Abstract
Purpose
Cisplatin (DDP) based chemotherapy regimens are widely used in advanced gastric cancer (GC). Drug resistance often limited the clinical benefits of cisplatin regimen. The mechanisms of cisplatin resistance have not been fully revealed. Therefore, further exploration of the relevant molecular mechanisms is urgently needed.
Patients and methods
DDP resistance associated miRNA of GC microarray dataset GSE86195 was obtained from the National Center for Biotechnology Information (NCBI) GEO database, GEO2R was applied to compare the samples in two different groups under the same experimental conditions. |log2(Fold Change) | (log2(FC)) was selected as the criteria to screen the statistically significant DE-miRNAs. StarBaseV3.0 was used to predict the target genes of the DE-miRNAs. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of target genes of DE-miRNAs were carried out using DAVID. The STRING database was applied to estimate the correlations between target genes. Analysis of hubgenes by coremine and The Human Protein Atlas (THPA). Initial expression validations of miR-424 and miR-491-5p, SMURF1 and BCL2L1 were carried out using clinical pathological specimens by RT-PCR.
Results
A total of 13 Differential expression-miRNAs (DE-miRNAs) were identified in DDP chemoresistant cells, including 9 upregulated miRNAs and 4 downregulated miRNAs. SMURF1 and BCL2L1 were screened as the critical genes in DDP-resistant GC, which were regulated by miR-424 and miR-491-5p respectively. The results of validation of hub genes expression in GC tissues indicated that in DFS<1-year group, the expression of miR-424 decreased significantly, notably upregulated expression of SMURF1 was also detected.
Conclusion
Our results implied that miR-424, as a tumor suppressor, could deregulate SMURF1 in DDP-resistant GC cells.
Keywords: gastric cancer, cisplatin drug resistance, miRNAs
Introduction
Gastric cancer (GC) is the fifth commonest cancer worldwide and the third commonest cause of cancer-related death.1 Although the treatment modalities of GC have gradually improved in the past few decades, the cure rate is still unsatisfactory, leading to more effective treatments remain a major clinical challenge. At the present time, chemotherapy plays an important role in the comprehensive treatment of GC.2 Platinum-based chemotherapy is one of the prominent chemotherapies for advanced GC.3–5 However, due to the often-emerging occurrence of drug resistance, the clinical applications of cisplatin (DDP)-based chemotherapy drugs have been limited,6,7 which is one of the leading causes of death in GC patients.7 Thus, reversing drug resistance of DDP offers potential for dramatic improvement of chemotherapy efficacy, as well as the prognosis of patients.9 However, mechanisms of DDP resistance are extremely complicated and have not yet been clearly established. Therefore, it is imperative to explore the relevant molecular biomarkers and mechanisms at the current stage.
MicroRNAs (miRNAs) are small, non-coding RNAs ranging in length from 21 to 23 nucleotides, which can act as post-transcriptional regulators to regulate downstream gene expression.10 Mature miRNAs can inhibit target protein expression by binding to the 3ʹ-untranslated region of the target gene mRNA in the cytoplasm. Aberrant miRNAs have been confirmed to regulate the biological roles of various cancers’ occurrence and progression11–17 Some studies suggested that several dysregulated miRNAs in GC involved in many biological processes,18–20 including cell proliferation, invasion and metastasis.
However, the exact function of miRNAs in DDP resistance has not been thoroughly studied yet, comprehensive analysis of miRNA expression profile between DDP resistance and DDP sensitive GC is required. Microarray and next-generation sequence data screened from high-throughput platforms may provide a wealth of useful information after bioinformatics analysis, which provides a promising opportunity to clarify the mechanism of cancer progression and its regulatory network.
Recently, in-depth bioinformatics analysis of integrated microarray data from shared public data platforms (for example GEO database) has been a hotspot of research, which has stimulated the development of biological interpretation. With the gradual accumulation of available biomedical data, biology computational technology has rapidly promoted the development of precision medicine. In this study, key miRNAs in DDP-resistant GC were screened by using a range of bioinformatics techniques and explored their regulatory networks and underlying molecular mechanisms. On this basis, initial expression validations were carried out using clinical pathological specimens from Tianjin Medical University General Hospital.
Materials and methods
miRNA microarray
GC microarray dataset GSE86195 was obtained from the National Center for Biotechnology Information (NCBI) GEO database (http://www.ncbi.nlm.nih.gov/geo) to identify DDP resistance associated miRNAs. GSE86195 dataset was based on Affymetrix GeneChip miRNA 2.0 Array platform (CapitalBio Corporation, Beijing, China). Specifically, GSE86195 dataset included miRNA expression profiles of two cisplatin resistant GC cell lines BGC-823/DDP and SGC-7901/DDP and their sensitive parental cell lines, respectively. BGC-823/DDP and SGC-7901/DDP cells were produced from parental BGC-823 and SGC-7901 cells described as Yajie.21
Screening for differentially expressed-miRNAs (de-miRNAs)
In the present study, GEO2R (www.ncbi.nlm.nih.gov/geo/geo2r/), an interactive web tool was applied to compare the samples in two different groups under the same experimental conditions,22 and identify genes that are differentially expressed across experimental conditions. |log2(Fold Change)| (|log2(FC)|) was selected as the criteria to screen the statistically significant differentially expressed-miRNAs (DE-miRNAs).22 Only miRNA with |log2(FC)| >1.5 and P-value <0.05 were identified as statistically DE-miRNAs. Additionally, the selected DE-miRNAs were divided into up-regulated and down-regulated groups. The heat map of DE-miRNAs was constructed using Heml.23
Target genes prediction of DE-miRNAs
The StarBaseV3.0 database predicted potential miRNA-target genes by collecting information from 7 existing prediction software (PITA, RNA22, miRmap, microT, miRanda, PicTar, TargetScan).24 In the present study, StarBaseV3.0 was used to predict the target genes of the DE-miRNAs. The common genes can be identified as a downstream target gene of DE-miRNA, only the genes were predicted by at least 5 softwares.
Functional and pathway enrichment analyses
DAVID (http://david.abcc.ncifcrf.gov/) is an online database for annotation, visualization and integrated discovery. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of target genes of DE-miRNAs were carried out using DAVID (version 6.8). GO analysis is widely used for a short list of genes with significant expression differences, which included three categories: biological process (BP), molecular function (MF) and cellular component (CC).25 While KEGG is a knowledge base for the systematic study of gene functions.26 P<0.05 was set as the threshold.
Protein-protein interaction (PPI) network and DE-miRNA target genes network construction
The STRING database (http://string-db.org) was applied to estimate the correlations between target genes,27 the combined score >0.9 were considered to have significant interactions. The important gene modules and key genes can be identified through PPI network analysis, Cytoscape (http://www.cytoscape.org/), as a network visualization tool was utilized to draw the network diagram.28 In order to identify the most valuable module, the network complex was further analyzed by MCODE package of Cytoscape. A plugin of Cytoscape, CytoHubba was used to rank nodes by their network features and select the top 10 genes from maximal clique centrality (MCC) as the hubgenes.29
Analysis of hubgenes by coremine
Annotation of biological processes involving hubgenes was performed by consulting the Coremine Medical online database, (www.coremine.com/medical/), the hubgenes included in the intersection of “stomach neoplasm” and “drug resistance” were analyzed successively.
