Abstract
BACKGROUND:
Beta-blockers improve outcomes in patients with systolic heart failure. However, it is unknown whether their initial negative inotropic effect may increase 30-day all-cause readmission, a target outcome for Medicare cost reduction and financial penalty for hospitals under the Affordable Care Act.
METHODS:
Of the 3067 Medicare beneficiaries discharged alive from 106 Alabama hospitals (1998-2001) with a primary discharge diagnosis of heart failure and ejection fraction (EF) <45%, 2202 were not previously on beta-blocker therapy, of which 383 received new discharge prescriptions for beta-blockers. Propensity scores for beta-blocker use, estimated for each of the 2202 patients, were used to assemble a matched cohort of 380 pairs of patients receiving and not receiving beta-blockers who were balanced on 36 baseline characteristics (mean age 73 years, mean EF 27%, 45% women, 33% African American)
RESULTS:
Beta-blocker use was not associated with 30-day all-cause readmission (hazard ratio {HR}, 0.87; 95% confidence interval {CI}, 0.64-1.18) or heart failure readmission (HR, 0.95; 95% CI, 0.57-1.58)., but was significantly associated with lower 30-day all-cause mortality (HR, 0.29; 95% CI, 0.12-0.73). During 4-year post-discharge, those in the beta-blocker group had lower mortality (HR, 0.81; 95% CI, 0.67-0.98) and combined outcome of all-cause mortality or all-cause readmission (HR, 0.87; 95% CI, 0.74-0.97) but not with all-cause readmission (HR, 0.89; 95% CI, 0.76-1.04).
CONCLUSIONS:
Among hospitalized older patients with systolic heart failure, discharge prescription of beta-blockers was associated with lower 30-day all-cause mortality and 4-year combined death or readmission outcomes without higher 30-day readmission.
Keywords: Systolic heart failure, Older adults, Beta blockers, Hospitalization, Readmission
Heart failure is a leading cause of hospital readmission for Medicare beneficiaries, many of which are considered potentially preventable.1,2 The Affordable Care Act, the new United States healthcare reform law, has identified 30-day all-cause readmission in hospitalized Medicare beneficiaries aged ≥65 years as a target outcome for reduction of Medicare costs. It is projected that hospitals may collectively lose about $7 billion over next 10 years for above-average 30-day all-cause readmission. In patients with systolic heart failure, beta-blockers reduce mortality and hospitalization in the randomized controlled trial setting,3,4 and their use has been associated with lower risk of one-year mortality and readmission in the post-discharge setting.5 However, beta-blockers have an early transient negative inotropic effect and concern remains that discharge initiation of these drugs in hospitalized patients with heart failure and reduced ejection fraction may adversely affect short-term outcomes and this has lately been heightened by the focus on 30-day all-cause hospital readmission in the Affordable Care Act. Therefore, the objective of the current study was to examine the association of discharge initiation of beta blockers with 30-day all-cause hospital readmission in hospitalized systolic heart failure patients.
MATERIALS AND METHODS
Data Source and Study Patients
We used the Alabama Heart Failure Project data for the current study, the details of which have been described previously.6 Briefly, medical records of fee-for-service Medicare beneficiaries discharged with a principal diagnosis of heart failure from 106 Alabama hospitals between July 1, 1998 and October 31, 2001 were identified.6–9 A diagnosis of heart failure was based on the International Classification of Diseases, 9th Revision, Clinical Modification, codes for heart failure. Copies of the 9649 charts were abstracted by trained technicians who directly entered data into a computer database. The 9649 hospitalizations occurred in 8555 unique patients. For patients with multiple hospitalizations, charts from the first hospitalization were used. The selected medical records were then transferred from participating hospitals to the Central Clinical Data Abstraction Centers (CDAC) where trained technicians abstracted data from charts directly into a computer database using a data collection tool programmed by MedQuest Software. CDAC ensured reliability of the abstraction process through internal and external re-abstractions of 40 charts monthly. Reliability findings demonstrated agreement values >80% and Kappa values >0.60.6
New Use of Beta-Blockers: Assembly of an Inception Cohort
Of the 8555 patients, 8049 were discharged alive, of these, 5479 (68%) had data on left ventricular ejection fraction, of which 3067 (68%) had ejection fraction <45%. Of these 3067 patients, 2202 (72%) were not on beta-blockers at admission. Of these, 383 (17%) received a discharge prescription for beta-blocker. Extensive data on baseline demographics, medical history including use of medications, hospital course, discharge disposition including medications, and physician specialty were collected.
