Abstract
In the setting of neoadjuvant therapy (NAT) for pancreatic adenocarcinoma (PDAC), accurate measurement of tumor size, and consequently, staging based on AJCC 8th edition, is difficult. Attempts to address the limitations of tumor size in the NAT setting have included correlation of residual tumor percent with survival. However, only cases with complete pathologic response or minimal residual disease have shown better prognosis compared to all other groups. To date, no studies have simultaneously evaluated the prognostic value of tumor size and tumor regression in the setting of PDAC status post NAT (NAT-PDAC). Our aim was to study the prognostic value of residual tumor index (RTI), a metric combining residual tumor percent and tumor bed size as an interaction term (% residual tumor x cm tumor bed size). In a cohort of 105 cases of NAT-PDAC, we show that RTI supersedes the prognostic value of AJCC 8th edition T staging via multivariate cox regression. At a binary cutoff of 0.35 for RTI, the hazard ratio for recurrence free survival is 3.26 (95% confidence interval 1.51–7.04), p<0.01. We further identified cutoffs of <=0.2, 0.2 to 2, and >2 that stratified our cases into three groups via RTI, which were statistically significant in Kaplan-Meier curve analysis of recurrence free survival (p<0.01) and overall survival (p<0.01). RTI represents a novel metric for combining the prognostic value of tumor size and residual tumor in NAT-PDAC.
Condensed abstract/ Precis
Applying the AJCC 8th edition staging to neoadjuvant treated pancreatic ductal adenocarcinomas is challenging. A combined pathologic parameter using tumor bed size and percentage of residual tumor, called residual tumor index, has been shown in this study to be a useful adjunct for pathologic assessment in such cases.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is an infiltrative tumor which is characterized by a prominent desmoplastic stroma. Infiltrative glands, tumor nests, or single tumor cells microscopically often extend beyond the grossly estimated margins(1). As a result of frequent co-existence of chronic pancreatitis in most patients(2), and the similarity of tumor desmoplasia and benign fibrosis, gross assessment of tumor size is challenging and error-prone. Neoadjuvant therapy (NAT) generates local inflammation which results in profound fibrosis in both tumor and surrounding stroma(3). Consequently, measuring the tumor/tumor bed size is even more challenging, and sometimes impossible in NAT-treated PDAC cases (NAT-PDAC). The current AJCC tumor (T) staging system (8th edition) is entirely based on tumor size, and therefore is difficult to apply in the setting of NAT. The largest multi-center validation study of the AJCC 8th edition staging system excluded NAT cases(4), which leaves practicing pathologists with a lack of guidance in the staging of NAT-treated cases. Our previous preliminary work suggests that tumor categories of the new AJCC staging do not correlate well with overall survival in NAT-PDAC patients(5). Therefore, new approaches for more effective quantification of tumor burden after NAT are needed.
Several studies have shown that tumor down-staging can be achieved following NAT, with a significant improvement in survival if tumors are subsequently resected(6). Response to NAT is variable, but approximately one third of patients show benefit from NAT. Regardless of the pathologic treatment response to NAT, a fibrotic tumor bed can usually be identified, which becomes the gross measured tumor size for this group of resected tumors. There is no standardized method to define the tumor size in NAT-PDAC. It is at the discretion of the reporting pathologist to use either the gross tumor bed size, or the microscopically measured tumor dimension(s) to report the T stage. A common practice is to include only the microscopic measurement in the event that there is a single focus of tumor remaining in the entire tumor bed (scenario 1). However, the gross tumor bed size may still be considered as the tumor size when there is more than one focus of viable tumor in the tumor bed identified microscopically (scenario 2). Although there is not much difference in the amount of tumor burden in these two scenarios, the sizing conundrum leads to drastic variations in the size-based T-staging. This issue is schematically illustrated in Figure 1.
Figure 1.
