Abstract
We examined patterns of relapse and prognostic factors in children with intracranial ependymoma. Records of 82 children diagnosed with localized intracranial ependymoma were reviewed. 52% first presented to our institution after relapse. Median age at initial diagnosis was 4 years (range 0–18 years). Gender was 55% male. Initial tumor location was infratentorial in 71% and supratentorial in 29%. Histology was WHO Grade II in 32% and Grade III in 68%. As part of definitive management, 99% had surgery, 70% received RT (26% 2D/3D-conformal RT[CRT], 22% intensity-modulated RT [IMRT], 22% proton), and 37% received chemotherapy. Median follow-up was 4.6 years (range 0.2–32.9). Overall, 74% of patients relapsed (50% local, 17% distant, 7% local + distant) at a median 1.5 (range 0.1–17.5) years. Five-year OS and FFS for patients presenting prior to relapse are 70% (95% confidence interval [CI], 50–83%) and 48% (95% CI 30–64%), respectively. On log-rank, superior overall survival (OS) was demonstrated for gross total resection (p = 0.03). Superior failure-free survival (FFS) was demonstrated for age < 5 years (p = 0.04). No difference in OS or FFS was found between 2D/3D-CRT versus IMRT/proton (p > 0.05). On multivariate analysis, age ≤ 5 was independently associated with a lower risk of death and failure versus older patients (p < 0.05). Contrary to previous reports, young age may not be a poor prognostic factor in patients who can tolerate intensive treatment. Future studies examining patients stratified by clinical and molecular attributes are warranted.
Keywords: Ependymoma, Radiation therapy, Chemotherapy, CNS tumors, Surgery
Introduction
Ependymoma is the third most common pediatric brain tumor and frequently affects the young, with one-third found in patients ≤ 3 years of age. Nearly 90% of pediatric ependymomas occur intracranially, with one-third supratentorial and two-thirds infratentorial [1–3].
Management of pediatric intracranial ependymoma typically involves maximal surgical resection followed by adjuvant radiation therapy (RT) to the tumor bed; although evidence exists that surgery alone may be curative in subsets of patients with radically resected supratentorial [4–7] and posterior fossa [8] ependymoma. The extent of surgical resection has been shown in multiple retrospective series to be the most important factor for both local control and survival, but gross total resection (GTR) historically has only been possible in about half of all patients [3, 9–11]. For patients with residual disease, the role and optimal timing of second-look surgery is uncertain [12]. While adjuvant RT has been shown to improve outcomes, the optimal dose and volume are uncertain, and several trials are aimed at assessing the role of conformal RT [13]. The use of chemotherapy has been explored in both the adjuvant and neo-adjuvant setting, including in very young children with the intent to delay RT, with limited success [9–12, 14, 15]. Unfortunately, randomized data concerning these multiple treatment modalities is currently lacking. More recently, it has become evident that ependymoma includes a spectrum of molecularly and biologically distinct entities with different clinical behavior, indicating that optimal therapy may vary depending on molecular subtype [16].
Despite significant advances in surgery and RT over the last several decades, morbidity and mortality in children with ependymoma remains high, with 5-year overall survival (OS) ranging from 50 to 85% and progression-free survival (PFS) ranging from 23 to 65% [17–19]. In this series, we report our institutional experience with ependymoma patients ≤ 18 years of age since 1984, focusing on patterns of relapse.
Methods and materials
Patient population
The study was approved by our internal Institutional Review Board and includes pediatric patients age ≤ 18 years at initial diagnosis of a primary intracranial ependymoma, who were treated at our institution with surgery, RT, and/or chemotherapy between 1984 and 2015. Histopathological diagnosis of ependymoma was confirmed on all patients by a neuropathologist at our institution. Patients receiving resection were deemed to have either GTR or subtotal resection (STR), as determined on post-operative imaging (5% computed tomography [CT] of the head, 95% magnetic resonance imaging [MRI] of the brain) after one or more resections as part of definitive management; no intermediate classification was used. We identified 82 consecutive patients meeting the above criteria.
Statistical analysis
Statistical analysis was performed with Stata Version 13.0 (StataCorp, College Station, TX, USA). Failure-free survival (FFS) was calculated from the time of diagnosis to first relapse at any site. The Kaplan-Meier method was used to assess FFS and OS. Univariate log-rank analysis was conducted to assess association of age, gender, tumor location, tumor grade, extent of resection, cerebrospinal fluid (CSF) diversion, use of chemotherapy, and use, modality, and dose of RT with OS and FFS. Multivariate Cox-proportional hazards analysis was conducted using age, gender, tumor location, grade, extent of resection, hydrocephalus requiring CSF diversion, use of chemotherapy, and use of RT to assess their association with risk of failure and mortality. Survival estimates are reported separately for patients receiving upfront management and for patients receiving management after relapse at our institution. A threshold p-value of ≤ 0.05 was used to determine statistical significance for all comparisons.
Results
Presentation
Baseline characteristics of the 82 patients are shown in Table 1. Of the sample, 48% of patients first presented to our institution before relapse and 52% presented after relapse. Among patients presenting to our institution before relapse, outside management prior to presentation included clinical evaluation only in 4%, radiographic diagnosis in 17%, biopsy in 1%, surgical resection in 20%, and surgery and adjuvant treatment in 6%. On initial presentation, the median age was 4.1 years (range 0.1–18.8 years); a total of 34 patients (41%) were aged 36 months or younger. Other key characteristics were 55% male, 71% infratentorial, and 68% WHO Grade III. The most commonly reported presenting symptoms were nausea/vomiting, headache, ataxia, and cranial nerve palsies. Hydrocephalus was present in 80% of patients. Median reported symptom duration prior to initial presentation was 1 month (range 0–12 months). Diagnosis was guided by imaging in all cases, with 5% diagnosed before 1988 on CT of the head and the remaining 95% diagnosed in 1988 or after on MRI of the brain. All patients had localized disease at time of initial diagnosis, evaluated by MRI spine and/or lumbar puncture. Molecular classification was conducted in 9 recent patients (11%), all initially diagnosed in 2009–2015, primarily using next generation sequencing with the MSK-IMPACT platform [20]. Of these, three patients with supratentorial tumors displayed RELA fusion and the remainder had indeterminate findings.
Table 1.
