Abstract
The mutant plumage color “extended brown (EB)” of the blue-breasted quail was genetically investigated. Mating experiments revealed that the EB plumage is controlled by an autosomal, incompletely dominant allele, for which we propose the symbol Eb. The EB plumage is characterized by dark brown color, and homozygotes for this mutation generally showed darker pigmentation than the heterozygotes. DNA sequencing and PCR-RFLP analyses of the EB mutants showed a rigid association between the EB plumage and a G-to-A nucleotide substitution at position 274 in the melanocortin 1-receptor gene (MC1R), clearly indicating that MC1R is the candidate gene for the EB plumage color in the blue-breasted quail.
Keywords: blue-breasted quail, extended brown, melanocortin 1-receptor, mutant, plumage color
Introduction
The blue-breasted quail (Coturnix chinensis) is sometimes reared as a pet owing to its small body size and varied plumage colors. Moreover, it is suitable for use as a laboratory research animal owing to characteristics including ease of handling, small body size, hardiness, high egg-laying performance, and short generation interval (Tsudzuki, 1994). It is desirable for a laboratory research animal to have many mutations to enable studies in various research fields, as is seen with chickens and Japanese quail (Coturnix japonica) (Smyth, 1990; Tsudzuki, 2008). However, few studies have been reported on mutations in the blue-breasted quail, except for those on the “light gray” and “brown” plumage mutations (Tsudzuki, 1995a, b). To increase the importance of the blue-breasted quail as a laboratory research animal, it is desirable to identify more mutations in this bird and analyze the molecular basis of the respective mutations.
In birds, plumage colors are manifested as a combination of structural and chemical colors. Structural color is produced by light reflection, which depends on the physical structure of the feathers. In contrast, chemical color is the result of pigments included in the feather follicles. Melanin is one of the typical chemical color-producing pigments (Oribe et al., 2009), and exists in two types, i.e., eumelanin (black to brown) and phaeomelanin (red to yellow). The colors of the multicolored avian plumage are mostly a manifestation of a combination of these melanin types (Akiyama et al., 2005). Several genes are associated with melanin production in various animal species (Jackson, 1994; Hzeiter and Schöneberg, 2010). Among them, the melanocortin 1-receptor gene (MC1R) is perhaps the most important; MC1R, a seven-transmembrane helix-bearing G-protein coupled receptor on melanocytes, plays a crucial role in determining the melanin type. MC1R responds to two ligands, the signaling molecules α-melanocyte stimulating hormone (α-MSH) and agouti signaling protein (ASIP) (Wolf Horrell et al., 2016). α-MSH activates MC1R as an agonist and increases the intracellular level of cyclic adenosine monophosphate (cAMP), leading to the generation of eumelanin in the melanocytes (Mountjoy et al., 1992; Robbins et al., 1993). In contrast, ASIP functions as an inverse agonist to inactivate MC1R, resulting in the reduction of the cAMP level and the subsequent production of phaeomelanin (Lu et al., 1994).
In chickens, the “extended black” (E) locus controls plumage color and pattern, and the molecular basis of its actions is rooted in the MC1R gene (Smyth, 1990; Takeuchi et al., 1996b). The E locus includes six alleles produced by nucleotide substitutions, which affect the phenotype by producing different plumage colors (Ling et al., 2003). Among these alleles, the most dominant allele, E, produces black plumage all over the body. Similarly, in Japanese quail, the well-known mutant called “extended brown” or “black” shows dark brown plumage (Somes, 1979; Cheng and Kimura, 1990; Tsudzuki et al., 1990; Tsudzuki, 2008) and this mutant also has its molecular basis rooted in MC1R (Nadeau et al., 2006). In chickens and Japanese quail, the black or dark brown colored plumage is caused by the nucleotide substitution c.274G>A in MC1R, which results in the non-synonymous amino acid substitution Glu92Lys (Takeuchi et al., 1996b; Nadeau et al., 2006). The association of MC1R with plumage color phenotypes is well studied in chickens and Japanese quail (Takeuchi et al., 1996b; Kerje et al., 2003; Ling et al., 2003; Nadeau et al., 2006). In contrast, no such studies focusing on the blue-breasted quail have been conducted.
We identified a mutant plumage color, that appeared as dark brown plumage, in the blue-breasted quail (Figs. 1 and 2), similar to the extended brown plumage of the Japanese quail. We named this mutant plumage phenotype of the blue-breasted quail as “extended brown” (EB) and developed a distinct strain. The similarity in the plumage colors between the blue-breasted quail and the Japanese quail suggested the possibility that the EB phenotype of the blue-breasted quails is caused by the same mutation in the MC1R gene as the one responsible for plumage color variation in the Japanese quail.