Propensity Matching: Assembly of a Balanced Cohort
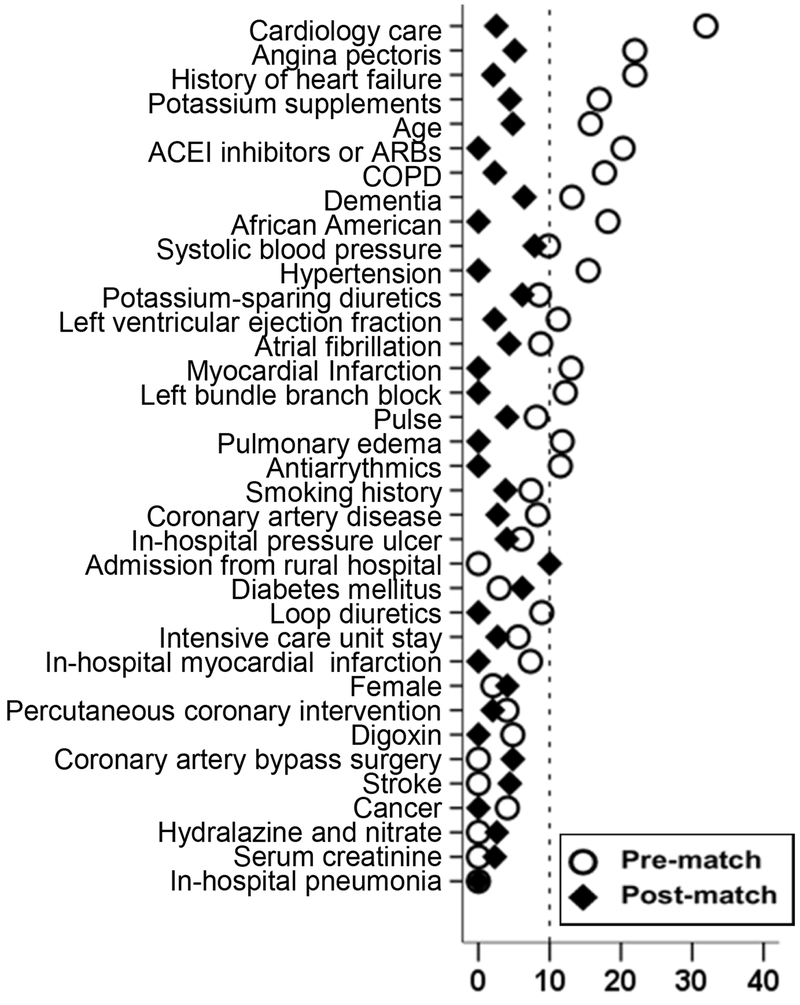
Because of the imbalances in baseline characteristics between patients receiving and not receiving beta-blockers (Table 1), we used propensity scores to assemble a cohort of patients in which those receiving and not receiving these drugs would be well-balanced on measured baseline characteristics.10–14 We began by estimating propensity scores or probability of receiving discharge prescription of beta-blockers for each of the 2202 patients using 36 measured baseline characteristics displayed in Figure 1. Using a greedy matching protocol, we were able to match 380 of the 383 patients receiving beta-blockers with 380 patients not receiving these drugs who had similar propensity scores.15–17 We then estimated absolute standardized differences of the 36 measured covariates for the two treatment groups, and presented the percentages of pooled standard deviations as Love plots. 18–21 An absolute standardized difference of 0% indicates no residual bias and differences <10% are considered inconsequential.
Table 1.