Summary of patient population characteristics
| Characteristic | Total cohort (n = 82) | No event (n = 17) | With event (n = 65) | p-value (no event vs. with event) |
|---|---|---|---|---|
| Median age at diagnosis, years (range) | 4.1 (0.1–18.8) | 3.5 (1.0–18.8) | 4.2 (0.1–17.3) | 0.974 |
| Sex | 0.467 | |||
| Male | 45 (55%) | 8 (47%) | 37 (55%) | |
| Female | 37 (45%) | 9 (53%) | 28 (45%) | |
| Primary site | 0.070 | |||
| Infratentorial | 58 (71%) | 9 (53%) | 49 (75%) | |
| Supratentorial | 24 (29%) | 8 (47%) | 16 (25%) | |
| WHO grade | 0.416 | |||
| II | 26 (32%) | 4 (24%) | 22 (34%) | |
| III | 56 (68%) | 13 (76%) | 43 (66%) | |
| Follow-up time (year) | 0.157 | |||
| Median (range) | 4.6 (0.1–32.9) | 7.2 (0.1–32.9) | 4.5 (0.6–32.1) | |
| Management of primary tumor | ||||
| Surgery | 82 (99%) | 17 (100%) | 64 (98%) | 0.607 |
| Extent of resection | 0.184 | |||
| Gross total resection | 56 (68%) | 14 (82%) | 42 (65%) | |
| Subtotal resection | 25 (30%) | 3 (18%) | 22 (34%) | |
| Number of surgeries | 0.967 | |||
| ≤ 1 | 77 (94%) | 16 (94%) | 61 (94%) | |
| > 1 | 5 (6%) | 1 (6%) | 4 (6%) | |
| CSF diversion | 31 (38%) | 6 (35%) | 25 (38%) | 0.810 |
| Radiation | 57 (70%) | 12 (71%) | 45 (70%) | 0.914 |
| Median dose (range, Gy) | 55.8 (14.4–59.4) | 57.6 (50.0–59.4) | 55.8 (14.4–59.4) | 0.813 |
| Median duration of RT in days (range) | 44 (24–61) | 44 (39–51) | 44 (24–61) | 0.581 |
| Chemotherapy | 30 (37%) | 4 (24%) | 26 (40%) | 0.209 |
| Without RT | 19 (23%) | 3 (18%) | 16 (25%) | 0.578 |
| Pre-RT | 8 (10%) | 1 (6%) | 7 (11%) | |
| Post-RT | 3 (4%) | 0 (0%) | 3 (5%) | |
| Type of first event | ||||
| None | 17 (21%) | 17 (100%) | 0 (0%) | < 0.001 |
| Local failure | 41 (50%) | 0 (0%) | 41 (63%) | |
| Distant failure | 14 (17%) | 0 (0%) | 14 (22%) | |
| Local + distant failure | 6 (7%) | 0 (0%) | 6 (9%) | |
| Death | 4 (5%) | 0 (0%) | 4 (6%) | |
Event: relapse or death
Disease management
A summary of treatment approach is displayed in Fig. 1. As part of definitive management, 99% had surgery with 68% receiving GTR and 30% STR. One patient was unable to receive resection due to brainstem involvement and underwent biopsy only. Five patients (6%) had second-look surgeries, resulting in 4 GTR and 1 STR. At the time of initial surgery, 38% of patients required CSF diversion: 2% endoscopic third ventriculostomy, 9% external ventricular drain, and 27% ventriculoperitoneal shunt.
Fig. 1.
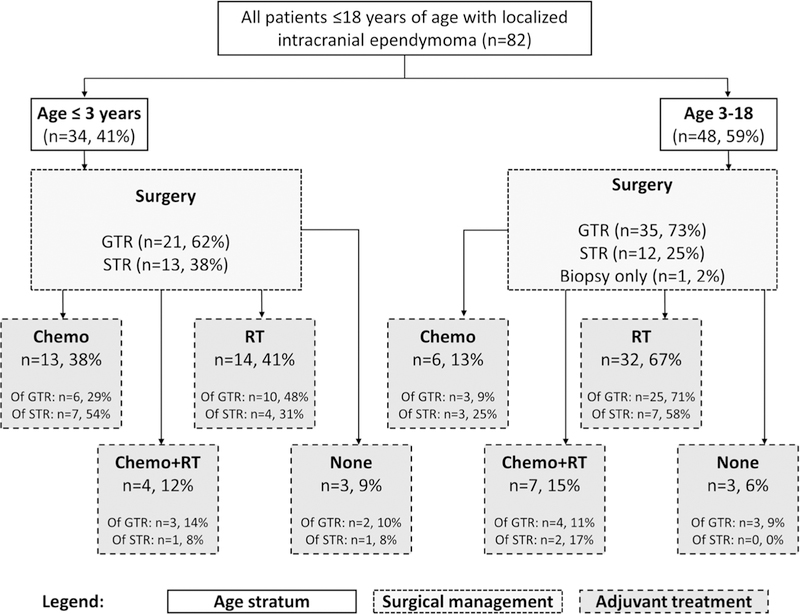
Summary of treatments used. GTR gross total resection, STR subtotal resection, RT radiotherapy, chemo chemotherapy
Adjuvant treatment was given to 93% of patients; 70% received RT and 37% received chemotherapy. Median RT dose in high dose region was 55.8 Gy (range Gy); 41% received > 54 Gy and 35% received 59.4 Gy. Modality was 2D/3D-conformal radiotherapy (CRT) in 26%, IMRT in 22%, and proton in 22%. Primary RT volume was involved field ± margin in 41% (24% infratentorial, 17% supratentorial), posterior fossa in 20%, and craniospinal axis in 6%. Median RT duration was 44 days (range 24–61). Median duration between first surgery and start of RT in patients who did not receive post-surgery chemotherapy was 48 days. Among all patients, 23% received chemotherapy without RT, 10% received chemotherapy before RT, and 4% received chemotherapy after RT. The most commonly used agents were cisplatin/carboplatin (32%), vincristine (30%), etoposide (27%), and cyclophosphamide (22%).
Outcomes
At a median follow-up of 4.6 years (range 0.1–32.9 years), relapse occurred for 74% (61/82) of all patients at a median of 1.5 years (range 0.1–17.5 years) after diagnosis. Among these patients, patterns of relapse included 67% local, 23% distant, 10% combined local and distant. Among patients receiving GTR as part of initial management, relapse occurred in 64% (36/56), of which 63% were local, 24% were distant, and 12% were combined local and distant. Among patients receiving subtotal resection, relapse occurred in 73% (19/26), of which 79% were local, 16% were distant, and 5% were combined local and distant. Among patients receiving adjuvant RT as part of initial management, relapse occurred in 75% (43/57), of which 65% were local, 28% were distant, and 7% were combined local and distant. Among patients not receiving adjuvant RT, relapse occurred in 72% (18/25), of which 72% were local, 11% were distant, and 17% were combined local and distant.