Fig. 1.
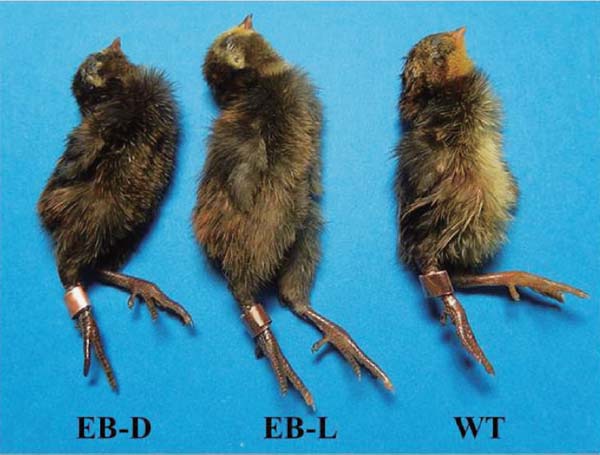
Newly hatched extended-brown (EB) mutant and wild-type (WT) chicks of the blue-breasted quail. The EB mutant occurs with two plumage color variations. “Extended brown-dark (EB-D)” is darker than “extended brown-light (EB-L).” WT chick shows yellowish color in the abdomen, whereas the abdominal pigmentation of the EB chick is apparently darker than that of the WT chick. Detailed explanations for EB-D and EB-L are provided in the text.
Fig. 2.

Dorsal (A–F) and ventral (G–L) views of wild-type (WT) and extended-brown (EB) mutant adult blue-breasted quails. EB-D and EB-L indicate darker and lighter plumage colors, respectively, seen in the EB mutant. The EB mutant shows darker plumage than WT, and the plumage pattern varies. Detailed explanations for EB-D and EB-L are provided in the text.
In the present study, we confirmed the inheritance mode of the EB mutation of the blue-breasted quail and examined whether the EB mutation is associated with mutation in MC1R.
Materials and Methods
Animals
Wild type (WT) and EB mutant blue-breasted quails were maintained in the Laboratory of Animal Breeding and Genetics, Hiroshima University. The EB mutant occurs in two types of plumage colors as described in detail in the “Results” section; one is extended brown-dark (EB-D) and the other extended brown-light (EB-L) (Figs. 1 and 2). Animal care and use in this study were in accordance with the animal experimentation guidelines of the Hiroshima University Animal Research Committee.
Mating Experiments
First, WT and EB-D birds were reciprocally mated. The F1 progeny birds were mated inter se to produce an F2 generation. In addition, F1 males were mated with WT females to obtain a testcross generation. Segregation ratios of the plumage color phenotypes in all generations were recorded and analyzed by the chi-square test.
Sequencing of the MC1R Gene
The DNA sequence of MC1R was determined using three EB-D and WT birds each that were previously used in the mating experiments as the parental birds (Table 1). DNA was extracted from the blood or liver using a Blood & Tissue Kit (Qiagen, Germantown, MD, USA) following the manufacturer's protocol. A 1,057-bp DNA fragment that contained the whole coding region of MC1R was amplified with specific primers (forward: 5′-TAGGGCACACGGGGGCTTT-3′; reverse: 5′-TCCTCTTCCTGTCTGTGCCACTGC-3′) using the Gene Amp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). The reaction mixture with a total volume of 25. 0 µl contained 1.0 µl of DNA, 12.5 µl of 2× buffer, 0.5 µl of each primer, 10 µl of ultrapure water, and 0.5 µl (0.625 U) of Tks G flex DNA Polymerase (Takara, Shiga, Japan). The thermal cycles were as follows: denaturation at 94°C for 1 min followed by 29 cycles of denaturation at 98°C for 10 s, annealing at 55°C for 15 s, and extension at 68°C for 1 min. The PCR products were purified by ethanol precipitation. The reaction mixture for sequencing had a total volume of 14 µl containing 0.64 µl primer, 20–80 ng PCR product, and an adequate volume of ultrapure water. A ∼1,000 bp fragment including the MC1R coding region was sequenced using the following four primers (forward: 5′-TAGGGCACACGGGGGCTTT-3′; reverse: 5′-TCCTCTTCCTGTCTGTGCCACTGC-3′; forward: 5′-TGCGCTACCACAGCATCATG-3′; reverse: 5′-CATGATGCTGTGGTAGCGCA-3′) by Fasmac Co., Ltd. (Atsugi, Japan). The sequence data were first confirmed using the BioEdit Sequence Alignment Editor (Ibis Biosciences, Carlsbad, CA, USA), and then sequence alignment was performed using MEGA 5 (Tamura et al., 2011).