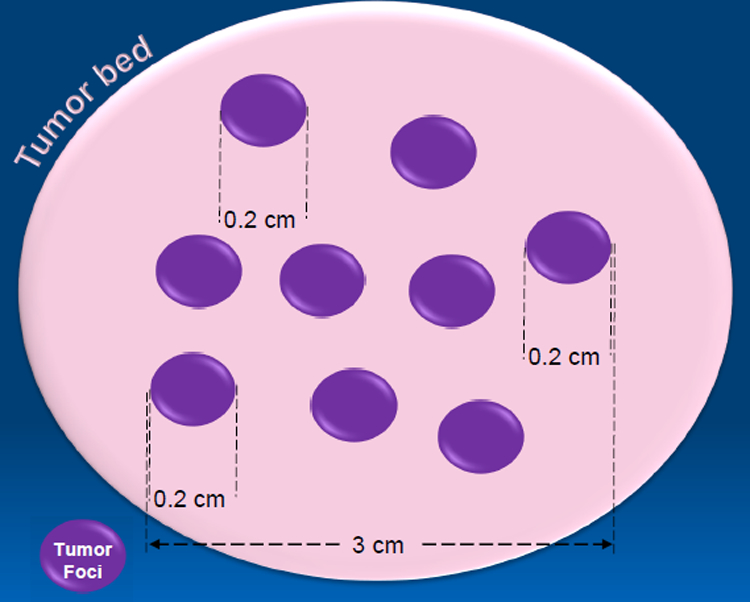
Conceptual figure showing the dilemma of measuring tumor size, with residual islands of tumor (purple) in a fibrotic tumor bed (beige). In this example, the tumor bed measures larger than the farthest extent between two tumor foci (3.0 cm). Currently, there are no guidelines of how to address the dilemma of measuring tumor size. Many pathologists continue to use gross size (4.0 cm in this example), while others use a mapped grossing approach, which in this case would yield a tumor size of 3.0 cm. In case of a single focus of residual tumor, tumor size is measured microscopically (0.2 cm). Some pathologists like to add up the microscopic dimensions of individual tumor foci (e.g. 0.2 cm + 0.2 cm + …) for the tumor size.
Since the current AJCC T-staging is not ideal for NAT-PDAC resection specimens, other pathologic parameters should be evaluated to aid in the current staging difficulties. Assessing the amount of residual tumor has been well-studied in NAT-PDAC. While the estimated residual tumor after neoadjuvant therapy correlates well with survival in rectal adenocarcinomas(7), this is not the case with PDAC(8,9). Multiple grading schemes for grading residual tumor in NAT-PDAC have been devised, the most popular being those proposed by the College of American Pathologists (CAP)(10) and Evans(11). Multiple studies have shown that there is only a prognostic difference in the relatively rare group of tumors with complete pathologic response and tumors with near-complete response (occasional microscopic foci of residual tumor), versus all other tumors with any higher tumor burden(8,9).
Assessment of the percentage of residual tumor alone cannot stratify these patients well, and clearly, better strategies are needed. Therefore, to get a more comprehensive pathologic parameter over the percentage of residual tumor alone, or just the tumor bed size, we combined these pathologic factors into what we defined as a residual tumor index (RTI), which is the product of viable residual tumor percentage and the greatest dimension of the tumor bed(% residual tumor x cm tumor bed size). The goal of our study is to test if RTI is a meaningful pathologic prognosticator that could be used as an adjunct to current T-staging, in order to accurately predict the risk of recurrence and overall survival in NAT- PDAC patients.
MATERIALS AND METHODS
Patient Population:
A retrospective cohort of 105 consecutive patients was identified from a prospectively maintained database, with a confirmed cytologic or histologic diagnosis of PDAC prior to NAT followed by definitive surgical resection. Approval from the Institutional Review Board was obtained prior to initiating the study. All patients had tumors located in the head of pancreas and underwent NAT followed by surgical resection at our institution, from December 1999 to July 2017. Cases with complete medical records, including availability of all archival H&E slides and clinical follow-up data, were included in the study.