Immunohistochemistry and prognostic analysis using the human protein atlas (THPA)
The images of Immunohistochemistry were obtained from the THPA (www.proteinatlas.org/), a publicly available database includes >5 million images of immunohistochemically stained tissues and cells.30 Data regarding the expression levels of LMO7, SMURF1, STAT3, BCL2L1 in gastric cancer tissues were obtained from THPA. In the meantime, the prognosis of high-expression and low-expression patients of the above genes was examined by Kaplan-Meier survival estimators, and the survival results of the two groups were compared by log-rank tests.
Expression validation by quantitative reverse transcription-polymerase chain reaction
The patients’ information who received R0 decision and standard postoperative adjuvant chemotherapy in the department of general surgery of Tianjin medical university general hospital since 2016/1 to 2018/12 was collected. This study was approved by the medical ethics committee of Tianjin medical university general hospital and consent form was completed before starting the study. And the patients were divided into two groups according to DFS≥1 year and DFS<1 year. Ten patients were selected randomly in each group. TRIzol reagent (Invitrogen) was utilized to extract total RNA from gastric cancer tissues in accordance with the manufacturer’s procedure, and then the total RNA (500ng) was reverse transcribed. Subsequently, we performed real-time PCR (RT-PCR) on applied biosystems 7900HT instrument. U6 (small nuclear RNA) for miRNA or GAPDH for mRNA were applied to normalize the result. All reactions were repeated 3 times and relative gene expressions were evaluated by the 2-ΔΔCt method. Primer sequences were as follows: SMURF1: 5ʹ-CTGGATGCTTTTGGTCTGGT-3ʹ(F);5ʹ-CCTGATAGACGCGAACACAG-3ʹ(R). BCL2L1:5ʹ-GGACAGCATATCAGAGCTTTGAACA-3ʹ(F),5ʹ-TTGTCTACGCTTTCCACGCA-3ʹ (R); miR-424-5p: 5ʹ-CCAGCAGTTCAAAAC ATGAATTG-3ʹ(F), 5ʹ-TATGGTTGTTCTCGACTCCTTGAC-3ʹ(R); miR-491-5p: 5ʹ-ACACTCCAGCTGGGAGTGGGGAACCCTTC-3ʹ(F)5ʹ-CTCAACTGGTGTCGTGGAGTCGGCAATTC AGTTGAGCCTCATG-3ʹ(R)
Statistical analysis
SPSS software (version 17.0; SPSS, Chicago, IL, USA) was applied to statistical analysis. Differences between the two groups were assessed by Student’s t-test. Pearson correlation analysis was performed to explore the relationship between miRNA and target genes in clinicopathologic specimen. P<0.05 was considered statistically significant.
Results
Microarray data
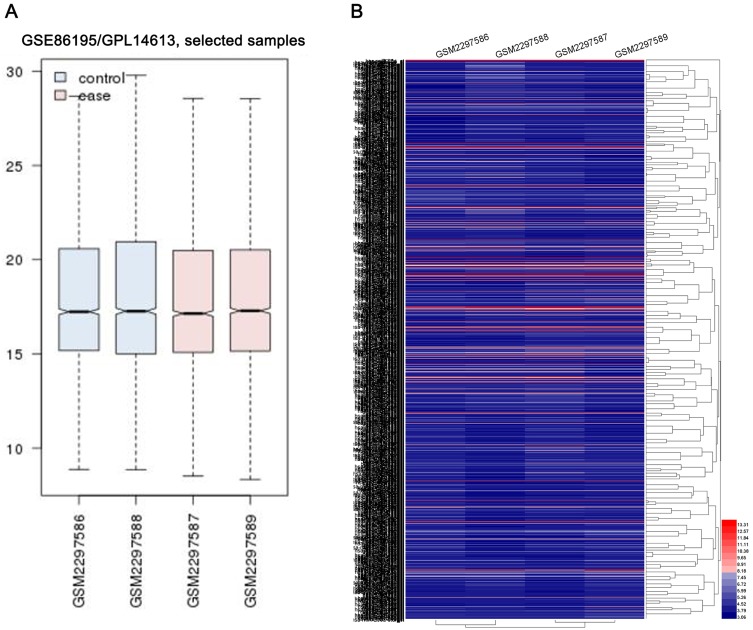
The miRNA expression profiles of GSE86195 were downloaded from GEO. The platform for GSE86195 was GPL14613 [miRNA-2] Affymetrix Multispecies miRNA-2 Array. GSE86195 included 2 kinds of GC cell lines, SGC7901 and BGC-823. The miRNA differences between Cisplatin (DDP)-sensitive cell lines and DDP-resistant cell lines were detected using the following datasets: GSM2297586 SGC-7901, parent; GSM2297587 SGC-7901/DDP, resistance; GSM2297588 BGC-823, parent; GSM2297589 BGC-823/DDP, resistance. The homogeneity of each datasets was evaluated by GEO2R, which indicated that all datasets had a satisfied homogeneity (Figure 1A). Data abundance and differences among datasets were displayed by heat map with 1681 upregulated miRNAs and 1756 downregulated miRNAs (Figure 1B).
Figure 1.
The miRNA expression profiles of GSE86195. (A) GEO2R was applied to evaluate the homogeneity of each datasets. (B) the heat map of all miRNAs in GSE86195.
Identification of DE-miRNAs and their target genes
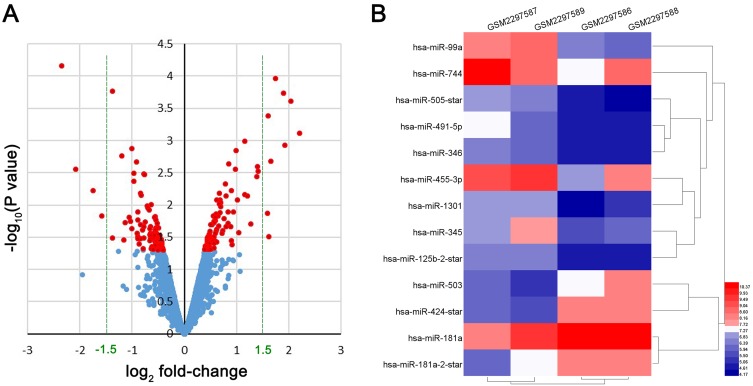
Relying on the criteria of |log2(FC)|>1.5 and P<0.05, a total of 13 DE-miRNAs were identified, including 9 upregulated miRNAs (hsa-miR-99a, hsa-miR-744, hsa-miR-505-star, hsa-miR-491-5p, hsa-miR-455-3p, hsa-miR-346, hsa-miR-345, hsa-miR-1301, hsa-miR-125b-2-star) and 4 downregulated miRNAs (hsa-miR-424-star, hsa-miR-181a-5p, hsa-miR-503, hsa-miR-181a-2-star) (Figure 2) in DDP chemoresistant cells, compared to DDP chemosensitive cells. StarBaseV3.0 was applied to predict the target genes of each DE-miRNAs, the results indicated that 1,159 potential target genes were generated, including 945 genes for downregulated miRNAs and 214 genes for upregulated miRNAs.
