. Second, the remaining theca tissues were cut into small specimens (approximately 5 mm × 5 mm) and placed in polystyrene tubes (Bio-One Ltd., Tokyo, Japan) containing 2 mL TCM-199 medium (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) with 10 U/mL penicillin and 10 µg/mL streptomycin. Different doses of TLR ligands were then added into the culture medium to produce the following concentration ranges: Pam3CSK4 (0–100 ng/mL), poly I:C (0–100 µg/mL), LPS (0–1000 ng/mL), flagellin (0–100 ng/mL), R837 (0–100 µg/mL), and CpG-ODN (0–10 µg/mL). The theca tissues were incubated for 3 h at 39°C under 5% CO2 and 95% air in a CO2 incubator. Seven trials were performed for each TLR ligand.
Experiment 2. Effect of NFκB Inhibition on the Induction of Cytokines in Response to TLR Ligands in the Theca
The theca tissues of the largest follicles were cultured in polystyrene tubes in the same manner as that described in experiment 1. Different doses of the NFκB inhibitor, BAY 11-7085 (0–10 µM), and different TLR ligands, such as Pam3CSK4 (100 ng/mL), poly I:C (100 µg/mL), LPS (1000 ng/mL), and CpG-ODN (10 µg/mL), were then added into the culture medium. BAY 11-7085 is an inhibitor of IκB phosphorylation, resulting in the prevention of NFκB activation, and has previously been used to inhibit NFκB in chicken vaginal cells (Kamimura et al., 2017). The TLR ligands were selected as they were found to upregulate the expression of cytokines in experiment 1. Tissues were incubated for 3 h at 39°C under 5% CO2 and 95% air. The experiment was repeated five times.
RNA Extraction and cDNA Preparation
Total RNA was extracted from cultured follicular theca tissues in experiments 1 and 2 using Sepasol RNA I Super (Nacalai Tesque Inc., Kyoto, Japan) and a Polytron homogenizer (Polytron PT1200ci Kinematica AG, Switzerland), and then dissolved in TE buffer (10 mM Tris, pH8.0, with 1 mM EDTA). The concentration of RNA in each sample was measured using Gene Quant Pro (Amersham PharmaciaBiotech, Cambridge, UK). RNA samples were mixed with RQ1 RNase-free DNase (Promega Co., Madison, WI, USA) in a 10 µL reaction mixture (10 µg of total RNA, 1× DNase buffer, and 1U DNase) and incubated in a PTC-100 programmable thermal controller (MJ Research, Inc., Waltham, MA, USA) at 37°C for 45 min and at 65°C for 10 min. The RNA samples were reverse-transcribed using ReverTra Ace (Toyobo Co. Ltd., Osaka, Japan), according to the manufacturer's instructions. Reverse transcription (RT) was performed at 42°C for 30 min, followed by heat inactivation at 99°C for 5 min, using the PTC-100 Programmable Thermal Controller (MJ Research, Inc.). The reaction mixture (10 µL) contained 1 µg of purified RNA, 1× RT buffer, 1 mM dNTP mixture, 20U RNase inhibitor, 0.5 mM oligo (dT) 20 primer, and 50 U ReverTra Ace.
PCR and Agarose Gel Electrophoresis (AGE) for Identifying TLRs
PCR was performed to identify the expression of TLRs in the theca interna and externa using the PTC-100 thermal controller (MJ Research, Inc.) in experiment 1. The reaction mixture (10 µL) contained 0.3 µL cDNA, 1× PCR buffer, 0.2 mM of each dNTP, 0.4 mM of each primer, and 0.625U Takara Taq (Takara Bio Inc., Shiga, Japan). Primer sequences used are shown in Table 1. The cycle parameters were as follows: initial denaturation at 94°C for 30 s; 38 cycles of denaturation at 94°C for 30 s, annealing at 56°C (for TLR1-2, 2-1, 2-2, 3, 4, 5, 15, and 21) or 58°C (TLR1-1 and 7) for 30 s, and extension at 72°C for 1 min; and a final extension at 72°C for 10 min. The PCR products were separated by electrophoresis on 1.5% (w/v) agarose gels with ethidium bromide (0.5 µg/mL) and photographed under UV illumination.
Table 1. Primer sequences of TLRs, cytokines, and RPS17 for PCR.
