Abstract
Autophagy, an intracellular bulk protein degradation system in skeletal muscle, is increased under catabolic conditions resulting in muscle atrophy. This study aimed to investigate the effects of insulin, insulin-like growth factor (IGF)-I, and amino acids on autophagy (LC3-II content and expression of autophagy-related genes) in chick myotubes. Chick myotubes were incubated with insulin (1 µg/ml), IGF-I (100 ng/ml), and amino acids for 3 h. The LC3-II content, an index of autophagosome formation, and mRNA expression of LC3B and GABARAPL1 were significantly decreased by insulin. The LC3-II content, but not mRNA expression of autophagy-related genes, was also significantly decreased by IGF-I. The LC3-II content and LC3B mRNA level were also significantly decreased by amino acids. The mRNA expression of atrogin-1/MAFbx, a muscle-specific ubiquitin ligase, was also significantly decreased by insulin, IGF-I, and amino acids in chick myotubes. These results indicated that insulin, IGF-I, and amino acids regulate autophagy as well as the ubiquitin–proteasome proteolytic pathway in chick myotubes.
Keywords: amino acids, autophagy, chick myotubes, IGF-I, insulin, LC3
Introduction
In skeletal muscle, protein mass is determined by the balance between the rate of protein synthesis and that of protein degradation. Similar to other tissues, protein degradation in skeletal muscle is controlled by autophagy and the ubiquitin–proteasome proteolytic pathway (Masiero et al., 2009). These proteolytic pathways are increased under several catabolic conditions that result in muscle atrophy (Sandri, 2010, 2013).
Macroautophagy, hereafter referred to as autophagy, is a lysosomal protein degradation pathway in the cell and a bulk degradative process involving protein aggregates and organelles. During autophagy, cytosolic materials, such as protein and organelles, are enclosed by the autophagosome. The autophagosome fuses with the lysosome, which provides lysosomal hydrolases, to form an autolysosome (Mizushima, 2007; Tooze and Yoshimori, 2010). Autophagy provides amino acids, which can be used for protein synthesis, energy production, and gluconeogenesis in physiological and nutritional conditions (Kuma and Mizushima, 2010). In addition, autophagy is essential to maintain skeletal muscle mass under catabolic conditions (Masiero et al., 2009; Seiliez et al., 2010). However, the regulation of autophagy in chicken skeletal muscle is largely unknown.
Muscle proteolysis under catabolic conditions is primarily due to the activation of the ubiquitin–proteasome proteolytic pathway (Lecker et al., 2004), whereby proteins destined to be degraded are linked to a chain of ubiquitin molecules that target these proteins for rapid breakdown by the proteasome (Glickman and Ciechanover, 2002). Evidence suggests that atrogin-1, an E3 ubiquitin ligase that is also referred to as MAFbx (muscle atrophy F-box), plays a pivotal role in muscle atrophy (Gomes et al., 2001). Its expression is increased in catabolic conditions that result in muscle atrophy (Gomes et al., 2001). The factors and mechanisms regulating the ubiquitin–proteasome proteolytic pathway in chicken skeletal muscle remain poorly understood.
Insulin, insulin-like growth factor (IGF) -I, and amino acids regulate protein synthesis and protein degradation in skeletal muscle (Prod'homme et al., 2004; Liu et al., 2006). They play an important role in growth and maintain anabolic effects in animals. Insulin and IGF-I inhibit overall protein breakdown, the degradation of myofibrillar proteins (Sacheck et al., 2004), and the expression of muscle-specific ubiquitin ligases (atrogin-1/MAFbx and MuRF1) (Sacheck et al., 2004; Tesseraud et al., 2007; Dupont et al., 2008; Saneyasu et al., 2017; Nakashima et al., 2017). Furthermore, insulin and IGF-I inhibit autophagy in skeletal muscle and muscle cells (Seiliez et al., 2010; Naito et al., 2013; Suryawan and Davis, 2014). Amino acids inhibit protein degradation by suppressing the ubiquitin–proteasome pathway (the expression of atrogin-1/MAFbx and MuRF1) in skeletal muscles (Tesseraud et al., 2007; Suryawan and Davis, 2014). However, the mechanism of protein degradation inhibition by amino acids in chicken skeletal muscle is not well understood. Although autophagy is regulated by amino acids in mammalian muscle (Naito et al., 2013; Suryawan and Davis, 2014), this effect has not been reported in chicken skeletal muscle. Thus, although the regulatory effects of insulin, IGF-I, and amino acids on autophagy in skeletal muscle are well known, such effects in chicken skeletal muscle are largely unknown. The present study investigated the effects of insulin, IGF-I, and amino acids on the autophagy–lysosome proteolytic pathway in chick myotubes.