Pathologic parameters:
The H&E slides for all cases were reviewed to assess the percentage of residual tumor, compared to surrounding fibrosis in the tumor bed. To assess reproducibility in estimating the percentage of residual tumor, and therefore in calculating the RTI, the entire review was conducted separately by two pathologists, blindfolded to each other’s assessment (DC and IG). There was an initial training session by the original reviewing pathologist (DC). Only H&E stained slides were used for this purpose, and eyeball estimation was used. The final percentage of residual tumor assigned on a particular case was an estimated mean value after looking at all slides which contained tumor (Figure 2). The tumor bed in NAT-PDAC includes areas of desmoplasia extending even into and between the normal components of the parenchyma, making it challenging to digitally measure the exclusive tumor component only. Moreover, akin to estimating macrovesicular steatosis in a liver biopsy, where a pathologist’s eyeballing to assess the percentage of fat is a standard practice, and is known to be reproducible despite its semi-quantitative nature, we chose this similar way to assess the percentage of residual tumor in NAT-PDAC. Other usual pathologic parameters, such as tumor differentiation, lymphovascular invasion, perineural invasion, margin status, and lymph node status, were also verified. A record of tumor size (tumor bed size) was noted from the pathology reports, and in ambiguous cases, verified by histologic review.
Figure 2.

Representative photomicrographs (20x H&E) demonstrating variable treatment response of pancreatic ductal adenocarcinoma to neoadjuvant therapy, estimated at 15% in (A), 40% in (B), and 90% in (C).
Statistical Analysis:
The clinical characteristics were summarized using descriptive statistics. Overall survival (OS) was defined as the period of time from the date of surgery to the date of death, expressed in years. Alive patients are censored at the last follow-up. Recurrence free survival (RFS) was defined as the period of time from the date of surgery to the date of detection of recurrence, expressed in years. Patients without recurrence are censored at the last follow-up. Recurrence free probabilities were calculated using Kaplan-Meier plots.
The intra-class correlation (between two pathologists’ assessments) was computed using the SAS Macro %INTRACC. Patients were divided into three groups based on RTI and their RFS and OS were compared. Differences between index categories were determined by log-rank test. The macro % findcut was used to define the best cut point for RTI, and residual tumor percent by finding the largest difference of log-rank test statistic between subjects in two groups(12).
Cox proportional-hazards models were used to evaluate the relationship of the interested variables for OS and RFS analysis, respectively. The proportionality assumption was tested by adding a time-dependent covariate for each variable. The variables with p<0.20 from univariate models are considered in the multivariable model. The final multivariable model was built using the backward stepwise selection approach to identify all significant risk factors. Factors significant at a 10% level were kept in the final model. All statistical tests were two-sided using α = 0.05 level of significance. SAS Version 9.4 (Cary, NC) was used to perform all statistical analyses.
RESULTS
Patient and Tumor characteristics:
The mean age of the patients in the study cohort was 63.4 years (±11.5), 54.3% were females, and the patient population was mostly Caucasians (91.4%) (Table 1). 44.8% of all tumors were classified as poorly-differentiated on histology, 50.5% were moderately-differentiated and 2.8% were well-differentiated. Tumor was present at the resection margins in 20% of cases and 21.9% of cases had tumor within 1 mm of resection margin. Using AJCC 8th edition staging guidelines, 30.4% of the cases were classified as T1c, 61.9% were T2 and 6.7% were T3. In one case, no definitive mass was identified on gross pathologic exam. 34.3% of patients were classified as having N1 disease, 21.9% as N2 and 43.8% were classified as N0. Lymphatic invasion was present in 45.7% of the tumors and perineural invasion was identified in 76.2% of the tumors. 47.6% patients received neoadjuvant FOLFIRINOX, 26.6% patients received Gemcitabine plus Abraxane, 20.9% patients received Gemcitabine alone and 2.9% received other 5-FU based regimens. Neoadjuvant radiation therapy was performed in 96.2% patients. Standard Whipple procedure was performed in 68.6% patients, pylorus sparing Whipple in 24.7%, Whipple at splenic artery (WATSA)(13) in 4.8% and total pancreatectomy in 1.9% patients. Patient and tumor characteristics are summarized in Table 1.