| Target genes | Sequences 5′-3′ | Accession No. |
|---|---|---|
| TLR1-1 | F: AGGTTGGACTTCTTATTGAGGCATAC | |
| R: AGATGAATCCCAAACTAGCAGAAAAA | NM_001007488 | |
| TLR1-2 | F: AGTCCATCTTTGTGTTGTCGCC | |
| R: ATTGGCTCCAGCAAGATCAGG | NM_001081709 | |
| TLR2-1 | F: ACATGTGTGAATGGCCTGAA | |
| R: TTGAGAAATGGCAGTTGCAG | NM_204278 | |
| TLR2-2 | F: AGGCACTTGAGATGGAGCAC | |
| R: CCTGTTATGGGCCAGGTTTA | AB046533 | |
| TLR3 | F: TCAGTACATTTGTAACACCCCGCC | |
| R: GGCGTCATAATCAAACACTCC | NM001011691 | |
| TLR4 | F: AGTCTGAAATTGCTGAGCTCAAAT | |
| R: GCGACGTTAAGCCATGGAAG | NM_001020693 | |
| TLR5 | F: CCACATCTGACTTCTGCCTTT | |
| R: TGCACATGTTTTCTCCTAGGT | NM_001024586 | |
| TLR7 | F: CCTGACCCTGACTATTAACCATT | |
| R: CGTAAAGTAGCAGGAAGACCC | NM_001011688 | |
| TLR15 | F: GTTCTCTCTCCCAGTGGGTGAAATAGC | |
| R: GTGGTTCATTGGTTGTTTTTAGGAC | NM_001037835 | |
| TLR21 | F: TGCCCCTCCCACTGCTGTCCACT | |
| R: AAAGGTGCCTTGACATCCT | NM_001030558 | |
| IL-1β | F: GTGAGGCTCAACATTGCGCTGTA | |
| R: TGTCCAGGCGGTAGAAGATGAAG | NM_204524 | |
| IL-6 | F: AGAAATCCCTCCTCGCCAAT | |
| R: AAATAGCGAACGGCCCTCA | NM_204628.1 | |
| TNFSF15 | F: CCTGAGTTATTCCAGCAACGCA | |
| R: ATCCACGAGCTTGATGTCACTAAC | NM_001024578 | |
| CXCLi2 | F: GGCTTGCTAGGGGAAATGA | |
| R: AGCTGACTCTGACTAGGAAACTGT | AJ009800 | |
| IFN-α | F: ATCCTGCTGCTCACGCTCCTTCT | |
| R: GGTGTTGTGGTGTCCAGGATG | XM_004937096 | |
| IFN-β | F: GCTCTCACCACCACCTTCTC | |
| R: GCTTGCTTCTTGTCCTTGCT | NM_001024836 | |
| RPS17 | F: AAGCTGCAGGAGGAGGAGAGG | |
| R: GGTTGGACAGGCTGCCGAAGT | NM_204217 |
F, forward; R, reverse.
Analysis of Expression of Cytokine Genes
The expression of cytokines (IL-1β, IL-6, tumor necrosis factor superfamily 15 (TNFSF15), CXCLi2, interferon-α (IFN-α), and IFN-β) in response to the TLR ligands and NFκB was analyzed by real-time PCR using a Roche Light Cycler Nano System (Roche Applied Science, Indianapolis, IN, USA) in experiments 1 and 2. The reaction mixture (10 µL) containing 1 µL cDNA, 5 µL Thunderbird SYBR qPCR mix (Toyobo Co. Ltd., Osaka, Japan), and 0.5 µM of each primer was mixed in the PCR tubes (Roche Diagnostics GmbH, Mannheim, Germany). Primer sequences used in this study are shown in Table 1. The amplification was carried out with 45 cycles at 95°C for 10 s and 62°C for 30 s (for IL-6 and ribosomal protein S17 (RPS17)), 63°C for 30 s (for IL-1β), 64°C for 30 s (for TNFSF15), 60°C for 30 s (for IFN-α), or 58°C for 30 s (for IFN-β). Real-time PCR data were analyzed by the 2−ΔΔCT method to calculate the relative level of each gene in each sample and were expressed as a ratio in relation to the RPS17 housekeeping gene (Livak and Schmittgen, 2001). A sample cultured without TLR ligands was used as a standard for analyzing the effects of TLR ligands (experiment 1). A sample cultured with each TLR ligand, but without the NFκB inhibitor, was used as a standard for the analysis of the effect of the inhibitor on cytokine expression by the TLR ligand (experiment 2).
Statistical Analysis
The relative expression levels of the cytokines were shown as the mean±SEM (n=7 in experiment 1, and n=5 in experiment 2). Significant differences among different treatments were assessed by the Kruskal-Wallis test, followed by multiple comparisons (Steel-Dwass test). Differences were considered significant at P<0.05.
Results
Experiment 1. Effects of TLR Ligands on Cytokine Expression in the Theca
In both the theca interna and externa of preovulatory follicles, the PCR products of TLR1 (type 1 and 2), 2 (type 1 and 2), 3, 4, 5, 7, 15, and 21 were detected, whereas those of TLR1 (type 2) and 21 were faint (Fig. 1a, b).
Fig. 1.
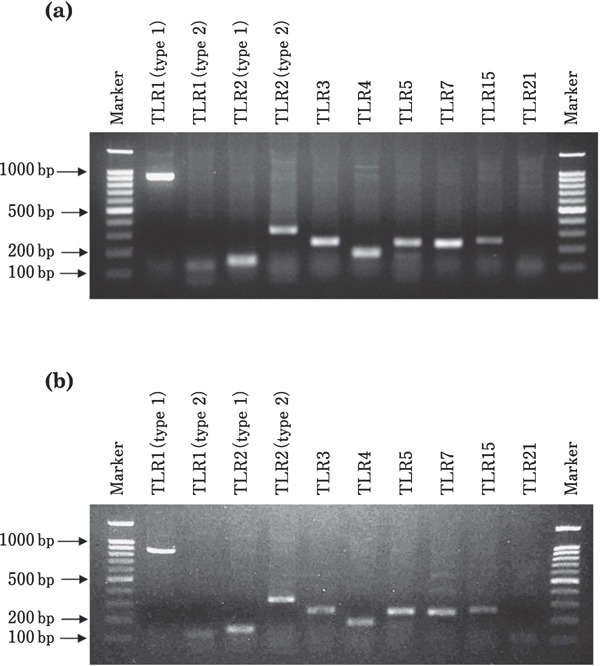
Reverse transcription-PCR products of TLRs in the theca interna (a) and externa (b) of the largest preovulatory follicle of a hen. The products of TLR1 (type 1), 2 (type 1 and 2), 3–5, 7, and 15 are clearly visible. The products of TLR1 (type 2) and TLR21 are faintly visible.