This study demonstrated that insulin, IGF-I, and amino acids suppress autophagy at the post-translational (LC3-II content, an index of autophagosome formation) and transcriptional levels (expression of autophagy-related genes) as well as the ubiquitin–proteasome proteolytic pathway (expression of atrogin-1/MAFbx) in chick myotubes.
Materials and Methods
Cell Culture
Myoblasts were isolated from the thigh muscles of 13-day-old chick embryos (Nakashima et al., 2017). Briefly, the muscle tissue obtained from the embryo was digested with dispase (Gibco, Waltham, MA USA), and the cell suspension was transferred to an uncoated culture dish to allow fibroblast attachment. Cells were counted and then plated onto gelatin-coated 6-well plates (Iwaki SciTech, Yoshida-cho, Shizuoka, Japan). Chick myoblasts were cultured in M-199 medium containing 15% calf serum and 2.5% chicken embryo extract and were grown at 37°C in a humidified atmosphere of 5% CO2 in air for 7 days. On day 7, the cells had formed myotubes and were incubated for 3 h in serum-free M-199 medium containing insulin (1 µg/ml, from porcine pancreas: Sigma-Aldrich, St. Louis, MO, USA) or IGF-I (100 ng/ml, R3-IGF-I: Sigma-Aldrich). To assess the effects of amino acids on myotubes, they were incubated for 3 h with amino acid-free minimum essential medium (0× amino acids in MEM) containing Earle's balanced salt solution and MEM vitamin solution (Gibco) or complete MEM (1× amino acids in MEM) containing Earle's balanced salt solution, MEM vitamin solution (Gibco), glutamine (0.292 g/l), MEM amino acids solution (Gibco), and non-essential amino acids solution (Gibco).
All experimental procedures were conducted in accordance with the guidelines of the Animal Care and Use Committee of the NARO Institute of Livestock and Grassland Science.
Real-time Polymerase Chain Reaction (PCR)
Total RNA was extracted from chick myotubes using TRIzol reagent (Invitrogen, USA) according to the manufacturer's directions. Complementary DNA was synthesized from total RNA using a random primer (TaKaRa, Shiga, Japan) and ReverTra Ace (TOYOBO, Tokyo, Japan). The sequences of the primers were as follows: atrogin-1/MAFbx, forward: 5′-CCAACAACCCAGAGACCTGT-3′ and reverse: 5′-GGAGCTTCACACGAACATGA-3′; microtubule-associated protein 1 light chain 3B (LC3B), forward: 5′-TCCGAGATCAGCATCCAACT-3′ and reverse: 5′-CACCATGCTGTGTCCGTTC-3′; GABA(A) receptor-associated protein like 1 (GABARAPL1), forward: 5′-CCGACAGAGTCCCCGTAATT-3′ and reverse: 5′-ATGGTAGCACTTGTGGGAGG-3′; autophagy-related 12 (ATG12), forward: 5′-CGGAAAGGACCCCAGAGAG-3′ and reverse: 5′-CTTGATGAAGTCGCACAGGC-3′; cathepsin B, forward: 5′-CAAGCTCAACACCACTGGAA-3′ and reverse: 5′-TCAAAGGTATCCGGCAAATC-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward: 5′-CCTCTCTGGCAAAGTCCAAG-3′ and reverse: 5′-CATCTGCCCATTTGATGTTG-3′; 18S rRNA, forward: 5′-AAACGGCTACCACATCCAAG-3′ and reverse: 5′-CCTCCAATGGATCCTCGTTA-3′. The mRNA levels were measured by real-time PCR analysis using a LightCycler® instrument (Roche Diagnostics, Basel, Switzerland) and the QuantiTect SYBR Green PCR system (Qiagen, Hilden, Germany). The level of GAPDH or 18S rRNA was measured as an internal control.
Western Blotting
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in RIPA lysis buffer system (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The lysate was centrifuged at 14,000 rpm for 5min at 4°C, and the supernatant was collected. Total protein concentration was estimated by bicinchoninic acid assay using a commercial kit (Pierce, Waltham, MA, USA) with bovine serum albumin as a standard.