Figure 2.
DE-miRNA expression analysis by Volcano Plot and heat map. (A) the volcano plot of all miRNAs in GSE86195, red color represented P<0.05, blue color represented P≥0.05. (B) the heat map of DE-miRNAs in GSE86195.
Functional and pathway enrichment analyses
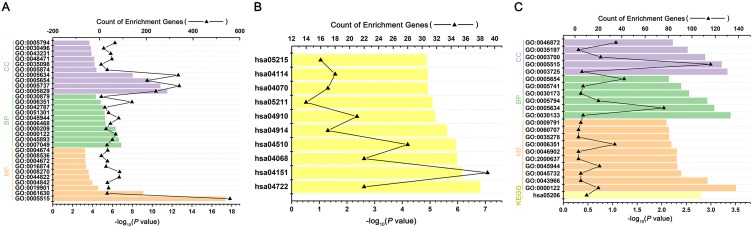
We performed GO functional and KEGG pathway enrichment analyses on the target genes generated previously. We performed the GO analysis at the following three different GO levels: biological process (BP) category; cell component (CC) category; and Molecular function(MF) category. The top 10 items that were significantly enriched by the target genes of downregulated miRNAs group at each of the above GO levels and KEGG pathway were outlined in Figure 3A, B. Intriguingly, ubiquitination activity was significantly upregulated in SGC-7901/DDP. In the BP category, the target genes were enriched in GO:0000209~protein polyubiquitination, GO:0042787~protein ubiquitination involved in ubiquitin-dependent protein catabolic process. In the CC category, the target genes were mainly enriched in the nucleus. In the MF category, the target genes were mainly enriched in GO:0061630~ubiquitin protein ligase activity, GO:0004842~ubiquitin-protein transferase activity. The above results indicated that ubiquitination activity might play an important role in the process of DDP drug resistance (Table 1). Then, GO and KEGG enrichment analyses were also performed on the target genes of upregulated miRNAs. The results were shown in Figure 3C and Table 2. In the BP category, the target genes were mainly enriched in negative regulation of transcription from RNA polymerase II promoter, positive regulation of transcription, DNA-templated. In the CC category, the target genes were mainly enriched in the nucleus. In the MF category, the target genes were mainly enriched in protein binding. Enriched KEGG pathways for target genes of upregulated DE-miRNA included microRNAs in cancer.
Figure 3.
GO and KEGG pathway annotation of the predicted target genes from the DE-miRNA. (A) Gene ontology (GO) enrichment of the target genes from the downregulated DE-miRNAs, including Biological function (BF) enrichment, Cellular component (CC) enrichment and Molecular Function (MF) enrichment. (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of target genes from the downregulated DE-miRNAs. (C) GO and KEGG enrichment of the target genes from the upregulated DEmiRNAs, including BF enrichment, CC enrichment and MF enrichment.
Table 1.
Enriched functions for the target genes of the downregulated miRNAs
| Terms | Count | P-value |
|---|---|---|
| Biological process (BP) | ||
| GO:0007049~cell cycle | 32 | 1.33E-07 |
| GO:0045893~positive regulation of transcription, DNA-templated | 55 | 2.23E-07 |
| GO:0000122~negative regulation of transcription from RNA polymerase II promoter | 69 | 3.35E-07 |
| GO:0000209~protein polyubiquitination | 28 | 4.95E-07 |
| GO:0006468~protein phosphorylation | 47 | 5.05E-06 |
| GO:0045944~positive regulation of transcription from RNA polymerase II promoter | 82 | 5.62E-06 |
| GO:0051301~cell division | 39 | 6.26E-06 |
| GO:0042787~protein ubiquitination involved in ubiquitin-dependent protein catabolic process | 23 | 7.78E-06 |
| GO:0006351~transcription, DNA-templated | 139 | 1.50E-05 |
| GO:0030879~mammary gland development | 8 | 4.83E-05 |
| Cellular component (CC) | ||
| GO:0005829~cytosol | 241 | 3.09E-12 |
| GO:0005737~cytoplasm | 341 | 1.53E-11 |
| GO:0005654~nucleoplasm | 204 | 1.61E-10 |
| GO:0005634~nucleus | 337 | 9.46E-09 |
| GO:0005874~microtubule | 33 | 4.18E-05 |
| GO:0035098~ESC/E (Z) complex | 7 | 6.33E-05 |
| GO:0048471~perinuclear region of cytoplasm | 53 | 6.82E-05 |
| GO:0043231~intracellular membrane-bounded organelle | 48 | 1.32E-04 |
| GO:0030496~midbody | 18 | 1.46E-04 |
| GO:0005794~Golgi apparatus | 66 | 2.07E-04 |
| Molecular function (MF) | ||
| GO:0005515~protein binding | 558 | 4.40E-18 |
| GO:0061630~ubiquitin protein ligase activity | 33 | 8.23E-10 |
| GO:0019901~protein kinase binding | 39 | 2.81E-05 |
| GO:0004842~ubiquitin-protein transferase activity | 34 | 1.07E-04 |
| GO:0044822~poly (A) RNA binding | 84 | 2.09E-04 |
| GO:0008270~zinc ion binding | 86 | 2.56E-04 |
| GO:0016874~ligase activity | 28 | 4.63E-04 |
| GO:0004672~protein kinase activity | 34 | 5.33E-04 |
| GO:0008536~Ran GTPase binding | 8 | 5.52E-04 |
| GO:0004674~protein serine/threonine kinase activity | 35 | 5.92E-04 |
| KEGG_PATHWAY | ||
| hsa04722: Neurotrophin signaling pathway | 22 | 1.53E-07 |
| hsa04151: PI3K-Akt signaling pathway | 39 | 7.09E-07 |
| hsa04068: FoxO signaling pathway | 22 | 1.04E-06 |
| hsa04510: Focal adhesion | 28 | 1.18E-06 |
| hsa04914: Progesterone-mediated oocyte maturation | 17 | 2.42E-06 |
| hsa04910: Insulin signaling pathway | 21 | 6.52E-06 |
| hsa05211: Renal cell carcinoma | 14 | 8.29E-06 |
| hsa04070: Phosphatidylinositol signaling system | 17 | 1.20E-05 |
Table 2.