Table 1.
Patient and Tumor Characteristics (n=105)
| n | % | |
|---|---|---|
| Age (years) | ||
| mean+/− SD | 63.4 | 11.5 |
| Race | ||
| Caucasian | 96 | 91.4 |
| African American | 9 | 8.6 |
| Gender | ||
| Female | 57 | 54.3 |
| Male | 48 | 45.7 |
| Tumor differentiation | ||
| well | 3 | 2.8 |
| moderate | 53 | 50.5 |
| poor | 47 | 44.8 |
| Could not be assessed | 2 | 1.9 |
| Resection margin | ||
| Negative | 61 | 58.1 |
| Positive | 21 | 20.0 |
| Tumor within 1mm | 23 | 21.9 |
| Positive margin location | ||
| Uncinate | 11 | 25.0 |
| Portal vein groove | 14 | 31.8 |
| Neck | 7 | 15.9 |
| Others | 12 | 27.2 |
| T stage | ||
| T1c | 32 | 30.4 |
| T2 | 65 | 61.9 |
| T3 | 7 | 6.7 |
| No definite mass identified | 1 | 0.9 |
| N stage | ||
| N0 | 46 | 43.8 |
| N1 | 36 | 34.3 |
| N2 | 23 | 21.9 |
| AJCC stage | ||
| IA | 5 | 4.8 |
| IB | 6 | 5.7 |
| IIA | 36 | 34.3 |
| IIB | 57 | 54.3 |
| III | 1 | 0.9 |
| Lymphatic invasion | ||
| 48 | 45.7 | |
| Perineural invasion | ||
| 80 | 76.2 | |
| Treatment response group | ||
| 0 | 23 | 21.9 |
| 1 | 82 | 78.1 |
| Neoadjuvant chemotherapy | ||
| *FOLFIRINOX | 50 | 47.6 |
| Gemcitabine + Abraxane | 28 | 26.6 |
| Gemcitabine | 22 | 20.9 |
| *FOLFOX | 3 | 2.9 |
| Unknown | 2 | 1.9 |
| Neoadjuvant radiation | ||
| Radiation | 101 | 96.2 |
| Type of procedure | ||
| Pylorus sparing | 26 | 24.7 |
| Standard | 72 | 68.6 |
| Total pancreatectomy | 2 | 1.9 |
| *WATSA | 5 | 4.8 |
FOLFIRINOX: 5 Fluorouracil, Irinotecan, Oxaliplatin, Leucovorin
FOLFOX: 5 Fluorouracil, oxaliplatin
WATSA: Whipple at Splenic artery.
Correlation of assessment of residual tumor between pathologists:
The intra-class correlation, computed using the SAS Macro %INTRACC, included a dataset of 107 patients with two pathologists’ readings. The intra-class correlation showed a Shrout-Fleiss reliability single score of 0.97, strongly supporting interobserver agreement.
RTI and Recurrence Free Survival
Patient variables including age, gender, race, and preoperative chemotherapy and pathologic variables including tumor grade, lymphatic invasion, peri-neural invasion, margin status, involvement of lymph nodes, residual tumor percent (binary cutoff at 10%), and T stage were tested on univariate analysis for association with RFS. On Cox univariate analysis, N stage (p=0.01), number of lymph nodes involved (p=0.01) and RTI (p<0.01) were significantly associated with RFS. Age, gender, race, tumor grade, margin status, perineural invasion and lymphatic invasion were not significantly associated with RFS. On multivariate analysis, only RTI was significantly associated with RFS (Table 2). Interestingly, on a separate analysis, both components of RTI i.e. residual tumor percent and tumor bed size were independently associated with OS (p-value 0.03 and <0.01 respectively) Patients were divided into three categories: low, mid, and high, based on the RTI cutoffs of 0.2 and 2.0. Patients with high RTI had shorter RFS (p-value<0.01) and OS (p-value<0.01) compared to patients with low or mid RTI (Figures 3 and 4).