Western blot analysis was conducted as previously described (Shimamoto et al., 2016). In brief, equal amounts of protein were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% (v/v) Tween-20 for 1 h at room temperature. Subsequently, the blocked membranes were incubated with primary antibody overnight at 4°C. After incubation with primary antibody, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Chicago, IL, USA) for 1 h at room temperature. The bands were visualized using Western Blotting Detection Reagent (GE Healthcare, UK) and LAS-3000 mini (Fujifilm, Tokyo, Japan). Relative band intensity was quantified using ImageJ software (National Institutes of Health, USA). Antibodies against LC3B (#2775) and actin (A2066) were purchased from Cell Signaling Technology (USA) and Sigma-Aldrich (USA), respectively.
Statistical Analysis
Data were analyzed using the Student's t-test. A P value of <0.05 was considered to be statistically significant. Data were expressed as meansµstandard deviation (SD).
Results and Discussion
This study investigated the effects of insulin, IGF-I, and amino acids on autophagy in chick myotubes. Insulin, IGF-I, and amino acids increase protein synthesis and decrease protein degradation in skeletal muscle (Liu et al., 2006). However, the role of insulin, IGF-I, and amino acids on the skeletal muscle proteolytic systems (autophagy–lysosome and ubiquitin–proteasome proteolytic pathways) is poorly understood in chicken skeletal muscle.
The effect of insulin on the LC3-II content, an index of autophagosome formation, is shown in Fig. 1A. The LC3-II content was significantly decreased in chick myotubes incubated with insulin (1 µg/ml) for 3 h, indicating that insulin suppresses autophagy in chick skeletal muscle cells. We also examined the effect of insulin on the mRNA expression of autophagy-related genes (LC3B, GABARAPL1, ATG12, and cathepsin B) and atrogin-1/MAFbx in chick myotubes (Fig. 1B). The mRNA expression of LC3B and GABARAPL1, but not ATG12 and cathepsin B was significantly decreased by insulin, indicating that insulin suppresses autophagy at the transcriptional level in chick myotubes. Atrogin-1/MAFbx mRNA expression was significantly decreased by insulin, indicating that insulin suppresses autophagy and ubiquitin–proteasome proteolytic pathways in chick myotubes. Autophagy plays an important role under catabolic conditions, wherein protein aggregates and organelles are degraded via the formation of autophagosomes, which fuse with lysosomes (Mizushima, 2007; Tooze and Yoshimori, 2010). During autophagosome formation, LC3 is lipidated, converting LC3-I to LC3-II (lipidation) (Kabeya et al., 2000). Therefore, the LC3-II content is an index of the autophagy. Measuring the conversion of LC3-I to LC3-II by western blotting is considered a reliable assay to determine autophagosome formation (Mizushima et al., 2010; Naito et al., 2013).
Fig. 1.
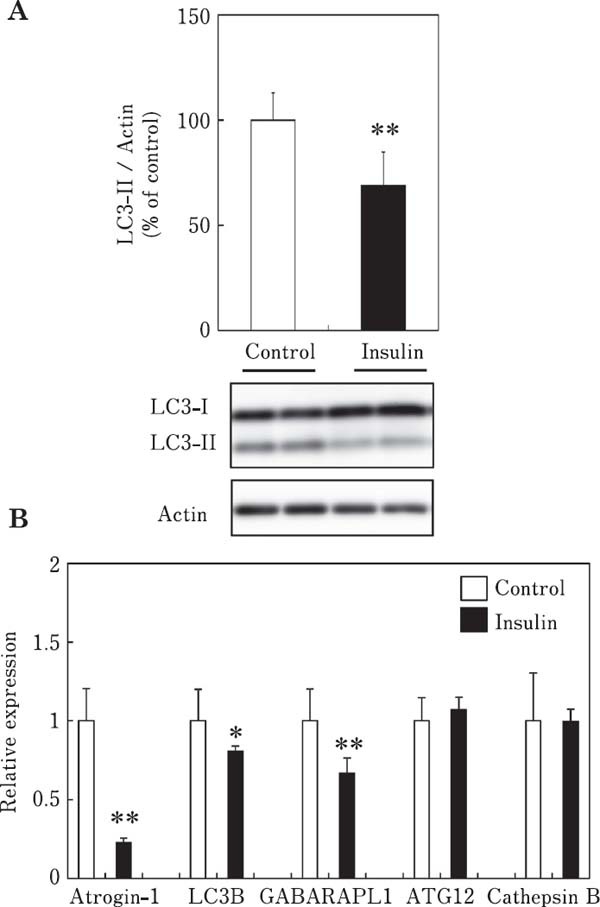
Effects of insulin on the LC3-II content (A) and mRNA expression of atrogin-1/MAFbx and autophagy-related genes (LC3B, GABARAPL1, ATG12, and cathepsin B) (B) in chick myotube cultures. Cells were incubated for 3 h in serum-free M-199 medium with insulin (1 µg/ml). Data are expressed as the mean±SD (n=5–6). Values are significantly different at * P<0.05; ** P<0.01.