Baseline characteristics of Medicare beneficiaries hospitalized for heart failure and ejection fraction <45, not receiving prior betta-blocker therapy, by the receipt of a new discharge prescription for beta-blockers, before and after propensity score matching
| Variables Mean ± SD or n (%) | Pre-match (N=2202) | Post-match (N=760) | ||||
|---|---|---|---|---|---|---|
| Beta blocker on
discharge |
P value | Beta blocker on
discharge |
P value | |||
| No (n=1819) | Yes (n=383) | No (n=380) | Yes (n=380) | |||
| Age (years) | 75 (±11) | 73 (±11) | 0.004 | 73 (±11) | 73(±11) | 0.974 |
| Female | 855 (47) | 172 (45) | 0.455 | 166 (44) | 171 (45) | 0.770 |
| African American | 470 (26) | 126 (33) | 0.005 | 127 (33) | 125 (33) | 0.939 |
| Admission from nursing homes | 83 (5) | 15 (4) | 0.577 | 13 (3) | 15 (4) | 0.845 |
| Smoking | 257 (14) | 65 (17) | 0.152 | 61 (16) | 65 (17) | 0.770 |
| Ejection fraction (%) | 28 (±9) | 27 (±8) | 0.008 | 27 (±9) | 27 (±9) | 0.391 |
| Past medical history | ||||||
| Prior heart failure | 1367 (75) | 250 (65) | <0.001 | 250 (66) | 248 (65) | 0.939 |
| Hypertension | 1204 (66) | 276 (72) | 0.026 | 273 (72) | 273 (72) | 1.000 |
| Coronary artery disease | 1110 (61) | 218 (57) | 0.136 | 208 (55) | 215(57) | 0.661 |
| Prior myocardial infarction | 541 (30) | 97 (25) | 0.940 | >104 (27) | 97 (26) | 0.622 |
| Angina pectoris | 222 (12) | 78 (20) | <0.001 | 69 (18) | 75(20) | 0.644 |
| Diabetes mellitus | 791 (44) | 181 (47) | 0.177 | 185 (49) | 179 (47) | 0.717 |
| Percutaneous coronary intervention | 253 (14) | 49 (13) | 0.624 | 40(11) | 49(13) | 0.367 |
| Coronary artery bypass surgery | 538 (30) | 113 (30) | 1.000 | 105 (28) | 112(30) | 0.630 |
| Stroke | 341 (19) | 73 (19) | 0.887 | 70 (18) | 73 (19) | 0.853 |
| Chronic obstructive pulmonary disease | 684 (38) | 113 (30) | 0.003 | 105 (28) | 113 (30) | 0.575 |
| Dementia | 158 (9) | 20 (5) | 0.024 | 16 (4) | 20 (5) | 0.609 |
| Cancer | 52 (3) | 10 (3) | 0.790 | 12 (3) | 10 (3) | 0.829 |
| Atrial fibrillation | 516 (28) | 88 (23) | 0.032 | 49 (23) | 51 (23) | 0.931 |
| Left bundle branch block | 409 (23) | 73 (19) | 0.141 | 62 (16) | 73 (19) | 0.343 |
| Pneumonia | 477 (26) | 100 (26) | 1.000 | 96 (25) | 99 (26) | 0.868 |
| Clinical findings | ||||||
| Pulse (beats/minute) | 94 (±22) | 96 (±23) | 0.104 | 97 (±22) | 97 (±24) | 0.775 |
| Systolic blood pressure (mmHg) | 144 (±30) | 148 (±31) | 0.056 | 151 (±31) | 149 (±31) | 0.508 |
| Respiration (breaths/minute) | 24 (±6) | 25 (±7) | 0.114 | 24 (±6) | 25 (±7) | 0.605 |
| Pulmonary edema by chest x-ray | 1305 (72) | 262 (68) | 0.190 | 266 (70) | 260 (68) | 0.694 |
| Tests and procedures | ||||||
| Serum creatinine (mEq/L) | 1.6 (±1) | 1.6 (±1) | 0.819 | 1.7 (±1) | 1.6(±1) | 0.272 |
| Glomerular filtration rate (ml s/min/1.73m2) | 54 (±24) | 57 (±25) | 0.047 | 56 (±27) | 57 (±25) | 0.798 |
| In-hospital events | ||||||
| Acute myocardial infarction | 103 (6) | 26 (7) | 0.394 | 25 (7) | 26 (7) | 1.000 |
| Pneumonia | 477 (26) | 100 (26) | 0.963 | 84 (22) | 98 (26) | 0.268 |
| Pressure ulcer | 163 (9) | 28 (7) | 0.319 | 30 (8) | 28 (7) | 0.891 |
| Hospital and care characteristics | ||||||
| Rural hospital | 410 (23) | 75 (20) | 0.204 | 78 (21) | 75 (21) | 0.856 |