Among 39 patients presenting to our institution for primary tumor management, median follow-up duration was 4.4 years (range 0.2–32.9 years) and 46% of patients relapsed. Of these, 78% of relapses were local, 22% were distant, and 0% were combined local and distant. Among those receiving management prior to relapse at our institution, 44% of patients expired at a median of 4.9 years (range 0.6–20.2 years) after initial diagnosis. Kaplan-Meier estimates of the 5-year OS and FFS are 70% (95% confidence interval [CI] 50–83%) and 48% (95% CI 30–64%), respectively (Fig. 2)
Fig. 2.
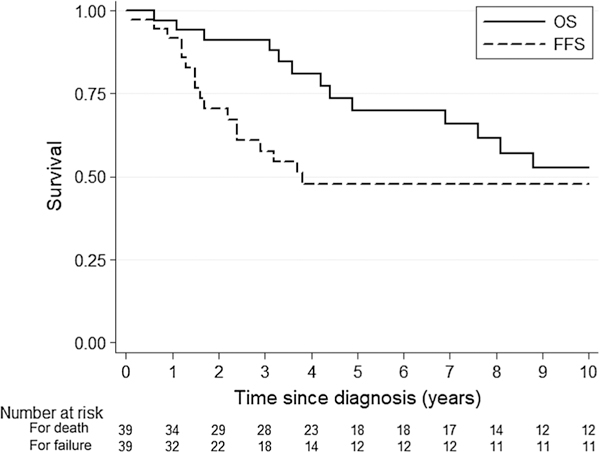
Kaplan-Meier OS and FFS curves for patients presenting to our institution for upfront management. Median follow-up duration was 4.4 years (range 0.2–32.9 years). OS overall survival, FFS failure-free survival
Among 43 patients presenting to our institution for management after relapse, median follow-up duration after initial diagnosis was 4.7 years (range 0.9–32.1 years). Among these patients, 70% expired at a median of 4.2 years (range 0.9–30.8 years) after initial diagnosis. For patients presenting to our institution after relapse, the Kaplan-Meier estimate of the OS at 5 years after initial diagnosis is 49% (95% CI 33–63%).
Among all patients, univariate log-rank analysis yielded superior OS for GTR (p = 0.03) and superior FFS for age < 5 years (p = 0.04). Gender, tumor location, tumor grade, CSF diversion, use of chemotherapy, and use, modality (2D/3D-CRT vs. IMRT/proton), and dose of RT were not significantly associated with OS or FFS. (Table 2) Among all patients, multivariate Cox proportional hazards analysis (Table 3) revealed a significantly lower risk of mortality associated independently with age ≤ 5 at diagnosis (hazard ratio [HR] 0.47, p = 0.040) and GTR (HR 0.53, p = 0.042) and higher risk of mortality associated with use of chemotherapy (HR 2.22, p = 0.038) and increasing year of diagnosis (HR 1.08, p = 0.002). Multivariate analysis yielded a significantly lower risk of relapse associated with age ≤ 5 at diagnosis (HR 0.38, p = 0.002) and higher risk of relapse associated with increasing year of diagnosis (HR 1.08, p < 0.001). On subgroup analysis of 39 patients presenting to our institution prior to relapse, multivariate analysis yielded a significantly lower risk of mortality associated with GTR (HR 0.21, p = 0.044) and higher risk of mortality associated hydrocephalus requiring CSF diversion (HR 4.89, p = 0.026). Subgroup analysis also yielded a higher risk of relapse associated with increasing year of diagnosis (HR 1.17, p = 0.030).
Table 2.
Univariate log-rank analysis results for OS and FFS among all patients
| Attribute (vs. comparison group) | Overall survival p-value |
Failure-free survival p-value |
|---|---|---|
| Gender: female (vs. male) | 0.92 | 0.60 |
| Age: ≤3 years (vs. >3 years) | 0.13 | 0.63 |
| Age: ≤5 years (vs. >5 years) | 0.71 | 0.04 |
| Location: supratentorial (vs. infratentorial) | 0.20 | 0.49 |
| Grade: WHO Grade III (vs. WHO Grade II) | 0.88 | 0.77 |
| Surgery: gross total resection (vs. subtotal resection) | 0.03 | 0.72 |
| Surgery: CSF diversion (vs. no CSF diversion) | 0.48 | 0.37 |
| Adjuvant therapy: chemotherapy (vs. none) | 0.12 | 0.53 |
| Adjuvant therapy radiation (vs. none) | 0.31 | 0.53 |
| RT modality: IMRT or proton (vs. 2D/3D-CRT) | 0.15 | 0.08 |
| RT dose: ≥54 Gy (vs. <54 Gy) | 0.08 | 0.72 |
Bolded values are significant at the 5% level
Table 3.