Atrogin-1/MAFbx, a muscle-specific ubiquitin ligase, is highly expressed in skeletal muscles undergoing atrophy, and its expression is related to the rate of muscle protein degradation and thus, muscle size. Atrogin-1/MAFbx plays a critical role in muscle proteolysis, and the level of atrogin-1/MAFbx gene expression is a reliable index of muscle proteolysis (Ohtsuka et al., 2011).
The effect of IGF-I on the LC3-II content is shown in Fig. 2A. The LC3-II content was significantly decreased in chick myotubes incubated with IGF-I (100 ng/ml) for 3 h, indicating that IGF-I suppresses autophagy in chick skeletal muscle cells. We examined the effect of IGF-I on the mRNA expression of the above-mentioned autophagy-related genes and atrogin-1/MAFbx in chick myotubes (Fig. 2B). The mRNA expression of LC3B, GABARAPL1, ATG12, and cathepsin B was not affected by IGF-I, indicating that IGF-I had no effect on autophagy at the transcriptional level in chick myotube within 3 h of incubation. However, atrogin-1/MAFbx mRNA expression was significantly decreased by IGF-I, indicating that IGF-I suppresses autophagy as well as the ubiquitin–proteasome proteolytic pathways in chick myotubes.
Fig. 2.
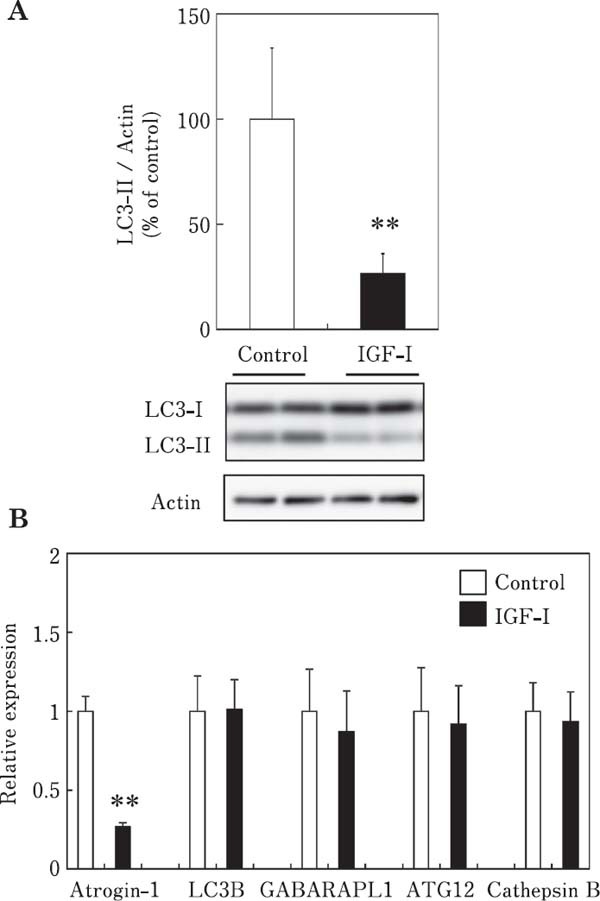
Effects of IGF-I on the LC3-II content (A) and mRNA expression of atrogin-1/MAFbx and autophagy-related genes (LC3B, GABARAPL1, ATG12, and cathepsin B) (B) in chick myotube cultures. Cells were incubated for 3 h in serum-free M-199 medium with IGF-I (100 ng/ml). Data are expressed as the mean±SD (n=6). Values are significantly different at ** P<0.01.