Table 1. Incidence of the extended brown (EB) mutation in the F1, F2, and testcross generations obtained from the mating experiments between the wild-type and EB mutant birds of the blue-breasted quail (Coturnix chinensis).
| Matingsa M×F | No. of pairs mated | No. of progeny observed | Phenotypes of progenyb | Expected ratioc | χ2 | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | EB-L | EB-D | WT:EB-L:EB-P | ||||||||
| M | F | M | F | M | F | ||||||
| EB-D×WT | 2 | 41 | 0 | 0 | 18 | 23 | 0 | 0 | 0:1:0 | — | — |
| WT×EB-D | 1 | 9 | 0 | 0 | 6 | 3 | 0 | 0 | 0:1:0 | — | — |
| Total | 3 | 50 | 0 | 0 | 24 | 26 | 0 | 0 | 0:1:0 | — | — |
| F1 × F1 | 10 | 35 | 7 | 17 | 11 | 1:2:1 | 0.95 | .70>P>.60 | |||
| F1 × WT | 3 | 37 | 16 | 21 | 0 | 1:1:0 | 0.30 | .60>P>.50 | |||
a M=male, F=female, WT=wild type, and EB-D=extended brown-dark.
b Phenotypes were classified in newly hatched chicks. EB-L=extended brown-light.
c Based on simple autosomal, incompletely dominant inheritance.
PCR-restriction Fragment Length Polymorphism (RFLP) Analysis
In chickens and Japanese quail, black or dark brown plumage color mutations are thought to be caused by the c.274G>A substitution in MC1R, and the substitution results in the production of a recognition site for the restriction enzyme MscI (5′-TGGCCA-3′) (Ugrankar, 2003). We examined the presence or absence of the recognition site (i.e., presence or absence of the mutation) in all the 122 birds (23 WT and 99 EB) of the F1, F2, and testcross generations using PCR-RFLP analysis. In theory, for birds without c.274G>A substitution, there would be only one band in electrophoresis, while for birds having the substitution in the homozygous or heterozygous condition, two or three electrophoretic bands, respectively, would appear.
DNA was extracted from the blood or liver of each bird according to the afore-mentioned method. The 462-bp DNA fragment around the recognition site of MscI was amplified by PCR using specific primers (forward: 5′-AGCCCTGGAATGCCAGTGA-3′; reverse: 5′-ACTAGGCCATGGTGACCACG-3′). The reaction mixture (20 µl) contained 1.0 µl of DNA, 2.0 µl of 10 × buffer, 2.0 µl of dNTP mixture, 1.0 µl of each primer, 12.9 µl of ultrapure water, and 0.1 µl (0.5 U) of AmpliTaq Gold DNA Polymerase (Applied Biosystems). The PCR conditions were as follows: denaturation at 95°C for 5 min, followed by 34 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min. After the purification of the PCR products, RFLP analysis was performed with 25.0 µl of the reaction solution that contained 500 ng of the PCR products, 2.5 µl of 10 × CutSmart Buffer (New England Biolabs, MA, USA), 1.0 µl of MscI (New England Biolabs Japan, Tokyo, Japan), and an adequate volume of ultrapure water. The solution was first incubated for 60 min at 37°C. After the incubation, 5.0 µl of 6× loading buffer (Takara) was added and the PCR products were electrophoresed on a 2.0% agarose gel with 1× Tris-acetic acid-EDTA running buffer using an electrophoresis apparatus (ATTO, Tokyo, Japan). The electrophoretic bands were visualized by staining with GelRed™ (Biotium, Fremont, CA, USA) for 30 min and then photographed.