Adjusted mortality and failure hazard ratios for intracranial ependymoma patients
| Attributes | For death |
For failure |
||||
|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| All patients (n = 82) | ||||||
| Gender | ||||||
| Male | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| Female | 0.95 | 0.49–1.87 | 0.893 | 1.16 | 0.66–2.05 | 0.612 |
| Age ≤ 5 | 0.47 | 0.23–0.97 | 0.040 | 0.38 | 0.20–0.70 | 0.002 |
| Location | ||||||
| Infratentorial | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| Supratentorial | 0.56 | 0.25–1.22 | 0.143 | 0.73 | 0.39–1.34 | 0.308 |
| WHO grade | ||||||
| II | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| III | 0.91 | 0.47–1.75 | 0.778 | 0.74 | 0.41–1.33 | 0.317 |
| Resection type | ||||||
| STR | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| GTR | 0.53 | 0.28–0.98 | 0.042 | 0.78 | 0.44–1.40 | 0.406 |
| CSF diversion used | 1.73 | 0.90–3.29 | 0.098 | 1.12 | 0.64–1.97 | 0.688 |
| Chemotherapy used | 2.22 | 1.07–4.64 | 0.033 | 1.86 | 0.87–3.99 | 0.110 |
| Radiation used | 0.55 | 0.24–1.27 | 0.162 | 0.48 | 0.21–1.14 | 0.098 |
| Year of diagnosis | 1.08 | 1.03–1.13 | 0.002 | 1.08 | 1.03–1.13 | < 0.001 |
| Patients presenting to our institution before relapse (n = 39) | ||||||
| Gender | ||||||
| Male | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| Female | 0.66 | 0.18–2.46 | 0.534 | 1.11 | 0.34–3.64 | 0.866 |
| Age ≤ 5 | 0.33 | 0.07–1.46 | 0.144 | 0.28 | 0.07–1.07 | 0.063 |
| Location | ||||||
| Infratentorial | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| Supratentorial | 0.75 | 0.20–2.77 | 0.661 | 1.10 | 0.36–3.30 | 0.869 |
| WHO grade | ||||||
| II | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| III | 0.99 | 0.29–3.42 | 0.992 | 0.73 | 0.18–2.98 | 0.663 |
| Resection type | ||||||
| STR | 1.00 | (Ref.) | (Ref.) | 1.00 | (Ref.) | (Ref.) |
| GTR | 0.21 | 0.04–0.96 | 0.044 | 0.36 | 0.09–1.47 | 0.154 |
| CSF diversion used | 4.89 | 1.21–19.77 | 0.026 | 1.27 | 0.35–4.56 | 0.714 |
| Chemotherapy used | 2.26 | 0.43–11.86 | 0.336 | 4.96 | 0.69–35.84 | 0.113 |
| Radiation used | 0.32 | 0.06–1.83 | 0.201 | 0.37 | 0.07–2.04 | 0.254 |
| Year of diagnosis | 1.07 | 0.96–1.19 | 0.197 | 1.17 | 1.02–1.64 | 0.030 |
HR hazard ratio, CI confidence interval, GTR gross total resection, STR subtotal resection, CSF cerebrospinal fluid
HR > 1 indicates increased likelihood of mortality/failure and HR < 1 indicates decreased likelihood of mortality/failure
Significant p-values are listed in bold
Relapse management
Of patients who relapsed, 74% experienced a subsequent relapse; the median number of total relapses with an intervening period of apparently controlled disease was 2 (range 1–11). Among all patients, cumulative proportions of eventual relapse were 63% local, 32% distant brain sites, 30% spine (4% spine as only site), and 2% extra-axial (n = 2, peritoneal and vertebral). Relapse management included surgical resection in 89%, RT in 82% (25% CSI), chemotherapy in 73%, and intrathecal radioimmunotherapy in 20%. Of 61 patients experiencing relapse, 43 expired at a median 2.4 years (range 0.3–30.2 years) after relapse.
Discussion
Relapse patterns and survival
In this study of pediatric patients treated at a single institution for newly diagnosed or recurrent intracranial ependymoma over a 31-year period, patterns of relapse were as follows: 67% local, 23% distant, and 10% combined local and distant relapse. Among patients presenting to our institution prior to relapse, 5-year OS and FFS were 70 and 48%, respectively. These data are consistent with previous reports of intracranial ependymoma, which have shown that local failure accounts for the vast majority of recurrences [11, 18, 19, 21–31]. Among series involving postoperative RT, 5-year OS and event-free survival (EFS) rates have ranged from 54 to 85% and 41–74%, respectively [11, 14, 21–25, 27, 28]. While multivariate analysis in this series among all patients revealed a significantly higher risk of death and relapse with each passing year, only a higher risk of relapse was found on subgroup analysis of patients presenting to our institution prior to relapse. A higher risk of relapse and/ or death may be explained in part by patients with adverse disease characteristics preferentially presenting to our institution in more recent years.
In the era of postoperative RT, the most comprehensive survival data has been published by St. Jude Children’s Hospital, as well as US and European cooperative groups. The prospective study from Merchant et al. of 153 patients spanning 1997–2003 showed 7-year local control and OS rates of 87% (95% CI 78–97%) and 81% (71–91%), respectively. Sites of relapse included 14% local, 15% distant, and 7% combined local and distant. Evidence from the Children’s Oncology Group is available from ACNS0121, which enrolled 378 patients aged 1–22 years from 2003 to 2007 and compared several strata: (1) observation for gross totally resected, differentiated supratentorial ependymoma, (2) chemotherapy + second-look surgery, and RT for incompletely resected ependymoma, (3) conformal RT after near total or macroscopic GTR, independent of tumor grade or location, and (4) conformal RT after microscopic GTR, regardless of tumor grade or location, excluding supratentorial, differentiated tumors. The 5-year EFS (± standard error) of these strata were 61 ± 14, 39 ± 7, 67 ± 5, and 70 ± 4%, respectively [4, 5]. Additionally, ACNS0121 showed that treatment of patients age < 3 years with early RT was feasible and associated with superior disease control. ACNS0831 is a randomized phase III trial, which opened in March 2010 and aims to compare 5-year EFS and OS for patients randomized to adjuvant chemotherapy or observation after irradiation [32].
While younger age at diagnosis has historically been associated with poorer outcomes, data from this series suggest that age < 5 at diagnosis may be independently associated with a decreased risk of mortality. This may be influenced by achievement of relatively high rates of GTR, adjuvant radiotherapy, and/or adjuvant chemotherapy among these patients at our institution relative to other institutions. While more aggressive management including radiotherapy may be deferred or omitted preferentially for young patients, recent studies have suggested that the benefits of superior disease control may outweigh the risks of radiotherapy-related sequelae, particularly with conformal treatment techniques such as IMRT or proton therapy [31, 33]. Another potential explanation for the observed findings is differences in molecular subtype composition between patients < 5 years of age and those ≥ 5 years of age; a disproportionately high proportion of subtypes associated with a favorable prognosis, such as ST-EPN-YAP1 or PF-EPN-B, could explain the outcomes observed in patients < 5 years of age. While subtyping information is not yet available for a majority of patients in the present study, a future analysis is planned.
Surgery
Extent of surgical resection has been shown in multiple series to be the most important factor for local control and survival [11, 12, 14, 15, 18, 21, 23, 25]. Additionally, second-look surgeries to enable maximal resection have shown promise in several series without significant additional morbidity [12, 18, 19, 34]. GTR was shown to be prognostic in the present series for OS on univariate log-rank and for OS and FFS on multivariate Cox analysis. However, only 5% of patients received > 1 surgery in the present series. Possible explanations for this include upfront management at outside institutions and more recent acceptance of the effectiveness of this technique; half of our sample was diagnosed before 2004. While all patients in this series were evaluated for extent of resection using post-operative imaging, recent studies have demonstrated that microscopic GTR may further facilitate disease control and survival [5, 35]. Additionally, while most patients had extent of resection evaluated with MRI, patients diagnosed before 1988 were evaluated by post-operative CT, representing a possible confounder within the analysis.
While hydrocephalus and the requirement for CSF diversionary procedures have been linked to endocrine and cognitive outcomes among patients with ependymoma [33, 36], we found an independent association between hydrocephalus requiring CSF diversion at initial surgery with risk of death among patients receiving primary disease management at our institution. However, these results may also be linked to tumor size, specific location, and involvement of adjacent structures, which could not be accounted for in our analysis.