Enriched functions for the target genes of the upregulated miRNAs
| Terms | Count | P-Value |
|---|---|---|
| Biological process (BP) | ||
| GO:0000122~negative regulation of transcription from RNA polymerase II promoter | 20 | 3.13E-04 |
| GO:0043966~histone H3 acetylation | 5 | 0.001185 |
| GO:0045732~positive regulation of protein catabolic process | 5 | 0.004059 |
| GO:0045944~positive regulation of transcription from RNA polymerase II promoter | 21 | 0.004878 |
| GO:2,000,637~positive regulation of gene silencing by miRNA | 3 | 0.004965 |
| GO:0046902~regulation of mitochondrial membrane permeability | 3 | 0.004965 |
| GO:0006351~transcription, DNA-templated | 34 | 0.00638 |
| GO:0035278~miRNA mediated inhibition of translation | 3 | 0.007179 |
| GO:0060707~trophoblast giant cell differentiation | 3 | 0.007179 |
| GO:0009791~post-embryonic development | 5 | 0.008128 |
| Cellular component (CC) | ||
| GO:0030133~transport vesicle | 7 | 4.03E-04 |
| GO:0005634~nucleus | 76 | 8.63E-04 |
| GO:0005794~Golgi apparatus | 20 | 0.001193 |
| GO:0030173~integral component of Golgi membrane | 5 | 0.00279 |
| GO:0005741~mitochondrial outer membrane | 7 | 0.004076 |
| GO:0005654~nucleoplasm | 42 | 0.007228 |
| Molecular function (MF) | ||
| GO:0003725~double-stranded RNA binding | 6 | 4.66E-04 |
| GO:0005515~protein binding | 116 | 6.09E-04 |
| GO:0003700~transcription factor activity, sequence-specific DNA binding | 22 | 0.001318 |
| GO:0035197~siRNA binding | 3 | 0.003002 |
| GO:0046872~metal ion binding | 35 | 0.005995 |
| KEGG_PATHWAY | ||
| hsa05206: microRNAs in cancer | 10 | 0.001587 |
PPI and mirRNA-target network analysis
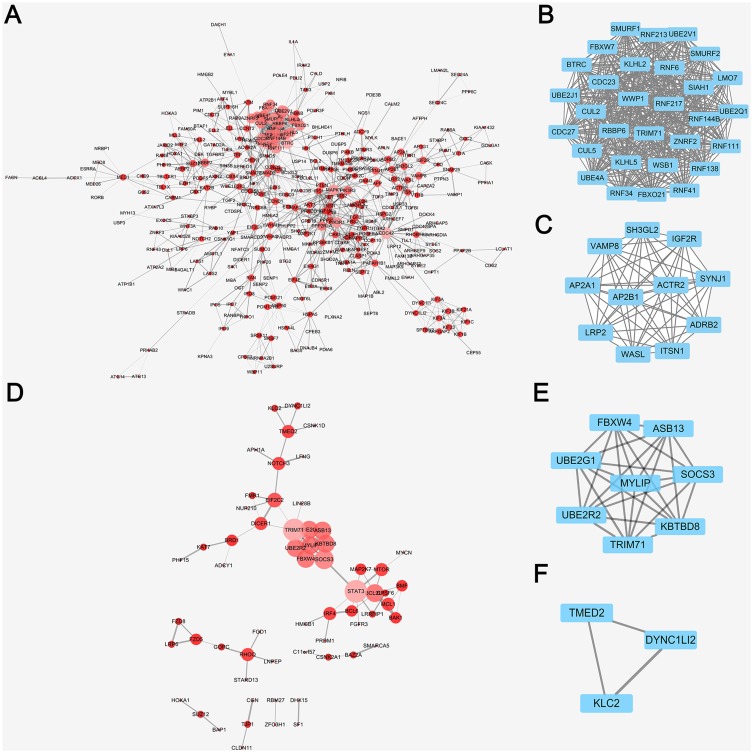
In order to forecast protein interactions, the target genes in top 10 of each GO levels and KEGG analysis were merged respectively. The results revealed that 779 target genes in downregulated miRNAs and 214 target genes in upregulated miRNAs were screened, which were submitted to the Search Tool for the Retrieval of Interacting Gene (STRING) database. The PPI network of downregulated miRNAs group consisted of 777 nodes and 1,372 edges, while the PPI network of upregulated miRNAs group consisted of 202 nodes and 63 edges. Meanwhile, the network complex was further analyzed, and the most significant module was screened out using MCODE package of Cytoscape (Figure 4). We screened out the top 10 target genes with higher MCC score as the hubgenes ultimately. The hubgenes were listed as follows: downregulated miRNAs group CDC27, CDC23, KLHL2, RNF213, SMURF1, SMURF2, UBE4A, LMO7, WSB1 and RNF6; upregulated miRNAs group MYLIP, UBE2R2, UBE2G1, ASB13, KBTBD8, SOCS3, FBXW4, TRIM71, STAT3 and BCL2L1.
Figure 4.
PPI network analysis by cytoscape of DE-miRNAs in downregulated and upregulated group. (A) PPI network analysis of all the target genes from the downregulated DE-miRNAs, the node size and color represent the degree of target genes (low value to small sizes and dark colors). (B) The top one tetwork complex analysis of target genes from the downregulated DE-miRNAs by MCODE, which included 30 nodes and 435 edges. (C) The top two network complex analysis of target genes from the downregulated DEmiRNAs by MCODE, which included 11 nodes and 55 edges. (D) PPI network analysis of all the target genes from the upregulated DE-miRNAs, the node size and color represent the degree of target genes (low value to small sizes and dark colors). (E) The top one network complex analysis of target genes from the downregulated DE-miRNAs by MCODE, which included 8 nodes and 28 edges. (F) The top two network complex analysis of target genes from the downregulated DE-miRNAs by MCODE, which included 3 nodes and 3 edges.
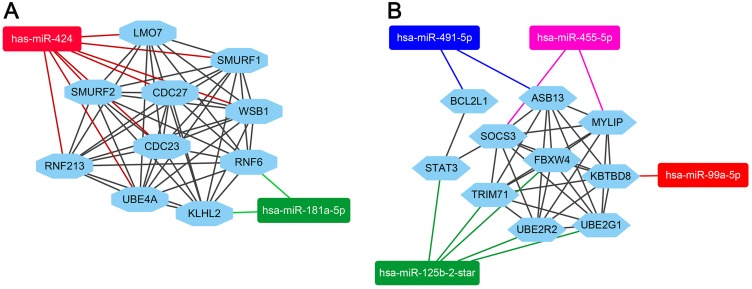
As shown in Figure 5, the miRNA-hubgenes network was constructed by cytoscape. The hubgenes of the downregulated miRNAs could be potentially regulated by hsa-miR-424 (CDC27, CDC23, RNF213, SMURF1, SMURF2, UBE4A, LMO7, WSB1) and hsa-miR-181a-5p (KLHL2, RNF6). The hub target genes of the upregulated miRNAs could be potentially regulated by hsa-miR-99a(KBTBD8), hsa-miR-491-5p (ASB13, BCL2L1), hsa-miR-455-5p (MYLIP, SOCS3) and hsa-miR-125b-2-star (UBE2R2, UBE2G1, FBXW4, TRIM71, STAT3, BCL2L1).