Results
Characteristics of the EB Mutant
At a glance, the EB mutant appeared to possess dark plumage all over the body. However, the plumage color could be classified into two types, both in the chicks and adults, on the basis of the extent of pigmentation, one being darker than the other (Figs. 1 and 2). For convenience of reporting, we refer to the darker one as EB-D (extended brown-dark) and the other as EB-L (extended brown-light) in this manuscript. Newly hatched EB-D chicks showed blackish brown plumage all over the body, and sometimes had obscure brown stripes on the back. Their beak color was dark brown, and their shank color was blackish. The down plumage color of the EB-L chicks was similar to but lighter than that of the EB-D chicks, and the latter generally had light brown stripes on their back. Their beak and shank colors were also similar to but somewhat lighter than those of the EB-D chicks. Adult EB males lacked the black and white markings that were typically seen on the cheeks and throat of the WT males; both the EB-D and EB-L males showed dark brown plumage with a grayish-blue tinge all over the body, which was slightly lighter in shade in the EB-L males than in the EB-D males. In the EB-L males, unlike in the EB-D males, rust-colored plumage was seen in the ventral region around the cloaca. The adult EB-D and EB-L females exhibited dark-brown plumage all over the body as seen in the males, and scale-like markings were seen on the breasts of the females. The pigmentation and markings were lighter and clearer, respectively, in the EB-L females than in the EB-D females.
Mode of Inheritance of the EB Mutation
The results of the mating experiments between the WT and EB-D birds are summarized in Table 1. All F1 birds derived from reciprocal matings showed EB-L plumage. In the F2 generation, the WT, EB-L, and EB-D phenotypes segregated in a ratio of 7:17:11. This segregation ratio was in good agreement with the expected ratio of 1:2:1 (χ2=0.95, 0.70>P>0.60), which was based on the assumption that the EB mutation is simple autosomal and incompletely dominant, and the EB-D and EB-L phenotypes represent individuals that are homozygous and heterozygous for the mutation, respectively. In the testcross generation obtained from matings between the F1 (EB-L) male and WT female, 16 offspring were WT and 21 were EB-L. This segregation ratio was in good accordance with the expected ratio of 1:1 (χ2=0.30, 0.60>P>0.50), which was based on the above hypothesis.
Association of the EB Plumage and MC1R Mutation
DNA sequencing revealed that the MC1R coding region of the blue-breasted quail is composed of 945 bp, and that the three EB-D mutant birds used as parental birds in the mating experiment (Table 1) have a single nucleotide substitution from G to A at position 274 (c.274G>A), while the three parental WT birds did not possess the substitution. In the PCR-RFLP analysis (Fig. 3) for the F1, F2, and testcross generations (Table 1), all 50 F1 birds that had the EB-L plumage exhibited three bands (225, 237, and 462 bp). In the F2 generation, seven WT, 17 EB-L, and 11 EB-D birds showed one (462 bp), three (225, 237, and 462 bp), and two (225 and 237 bp) electrophoretic bands, respectively. In the testcross generation also, 16 WT and 21 EB-L birds showed one (462 bp) and three (225, 237, and 462 bp) bands, respectively.
Fig. 3.
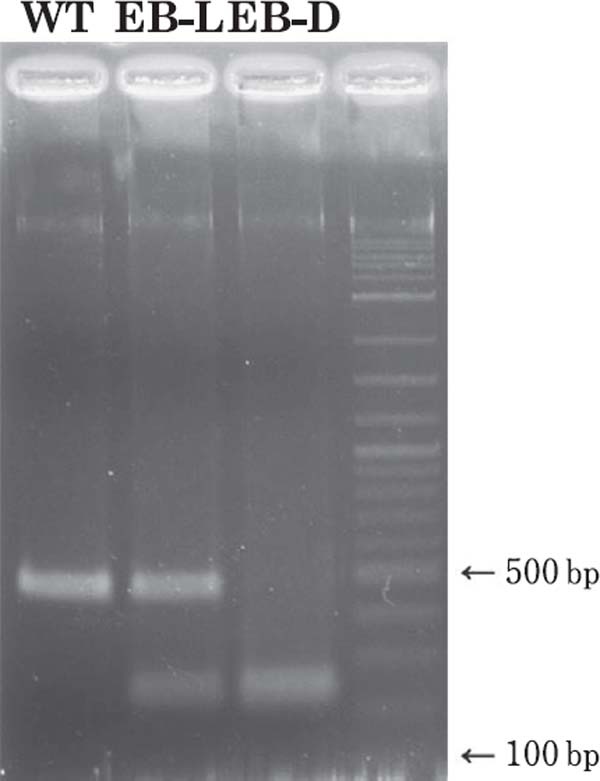
PCR-RFLP analysis of the MC1R gene of the blue-breasted quail. WT=wild type, EB-L=extended brown-light, and EB-D=extended brown-dark. The 462-bp amplicon around the c.274Ggt;A substitution in MC1R was digested with the restriction enzyme MscI. WT had only one non-cleaved band of 462 bp. EB-L produced three bands of 462, 237, and 225 bps, while EB-D had two bands of 237 and 225 bps. In the photo, the bands of 225 and 237 bps are merged because of similar amplicon sizes. Despite the merged bands, WT, EB-L, and EB-D are clearly and easily distinguishable from each other by PCR-RFLP.