Adjuvant radiotherapy
Adjuvant RT has been shown to improve outcomes for patients with intracranial ependymoma [33]. While early studies suggested that ependymoma had a predilection for spinal failure, modern studies in the era of improved imaging have shown that spinal failures are rare even in the absence of CSI [14, 37]. As a result, local field RT has become standard. While the appropriate volume for posterior fossa tumors has been disputed, recent evidence has shown favorable results with irradiation to the involved field + a 1 cm margin surrounding the post-operatively defined tumor bed [18]. The COG trials have also tested the hypothesis that reducing of the target volume of RT is associated with a decrease in side effects without compromise of local control rates, and results from ACNS0121 support the use of limited-volume irradiation, even for patients as young as 12 months old [13].
While patients in the current study were heterogeneously managed, we did not find differences in FFS or OS by RT volume, which may support the use of involved field RT to reduce short- and long-term sequelae such as cognitive decline, hearing loss, endocrine deficits, and growth abnormalities. Dose has also been shown to be an important factor in disease control, particularly since the primary pattern of relapse in ependymoma is local. In particular, doses > 54 Gy have been linked to superior outcomes [38]. A total of 41% of patients in the present study received > 54 Gy in this series, with most of these receiving treatment in the last 15 years. RT technique has evolved over the last several decades, with intensity-modulated radiotherapy (IMRT) and proton therapy offering greater conformality as compared to 2D/3D-CRT. Recent series have shown that patients treated with IMRT or proton therapy have similar progression-free survival to conventional RT methods with lower rates of toxicity [29, 31, 39]. We did not find differences in FFS or OS when comparing IMRT/proton therapy to 2D/3D-CRT, indicating that disease control is not compromised with greater conformality.
Adjuvant chemotherapy
Evidence is mixed regarding the role of chemotherapy in the treatment of localized intracranial ependymoma. The best response rates to chemotherapy have been noted in young children who receive chemotherapy after initial resection [40]. Platinum-based agents have shown the most activity in phase II trials of ependymoma patients, and cisplatin/car-boplatin were among the most frequently used agents used in the present study. Adjuvant chemotherapy trials have not shown any FFS or OS benefit, nor has there been a conclusively proven benefit shown to using chemotherapy to delay RT [21, 41, 42]. While the UKCCSG/SIOP CNS 9204 study showed that patients < 3 years of age could potentially avoid or delay RT, results from the AIEOP protocol demonstrated disappointingly low EFS and OS rates [43, 44]. In the present study, adjuvant chemotherapy was used in over half of patients < 3 years of age, often in an attempt to avoid RT. However, chemotherapy did not confer a benefit in terms of OS or FFS on univariate log-rank analysis and was associated with a roughly two-fold increase in risk of mortality on Cox analysis, possibly reflecting the impact of deferred or omitted RT. Adjuvant chemotherapy has shown a potential role in bridging to subsequent tumor resection [12]. This approach was also investigated in ACNS0121, which showed low rates of surgical morbidity, and continues to be investigated in ACNS0831 [5, 32].
Relapse development and management
The majority of patients experienced more than one local relapse, with many patients experiencing > 2 with intervening periods of apparently controlled disease. Cumulatively, 30% of patients eventually experienced spinal relapse, though this was concomitant with relapse at local or distant sites in the brain in all but 4% of patients. These data support the use of local field RT as upfront therapy but simultaneously highlight the need for improved local control with novel treatment strategies. Similar to initial management, successful management of relapsed patients has centered around maximal safe resection and re-irradiation, whereas chemotherapy has not shown a proven benefit [45, 46]. The most common therapies used for relapsed patients in the present study were surgery and re-RT. However, the optimal RT volume, fraction size, and technique, as well as the role of novel therapies such as vaccines, hypomethylating agents, and radioimmunotherapy remain active areas of investigation [47–49].
Molecular subtyping
Molecular, genetic, and epigenetic analysis of tumor may help to guide prognosis and treatment for intracranial ependymoma. Recent studies have identified nine subtypes based on DNA methylation profiling, of which three apply to supratentorial tumors. Among supratentorial tumors, the RELA fusion subtype (ST-EPN-RELA) is typically found in preschool-aged children and associated with an inferior survival outcome, whereas the YAP1 subtype (ST-EPN-YAP1) is much rarer, found in young children, and appears to be associated with a superior survival outcome. A third subtype of supratentorial ependymoma, subependymoma (ST-SE), is found predominantly in adults and associated with superior survival [16, 50]. In our study, three supratentorial patients (aged 2, 6, and 12 at initial diagnosis) were found to be ST-EPN-RELA subtype and relapsed 1.3 (local), 1.2 (local), and 3.2 (distant brain) years after initial diagnosis, respectively. Future directions may examine the roles of more aggressive therapy in high risk groups, such as ST-EPN-RELA patients, and therapy de-escalation in lower-risk groups, such as ST-EPN-YAP1 patients. Posterior fossa subtypes identified include Group A (PF-EPN-A), Group B (PF-EPN-B), and subependymoma (PF-SE), but subtyping of the posterior fossa ependymoma patients in our series was not available during the period of the present study. Routine subtype testing for all ependymomas was implemented at our institution in 2015 after Pajtler et al. published the seminal paper on molecular classification of ependymal tumors [50]. Future directions may examine the roles of more aggressive therapy in high risk groups, such as ST-EPN-RELA patients, and therapy de-escalation in lower-risk groups, such as PF-EPN-B patients [8].
In addition to molecular subtype, chromosome copy number alterations are associated with prognosis. Gains in chromosome 1q have been shown to be an independent poor prognostic factor for progression, particularly at distant sites [16, 35]; studies examining the role of CSI in these patients are ongoing and future studies identifying other copy number alterations may elucidate targets for therapy. Risk stratification by molecular subtype has been shown to be superior to histopathologic grading and is expected to facilitate future risk-tailored, prospective clinical trials in ependymoma [16, 50].
Conclusions
Limitations of the present study include the retrospective design spanning multiple decades, heterogeneous patient population, management, and histopathologies represented, lack of molecular data, and probable confounders within the analysis. Though this series represents a relatively large cohort of ependymoma patients, subgroup analysis may be prone to Type I and Type II errors. Nonetheless, the data presented adds to our current understanding on relapse patterns, prognostic factors, and outcomes of children with localized intracranial ependymoma. In particular, the results of this series generate the hypothesis that patients < 5 years of age may preferentially benefit from GTR followed by adjuvant RT. In addition, the results also suggest that more conformal RT techniques, including IMRT and proton therapy, are not associated with increased risk of failure or death despite a high likelihood of local failure among intracranial ependymoma patients. Ongoing multi-institutional prospective trials will provide a clearer depiction of optimal management for these patients, particularly as molecular subtyping is more uniformly incorporated. Given the potential morbidity of treatment, therapy modulation based on risk may be important in enabling not only good disease control but also excellent functional outcomes.