Stimulation of the theca by Pam3CSK4 increased the expression level of proinflammatory cytokines (IL-1β, IL-6, TNFSF15, and CXCLi2) (Fig. 2a–d) and interferons (IFN-α and IFN-β) (Fig. 2e and f), as compared to that in the control, in which no TLR ligands were added. Poly I:C significantly increased the expression of IL-6, CXCLi2, and IFN-β at a concentration of 100 µg/mL (Fig. 3b, d, and f), but had no effect on the expression of IL-1β, TNFSF15, IFN-α, and IFN-β (Fig. 3a, c, and e) when compared with the control. LPS significantly upregulated the expression of IL-1β, IL-6, TNFSF15, CXCLi2, and IFN-β as compared with the control (Fig. 4a, b, c, d, and f), but not that of IFN-α (Fig. 4e). Flagellin and R837 had no significant effect on the expression of the six cytokines (data not shown). Only IL-1β expression increased significantly by CpG-ODN (10 µg/mL) as compared to the control (Fig. 5a).
Fig. 2.
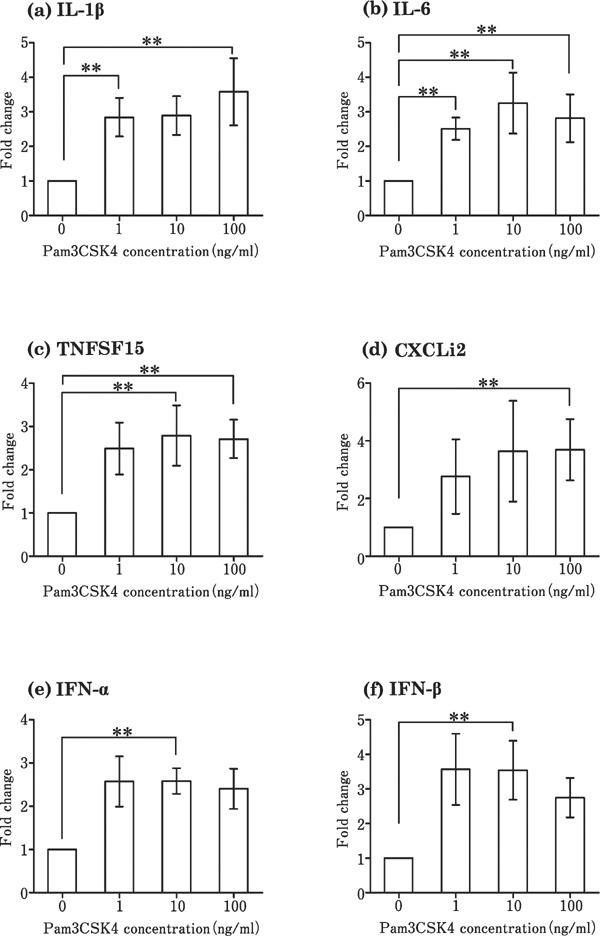
Changes in the expression of cytokines in cultured follicular theca from laying hens in response to Pam3CSK4 stimulation. Cultured tissues were stimulated with 0–100 ng/mL Pam3CSK4. Values are the mean±SEM (n=7). Asterisks indicate significant differences (** P<0.01).
Fig. 3.
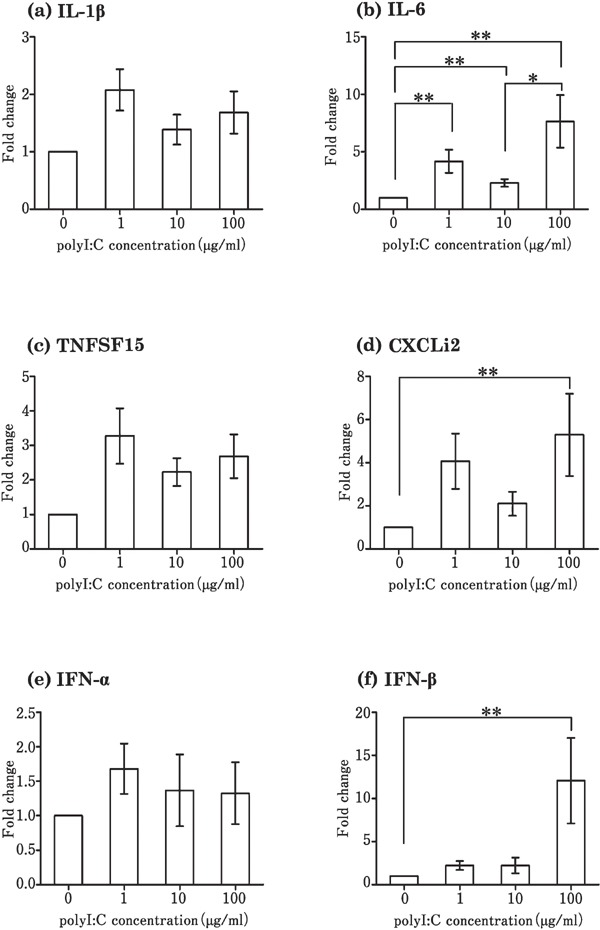
Changes in the expression of cytokines in cultured follicular theca from laying hens in response to poly I:C stimulation. Cultured tissues were stimulated with 0–100 µg/mL poly I:C. Values are the mean±SEM (n=7). Asterisks indicate significant differences (* P<0.05, ** P<0.01).