Table 2.
Cox Regression Analysis of Recurrence Free Survival in Relation to Clinicopathologic Variables
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Characteristics | p-value | HR (95% CI) | p-value | |
| Gender | 0.18 | - | - | |
| Age | 0.86 | - | - | |
| Race | 0.86 | - | - | |
| Tumor grade | 0.25 | - | - | |
| T-stage | 0.14 | - | - | |
| N stage | 0.01 | - | - | |
| Margin status (tumor within 1mm) | 0.76 | - | - | |
| Margin status (Uncinate) | 0.82 | - | - | |
| Lymph nodes involved | 0.01 | - | - | |
| Residual Tumor Index | <0.01 | Residual Tumor Index | ||
| >0.35 | 3.26 (1.51–7.04) | <0.01 | ||
| <=0.35 | ref | |||
| Perineural invasion | 0.08 | - | - | |
| Lymphatic invasion | 0.09 | - | - | |
Figure 3.

Kaplan Meier survival curves of patients who underwent neoadjuvant therapy followed by surgical resection for PDAC categorized into 3 RTI groups (≤0.2, 0.2 to 2, and >2). There is a significant difference in recurrence free survival for patients with low RTI compared to high RTI (p<0.01).
Figure 4.
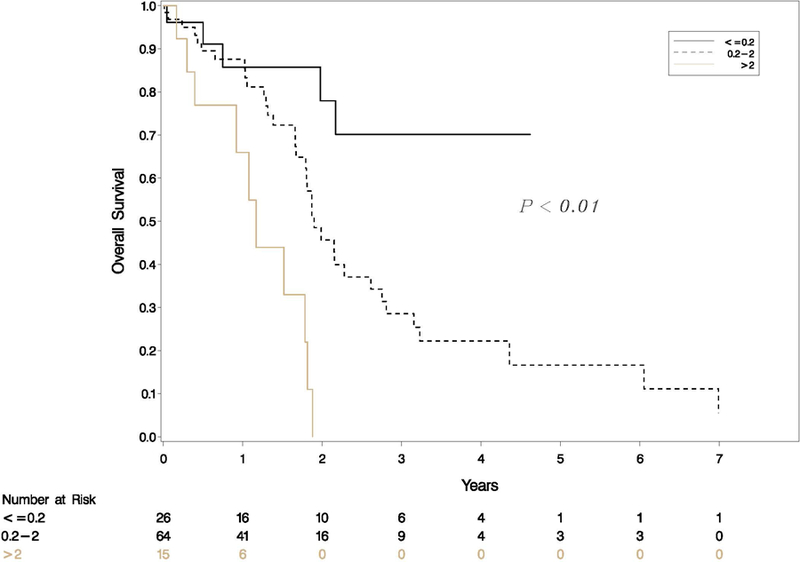
Kaplan Meier survival curves of patients who underwent neoadjuvant therapy followed by surgical resection for PDAC categorized into 3 RTI groups (≤0.2, 0.2 to 2, and >2). There is a significant difference in overall survival for patients with low RTI compared to high RTI (p<0.01).
To understand this better, we further categorized patients into two groups, high and low RTI, as described in statistical methods above. A threshold of 0.35 for RTI was identified and patients were divided into two groups. RTI >0.35 was significantly associated with shorter recurrence free survival (Table 2 & Figure 5). The median RFS in RTI >0.35 group was 1.22 years (95% CI: 0.83–1.49) and in RTI ≤ 0.35 group was 3.08 years (95% CI: 1.77-upper bound not estimated) which was significantly different from RTI <0.35 (p value<0.01, Figure 5).
Figure 5.