| Cardiology care | 1191 (66) | 307 (80) | <0.001 | 308 (81) | 304 (80) | 0.784 |
| Intensive care unit stay | 75 (4) | 19 (5) | 0.461 | 17 (5) | 18 (5) | 1.000 |
| Length of stay (days) | 7 (±5) | 8 (±5) | 0.009 | 8 (±7) | 8 (±5) | 0.929 |
| Discharge medications | ||||||
| Renin-angiotensin inhibitors | 1224 (67) | 286 (75) | 0.005 | 282 (74) | 283 (75) | 1.000 |
| Digoxin | 1037 (57) | 221 (58) | 0.803 | 225 (59) | 218 (57) | 0.670 |
| Loop diuretics | 1567 (86) | 322 (84) | 0.291 | 317 (83) | 319 (84) | 0.922 |
| Potassium-sparing diuretics | 345(19) | 91 (24) | 0.032 | 88 (23) | 89 (23) | 1.000 |
| Potassium supplement | 865 (48) | 144 (38) | <0.001 | 146 (38) | 143 (38) | 0.881 |
| Hydralazine and nitrate | 73 (4) | 15 (4) | 1.000 | 18 (5) | 15(4) | 0.722 |
| Antiarrhythmics | 293 (16) | 51 (13) | 0.188 | 43 (11) | 51 (13) | 0.441 |
Figure 1.
Absolute standardized differences comparing 36 baseline characteristics between patients receiving and not receiving discharge prescription of Beta-blocker, before and after propensity score matching (ACE=angiotensin-converting enzyme, ARB=angiotensin receptor blockers, COPD=Chronic obstructive pulmonary disease)
Outcomes
The primary outcome of the current analysis was 30 day all-cause hospitalization. Secondary outcomes included all-cause mortality, heart failure hospitalizations and the combined end point of all-cause readmission or all-cause mortality. Time to all outcomes started from the date of discharge from the index hospitalization. Data on outcomes and time to events were obtained from the Centers for Medicare and Medicaid Services Denominator File, Medicare Provider Analysis and Review File and Inpatient Standard Analytical File.
Statistical Analysis
For descriptive analyses, we used Pearson Chi square and Wilcoxon rank-sum tests for comparisons. Kaplan-Meier plots and Cox regression analyses were used to determine the associations of discharge initiation of beta-blocker therapy with 30-day all-cause readmission. We repeated the Cox model for other outcomes at 30 days and 4 years post-discharge. All outcomes analyses were based on matched cohort. All statistical tests were two-tailed with a p-value <0.05 considered significant. Statistical analyses were performed using SPSS-22 for Windows (IBM Corp. 2013. IBM SPSS Statistics for Windows, Armonk, NY).
Results
Baseline Characteristics
Matched patients had a mean age (±SD) of 73 (±11) years, 44% were women, and 33% were African American. Pre-match imbalances in the distribution of age, race, history of prior heart failure, comorbidities and treatment between patients receiving and not receiving beta blockers were well-balanced after matching (Table 1 and Figure 1).
Beta-Blocker Use and 30-Day All-Cause Readmission
30-day all-cause hospital readmission occurred in 20% and 23% of matched patients receiving and not receiving beta-blockers, respectively (hazard ratio {HR} when beta-blocker use is compared with their non-use, (hazard ratio {HR}, 0.87; 95% confidence interval {CI}, 0.64–1.18; p=0.337; Table 2). Beta-blocker use also had no association with heart failure readmission – occurring in 8% of matched patients receiving and not receiving beta-blockers, (HR 0.95; 95% CI, 0.57–1.58; Table 2).
Table 2.