Acknowledgements
Funding was provided by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748). This work is also supported by a gift from Jack and Susan Rudin and the Louis and Rachel Rudin Foundation.
Footnotes
Compliance with ethical standards
Ira Dunkel reports personal fees from Bayer Health Care Pharmaceuticals, Ipsen, Celgene, and Apexigen, and grants from Genentech and Parexel (GSK, Novartis), all outside the submitted work. Dr. Souweidane reports personal fees from Aesculap outside the submitted work.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent For this type of study, formal consent is not required.
Conflict of interest
All other authors declare no conflicts.
References
- 1.Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro-Oncology 18 (suppl_5):v1–v75. 10.1093/neuonc/now207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pejavar S, Polley MY, Rosenberg-Wohl S, Chennupati S, Prados MD, Berger MS, Banerjee A, Gupta N, Haas-Kogan D (2012) Pediatric intracranial ependymoma: the roles of surgery, radiation and chemotherapy. J Neuro-Oncol 106(2):367–375. 10.1007/s11060-011-0671-9 [DOI] [PubMed] [Google Scholar]
- 3.Rousseau P, Habrand JL, Sarrazin D, Kalifa C, Terrier-Lacombe MJ, Rekacewicz C, Rey A (1994) Treatment of intracranial ependymomas of children: review of a 15-year experience. Int J Radiat Oncol Biol Phys 28(2):381–386 [DOI] [PubMed] [Google Scholar]
- 4.Merchant TE, Bendel AE, Sabin N, Burger PC, Wu S, Boyett JM (2015) A phase II trial of conformal radiation therapy for pediatric patients with localized ependymoma, chemotherapy prior to second surgery for incompletely resected ependymoma and observation for completely resected, differentiated, supratentorial ependymoma. Int J Radiat Oncol Biol Phys 93(3):S1 10.1016/j.ijrobp.2015.07.009 [DOI] [Google Scholar]
- 5.Group CsO, Institute NC (2003) Observation or radiation therapy and/or chemotherapy and second surgery in treating children who have undergone surgery for ependymoma. https://ClinicalTrials.gov/show/NCT00027846
- 6.Palma L, Celli P, Mariottini A, Zalaffi A, Schettini G (2000) The importance of surgery in supratentorial ependymomas. Long-term survival in a series of 23 cases. Childs Nerv Syst 16(3):170–175. 10.1007/s003810050487 [DOI] [PubMed] [Google Scholar]
- 7.Venkatramani R, Dhall G, Patel M, Grimm J, Hawkins C, McComb G, Krieger M, Wong K, O’Neil S, Finlay JL (2012) Supratentorial ependymoma in children: to observe or to treat following gross total resection? Pediatr Blood Cancer 58(3):380–383. 10.1002/pbc.23086 [DOI] [PubMed] [Google Scholar]
- 8.Ramaswamy V, Hielscher T, Mack SC, Lassaletta A, Lin T, Pajtler KW, Jones DT, Luu B, Cavalli FM, Aldape K, Remke M, Mynarek M, Rutkowski S, Gururangan S, McLendon RE, Lipp ES, Dunham C, Hukin J, Eisenstat DD, Fulton D, van Landeghem FK, Santi M, van Veelen ML, Van Meir EG, Osuka S, Fan X, Muraszko KM, Tirapelli DP, Oba-Shinjo SM, Marie SK, Carlotti CG, Lee JY, Rao AA, Giannini C, Faria CC, Nunes S, Mora J, Hamilton RL, Hauser P, Jabado N, Petrecca K, Jung S, Massimi L, Zollo M, Cinalli G, Bognar L, Klekner A, Hortobagyi T, Leary S,Ermoian RP, Olson JM, Leonard JR, Gardner C, Grajkowska WA, Chambless LB, Cain J, Eberhart CG, Ahsan S, Massimino M, Giangaspero F, Buttarelli FR, Packer RJ, Emery L, Yong WH, Soto H, Liau LM, Everson R, Grossbach A, Shalaby T, Grotzer M, Karajannis MA, Zagzag D, Wheeler H, von Hoff K, Alonso MM, Tunon T, Schuller U, Zitterbart K, Sterba J, Chan JA, Guzman M, Elbabaa SK, Colman H, Dhall G, Fisher PG, Fouladi M, Gajjar A, Goldman S, Hwang E, Kool M, Ladha H, Vera-Bolanos E, Wani K, Lieberman F, Mikkelsen T, Omuro AM, Pollack IF, Prados M, Robins HI, Soffietti R, Wu J, Metellus P, Tabori U, Bartels U, Bouffet E, Hawkins CE, Rutka JT, Dirks P, Pfister SM, Merchant TE, Gilbert MR, Armstrong TS, Korshunov A, Ellison DW, Taylor MD (2016) Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J Clin Oncol 34(21):2468–2477. 10.1200/JCO.2015.65.7825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horn B, Heideman R, Geyer R, Pollack I, Packer R, Goldwein J, Tomita T, Schomberg P, Ater J, Luchtman-Jones L, Rivlin K, Lamborn K, Prados M, Bollen A, Berger M, Dahl G, McNeil E, Patterson K, Shaw D, Kubalik M, Russo C (1999) A multi-institutional retrospective study of intracranial ependymoma in children: identification of risk factors. J Pediatr Hematol/Oncol 21(3):203–211 [DOI] [PubMed] [Google Scholar]
- 10.Sutton LN, Goldwein J, Perilongo G, Lang B, Schut L, Rorke L, Packer R (1990) Prognostic factors in childhood ependymomas. Pediatr Neurosurg 16(2):57–65 [DOI] [PubMed] [Google Scholar]
- 11.Pollack IF, Gerszten PC, Martinez AJ, Lo KH, Shultz B, Albright AL, Janosky J, Deutsch M (1995) Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery 37(4):655–666 (discussion 666–657) [DOI] [PubMed] [Google Scholar]
- 12.Foreman NK, Love S, Gill SS, Coakham HB (1997) Second-look surgery for incompletely resected fourth ventricle ependymomas: technical case report. Neurosurgery 40(4):856–860 (discussion 860) [DOI] [PubMed] [Google Scholar]
- 13.Merchant TE, Hodgson D, Laack NN, Wolden S, Indelicato DJ, Kalapurakal JA (2013) Children’s Oncology Group’s 2013 blueprint for research: radiation oncology. Pediatr Blood Cancer 60(6):1037–1043. 10.1002/pbc.24425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perilongo G, Massimino M, Sotti G, Belfontali T, Masiero L, Rigobello L, Garre L, Carli M, Lombardi F, Solero C, Sainati L, Canale V, del Prever AB, Giangaspero F, Andreussi L, Mazza C, Madon E (1997) Analyses of prognostic factors in a retrospective review of 92 children with ependymoma: Italian Pediatric Neurooncology Group. Med Pediatr Oncol 29(2):79–85 [DOI] [PubMed] [Google Scholar]