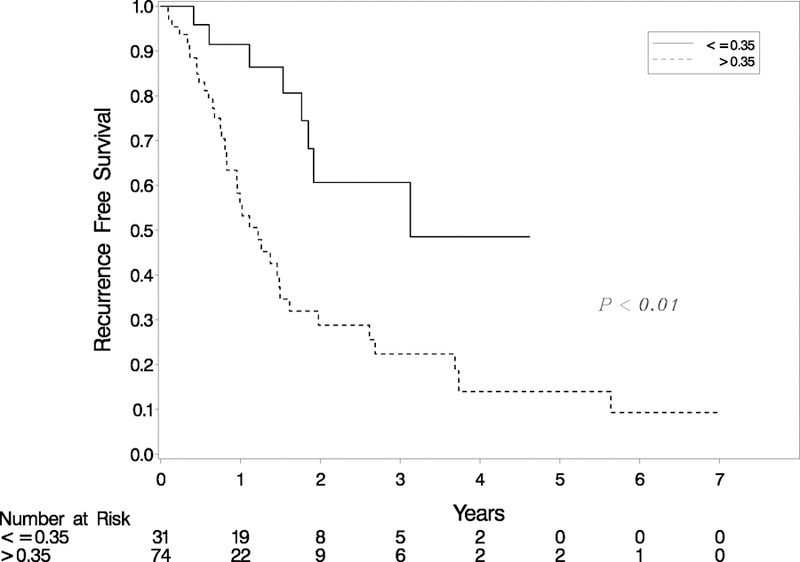
Kaplan Meier survival curves of patients who underwent neoadjuvant therapy followed by surgical resection for PDAC categorized based on two statistically derived RTI groups. There is a significant difference in recurrence free survival for patients with low RTI compared to high RTI (p<0.01).
RTI and Overall Survival:
On univariate analysis, lymphatic invasion (p-value=0.05), N stage (p-value<0.01), number of lymph nodes involved (p<0.01), T stage (p-value=0.04), and RTI (p-value<0.01) were significant predictors of overall survival post resection (Table 3). However, on multivariate analysis RTI > 0.35 (HR=2.40, 95% CI: 1.01–5.66, p-value=0.05) and N stage (p-value=0.04) were the only independent predictors of overall survival. This suggests that an RTI of 0.35 or higher is independently associated with poor overall survival (Table 3). The median OS in the RTI >0.35 group was 1.82 years (95% CI: 1.52–2.15) which was significantly different from RTI<0.35 group (median survival not achieved, p value<0.01, Figure 6).
Table 3.
Cox Regression Analysis of Overall Survival in Relation to Clinicopathologic Variables
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Characteristics | p-value | HR (95% CI) | p-value | |
| Gender | 0.52 | - | - | |
| Age | 0.23 | - | - | |
| Race | 0.70 | - | - | |
| Tumor grade | 0.20 | - | - | |
| T-stage | 0.04 | - | - | |
| N stage | <0.01 | N stage | ||
| N1 | 2.24 (1.10–4.60) | 0.04 | ||
| N2 | 2.46 (1.13–5.35) | |||
| N0 | Ref | |||
| Margin status (tumor within 1mm) | 0.11 | - | - | |
| Margin status (Uncinate) | 0.17 | - | - | |
| Lymph nodes involved | <0.01 | - | - | |
| Residual Tumor Index | <0.01 | Residual Tumor Index | ||
| >0.35 | 2.40 (1.01– 5.66) | 0.05 | ||
| <=0.35 | Ref | |||
| Perineural invasion | 0.06 | - | - | |
| Lymphatic invasion | 0.05 | - | - | |
Figure 6.

Kaplan Meier survival curves of patients who underwent neoadjuvant therapy followed by surgical resection for PDAC categorized based on two statistically derived RTI groups. There is a significant difference in overall survival for patients with low RTI compared to high RTI (p<0.01).