Association between a new discharge prescription for beta-blocker and 30-day post-discharge outcomes in a propensity-matched cohort of Medicare beneficiaries hospitalized for heart failure
| (events) |
||||
|---|---|---|---|---|
| Outcomes | New discharge prescription for
beta-blocker |
Absolute risk diff.* | Hazard ratio† (95 confidence interval) | |
| No (n=380) %,N | Yes (n=380) %,N | |||
| All-cause hospital readmission | 23 (86) | 20 (77) | −3 | 0.87 (0.64–1.18); p=0.377 |
| Heart failure readmission | 8 (30) | 8 (29) | 0 | 0.95 (0.57–1.58); p=0.837 |
| All-cause mortality | 5(20) | 2(6) | −3 | 0.29 (0.12–0.73); p=0.008 |
| All-cause mortality or all-cause readmission | 26 (97) | 21 (81) | −5 | 0.81 (0.60–1.09); p=0.166 |
Absolute risk differences(%) were calculated by subtracting percent events in patients receiving no beta-blocker from those receiving those drugs
The hazard ratios compared patients receiving beta-blocker versus those not receiving beta-blocker. These hazard ratios were calculated by treating patients without events during the first 30 days as censored
Other Outcomes
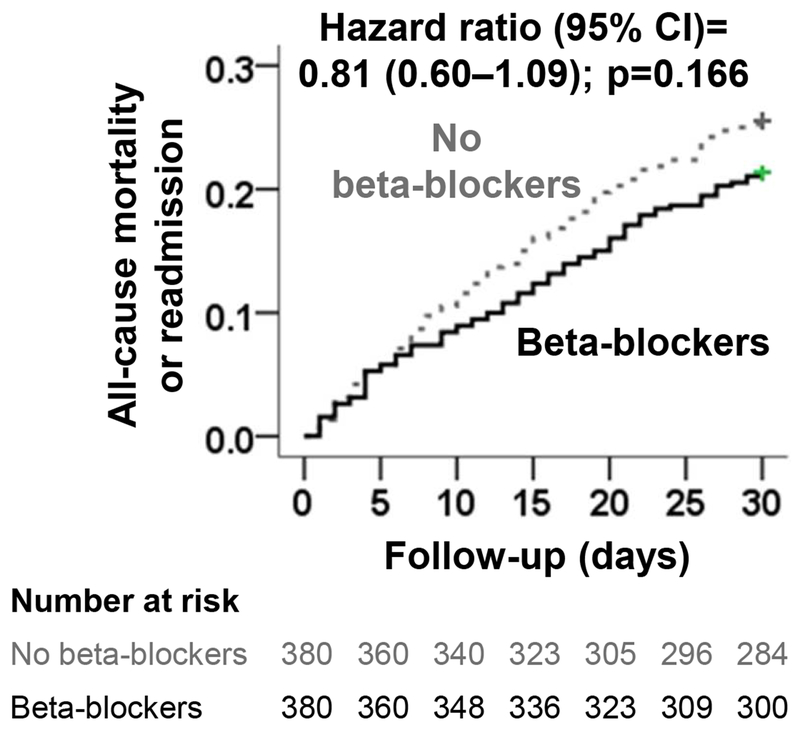
There were only 26 (3.5%) deaths during the first 30 days of post-discharge follow-up. All-cause mortality occurred in 2% and 5% of those receiving and not receiving beta blockers, respectively (HR, 0.29; 95% CI, 0.12–0.73). When stratified by EF< 20 patients, all cause readmission occurred in 12% vs 23% of those receiving beta blockers vs not receiving beta blockers,(HR,0.5;95% CI,0.20–1.24). Combined outcome of 30 day all cause readmission and allcause mortality occurred in 21 % and 26 % of those receiving and not receiving discharge prescription of beta blockers, respectively (HR, 0.81; 95% CI, 0.60–1.09; p-0.166; Figure 2 and Table 2). This latter association did not vary between patients with EF <20% or 20–44%.
Figure 2.