Fig. 4.
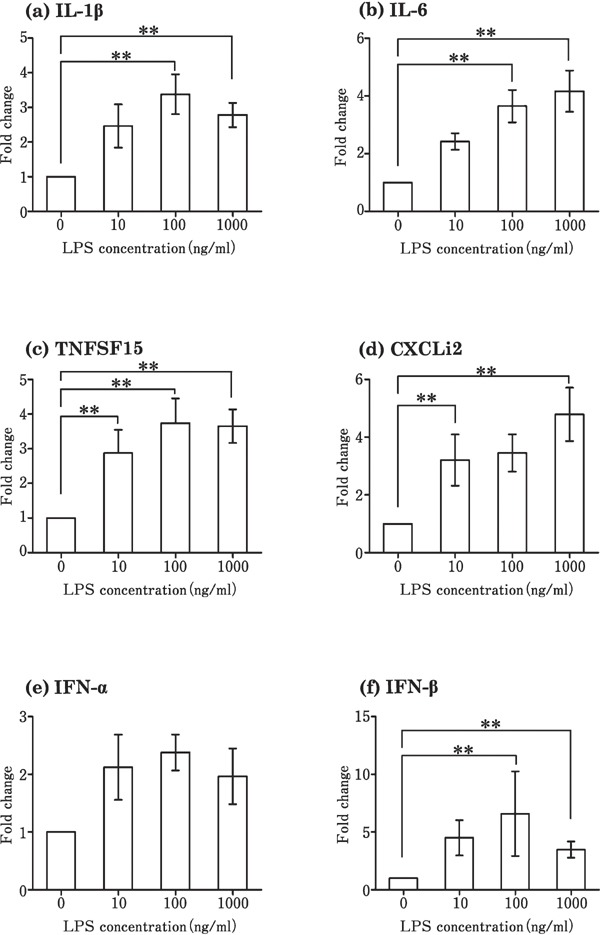
Changes in the expression of cytokines in cultured follicular theca from laying hens in response to LPS stimulation. Cultured tissues were stimulated with 0–1000 ng/mL LPS. Values are the mean±SEM (n=7). Asterisks indicate significant differences (** P<0.01.).
Fig. 5.
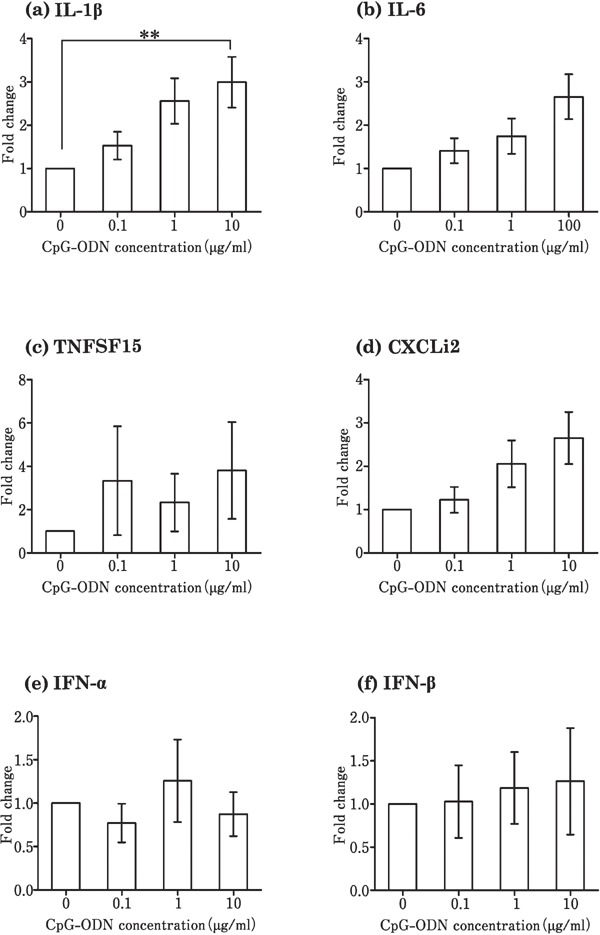
Changes in the expression of cytokines in cultured follicular theca from laying hens in response to CpG-ODN stimulation. Cultured tissues were stimulated with 0–10 µg/mL CpG-ODN. Values are the mean±SEM (n=7). Asterisks indicate significant differences (** P<0.01).