Figure 5.
mircoRNA-target hubgenes network analysis by cytoscape. (A) The hubgenes of downregulated miRNAs group included CDC27, CDC23, RNF213, SMURF1, SMURF2, UBE4A, LMO7, WSB1 which were regulated by miR-424, KLHL2 and RNF6 were regulated by miR-181a-5p. (B) The hubgenes of upregulated miRNAs group included MYLIP, SOCS3 which were regulated by miR-455-5p, FBXW4, TRIM71, STAT3, UBE2R2, UBE2G1 which were regulated by miR-125b-2-star, ASB13, BCL2L1 which were regulated by miR-491-5p and KBTBD8, which were regulated by miR-99a-5p.
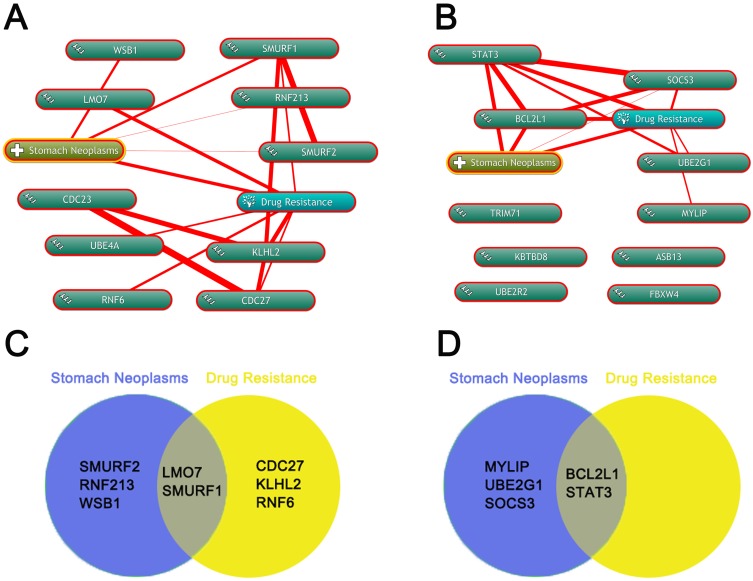
Annotation of biological processes involving hubgenes were performed by consulting the Coremine Medical online database. In the downregulated miRNAs group, 5 hubgenes were related to stomach neoplasms including CDC27, LMO7, KLHL2, RNF6 and SMURF1, while 5 hubgenes were related to drug resistance including LMO7, SMURF2, RNF213, SMURF1, WSB1. Therefore, LMO7 and SMURF1 were treated as the target genes related to stomach neoplasms drug resistance (Figure 6 A and C). In upregulated miRNAs group, 5 hubgenes were related to stomach neoplasms including BCL2L1, MYLIP, STAT3, UBE2G1 and SOCS3, while 2 hubgenes were related to drug resistance including BCL2L1 and STAT3. Therefore, BCL2L1 and STAT3 were treated as the other two target genes related to stomach neoplasms drug resistance (Figure 6 B and D).
Figure 6.
Annotation of biological processes involving hubgenes by Coremine. (A) Annotation of biological processes of hubgenes in downregulted DE-miRNA group by the Coremine database. (B) Annotation of biological processes of hubgenes in upregulted DE-miRNA group by the Coremine database. (C) screen the hubgenes in downregulted DE-miRNA group both associated with stomach neoplasms and drug resistance by Venny. (D) screen the hubgenes in downregulted DE-miRNA group both associated with stomach neoplasms and drug resistance by Venny.
Validation of hub genes expression and prognostic analyses by THPA
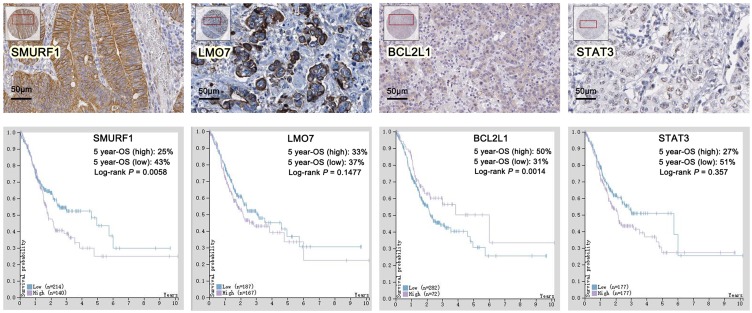
We continued to examine the protein expression of hub genes in GC tissues, in the meantime, prognostic analyses were extracted from THPA. The results revealed that, in GC patients, high expression of SMURF1 contributed to an unfavorable prognosis in comparison with low expression group (P=0.0058). Low expression of BCL2L1 contributed to poor prognosis remarkably in comparison with high expression group (P=0.0014). While no significance was observed between high and low expression groups of LMO7 and STAT3. Therefore, we chose SMURF1 and BCL2L1 as the critical genes in DDP resistant GC. Based on the mircoRNA-target hubgenes network analysis, SMURF1 and BCL2L1 were regulated by miR-424 and miR-491-5p respectively. (Figure 7).
Figure 7.
Validation of hubgenes expression and prognostic analyses of hubgenes by THPA.
Validation of hub genes expression in GC tissues by RT-PCR
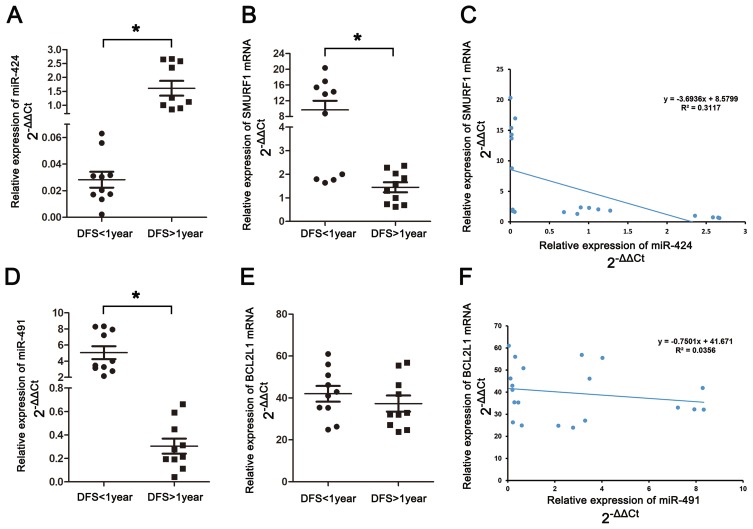
The expression of miR-424, miR-491, SMURF1 and BCL2L1 in GC tissues of our department were evaluated by RT-PCR. The results indicated that in DFS<1-year group, the expression of miR-424 decreased significantly (P<0.001) (Figure 8A), in the meantime, notably upregulated expression of miR-491 (P<0.001) (Figure 8D) and SMURF1 (P=0.006) (Figure 8B) was also detected. While, no significant difference of BCL2L1 expression could be found between DFS<1-year group and DFS≥1-year group (Figure 8E). Relying on the above results, miR-424 and SMURF1 exhibited a significant negative linear correlation relationship (Figure 8C), while no significant linear correlation relationship was founded between miR-491 and BCL2L1(Figure 8F).