DISCUSSION
Evaluation of Residual PDAC after NAT
The assessment of residual tumor following NAT is addressed by a growing body of literature. Multiple groups, including CAP, have proposed grading systems with varying quantitative or qualitative cutoffs of residual tumor percent(10,11,14,15). Of these, White et al. demonstrated a survival difference based on residual tumor percent, but this was a single center study with 54 patients(15). Evaluation of the Evans and CAP residual tumor grading systems in 223 NAT-PDAC patients showed that a binary classification of patients with pathologic complete response and minimal residual tumor (Evans grades IV and III/ CAP grades 0 and 1) had better survival rates than those with moderate and poor response (Evans grades IIb, IIa, and I/ CAP grades 2 and 3)(8). In the current study, univariate cox regression model shows that residual tumor correlates with overall and recurrence free survival at a cutoff of 10%, which recapitulates the binary classification of our previous study(8). Additionally, this result supports the findings of White et al. that residual tumor percent correlates with overall survival. However, none of the residual tumor grading systems incorporate tumor bed size.
AJCC 8th Edition T Stage Validity in Pancreatic Ductal Adenocarcinoma Status Post Neoadjuvant Therapy
The change in T staging of PDAC in the AJCC 8th edition suggests that tumor size alone supersedes the sometimes subjective evaluation of extrapancreatic extension, a criterion that was used in the previous T-staging (AJCC 7th edition). A multi-institutional validation study by Allen et al. supporting this change shows that tumor size alone correlates with survival in patients undergoing surgical resection of PDAC(4). However, this study excluded patients who received NAT. Another recent validation study that used the Surveillance, Epidemiology and End Results (SEER) data set showed that the AJCC 8th edition staging was comparable to the AJCC 7th edition staging in NAT-PDAC cases(16). Our results show that tumor bed size (as a continuous variable), and also AJCC 8th edition T stage correlate with survival in univariate cox regression, which offers additional support to the validity of the use of the AJCC 8th edition T stage in NAT-PDAC(5).
Why Use Residual Tumor Index (RTI)?
If residual tumor percent and AJCC 8th edition T stage both correlate with survival in NAT- PDAC, both parameters could be used separately for prognostication. However, these findings are difficult to combine into a discrete adjunctive tumor classification system for prognostication. Even if tumor bed size is reduced to a 4-tier system, and residual tumor is grouped in 4 categories as in the CAP system, the result would be 16 possible combinations of T stage and residual tumor percent. One possible solution is the use the product of tumor bed size and residual tumor percent, defined here as RTI. The RTI represents an efficient and simple means to combine the prognostic information contained in tumor bed size and tumor regression simultaneously. In this limited dataset, on multivariate analysis, RTI is superior to the predictive value of the AJCC 8th edition T stage, which is no longer significantly correlated with survival when RTI is included in the model.
The lack of simultaneous comparison of tumor bed size and residual tumor percent in previous studies has several major limitations. For example, in a system that only compares residual tumor percent, a 5.0 cm tumor bed with 20% residual tumor would be regarded as the same as a 0.5 cm tumor with 20% residual tumor, whereas the RTI would be 1.0 in the former and 0.1 in the latter – a difference of an order of magnitude. Table 4 displays the RTI over a range of tumor bed sizes and residual tumor percent. The RTI has as its maximum value the gross tumor bed size. In cases where there is no treatment response, the RTI is equal to the tumor bed size. Regardless of tumor bed size, the RTI is 0 if there is no residual tumor, and this result is consistent with the fact that complete responders to NAT in PDAC have a remarkably superior prognosis(16).
Table 4.