Experiment 2. Effects of NFκB Inhibition on the Induction of Cytokines in Response to TLR Ligands in the Theca
To examine whether NFκB is the transcription factor responsible for the induction of cytokines by Pam3CSK4, poly I:C, LPS, and CpG-ODN in the theca, the theca tissues were incubated with TLR ligands along with 0–10 µM BAY 11-7085 (inhibitor of NFκB). The effect of Pam3CSK4 (100 ng/mL) on TNFSF15 was downregulated by BAY 11-7085 (Fig. 6c). However, BAY 11-7085 did not affect the expression of the other cytokines (IL-1β, IL-6, CXCLi2, IFN-α, and IFN-β) that were induced by Pam3CSK4 (Fig. 6a, b, d, e, and f). As shown in Fig. 7, the LPS-induced (1000 ng/mL) expression of IL-1β, IL-6, CXCLi2, and IFN-β in the theca was downregulated by BAY 11-7085 (Fig. 7a, b, d, and e). The poly I: C-induced (100 µg/mL) expression of IL-6, CXCLi2, and IFN-β in the theca was not suppressed by BAY 11-7085 (Fig. 8a–c), whereas the expression of IL-1β in response to CpG-ODN (10 µg/mL) increased following BAY 11-7085 exposure (Fig. 8d).
Fig. 6.
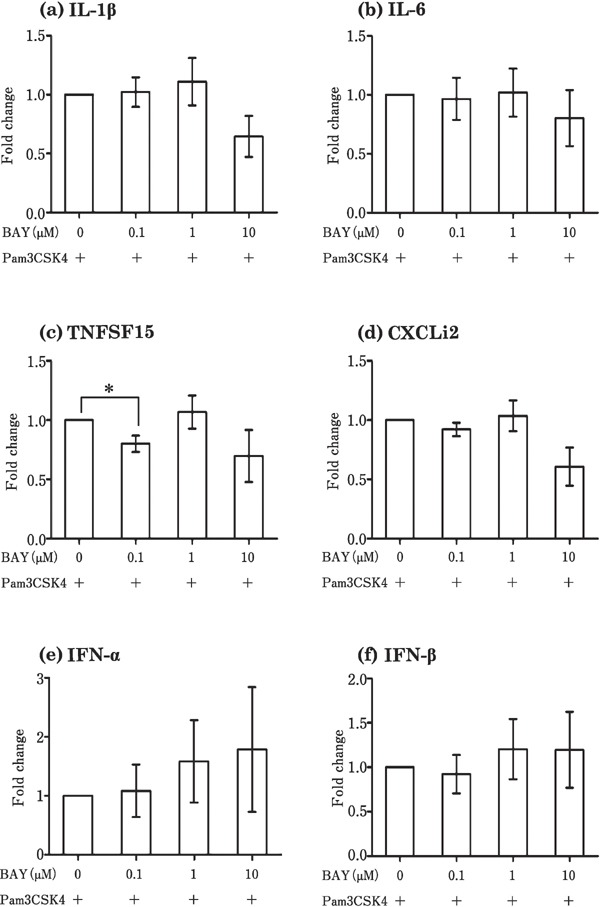
Effects of the NFκB inhibitor, BAY 11-7085, on the expression of genes induced by Pam3CSK4 in cultured follicular theca tissues. Cultured follicular theca tissues were treated with Pam3CSK4 (100 ng/mL) in the presence or absence of BAY 11-7085 (0–10 µM). Values are the mean±SEM (n=5). Asterisks indicate significant differences (* P<0.05).
Fig. 7.
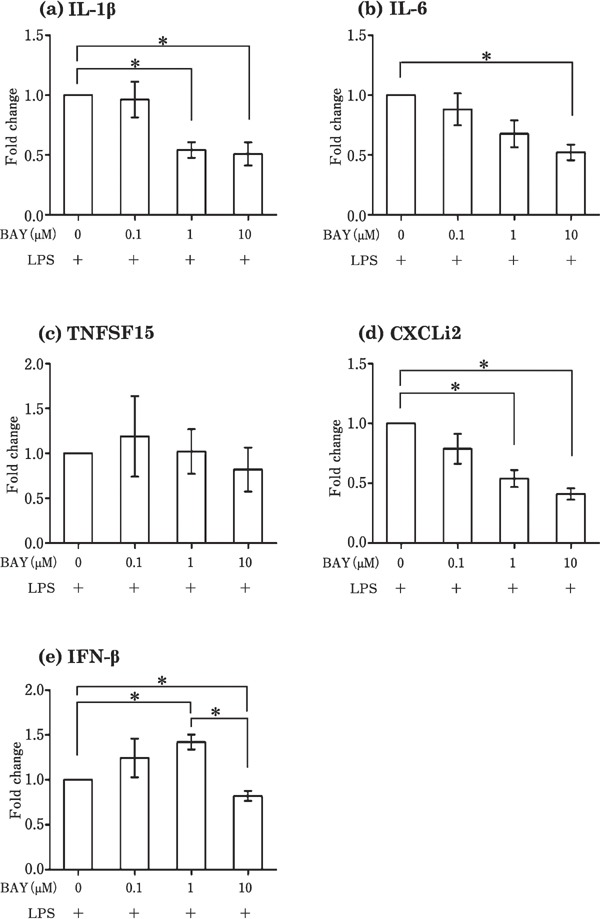
Effects of the NFκB inhibitor, BAY 11-7085, on the expression of genes induced by LPS in cultured follicular theca tissues. Cultured follicular theca tissues were treated with LPS (1000 ng/mL) in the presence or absence of BAY 11–7085 (0–10 µM). Values are the mean±SEM (n=5). Asterisks indicate significant differences (* P<0.05).