Discussion
To our knowledge, this is the first study investigating both the mode of inheritance of the EB plumage and the association between this phenotype and the c.274G>A substitution in the MC1R gene of the blue-breasted quail. The segregation ratios of the WT and EB birds in the F1, F2, and testcross generations clearly indicated that the EB phenotype is controlled by an autosomal, incompletely dominant allele. We propose the gene symbol Eb for the mutant allele. The phenotypes EB-D and EB-L are thought to be homozygous and heterozygous for Eb, respectively.
The F1 progeny obtained from the matings between the WT and EB-D birds had EB-L plumage and exhibited three electrophoretic bands in PCR-RFLP analysis. In the F2 generation also, all 17 EB-L birds exhibited three electrophoretic bands. In contrast, two bands were observed for all 11 EB-D birds, and one band was observed in all seven WT birds, clearly demonstrating that EB-D birds are homozygous for the c.274G>A substitution, while WT has no such substitution. In the testcross generation, the WT and EB-L offspring produced one and three electrophoretic bands, respectively, as seen in the cases of F1 and F2 birds. Judging from these complete associations between plumage color and the absence and/or presence ofc. 274G>A substitution in the MC1R gene, it appears that MC1R is the candidate gene for the EB plumage color mutation.
In Japanese quail, a mutant that has dark brown plumage quite similar to that of the blue-breasted quail, has been observed and is called the “extended brown (EB)” or “black” (Somes, 1979; Cheng and Kimura, 1990; Tsudzuki et al., 1990; Tsudzuki, 2008). In accordance with our findings, this phenotype is caused by a c.274G>A substitution in MC1R, which has a size of 945 bp (Takeuchi et al., 1996a), similar to the MC1R gene size in blue-breasted quail. The EB mutants of Japanese and blue-breasted quail have only the c.274G>A substitution in MC1R in common. In chickens, the MC1R gene is of the same size as in Japanese and blue-breasted quails, and the existence of dark-colored birds that have the same nucleotide substitution (c.274G>A) in MC1R has been well documented (Takeuchi et al., 1996b; Kerje et al., 2003; Ling et al., 2003; Dàvila et al., 2014). Based on these findings, it can be safely concluded that the three poultry species, i.e., chicken, Japanese quail, and blue-breasted quail, have a shared regulatory mechanism of the MC1R gene that is responsible for their dark plumage. In chickens, however, the birds that have c.274G>A mostly possess additional nucleotide substitutions, e.g., c.212T>C, c.376 G>A, and c.637G>A (Ling et al., 2003; Dàvila et al., 2014), and show dense black plumage all over the body, unlike the mutant EB quails. Thus, these additional substitutions might cause the differences (black or dark brown) in plumage colors between chickens and the two quail species. However, while some chickens only have the c.274G>A substitution in MC1R, and show dense black plumage all over the body (Dàvila et al., 2014), some chickens that carry the MC1R c.274G>A substitution only are partly covered with brown feathers on their black bodies. Therefore, details on the function of the c.274G>A substitution (Glu92Lys at the amino acid level) in the expression of dark (black or dark brown) plumage colors remain unknown. Although it has been revealed that the Glu92Lys mutation resides in the transmembrane 2 region of MC1R and results in constitutively active MC1R, which leads to increased eumelanin by increasing cAMP and successively enhancing tyrosinase activity (Ling et al., 2003; Benned-Jensen et al., 2011), further studies will be necessary to reveal the mechanisms through which dark brown and/or black plumage are expressed in quails and chickens.
Acknowledgments
The authors are grateful to all colleagues at the laboratory of Animal Breeding and Genetics, Graduate School of Biosphere Science, Hiroshima University, for their help with this study.