- 15.Chiu JK, Woo SY, Ater J, Connelly J, Bruner JM, Maor MH, van Eys J, Oswald MJ, Shallenberger R (1992) Intracranial ependymoma in children: analysis of prognostic factors. J Neuro-Oncol 13(3):283–290 [DOI] [PubMed] [Google Scholar]
- 16.Pajtler KW, Mack SC, Ramaswamy V, Smith CA, Witt H, Smith A, Hansford JR, von Hoff K, Wright KD, Hwang E, Frappaz D, Kanemura Y, Massimino M, Faure-Conter C, Modena P, Tabori U, Warren KE, Holland EC, Ichimura K, Giangaspero F, Castel D, von Deimling A, Kool M, Dirks PB, Grundy RG, Foreman NK, Gajjar A, Korshunov A, Finlay J, Gilbertson RJ, Ellison DW, Aldape KD, Merchant TE, Bouffet E, Pfister SM, Taylor MD (2017) The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol 133(1):5–12. 10.1007/s00401-016-1643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merchant TE (2002) Current management of childhood ependymoma. Oncology (Williston Park) 16(5):629–642 (644; discussion 645 – 626, 648) [PubMed] [Google Scholar]
- 18.Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA (2009) Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol 10(3):258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massimino M, Miceli R, Giangaspero F, Boschetti L, Modena P, Antonelli M, Ferroli P, Bertin D, Pecori E, Valentini L, Biassoni V, Garre ML, Schiavello E, Sardi I, Cama A, Viscardi E, Scarzello G, Scoccianti S, Mascarin M, Quaglietta L, Cinalli G, Diletto G, Genitori L, Peretta P, Mussano A, Buccoliero A, Calareso G, Barra S, Mastronuzzi A, Giussani C, Marras CE, Balter R, Bertolini P, Giombelli E, La Spina M, Buttarelli FR, Pollo B, Gandola L (2016) Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro-Oncology 18(10):1451–1460. 10.1093/neuonc/now108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O’Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF (2015) Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17(3):251–264. 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson PL, Zeltzer PM, Boyett JM, Rorke LB, Allen JC, Geyer JR, Stanley P, Li H, Albright AL, McGuire-Cullen P, Finlay JL, Stevens KR Jr, Milstein JM, Packer RJ, Wisoff J (1998) Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg 88(4):695–703. 10.3171/jns.1998.88.4.0695 [DOI] [PubMed] [Google Scholar]
- 22.Akyuz C, Emir S, Akalan N, Soylemezoglu F, Kutluk T, Buy-ukpamukcu M (2000) Intracranial ependymomas in childhood-a retrospective review of sixty-two children. Acta Oncol (Stockholm Sweden) 39(1):97–100 [DOI] [PubMed] [Google Scholar]
- 23.van Veelen-Vincent ML, Pierre-Kahn A, Kalifa C, Sainte-Rose C, Zerah M, Thorne J, Renier D (2002) Ependymoma in childhood: prognostic factors, extent of surgery, and adjuvant therapy. J Neu-rosurg 97(4):827–835. 10.3171/jns.2002.97.4.0827 [DOI] [PubMed] [Google Scholar]
- 24.Oya N, Shibamoto Y, Nagata Y, Negoro Y, Hiraoka M (2002) Postoperative radiotherapy for intracranial ependymoma: analysis of prognostic factors and patterns of failure. J Neuro-Oncol 56(1):87–94 [DOI] [PubMed] [Google Scholar]
- 25.Jaing TH, Wang HS, Tsay PK, Tseng CK, Jung SM, Lin KL, Lui TN (2004) Multivariate analysis of clinical prognostic factors in children with intracranial ependymomas. J Neuro-Oncol 68(3):255–261 [DOI] [PubMed] [Google Scholar]
- 26.Merchant TE, Mulhern RK, Krasin MJ, Kun LE, Williams T, Li C, Xiong X, Khan RB, Lustig RH, Boop FA, Sanford RA (2004) Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol 22(15):3156–3162. 10.1200/jco.2004.11.142 [DOI] [PubMed] [Google Scholar]
- 27.Mansur DB, Perry A, Rajaram V, Michalski JM, Park TS, Leonard JR, Luchtman-Jones L, Rich KM, Grigsby PW, Lockett MA, Wahab SH, Simpson JR (2005) Postoperative radiation therapy for grade II and III intracranial ependymoma. Int J Radiat Oncol Biol Phys 61(2):387–391. 10.1016/j.ijrobp.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Shu HK, Sall WF, Maity A, Tochner ZA, Janss AJ, Belasco JB, Rorke-Adams LB, Phillips PC, Sutton LN, Fisher MJ (2007) Childhood intracranial ependymoma: twenty-year experience from a single institution. Cancer 110(2):432–441. 10.1002/cncr.22782 [DOI] [PubMed] [Google Scholar]
- 29.Schroeder TM, Chintagumpala M, Okcu MF, Chiu JK, Teh BS, Woo SY, Paulino AC (2008) Intensity-modulated radiation therapy in childhood ependymoma. Int J Radiat Oncol Biol Phys 71(4):987–993. 10.1016/j.ijrobp.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 30.MacDonald SM, Sethi R, Lavally B, Yeap BY, Marcus KJ, Caruso P, Pulsifer M, Huang M, Ebb D, Tarbell NJ, Yock TI (2013) Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro-Oncology 15(11):1552–1559. 10.1093/neuonc/not121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ares C, Albertini F, Frei-Welte M, Bolsi A, Grotzer MA, Goitein G, Weber DC (2016) Pencil beam scanning proton therapy for pediatric intracranial ependymoma. J Neuro-Oncol 128(1):137–145. 10.1007/s11060-016-2090-4 [DOI] [PubMed] [Google Scholar]
- 32.Group CsO, Institute NC (2010) Maintenance chemotherapy or observation following induction chemotherapy and radiation therapy in treating younger patients with newly diagnosed ependymoma. https://ClinicalTrials.gov/show/NCT01096368