Fig. 8.
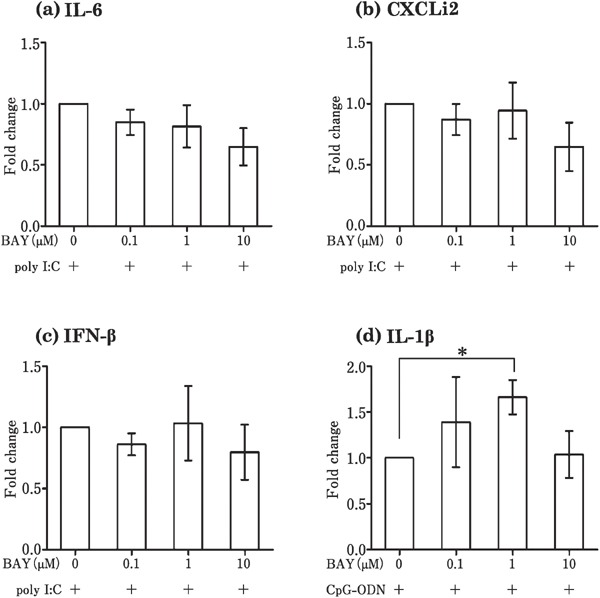
Effects of the NFκB inhibitor, BAY 11-7085, on the expression of genes induced by poly I:C (a, b, and c) and CpG-ODN (d) in cultured follicular theca tissues. Cultured follicular theca tissues were treated with poly I:C (100 µg/mL) or CpG-ODN (10 µg/mL) in the presence or absence of BAY 11-7085 (0–10 µM). Values are the mean±SEM (n=5). Asterisks indicate significant differences (* P<0.05).
Discussion
This study investigated whether the stimulation of TLRs in the theca could induce the expression of inflammatory cytokines (IL-1β, IL-6, TNSF15, and CXCLi2) and IFNs (IFN-α and IFN-β). The major findings included: (1) stimulation of the theca by TLR2 and TLR4 ligands (Pam3CSK4 and LPS, respectively) upregulated the expression of all cytokines and IFNs, except for IFN-α by LPS; (2) TLR3 ligand (poly I:C) induced the expression of IL-6, CXCLi2, and IFN-β; and (3) inhibition of NFκB suppressed the induction of IL-1β, IL-6, CXCLi2, and IFN-β by LPS. We also identified the expression of TLR1 (type 1 and 2), TLR2 (type 1 and 2), TLR3–5, 7, 15, and 21 in the theca interna and externa, whereas the PCR products of TLR1 (type 2) and TLR21 were faint. The results support the previous report that showed the expression of TLR2, 4, 5, and 7 in the theca among TLRs 1–5 and 7 (Subedi et al., 2007), and further demonstrate that TLR 3, 15, and 21 are also expressed in that tissue. These results suggested that these TLRs could recognize bacterial and viral patterns in the theca interna and externa.
The expressions of TLR2 and TLR4 suggest that they can recognize both Gram-positive and -negative bacteria. Increased expression of IL-1β, IL-6, TNSF15, CXCLi2, and IFNs by Pam3CSK4 and LPS supports that these TLRs are functional and could recognize the patterns of Gram-positive and -negative bacteria in the theca. However, stimulation with flagellin showed no significant effect on the expression of the detected cytokines, suggesting that recognition of flagellin by TLR5 may not play a significant role in cytokine induction. Generally, the real-time PCR data for each cytokine was highly variable. This may suggest the response to TLRs and the synthesis of cytokines may differ between individuals. Inflammatory cytokines are multifunctional in their modulation of immune cells. Salmonella enteritidis is a major bacterial species that can contaminate the egg yolk and other internal contents in the ovary and oviduct. Experimental inoculation of hens with Salmonella enteritidis resulted in the colonization of the ovarian stroma and the follicular wall, including the surface layers, theca, and occasionally, the granulosa layers (Takata et al., 2003). The LPS released by those Salmonella organisms may be recognized by TLR4 in the theca, inducing the expression of inflammatory cytokines and causing an immune response.