Figure 8.
Validation of hubgenes expression in GC tissues by RT-PCR. (A) The expression of miR-424 in gastric cancer patients. (B) The expression of SMURF1 in gastric cancer patients. (C) Linear correlation analysis between miR-424 and SMURF1. D) The expression of miR-491 in gastric cancer patients. (E) The expression of BCL2L1 in gastric cancer patients. (F) Linear correlation analysis between miR-491 and BCL2L1 (*Significant differences could be observed among the two groups).
Discussion
DDP is widely used in the treatment of a variety of solid tumors.31 However, repeated long-term clinical exposure to DDP typically results in tumor cells escaping the DDP-induced apoptosis program. Therefore, it is important to clarify the molecular mechanism of cisplatin resistance in reversing drug resistance in cancer therapy. Up to now, lots of studies have confirmed the association of miRNA expression profiles with the gastric carcinogenesis. These studies have reported miRNA function and confirmed the miRNA value as a diagnostic marker and prognostic analysis.32–35 In this study, we investigated the distribution of key miRNAs in DDP-resistant BGC-823 and SGC-7901 cells and their target gene profiles relying on bioinformatics differential analysis.
In this study, various miRNAs showed differential expression in the microarray analysis, including 1681 upregulated miRNAs and 1756 downregulated miRNAs. According to the DE-miRNA criteria (|log2FC|>1.5 and P<0.05), a total of 13 DE-miRNAs were identified, including 9 upregulated miRNAs and 4 downregulated miRNAs. In Wang’s study, |log2FC|>1 and P<0.05 were treated as the DE-miRNA criteria,36 while in Li’s study,37 |log2FC|>1.5 and P<0.05 were applied as the DE-miRNA criteria which were consisted with our research. Afterwards, StarBaseV3.0 was used to predict the target genes of each DE-miRNA. As an open-source platform for studying the miRNA-mRNA, StarBaseV3.0 has been used in many studies.6,38–40 In our study, in order to improve the accuracy of target gene prediction, we only select target genes that were simultaneously included in at least five types of databases as potential target genes. 1,159 potential target genes were generated, including 214 target genes for upregulated miRNAs and 945 genes for downregulated miRNAs. Afterwards, GO and KEGG analyses were generated for functional prediction. The top 10 functions and pathway enrichment were selected for further research. 779 target genes in downregulated miRNAs and 214 target genes in upregulated miRNAs were submitted to the STRING database to construct two PPI networks, respectively. We continued to screen the hub genes by the plug-in CytoHubba in Cytoscape, which was widely used in other research.41 After checked by Coremine database and THPA, SMURF1 and BCL2L1 were identified as the final hubgenes in the present research, which were regulated by miR-424 and miR-191a, respectively. However, after the validation of hub genes expression in GC tissues, no significant difference of BCL2L1 expression could be found between DFS<1-year group and DFS≥1-year group. Therefore, we speculated that miR-424 regulated the chemotherapy sensitivity by inhibiting the expression of SMURF1.
MicroRNAs (miRNAs) are small non-coding RNAs with lengths of 19–25 nucleotides that regulate the translation or degradation of target mRNA in the human body.42 MiRNAs play an important role in the proliferation, invasion and apoptosis of malignant tumour cells and may affect resistance to cisplatin.43,44 Many reports have testified that miR-424 was abnormally expressed in several human cancers.45–48 For instance, in non-small cell lung cancer, loss of miR-424-3p conferred chemoresistance through targeting YAP1.49 Besides, miR-424 could influence many biology processes by other target genes. In Baptista’s research, miR-424 promoted pulmonary arterial hypertension by directly targeting SMURF1.50 In our study, the expression of miR-424 in DDP resistant group was significantly decreased in compared with the DDP chemosensitive group, which was consistent with other research.51,52 In present study, hub genes were screened by the plug-in CytoHubba, among the top 10 hubgenes in downregulation miRNA group, 8 of them were identified as the target genes of miRNA-424(CDC27, CDC23, RNF213, SMURF1, SMURF2, UBE4A, LMO7, WSB1), the hub gens were continued to be screened by Coremine Medical online database. Coremine Medical is a domain-specific search engine for medical information, which was based on a new search concept that offers the user domain-specific networks. In previous studies, the Coremine database was widely used to find new therapeutic targets and mechanism analysis, especially in the analysis of chemotherapy resistance mechanisms.53,54 Therefore, after cross-alignment by coremine database, LMO7, SMURF1, BCL2L1 and STAT3 were identified as the primary hubgenes. Afterwards, we continued to evaluate the association between hubgenes and the prognosis of gastric cancer patients by THPA. As an online human proteome database, THPA has already contributed to several thousands of publications in the field of human biology and disease and it is selected by the organization ELIXIR (www.elixir-europe.org) as a European core resource due to its fundamental importance for a wider life science community,55 the results indicated that the expression of SMURF1 and BCL2L1 in gastric cancer is significantly associated with the poor prognosis in gastric cancer patient. Therefore, we identified SMURF1 and BCL2L1 as the hubgenes ultimately.As one of the hub genes, SMURF1 was also the target genes of miR-424.
SMURF1 belongs to the NEDD4 subfamily of HECT-type E3s and plays an important role in the ubiquitination process of the C2-WWHECT domain56 and is involved in several key cellular processes. SMURF1 has been shown to form an isomeric complex with the common-partner Smad (Co-Smad) Smad4 and ubiquitinate to degrade the BMP receptor-regulated Smad protein. This complex regulates the transcription of multiple target genes in the nucleus and is involved in many biological processes such as tumorigenesis, cancer progression and chemoresistance.57,58 In the present study, the results of functional and pathway enrichment analyses demonstrated that ubiquitination activity exhibited more active in SGC-7901/DDP, which also indicated that ubiquitination activity might perform an essential function in the process of DDP drug resistance.
Based on results of microarray, in DDP-resistant cells, the expression of miR-424 was significantly decreased in comparison with DDP-sensitive cells. In our validation tests, we detected the expression of SMURF1 and miR-424 in GC tissues derived from the patients who received R0 resection and standard postoperative adjuvant chemotherapy. The results showed miR-424 was downregulated in patients with DFS<1 year, while SMURF1 was upregulated. Statistically negative linear relationship between them implied SUMRF1 was negatively regulated by miR-424. These results were all consisted with the microarray sequencing and our analysis. However, the detail mechanism of SMURF1 inducing the drug resistance still needs to be studied. Lin’s research demonstrated that PKA/Smurf1 antagonizes the downregulating effect of JNK on Nur77, leading to the accumulation of Nur77 for apoptosis induction triggered by cisplatin.59 Vial’s research indicates that SMURF1 participated in the formation of lamellipodia by regulating peripheral RhoA/ROCK/MLC2 signaling and further regulate the plasticity and motility of tumor cells.60 RhoA belongs to the Rho GTPase family,61 which involved in the switching cycle of intracellular molecules between in the active form of GTP binding and the inactive form of GDP binding. Many studies have testified that RhoA was closely related to chemoresistance in GC and colorectal cancer.62 Therefore, we speculated that, in DDP resistant cancer cells, downregulation of miR-424 contributed to the upregulation expression of SMURF1, which induced the DDP resistance via stimulating the RhoA.