- 33.Merchant TE, Fouladi M (2005) Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neuro-Oncology 75(3):287–299. 10.1007/s11060-005-6753-9 [DOI] [PubMed] [Google Scholar]
- 34.Massimino M, Solero CL, Garre ML, Biassoni V, Cama A, Genitori L, Di Rocco C, Sardi I, Viscardi E, Modena P, Potepan P, Barra S, Scarzello G, Galassi E, Giangaspero F, Antonelli M, Gandola L (2011) Second-look surgery for ependymoma: the Italian experience. J Neurosurg Pediatr 8(3):246–250. 10.3171/2011.6.peds1142 [DOI] [PubMed] [Google Scholar]
- 35.Merchant TE (2017) Current clinical challenges in childhood ependymoma: a focused review. J Clin Oncol 10.1200/JCO.2017.73.1265 [DOI] [PubMed] [Google Scholar]
- 36.Merchant TE, Lee H, Zhu J, Xiong X, Wheeler G, Phipps S, Boop FA, Sanford RA (2004) The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg 101(2 Suppl):159–168. 10.3171/ped.2004.10L2.0159 [DOI] [PubMed] [Google Scholar]
- 37.Rezai AR, Woo HH, Lee M, Cohen H, Zagzag D, Epstein FJ (1996) Disseminated ependymomas of the central nervous system. J Neurosurg 85(4):618–624. 10.3171/jns.1996.85.4.0618 [DOI] [PubMed] [Google Scholar]
- 38.Ducassou A, Murraciole X, Chaltiel L, Bolle S, Claude L, Bernier V, Coche-Dequeant B, Supiot S, Huchet A, Kerr C, Nguyen TD, Alapetite C, Tensaouti F, Liceaga S, Filleron T, Laprie A (2015) OC-0309: role of age, grade and RT dose on outcome of 177 ependymoma—13 years experience of Child’s cancer French Society. Radiother Oncol 115:S155 10.1016/S0167-8140(15)40307-X [DOI] [Google Scholar]
- 39.Sato M, Gunther JR, Mahajan A, Jo E, Paulino AC, Adesina AM, Jones JY, Ketonen LM, Su JM, Okcu MF, Khatua S, Dauser RC, Whitehead WE, Weinberg J, Chintagumpala MM (2017) Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 10.1002/cncr.30623 [DOI] [PubMed] [Google Scholar]
- 40.Chan MD, McMullen KP (2012) Multidisciplinary management of intracranial ependymoma. Curr Probl Cancer 36(1):6–19. 10.1016/j.currproblcancer.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 41.Evans AE, Anderson JR, Lefkowitz-Boudreaux IB, Finlay JL (1996) Adjuvant chemotherapy of childhood posterior fossa ependymoma: cranio-spinal irradiation with or without adjuvant CCNU, vincristine, and prednisone: a Childrens Cancer Group study. Med Pediatr Oncol 27(1):8–14. [DOI] [PubMed] [Google Scholar]
- 42.Timmermann B, Kortmann RD, Kuhl J, Meisner C, Slavc I, Pietsch T, Bamberg M (2000) Combined postoperative irradiation and chemotherapy for anaplastic ependymomas in childhood: results of the German prospective trials HIT 88/89 and HIT 91. Int J Radiat Oncol Biol Phys 46(2):287–295 [DOI] [PubMed] [Google Scholar]
- 43.Grundy RG, Wilne SA, Weston CL, Robinson K, Lashford LS, Ironside J, Cox T, Chong WK, Campbell RH, Bailey CC, Gatta-maneni R, Picton S, Thorpe N, Mallucci C, English MW, Punt JA, Walker DA, Ellison DW, Machin D (2007) Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol 8(8):696–705. 10.1016/s1470-2045(07)70208-5 [DOI] [PubMed] [Google Scholar]
- 44.Massimino M, Gandola L, Barra S, Giangaspero F, Casali C, Potepan P, Di Rocco C, Nozza P, Collini P, Viscardi E, Bertin D, Biassoni V, Cama A, Milanaccio C, Modena P, Balter R, Tam-burrini G, Peretta P, Mascarin M, Scarzello G, Fidani P, Milano GM, Sardi I, Genitori L, Garre ML (2011) Infant ependymoma in a 10-year AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) experience with omitted or deferred radiotherapy. Int J Radiat Oncol Biol Phys 80(3):807–814. 10.1016/j.ijrobp.2010.02.048 [DOI] [PubMed] [Google Scholar]
- 45.Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D (2010) Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Child’s Nerv Syst 26(7):905–911. 10.1007/s00381-009-1067-4 [DOI] [PubMed] [Google Scholar]
- 46.Merchant TE, Boop FA, Kun LE, Sanford RA (2008) A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 71(1):87–97. 10.1016/j.ijrobp.2007.09.037 [DOI] [PubMed] [Google Scholar]
- 47.Pittsburgh Uo, Cancer SK, Institute NC (2012) Immunotherapy for recurrent ependymomas in children treatment for recurrent ependymomas using HLA-A2 restricted tumor antigen peptides in combination with imiquimod. https://ClinicalTrials.gov/show/NCT01795313
- 48.Urie M, Goitein M, Wagner M (1984) Compensating for heterogeneities in proton radiation therapy. Phys Med Biol 29:553 10.1088/0031-9155/29/5/008 [DOI] [PubMed] [Google Scholar]
- 49.Kramer K, Humm JL, Souweidane MM, Zanzonico PB, Dunkel IJ, Gerald WL, Khakoo Y, Yeh SD, Yeung HW, Finn RD, Wolden SL, Larson SM, Cheung NK (2007) Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol 25(34):5465–5470. 10.1200/jco.2007.11.1807 [DOI] [PubMed] [Google Scholar]
- 50.Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, Wani K, Tatevossian R, Punchihewa C, Johann P, Reimand J, Warnatz HJ, Ryzhova M, Mack S, Ramaswamy V, Capper D, Schweizer L, Sieber L, Wittmann A, Huang Z, van Sluis P, Vol-ckmann R, Koster J, Versteeg R, Fults D, Toledano H, Avigad S, Hoffman LM, Donson AM, Foreman N, Hewer E, Zitterbart K, Gilbert M, Armstrong TS, Gupta N, Allen JC, Karajannis MA, Zagzag D, Hasselblatt M, Kulozik AE, Witt O, Collins VP, von Hoff K, Rutkowski S, Pietsch T, Bader G, Yaspo ML, von Deimling A, Lichter P, Taylor MD, Gilbertson R, Ellison DW, Aldape K, Korshunov A, Kool M, Pfister SM (2015) Molecular classification of ependymal tumors across all CNS compartments, histo-pathological grades, and age groups. Cancer Cell 27(5):728–743. 10.1016/jxcell.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