Insulin and IGF-I are anabolic factors in skeletal muscle and also regulate autophagy in skeletal muscle (Seiliez et al., 2010; Naito et al., 2013; Suryawan and Davis, 2014). Although insulin and IGF-I have a regulatory effect on autophagy in skeletal muscle, their effects in chicken skeletal muscle have not been reported. In the present study, insulin inhibited autophagy at the transcriptional and post-translational levels in chick myotubes. In contrast, IGF-I inhibited autophagy at the post-translational level but not at the transcriptional level in chick myotubes. These results suggested that IGF-I, but not insulin, regulates autophagy at the post-translational rather than the transcriptional level in chicken skeletal muscle.
Although it is well known that insulin and IGF-I have a regulatory effect on the expression of atrogin-1/MAFbx in mammalian skeletal muscle, their effects in chicken muscle cells are largely unknown. We previously reported that IGFI suppresses the expression of atrogin-1/MAFbx in chick myotubes (Nakashima et al., 2017). In the present study, insulin suppressed the mRNA expression of atrogin-1/MAFbx in chick myotubes. Together, these findings indicate that insulin and IGF-I regulate the expression of atrogin-1/MAFbx in chicken skeletal muscle. Insulin reportedly suppresses atrogin-1/MAFbx expression in quail QT6 fibroblasts (Tesseraud et al., 2007) and chicken skeletal muscle (Dupont et al., 2008; Saneyasu et al., 2017). The results of the present study support these previous findings and extend them by demonstrating that insulin and IGF-I also regulate the mRNA expression of atrogin-1/MAFbx in chick skeletal muscle cells. The present study demonstrated that insulin and IGF-I regulate the ubiquitin–proteasome proteolytic pathway as well as autophagy in chicken skeletal muscle.
The effect of amino acids on the LC3-II content is shown in Fig. 3A. LC3-II content was significantly decreased in chick myotubes incubated with amino acids for 3 h, indicating that amino acids suppress autophagy in chick skeletal muscle cells. In addition, we examined the effects of amino acids on the expression of the afore-mentioned autophagy-related genes and atrogin-1/MAFbx in chick myotubes (Fig. 3B). The mRNA expression of LC3B, but not GABARAPL1, ATG12, and cathepsin B was significantly decreased by amino acids, indicating that amino acids suppress autophagy at the transcriptional level in chick myotubes. Atrogin-1/MAFbx mRNA expression was significantly decreased by amino acids, indicating that amino acids suppress the ubiquitin–proteasome proteolytic pathways in chick myotubes. Amino acids have been reported to downregulate the expression of the autophagy-related genes GABARAPL1, LC3B, and ATG4B, but not ATG12L, of and atrogin-1/MAFbx in rainbow trout myoblasts (Seiliez et al., 2012). This result is consistent with that of the present study. In the present study, amino acids inhibited autophagy at the posttranslational and transcriptional levels in chicken skeletal muscle.
Fig. 3.
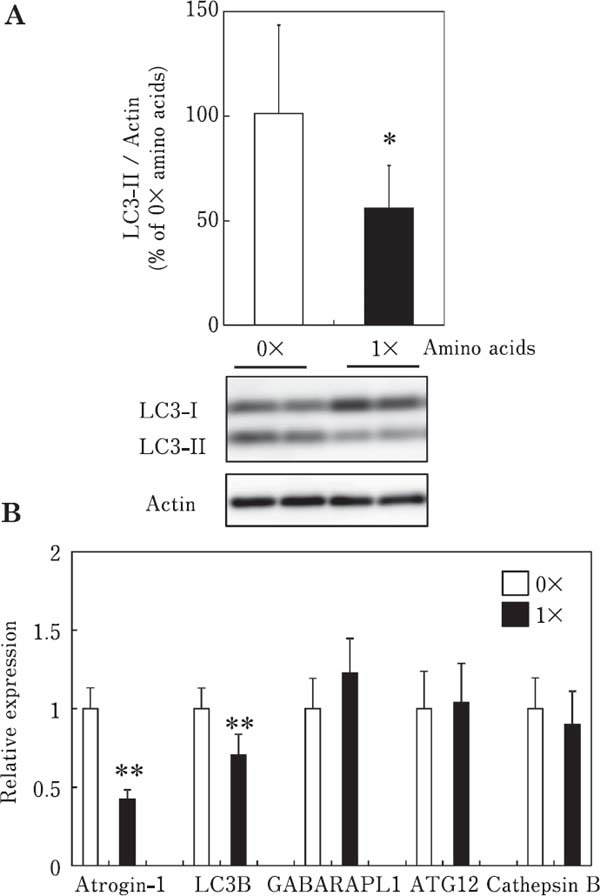
Effects of amino acids on the LC3-II content (A) and mRNA expression of atrogin-1/MAFbx and autophagy-related genes (LC3B, GABARAPL1, ATG12, and cathepsin B) (B) in chick myotube cultures. Cells were incubated for 3 h in 0× or 1× amino acids in serum-free MEM. Data are expressed as the mean±SD (n=5–6). Values are significantly different at * P<0.05; ** P<0.01.