We reported that the ligands of TLR3, 4, and 21 upregulated the expression of IL-1β, IL-6, and CXCLi2, and the ligands of TLR5 and 7 induced the expression of IL-1β in cultured oviduct tissues (Sonoda et al., 2013; Kamimura et al., 2017). AvBDs and cathelicidins (CATHs) are antimicrobial peptides that are expressed in the hen oviduct (Yoshimura, 2015). We found that, in cultured vaginal cells, the expression of AvBD1 and 3 was upregulated by IL-1β, and that of AvBD12 was upregulated by IL-6 (Sonoda et al., 2013). Furthermore, the expression of CATH1, 2, and 3 in the vaginal cells was upregulated by IL-1β, and that of CATH3 in the uterus cells was upregulated by IL-6 (Abdel-Mageed et al., 2017). In the theca of follicles, the synthesis of AvBD12 was upregulated by IL-1β (Abdelsalam et al., 2012). CXCLi2 is a chemokine responsible for attracting heterophils (Redmond et al., 2011), monocytes (Barker et al., 1993), and CD3 + T cells (Min et al., 2001) to infection sites. We had previously reported that the injection of hens with LPS caused the upregulation of CXCLi2 expression along with T cell recruitment in the oviduct (Nii et al., 2011). Thus, the observed synthesis of IL-1β and CXCLi2 in response to bacterial pattern recognition in the current study may upregulate AvBD synthesis and leukocyte recruitment in the theca for protection against infection.
Apoptosis of the granulosa and theca cells causes follicular atresia (Kitamura et al., 2002), and this apoptosis is attributed to tumor necrosis factors (TNF) (Bridgham and Johnson, 2001; Johnson et al., 2007). The follicles may undergo atresia when infected by pathogenic microbes. As the expression of TNFSF15, a member of the TNF family, was upregulated by Pam3CSK4 and LPS, it may induce atresia in the follicles infected with Gram-positive and -negative bacteria.
The expression of IL-6, CXCLi2, and IFN-β was upregulated in the theca by the TLR3 ligand, poly I:C. This result suggests that immunomodulation by IL-6 and leukocyte recruitment by CXCLi2 may be induced by TLR3 stimulation. IFN-β is an antiviral factor that may be involved in the defense against dsRNA viruses. However, stimulation of the theca by R837, a TLR7 ligand, had no significant effect on the expression of any of the cytokines studied. The PCR products of TLR21 in both theca interna and externa were faint, and stimulation by CpG-ODN, a TLR21 ligand, upregulated the expression of only IL-1β. This may suggest that the ability to recognize DNA viruses may be less developed in those tissues. The fact that fewer cytokines were induced in response to the ligands of TLR 3, 7, and 21 suggests that the pattern recognition system for viruses is less developed than that for bacteria in the theca.
Inhibition of NFκB using BAY 11-7085 resulted in the suppression of IL-1β, IL-6, CXCLi2, and IFN-β in the theca stimulated by LPS, suggesting that NFκB is one of transcription factors responsible for the effect of LPS on those cytokines. In vaginal cells, NFκB is responsible for the expression of inflammatory cytokines and chemokines, including IL-1β, IL-6, and CXCLi2 in response to the ligands of TLR3, 4, and 21, and IL-1β in response to the ligands of TLR5 and 7 (Kamimura et al., 2017). The expression of IL-1β in the culture with CpG-ODN was upregulated by 1 µM BAY 11-7085. Although the exact reason for these results is not known, we hypothesize that the inhibition of NFκB by BAY 11-7085 might positively affect the other transcription factors or signal transduction factors involved in the induction of theses cytokines. Since the transcription factors responsible for the cytokine expression induced by the other TLRs in the theca remain unknown, further studies are necessary.
In conclusion, inflammatory cytokines are induced by the stimulation of theca tissues with the ligands of TLR2 and 4, suggesting that Gram-positive and -negative bacteria are recognized by those TLRs in the theca. The number of cytokines whose expression was upregulated by TLR3, TLR7, and TLR21 was limited. These results suggest that the innate immune system, including pattern recognition by TLRs and cytokine synthesis, is induced in the theca, and the ability of these cells to recognize bacterial patterns is more developed than that for viruses.
Acknowledgments
This work was supported by a Grant-in-Aid for Challenging Research (Exploratory) from the Japan Society for the Promotion of Science (No. 17K19323) awarded to YY.