Kaplan-Meier plots for combined end point of 30-day all-cause readmission or all-cause mortality in a propensity-matched cohort of patients with heart failure and ejection fraction <45%, receiving and not receiving a new discharge prescription for beta-blocker (CI=confidence interval)
Beta-blocker use had no significant association with all-cause or heart failure readmission during 4 years post-discharge. However, there was significant reduction in both all-cause mortality (HR, 0.81; 95% CI, 0.67–0.98) and the combined outcome of all-cause readmission or all-cause mortality (HR, 0.86; 95% CI, 0.74–0.97) during 4 years after hospital discharge.
Discussion
Findings from the current study demonstrate that a discharge prescription for beta-blocker was associated with a significantly lower risk of 30-day and 4-year all-cause mortality without significantly increasing the risk of 30-day all-cause or heart failure readmissions. The use of beta-blockers was also associated with a similar lower risk of the combined end point of all-cause readmission or all-cause mortality at 30 days and 4 years; however, only the 4-year association was statistically significant, likely due to higher events. These results, based on a rigorously conducted study, using an inception-cohort propensity-matched design suggest that hospitalized older patients with systolic heart failure may be prescribed beta-blockers without concerns for adversely affecting 30-day all-cause readmission, an outcome with financial implications for nation’s hospitals.
Findings from laboratory animals and human studies suggest that beta-blockers prevent and reverse maladaptive ventricular remodeling in heart failure,22,23 which explain their beneficial effect on heart failure hospitalization and heart failure death.3,4 However, this effect of beta-blockers may not be apparent until after one month of therapy,24 which may in part explain the lack of association between beta-blocker use and lower 30-day readmissions. Further, the early beneficial effect of beta-blockers on disease progression and symptoms may also have been attenuated by the negative inotropic effects of these drugs. Older heart failure patients often restrict their physical activity to avoid symptoms and hospitalization.25 If more symptomatic patients in the non-beta-blocker group avoided symptoms by restricting mobility they are less likely to be readmitted, thus attenuating the between-group difference. However, in these same patient population, the use of digoxin and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was associated with lower readmission,17‘26 suggesting that the effect of beta-blockers on readmission maybe mild in hospitalized older systolic heart failure patients. Beta-blockers reduce both sudden cardiac death and pump failure death.3,4 The mode of death in hospitalized older patients with advanced systolic heart failure would be expected to be predominantly due to pump failure.27–31 However, because of the time-lag associated with ventricular remodeling,24 the lower 30-day all-cause-mortality in our study would be expected to be primarily due to reduction of sudden death. Given the small number of deaths during the 30-day post-discharge period, chance remains a possibility which might have inflated the hazard ratio.
Several studies have examined the short-term effect of beta-blockers on outcomes. In the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial, in clinically euvolemic patients with systolic heart failure, the mortality benefit of carvedilol appeared to begin early though it was not statistically significant, likely due to small number of events.32 In the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) registry, among hospitalized systolic heart failure patients, the use of beta-blockers was associated with an 11% (95% CI, 1%−20%) significantly lower all-cause readmission at one year post-discharge.5 Although data on 30-day all-cause readmission were not presented, the magnitude of the association between beta-blocker use and all-cause readmission in that study is similar to that observed in our study.
Although the use of beta-blockers in systolic heart failure has increased in recent years,5 concerns about how their initial negative inotropic effect may adversely affect readmission remain a barrier to their use before hospital discharge. Due to recent focus on 30-day all-cause readmission, hospitals and clinicians are under pressure to lower this outcome to avoid financial penalties, and this may further cause underutilization of these drugs during hospital discharge. However, findings from the current study should be reassuring. Heart failure patients not receiving evidence-based drugs during hospital discharge often do not receive them after discharge,33–35 and considering the mortality benefit of beta-blockers in older systolic heart failure patients, this guideline-recommended evidence-based therapy should be initiated before hospital discharge to all systolic heart failure patients without an absolute contraindication.36 A pre-discharge (vs. post-discharge) initiation of beta-blockers has also been shown to be associated with higher use of beta-blockers without higher readmission.37
Our study has several limitations. Despite the use of propensity matching to assemble a cohort that was balanced in measured baseline characteristics, as in any observational study, bias due to imbalances in unmeasured covariates is possible. Lack of data on cause-specific deaths, invasive hemodynamic measurements, and dosage of beta-blockers used are other limitations. We also had no data on post-discharge use of medications, but, treatment crossover is likely to be minimal.33–35 Although a resultant regression dilution may underestimate observed associations,38 it is unlikely to explain the null association with readmission as there was a significant association with mortality. Chance and lower numbers of mortality events in 30 days could have erroneously inflated hazard ratios.Although the current study is based on a single state from the early 2000s, outcomes in heart failure patients in Alabama is similar to national estimates,1,39 and management of hospitalized older systolic heart failure patients has not evolved much in the past decade.