Autophagy, an intracellular bulk protein degradation system, is increased under different catabolic conditions. LC3 lipidation (post-translational lipid modification of LC3 protein) is widely used as an index of autophagy (Mizushima et al., 2010; Naito et al., 2013). Recently, several studies have demonstrated that autophagy is involved in the transcriptional regulation of autophagy-related genes in the skeletal muscle (Mammucari et al., 2007; Zhao et al., 2007; Seiliez et al., 2010, 2012). The expression of autophagy-related genes is regulated by forkhead box O (FOXO) transcription factors such as FOXO1 and FOXO3a in skeletal muscle (Mammucari et al., 2007; Zhao et al., 2007). Therefore, expression induction (Mammucari et al., 2007; Zhao et al., 2007; Seiliez et al., 2010, 2012) and lipidation of the LC3 protein at the post-translational level (Kabeya et al., 2000) are essential for autophagy. The transcription factors FOXO1 and FOXO3a have been reported to regulate the expression of autophagy-related genes, such as LC3B, GABARAPL1, Vps34, ULk2, and ATG12 in skeletal muscle (Mammucari et al., 2007; Zhao et al., 2007). Furthermore, atrogin-1/MAFbx, a muscle-specific ubiquitin ligase, is highly expressed in skeletal muscles undergoing atrophy, and its expression is related to the rate of muscle protein degradation and, thus, muscle size. FOXO1 and FOXO3a also activate the transcription of atrogin-1/MAFbx in skeletal muscle (Sandri et al., 2004). Furthermore, FOXO transcription factors stimulate protein degradation and autophagy in skeletal muscle (Mammucari et al., 2007; Zhao et al., 2007). The FOXO transcription factors may be important in regulating the expression of autophagy-related genes and muscle-specific ubiquitin ligases (atrogin-1/MAFbx and MuRF1, E3 ligase in the ubiquitin–proteasome proteolytic pathway) in skeletal muscle.
We demonstrated for the first time that insulin, IGF-I, and amino acids suppress autophagy at the transcriptional and post-translational levels in primary chicken muscle cultures. Our findings indicate that LC3, at the transcriptional and post-translational levels, is a useful marker of autophagy in chicken skeletal muscles. With this marker, other factors such as hormones and nutrients that influence autophagy can be determined, allowing the regulation of avian muscle growth in poultry production.
Because this study was conducted using cultured chicken cells, which do not directly represent in vivo conditions, further investigations with the administration of insulin, IGF-I, and amino acids in vivo will be necessary. The present study showed that insulin, IGF-I, and amino acids suppress the autophagy–lysosome proteolytic pathway as well as the ubiquitin–proteasome proteolytic pathway in chick myotubes.
Acknowledgments
We are grateful to Dr. Daichi Ijiri (Kagosima University) and Saki Shimamoto (Kagoshima University) for helpful suggestions and technical advice.