References
- Akiyama T, Sasaki M and Takenaka Y. Body color and pattern formations in animals: pigment cell development, genes and a reaction-diffusion model. Hiyoshi Review of Natural Science Keio University, 37: 73-94. 2005. (in Japanese) [Google Scholar]
- Benned-Jensen T, Mokrosinski J and Rosenkilde M. The E92K melanocortin 1 receptor mutant induces cAMP production and arrestin recruitment but not ERK activity indicating biased constitutive signaling. PLoS One, 6: e24644. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KM and Kimura M. Mutations and major variants in Japanese quail. In: Poultry Breeding and Genetics. (Crawford RD ed.) pp. 333-362. Elsevier. Amsterdam. 1990. [Google Scholar]
- Dàvila SG, Gil MG, Resino-Talavàn P and Campo JL. Association between polymorphism in the melanocortin 1 receptor gene and E locus plumage color phenotype. Poultry Science, 93: 1089-1096. 2014. [DOI] [PubMed] [Google Scholar]
- Hofreiter M and Schöneberg T. The genetic and evolutionary basis of colour variation in vertebrates. Cellular and Molecular Life Sciences, 67: 2591-2603. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IJ. Molecular and developmental genetics of mouse coat color. Annual Reviews of Genetics, 28: 189-217. 1994. [DOI] [PubMed] [Google Scholar]
- Kerje S, Lind J, Schütz K, Jensen P and Andersson L. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Animal Genetics, 34: 241-248. 2003. [DOI] [PubMed] [Google Scholar]
- Ling MK, Lagerström MC, Fredriksson R, Okimoto R, Mundy NI, Takeuchi S and Schiöth HB. Association of feather colour with constitutively active melanocortin 1 receptors in chicken. European Journal of Biochemistry, 270: 1441-1449. 2003. [DOI] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO and Cone RD. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature, 371: 799-802. 1994. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT and Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science, 257: 1248-1251. 1992. [DOI] [PubMed] [Google Scholar]
- Nadeau NJ, Minvielle F and Mundy NI. Association of a Glu92Lys substitution in MC1R with extended brown in Japanese quail (Coturnix japonica). Animal Genetics, 37: 287-289. 2006. [DOI] [PubMed] [Google Scholar]
- Oribe E, Yoshihara C, Takahashi S and Takeuchi S. Molecular mechanism of melanin-based pigmentation in birds. Comparative Physiology and Biochemistry, 26: 3-11. 2009. (in Japanese) [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG and Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell, 72: 827-834. 1993. [DOI] [PubMed] [Google Scholar]
- Smyth JR Jr. Genetics ofplumage, skin and eye pigmentation in chickens. In: Poultry Breeding and Genetics (Crawford RD ed.). pp. 109-167. Elsevier Science Publishing Company Inc. New York. 1990. [Google Scholar]
- Somes RG Jr. Genetic bases for plumage color patterns in four varieties of Japanese quail. Journal of Heredity, 70: 205-210. 1979. [Google Scholar]
- Takeuchi S, Suzuki H, Hirose S, Yabuuchi M, Sato C, Yamamoto H and Takahashi S. Molecular cloning and sequence analysis of the chick melanocortin 1-receptor gene. Biochimica et Biophysica Acta, 1306: 122-126. 1996a. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Suzuki H, Yabuuchi M and Takahashi S. A possible involvement of melanocortin 1-recepter in regulating feather color pigmentation in the chicken. Biochimica et Biophysica Acta, 1308: 164-168. 1996b. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M and Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28: 2731-2739. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudzuki M, Fuji J and Wakasugi N. An allelism test for plumage color mutant genes, D (black) and ps (pansy), in Japanese quail (Coturnix coturnix japonica). Japanese Journal of Poultry Science, 27: 266-269. 1990. [Google Scholar]
- Tsudzuki M. Excalfactoria quail as a new laboratory research animal. Poultry Science, 73: 763-768. 1994. [DOI] [PubMed] [Google Scholar]
- Tsudzuki M. Light gray: a plumage color mutation of Chinese painted quail (Excalfactoria chinensis). Journal of Heredity, 86: 68-70. 1995a. [DOI] [PubMed] [Google Scholar]
- Tsudzuki M. Brown: a plumage color mutation in Chinese painted quail (Excalfactoria chinensis). Journal of Heredity, 86: 307-309. 1995b. [DOI] [PubMed] [Google Scholar]
- Tsudzuki M. Mutations of Japanese quail (Coturnix japonica) and recent advances of molecular genetics for this species. Journal of Poultry Science, 45: 159-179. 2008. [Google Scholar]
- Ugrankar RB. DNA sequence of melanocortin 1-receptor gene in Coturnix japonica: correlation with three E locus alleles, E, E+, and ERH. Inquiry: The University of Arkansas Undergraduate Research Journal, 4: Article 21. 2003. [Google Scholar]
- Wolf Horrell EM, Boulanger MC and D'Orazio JA. Melanocortin 1 receptor: structure, function, and regulation. Frontiers in Genetics, 7: 95. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