Conclusions
Among Medicare beneficiaries with heart failure and reduced ejection fraction hospitalized for acute decompensation, a discharge prescription for beta-blockers was associated with lower 30-day all-cause mortality without higher 30-day all-cause or heart failure readmissions suggesting that these drugs can be prescribed before hospital discharge without concerns for adversely affecting 30-day all-cause readmission, an outcomes of substantial financial consequences for nation’s hospitals.
Table 3.
Association between a new discharge prescription for beta-blocker and 4-year post-discharge outcomes in a propensity-matched cohort of Medicare beneficiaries hospitalized for heart failure
| (events) |
||||
|---|---|---|---|---|
| Outcomes | New discharge prescription for
b-blocker |
Absolute risk diff.* | Hazard ratio† (95 confidence interval) | |
| No (n=380) %,N | Yes (n=380) %,N | |||
| All-cause hospital readmission | 83 (315) | 81 (309) | −2 | 0.89 (0.76–1.04) |
| Heart failure readmission | 49 (186) | 49 (187) | 0 | 0.92 (0.75–1.13) |
| All-cause mortality | 60 (226) | 53 (202) | −7 | 0.81 (0.67–0.98) |
| All-cause mortality or all-cause readmission | 94 (357) | 89 (339) | −5 | 0.86 (0.74–0.97) |
Absolute risk differences(%) were calculated by subtracting percent events in patients receiving no beta-blocker from those receiving those drugs
The hazard ratios compared patients receiving beta-blocker versus those not receiving beta-blocker. These hazard ratios were calculated by treating patients without events during the first 30 days as censored
Clinical significance.
Beta-blocker use was associated with lower risk of 30-day all-cause mortality without higher 30-day all-cause or heart failure readmissions in older Medicare beneficiaries hospitalized for acute systolic heart failure.
This benefit of beta-blockers was observed throughout the first 4 years after discharge
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflict of interest to disclose
All authors had access to the data and a role in writing the manuscript
References:
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 2.Jiang HJ, Russo CA, Barrett ML. Nationwide Frequency and Costs of Potentially Preventable Hospitalizations, 2006: Statistical Brief #72 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD: )2006. [PubMed] [Google Scholar]
- 3.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 4.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feller MA, Mujib M, Zhang Y, Ekundayo OJ, Aban IB, Fonarow GC, et al. Baseline characteristics, quality of care, and outcomes of younger and older Medicare beneficiaries hospitalized with heart failure: findings from the Alabama Heart Failure Project. Int J Cardiol. 2012;162:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Fonarow GC, Sanders PW, Farahmand F, Allman RM, Aban IB, et al. A propensity-matched study of the comparative effectiveness of angiotensin receptor blockers versus angiotensin-converting enzyme inhibitors in heart failure patients age >/= 65 years. Am J Cardiol. 2011;108:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, Rich MW, Zile M, Sanders PW, Patel K, Zhang Y, et al. Renin-angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med. 2013;126:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed A, Fonarow GC, Zhang Y, Sanders PW, Allman RM, Arnett DK, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 11.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 12.Michels KB, Braunwald E. Estimating treatment effects from observational data: dissonant and resonant notes from the SYMPHONY trials. JAMA. 2002;287:3130–3132. [DOI] [PubMed] [Google Scholar]