References
- Dupont J, Tesseraud S, Derouet M, Collin A, Rideau N, Crochet S, Godet E, Cailleau-Audouin E, Metayer-Coustard S, Duclos MJ, Gespach C, Porter TE, Cogburn LA and Simon J. Insulin immuno-neutralization in chicken: effects on insulin signaling and gene expression in liver and muscle. Journal of Endocrinology, 197: 531-542. 2008. [DOI] [PubMed] [Google Scholar]
- Glickman MH and Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiological Reviews, 82: 373-428. 2002. [DOI] [PubMed] [Google Scholar]
- Gomes MD, Lecker SH, Jagoe RT, Navon A and Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proceedings of the National Academy of Sciences of the United States of America, 98: 14440-14445. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO Journal, 19: 5720-5728. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A and Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Seminers in Cell Developmental Biology, 21: 683-690. 2010. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE and Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB Journal, 18: 39-51. 2004. [DOI] [PubMed] [Google Scholar]
- Liu Z, Long W, Fryburg DA and Barrett EJ. The regulation of body and skeletal muscle protein metabolism by hormones and amino acids. Journal of Nutrition, 136: 212S-217S. 2006. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S and Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metabolism, 6: 458-471. 2007. [DOI] [PubMed] [Google Scholar]
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S and Sandri M. Autophagy is required to maintain muscle mass. Cell Metabolism, 10: 507-515. 2009. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes & Development, 21: 2861-73. 2007. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T and Levine B. Methods in mammalian autophagy research. Cell, 140: 313-326. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito T, Kuma A and Mizushima N. Differential contribution of insulin and amino acids to the mTORC1-autophagy pathway in the liver and muscle. Journal of Biological Chemistry, 288: 21074-21081. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Ishida A, Shimamoto S, Ijiri D and Ohtsuka A. Effects of insulin-like growth factor-I on the expression of atrogin-1/MAFbx in chick myotube cultures. Journal of Poultry Science, 54: 247-252. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka A, Kawatomi N, Nakashima K, Araki T and Hayashi K. Gene expression of muscle-specific ubiquitin ligase, atrogin-1/MAFbx, positively correlates with skeletal muscle protelysis in food-deprivated broiler chickens. Journal of Poultry Science, 48: 92-96. 2011. [Google Scholar]
- Prod'homme M, Rieu I, Balage M, Dardevet D and Grizard J. Insulin and amino acids both strongly participate to the regulation of protein metabolism. Current Opinion Clinical Nutrition and Metabolism Care, 7: 71-77. 2004. [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Ohtsuka A, McLary SC and Goldberg AL. IGF-1 stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin-ligases, atrogin-1 and MuRF1. American Journal of Physiology Endocrinology and Metabolism, 287: E591-E601. 2004. [DOI] [PubMed] [Google Scholar]
- Sandri M. Autophagy in skeletal muscle. FEBS Letters, 584: 1411-1416. 2010. [DOI] [PubMed] [Google Scholar]
- Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. International Journal of Biochemistry & Cell Biology, 45: 2121-2129. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH and Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell, 117: 399-412. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyasu T, Tsuchii N, Nakano Y, Kitashiro A, Tsuchihashi T, Shindo H, Honda K and Kamisoyama H. Effects of short-term fasting on the Akt-mediated pathway involved in protein metabolism in chicken skeletal muscle. Domestic Animal Endocrinology, 61: 54-61. 2017. [DOI] [PubMed] [Google Scholar]
- Seiliez I, Gabillard JC, Riflade M, Sadoul B, Dias K, Averous J, Tesseraud S, Skiba S and Panserat S. Amino acids downregulate the expression of several autophagy-related genes in rainbow trout myoblasts. Autophagy, 8: 364-375. 2012. [DOI] [PubMed] [Google Scholar]
- Seiliez I, Gutierrez J, Salmeron C, Skiba-Cassy S, Chauvin C, Dias K, Kaushik S, Tesseraud S and Panserat S. An in vivo and in vitro assessment of autophagy-related gene expression in muscle of rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology, 157: 258-266. 2010. [DOI] [PubMed] [Google Scholar]
- Shimamoto S, Ijiri D, Nakashima K, Kawaguchi M, Ishimaru Y, Furukawa A and Ohtsuka A. Clenbuterol changes phosphorylated FOXO1 localization and decreases protein degradation in the sartorius muscle of neonatal chicks. Bioscience, Biotechnology, and Biochemistry, 80: 1499-1504. 2016. [DOI] [PubMed] [Google Scholar]
- Suryawan A and Davis TA. Regulation of protein degradation pathways by amino acids and insulin in skeletal muscle of neonatal pigs. Journal of Animal Science and Biotechnology, 5: 8. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseraud S, Metayer-Coustard S, Boussaid S, Crochet S, Audouin E, Derouet M and Seiliez I. Insulin and amino acid availability regulate atrogin-1 in avian QT6 cells. Biochemical and Biophysical Research Communications, 357: 181-186. 2007. [DOI] [PubMed] [Google Scholar]
- Tooze SA and Yoshimori T. The origin of the autophagosomal membrane. Nature Cell Biology, 12: 831-835. 2010. [DOI] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH and Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metabolism, 6: 472-483. 2007. [DOI] [PubMed] [Google Scholar]