References
- Abdel-Mageed AM, Nii T, Isobe N and Yoshimura Y. Modulatory roles of proinflammatory cytokines on the expression of cathelicidins in the lower regions of the oviduct of laying hens. Cytokine, 99: 66-72. 2017. [DOI] [PubMed] [Google Scholar]
- Abdelsalam M, Isobe N and Yoshimura Y. Effects of lipopolysaccharide and interleukins on the expression of avian β-defensins in hen ovarian follicular tissue. Poultry Science, 91: 2877-2884. 2012. [DOI] [PubMed] [Google Scholar]
- Abdelsalam M, Isobe N and Yoshimura Y. Effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokines and influx of leukocytes in the hen ovary. Poultry Science, 90: 2054-2062. 2011. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R and Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature, 413: 732-738. 2001. [DOI] [PubMed] [Google Scholar]
- Barker KA, Hampe A, Stoeckle MY and Hanafusa H. Transformation-associated cytokine 9E3/CEF4 is chemotactic for chicken peripheral blood mononuclear cells. Journal of Virology, 67: 3528-3533. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua A, Michiue H and Yoshimura Y. Changes in the localization of MHC class II positive cells in hen ovarian follicles during the processes of follicular growth, postovulatory regression and atresia. Reproduction, 121: 953-957. 2001 [PubMed] [Google Scholar]
- Bridgham JT and Johnson AL. Expression and regulation of Fas antigen and tumor necrosis factor receptor type I in hen granulosa cells. Biology of Reproduction, 65: 733-739. 2001. [DOI] [PubMed] [Google Scholar]
- Brownlie R and Allan B. Avian Toll-like receptors. Cell and Tissue Research, 343: 121-130. 2011. [DOI] [PubMed] [Google Scholar]
- de Zoete MR, Bouwman LI, Keestra AM and van Putten JPM. Cleavage and activation of a Toll-like receptor by microbial proteases. Proceedings of the National Academy of Sciences of the United States of America, 108: 4968-4973. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AL, Ratajczak C, Haugen MJ, Liu HK and Woods DC. Tumor necrosis factor-related apoptosis inducing ligand expression and activity in hen granulosa cells. Reproduction, 133: 609-616. 2007. [DOI] [PubMed] [Google Scholar]
- Johnson AL. Reproduction in the Female. In: Sturkie's Avian Physiology (Whittow G.C ed.). pp. 569-596. Academic Press. New York. 2000. [Google Scholar]
- Kamimura T, Isobe N and Yoshimura Y. Effects of inhibitors of transcription factors, nuclear factor-κB and activator protein 1, on the expression of proinflammatory cytokines and chemokines induced by stimulation with Toll-like receptor ligands in hen vaginal cells. Poultry Science, 96: 723-730. 2017. [DOI] [PubMed] [Google Scholar]
- Keestra AM, de Zoete MR, van Aubel RA and van Putten JP. The central leucine-rich repeat region of chicken TLR16 dictates unique ligand specificity and species-specific interaction with TLR2. Journal of Immunology, 178: 7110-7119. 2007. [DOI] [PubMed] [Google Scholar]
- Kitamura A, Yoshimura Y and Okamoto T. Changes in the Populations of Mitotic and Apoptotic Cells in White Follicles During Atresia in Hens. Poultry Science, 81: 408-413. 2002. [DOI] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 -ΔΔCT method. Methods, 25: 402-408. 2001. [DOI] [PubMed] [Google Scholar]
- Michailidis G, Theodoridis A and Avdi M. Transcriptional profiling of Toll-like receptors in chicken embryos and in the ovary during sexual maturation and in response to Salmonella enteritidis infection. Animal Reproduction Science, 122: 294-302. 2010. [DOI] [PubMed] [Google Scholar]
- Min W, Lillehoj HS, Burnside J, Weining KC, Staeheli P and Zhu JJ. Adjuvant effects of IL-1β, IL-2, IL-8, IL-15, IFN-α, IFN-γ TGF-β4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine, 20: 267-274. 2001. [DOI] [PubMed] [Google Scholar]
- Nerren JR, He H, Genovese K and Kogut MH. Expression of the avian-specific toll-like receptor 15 in chicken heterophils is mediated by Gram-negative and Gram-positive bacteria, but not TLR agonists. Veterinary Immunology and Immunopathology, 136: 151-156. 2010. [DOI] [PubMed] [Google Scholar]
- Nii T, Sonoda Y, Isobe N and Yoshimura Y. Effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokines and the subsequent recruitment of immunocompetent cells in the oviduct of laying and molting hens. Poultry Science, 90: 2332-2341. 2011. [DOI] [PubMed] [Google Scholar]
- Redmond SB, Chuammitri P, Andreasen CB, Palić D and Lamont SJ. Proportion of circulating chicken heterophils and IL8 expression in response to Salmonella enteritidis are affected by genetic line and immune modulating diet. Veterinary Immunology and Immunopathology, 140: 323-328. 2011. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Abdel Mageed AM, Isobe N and Yoshimura Y. Induction of avian β-defensins by CpG oligodeoxynucleotides and proinflammatory cytokines in hen vaginal cells in vitro. Reproduction, 145: 621-631. 2013. [DOI] [PubMed] [Google Scholar]
- St Paul M, Brisbin JT, Abdul-Careem MF and Sharif S. Immunostimulatory properties of Toll-like receptor ligands in chickens. Veterinary Immunology and Immunopathology, 152: 191-199. 2013. [DOI] [PubMed] [Google Scholar]
- Subedi K, Isobe N, Nishibori M and Yoshimura Y. Changes in the expression of toll-like receptor mRNAs during follicular growth and in response to lipopolysaccharide in the ovarian follicles of laying hens. Journal of Reproduction and Development, 53: 1227-1235. 2007. [DOI] [PubMed] [Google Scholar]
- Takata T, Liang J, Nakano H and Yoshimura Y. Invasion of Salmonella Enteritidis in the tissues of reproductive organs in laying Japanese quail: An immunocytochemical study. Poultry Science, 82: 1170-1173. 2003. [DOI] [PubMed] [Google Scholar]
- Woods DC, Schorey JS and Johnson AL. Toll-like receptor signaling in hen ovarian granulosa cells is dependent on stage of follicle maturation. Reproduction, 137: 987-996. 2009. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y. Avian β-defensins expression for the innate immune system in hen reproductive organs. Poultry Science, 94: 804-809. 2015. [DOI] [PubMed] [Google Scholar]