Conclusion
In summary, our data revealed that miR-424 was a tumor suppressor and it could deregulate SMURF1 in DDP-resistant GC cells, which might provide a novel strategy for targeted therapy of patients with GC.
Acknowledgments
This research was funded by Tianjin Medical University General Hospital Youth Incubation Fund, grant number ZYYFY2017029, Youth Science Foundation of the Second Hospital Center Laboratory of Tianjin Medical University, grant number 2017ydey11 and Tianjin municipal education commission scientific research project, grant number 2018KJ055.
Author contributions
Weihua Fu and Weidong Li constructed the ideas and assumptions of the research. Tong Liu and Weidong Li designed a research plan to reach the conclusions. Li Lu and Yaoheng Lu were responsible for conducting experiments, patient’s follow-up, data management and statistics. Li Lu and Menglin Wu were responsible for the construction of the whole or the body of the manuscript and the production of the images. Reviewing the manuscript before submission not only for spelling and grammar but also for its intellectual content have been provided by Zhicheng Zhao and Weidong Li. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Hwang JH. Understanding gastric cancer risk factors: we need to close the gap. Gut Liver. 2018;12:1–2. doi: 10.5009/gnl17503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378 [DOI] [PubMed] [Google Scholar]
- 4.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717 [DOI] [PubMed] [Google Scholar]
- 5.Faghihloo E, Araei Y, Mohammadi M, Mirzaei H, Mohammadi HR, Mokhtari-Azad T. The effect of oxaliplatin on the E-cadherin expression in gastric cancer cell line. Cancer Gene Ther. 2016;23(11):396–399. doi: 10.1038/cgt.2016.52 [DOI] [PubMed] [Google Scholar]
- 6.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia JA, Dreicer R. Systemic chemotherapy for advanced bladder cancer: update and controversies. J Clin Oncol. 2006;24:5545–5551. doi: 10.1200/JCO.2006.08.0564 [DOI] [PubMed] [Google Scholar]
- 8.Peng PL, Zhou XY, Yi GD, Chen PF. Wang F Dong WG. Identifcation of a novel gene pairs signature in the prognosis of gastric cancer. Cancer Med. 2018;7:344–350. doi: 10.1002/cam4.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nienhüser H, Schmidt T. Angiogenesis and anti-angiogenic therapy in gastric cancer. Int J Mol Sci. 2018;19(43):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103 [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Zhang Y, Liu Z, Zhang X, Jia J. miRNA-532-5p functions as an oncogenic microRNA in human gastric cancer by directly targeting RUNX3. J Cell Mol Med. 2016;20(1):95–103. doi: 10.1111/jcmm.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi B, Liu SG, Qin XG, et al. Overregulation of microRNA-212 in the poor prognosis of esophageal cancer patients. Genet Mol Res. 2014;13:7800–7807. doi: 10.4238/2014.September.26.18 [DOI] [PubMed] [Google Scholar]
- 13.Jamali L, Tofigh R, Tutunchi S, et al. Circulating microRNAs as diagnostic and therapeutic biomarkers in gastric and esophageal cancers. J Cell Physiol. 2018;233(11):8538–8550. doi: 10.1002/jcp.26850 [DOI] [PubMed] [Google Scholar]
- 14.Bae HJ, Noh JH, Kim JK, et al. MicroRNA-29c functions as a tumor suppressor by direct targeting oncogenic SIRT1 in hepatocellular carcinoma. Oncogene. 2014;33:2557–2567. doi: 10.1038/onc.2013.216 [DOI] [PubMed] [Google Scholar]
- 15.Simonian M, Mosallayi M, Mirzaei H. Circulating miR-21 as novel biomarker in gastric cancer: diagnostic and prognostic biomarker. J Cancer Res Ther. 2018;14(2):475. [DOI] [PubMed] [Google Scholar]
- 16.Zhou R, Zhou X, Yin Z, et al. Tumor invasion and metastasis regulated by microRNA-184 and microRNA-574-5p in small-cell lung cancer. Oncotarget. 2015;6(42):44609–44622. doi: 10.18632/oncotarget.6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yau TO, Wu CW, Tang CM, et al. microRNA-20a in human faeces as a non-invasive biomarker for colorectal cancer. Oncotarget. 2016;7(2):1559–1568. doi: 10.18632/oncotarget.6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Z, Wang J, Zhao L, et al. Potential role of microRNA-21 in the diagnosis of gastric cancer: a meta-analysis. PLoS One. 2013;8:e73278. doi: 10.1371/journal.pone.0073278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XW, Wu Y, Wang D, Qin ZF. MicroRNA network analysis identifies key microRNAs and genes associated with precancerous lesions of gastric cancer. Genet Mol Res. 2014;13:8695–8703. doi: 10.4238/2014.October.27.10 [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto N, Naito Y, Oue N, et al. MicroRNA-148a is downregulated in gastric cancer, targets MMP7, and indicates tumor invasiveness and poor prognosis. Cancer Sci. 2014;105:236–243. doi: 10.1111/cas.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yajie Z, Wenxia X, Pan N, et al. MiR-99a and MiR-491 regulate cisplatin resistance in human gastric cancer cells by targeting CAPNS1[J]. Int J Bio Sci. 2016;12(12):1437–1447. doi: 10.7150/ijbs.16529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng W, Wang Y, Liu Z, Cheng H, Xue Y. HemI: A Toolkit for Illustrating Heat maps. PLoS One. 2014;9(11):e111988. doi: 10.1371/journal.pone.0111988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–97. doi: 10.1093/nar/gkt1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gene Ontology Consortium. The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 2006;34(Database Issue):D322–D326. doi: 10.1093/nar/gkj021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanehisa M, Skegg G. Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248 [DOI] [PubMed] [Google Scholar]
- 31.Muggia FM, Bonetti A, Hoeschele JD, Rozencweig M, Howell SB. Platinum antitumor complexes: 50 years since barnett rosenberg’s discovery. J Clin Oncol. 2015;33:4219–4226. doi: 10.1200/JCO.2015.60.7481 [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Tian J, Z J G, et al. Expression of microRNAs-106b in nonsmall cell lung cancer[J]. J Cancer Res Therap. 2018;14(9):295–298. doi: 10.4103/0973-1482.235344 [DOI] [PubMed] [Google Scholar]