- 13.Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banach M, Bhatia V, Feller MA, Mujib M, Desai RV, Ahmed MI, et al. Relation of baseline systolic blood pressure and long-term outcomes in ambulatory patients with chronic mild to moderate heart failure. Am J Cardiol. 2011;107:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shariff N, Desai RV, Patel K, Ahmed MI, Fonarow GC, Rich MW, et al. Rate-control versus rhythm-control strategies and outcomes in septuagenarians with atrial fibrillation. Am J Med. 2013;126:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed A, Bourge RC, Fonarow GC, Patel K, Morgan CJ, Fleg JL, et al. Digoxin use and lower 30-day all-cause readmission for medicare beneficiaries hospitalized for heart failure. Am J Med. 2014;127:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. Primer on statistical interpretation or methods report card on propensity-score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:62–67. [DOI] [PubMed] [Google Scholar]
- 19.Gheorghiade M, Fonarow GC, van Veldhuisen DJ, Cleland JG, Butler J, Epstein AE, et al. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur Heart J. 2013;34:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mujib M, Patel K, Fonarow GC, Kitzman DW, Zhang Y, Aban IB, et al. Angiotensin-converting enzyme inhibitors and outcomes in heart failure and preserved ejection fraction. Am J Med. 2013;126:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel K, Fonarow GC, Kitzman DW, Aban IB, Love TE, Allman RM, et al. Aldosterone Antagonists and Outcomes in Real-World Older Patients with Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2013;1:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–1953. [DOI] [PubMed] [Google Scholar]
- 23.Gwathmey JK, Kim CS, Hajjar RJ, Khan F, DiSalvo TG, Matsumori A, et al. Cellular and molecular remodeling in a heart failure model treated with the beta-blocker carteolol. Am J Physiol. 1999;276:H1678–1690. [DOI] [PubMed] [Google Scholar]
- 24.Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154–1161. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A, Fleg JL. Heart failure in older adults In: Aronow WS, Flaherty JD, Rich MW, eds. Tresch and Aronow’s Cardiovascular Disease in the Elderly, Fifth Edition Boca Raton, Florida: CRC Press; Taylor and Francis Group; 2013. [Google Scholar]
- 26.Sanam K, Bhatia V, Parvataneni S, Morgan CJ, Lloyd S, Hage F, et al. Discharge Initiation of ACE Inhibitors or ARBs is Associated with Significantly Lower 30-Day All-Cause Readmission in Hospitalized Older Patients with Heart Failure and Reduced Ejection Fraction J Am Coll Cardiol. 2014. [Google Scholar]
- 27.Carson P, Anand I, O’Connor C, Jaski B, Steinberg J, Lwin A, et al. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46:2329–2334. [DOI] [PubMed] [Google Scholar]
- 28.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. [DOI] [PubMed] [Google Scholar]
- 29.Kheirbek RE, Alemi F, Citron BA, Afaq MA, Wu H, Fletcher RD. Trajectory of illness for patients with congestive heart failure. J Palliat Med. 2013;16:478–484. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, et al. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159:841–849 e841. [DOI] [PubMed] [Google Scholar]
- 31.Konstam MA, Gheorghiade M, Burnett JC Jr., Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 32.Krum H, Roecker EB, Mohacsi P, Rouleau JL, Tendera M, Coats AJ, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289:712–718. [DOI] [PubMed] [Google Scholar]
- 33.Butler J, Arbogast PG, BeLue R, Daugherty J, Jain MK, Ray WA, et al. Outpatient adherence to beta-blocker therapy after acute myocardial infarction. J Am Coll Cardiol. 2002;40:1589–1595. [DOI] [PubMed] [Google Scholar]
- 34.Butler J, Arbogast PG, Daugherty J, Jain MK, Ray WA, Griffin MR. Outpatient utilization of angiotensin-converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. [DOI] [PubMed] [Google Scholar]
- 35.Curtis LH, Mi X, Qualls LG, Check DK, Hammill BG, Hammill SC, et al. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986 e971. [DOI] [PubMed] [Google Scholar]
- 36.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. [DOI] [PubMed] [Google Scholar]
- 37.Gattis WA, O’Connor CM, Gallup DS, Hasselblad V, Gheorghiade M, Investigators I-H, et al. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–1541. [DOI] [PubMed] [Google Scholar]
- 38.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
- 39.Mujib M, Zhang Y, Feller MA, Ahmed A. Evidence of a “heart failure belt” in the southeastern United States. Am J Cardiol. 2011;107:935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]