- 33.Shao Q, Xu J, Guan X, et al. In vitro and in vivo effects of miRNA-19b/20a/92a on gastric cancer stem cells and the related mechanism. Int J Med Sci. 2018;15(1):86–94. doi: 10.7150/ijms.21164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirzaei H, Khataminfar S, Mohammadparast S, et al. Circulating microRNAs as potential diagnostic biomarkers and therapeutic targets in gastric cancer: current status and future perspectives. Curr Med Chem. 2016;23(36):4135–4150. doi: 10.2174/0929867323666160818093854 [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Lu Z, Wang T, et al. Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: A miRNA expression analysis. Gene. 2018;673(5):181–193. doi: 10.1016/j.gene.2018.06.037 [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Zhang J. Identification of differential expression lncRNAs in gastric cancer using transcriptome sequencing and bioinformatics analyses. Mol Med Rep. 2018;17(6):8189–8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Huang C, Li Q, et al. Construction and comprehensive analysis for dysregulated long non-coding RNA (lncRNA)-associated competing endogenous RNA (ceRNA) network in gastric cancer. Med Sci Monit. 2018;24:37–49. doi: 10.12659/MSM.905410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong N, Zhang X, Liu Q. Identification of therapeutic targets for Parkinson’s disease via bioinformatics analysis. Mol Med Rep. 2017;15(2):731–735. doi: 10.3892/mmr.2016.6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Ning Y, Zhou B, et al. Integrated bioinformatics analysis of the osteoarthritis associated microRNA expression signature. Mol Med Rep. 2018;17(1):1833–1838. [DOI] [PubMed] [Google Scholar]
- 40.Xu F, Zhang J. Long non-coding RNA HOTAIR functions as miRNA sponge to promote the epithelial to mesenchymal transition in esophageal cancer. Biomed Pharmacother. 2017;90:888–896. doi: 10.1016/j.biopha.2017.03.103 [DOI] [PubMed] [Google Scholar]
- 41.Tang J, Kong D, Cui Q, et al. Bioinformatic analysis and identification of potential prognostic microRNAs and mRNAs in thyroid cancer. PeerJ. 2018;6(Suppl 4):e4674. doi: 10.7717/peerj.4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 43.Hollis M, Nair K, Vyas A, et al. MicroRNAs potential utility in colon cancer: early detection, prognosis, and chemosensitivity. World J Gastroenterol. 2015;21:8284–8292. doi: 10.3748/wjg.v21.i27.8284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Gao Y, Zhang K, et al. MicroRNAs as regulators of cisplatin resistance in lung cancer. Cell Physiol Biochem. 2015;37:1869–1880. doi: 10.1159/000438548 [DOI] [PubMed] [Google Scholar]
- 45.Oneyama C, Kito Y, Asai R, et al. MiR-424/503-mediated Rictor upregulation promotes tumor progression. PloS ONE. 2013;8(11):e80300. doi: 10.1371/journal.pone.0080300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long XH, Mao JH, Peng AF, ZHOU Y, HUANG SH, LIU ZL. Tumor suppressive microRNA-424 inhibits osteosarcoma cell migration and invasion via targeting fatty acid synthase. Exp Therap Med. 2013;5(4):1048–1052. doi: 10.3892/etm.2013.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu L, Ding GF, He C, Sun L, Jiang Y, Zhu L. MicroRNA-424 is down-regulated in hepatocellular carcinoma and suppresses cell migration and invasion through c-Myb. PloS ONE. 2014;9(3):e91661. doi: 10.1371/journal.pone.0091661 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Yang H, Zheng W, Shuai X, et al. MicroRNA-424 inhibits Akt3/E2F3 axis and tumor growth in hepatocellular carcinoma. Oncotarget. 2015;6(29):27736–27750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang M, Zeng J, Zhao Z, et al. Loss of MiR‐424‐3p, not miR‐424‐5p, confers chemoresistance through targeting YAP1 in non‐small cell lung cancer. Mol Carcinog. 2017;56(3):821–832. doi: 10.1002/mc.22536 [DOI] [PubMed] [Google Scholar]
- 50.Baptista R, Marques C, Catarino S, et al. MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovasc Res. 2018;114(1):53–64. doi: 10.1093/cvr/cvx187 [DOI] [PubMed] [Google Scholar]
- 51.Lu C, Wang H, Chen S, et al. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway[J]. J Cell Mol Med. 2018;22:2478–2487. doi: 10.1111/jcmm.13556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bieg D, Sypniewski D, Nowak E, et al. MiR-424-3p suppresses galectin-3 expression and sensitizes ovarian cancer cells to cisplatin. Arch Gynecol Obstetrics. 2019;299(4):1077–1087. doi: 10.1007/s00404-018-4999-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong Q, Ma Y, Yu J, et al. Predicted molecular targets and pathways for germacrone, curdione, and furanodiene in the treatment of breast cancer using a bioinformatics approach. Scientific Ports. 2017;7(1):15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei L, Yin F, Chen C, Li L. Expression of integrin α-6 is associated with multi drug resistance and prognosis in ovarian cancer. Oncol Lett. 2019;17(4):3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uhlen M, Zhang C, Lee S, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:6352. doi: 10.1126/science.aan2507 [DOI] [PubMed] [Google Scholar]
- 56.Zou X, Levy-Cohen G, Blank M. Molecular functions of NEDD4 E3 ubiquitin ligases in cancer. Biochim Biophys Acta. 2015;1856:91–106. [DOI] [PubMed] [Google Scholar]
- 57.Nakao A. Inhibitory smads: mechanisms of action and roles in human diseases In: Dijke P, Heldin CH, editors. Smad Signal Transduction. Proteins and Cell Regulation. Vol. 5 Dordrecht: Springer; 2006:379–395. [Google Scholar]
- 58.Murakami K, Mathew R, Huang J, et al. Smurf1 ubiquitin ligase causes downregulation of BMP receptors and is induced in monocrotaline and hypoxia models of pulmonary arterial hypertension. Exp Biol Med. 2010;235:805–813. doi: 10.1258/ebm.2010.009383 [DOI] [PubMed] [Google Scholar]
- 59.Lin H, Lin Q, Liu M, et al. PKA/Smurf1 signaling-mediated stabilization of Nur77 is required for anticancer drug cisplatin-induced apoptosis. Oncogene. 2014;33(13):1629–1639. doi: 10.1038/onc.2013.116 [DOI] [PubMed] [Google Scholar]
- 60.Sahai E, Garcia-Medina R, Pouysségur J, Vial E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J Cell Biol. 2007;176:35–42. doi: 10.1083/jcb.200605135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan R, Xu X, Chen M, et al. Advances in the studies of roles of Rho/Rhokinase in diseases and the development of its inhibitors. Eur J Med Chem. 2013;70:613–622. doi: 10.1016/j.ejmech.2013.10.048 [DOI] [PubMed] [Google Scholar]
- 62.Yoon C, Cho SJ, Aksoy BA, et al. Chemotherapy resistance in diffuse type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin Cancer Res. 2016;22(4):971–983. doi: 10.1158/1078-0432.CCR-15-1356 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]