Residual Tumor Index (RTI) Across a Range of Tumor Bed Sizes and Residual Tumor % in Deciles
| Residual Tumor % | 0.5 cm | 1.0 cm | 2.0 cm | 3.0 cm | 4.0 cm | 5.0 cm |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0.05 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| 20 | 0.1 | 0.2 | 0.4 | 0.6 | 0.8 | 1 |
| 30 | 0.15 | 0.3 | 0.6 | 0.9 | 1.2 | 1.5 |
| 40 | 0.2 | 0.4 | 0.8 | 1.2 | 1.6 | 2 |
| 50 | 0.25 | 0.5 | 1 | 1.5 | 2 | 2.5 |
| 60 | 0.3 | 0.6 | 1.2 | 1.8 | 2.4 | 3 |
| 70 | 0.35 | 0.7 | 1.4 | 2.1 | 2.8 | 3.5 |
| 80 | 0.4 | 0.8 | 1.6 | 2.4 | 3.2 | 4 |
| 90 | 0.45 | 0.9 | 1.8 | 2.7 | 3.6 | 4.5 |
| 100 | 0.5 | 1 | 2 | 3 | 4 | 5 |
Survival in NAT-PDAC Patients Eligible for Resection: RTI Applies to Patients with Better Prognosis
Our survival data relative to the RTI should be interpreted in the context that NAT in PDAC has been associated with an improved rate of R0 resections which offers improved long-term survival(17). Moreover, only selected patients who tolerate chemotherapy undergo the highly morbid abdominal surgery, which is associated with better post-operative outcomes(18,19). In this study we have selected NAT-treated patients who underwent surgical resection, therefore the overall prognosis of this cohort is expected to be better than stage-matched treatment-naïve patients undergoing surgical resection for PDAC.
Limitations of our study
Estimating RTI involves subjective assessment of the percentage of residual tumor. Although it would have been ideal to find a way to quantitate this parameter objectively, there are practical limitations. PDAC are very infiltrative tumors, and are also associated with a lot of desmoplasia. For a resection specimen, the tumor is often sampled with the adjacent parenchyma to show the relationship to margins, which is an important prognostic pathologic parameter. Therefore, in these tumor sections, which are multiple (often >10) per case, the tumor bed includes areas of desmoplasia extending even into and between the normal components of the parenchyma, making it challenging to quantitatively measure the exclusive tumor component only.
Since calculating the RTI involves estimation of the percentage of residual tumor by eyeballing, the question of interobserver variability always remains. To address this issue, two pathologists were involved in our study, and assessments were carried out independently and blindfolded to each other’s results. Subsequently, the intra-class correlation was computed using the SAS Macro %INTRACC. In accordance with previously accepted criteria by Landis and Koch, k values greater than 0.4 indicate ‘‘moderate agreement,’’ values greater than 0.6 indicate ‘‘substantial agreement’’ and values greater than 0.8 indicate ‘‘excellent’’ or ‘‘near perfect agreement’’(12). The intra-class correlation between two pathologists’ readings in our study showed a Shrout-Fleiss reliability single score of 0.97, strongly supporting interobserver agreement in assessing RTI.
Another potential shortcoming of our study is that it is not adequately powered for subset analysis including comparing outcomes of patients with similar RTI but different tumor bed size or tumor response to neoadjuvant therapy, for example, a non-responsive 2 cm tumor (RTI = 2) versus a 4 cm tumor with 50% regression (RTI = 0.5 × 4 = 2). This is a conceptual study and it needs to be validated in a large multi-institutional cohort of patients with long term recurrence and follow up data.
Conclusion
The RTI simplifies the relationship between tumor bed size and residual tumor percent in NAT-PDAC cases, and supersedes the predictive value of AJCC 8th edition T stage in multivariate analysis. With our study also demonstrating inter-observer reproducibility, assessment of RTI appears to be a practical method that can be used widely by pathologists in any practice setting. It does not require any special technique such as digital analysis, and does not consume more time than ordinarily taken to assess the other pathologic parameters for standardized reporting of such a specimen. Future studies and larger data sets are needed to validate the prognostic value of RTI, and may refine the subcategories beyond the cutoffs we defined with our dataset.
Acknowledgments
Funding:
WGH, GAW, JL supported by the SPORE grant 5P50CA196510-02.
REDCap Supported by Clinical and Translational Science Award (CTSA) Grant [UL1 TR000448] and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842
RZP supported by NIH5T32CA00962128.
Footnotes
Disclosure:
The authors report no conflicts of interest.
REFERENCES
- 1.Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: implications for resection margin assessment. Histopathology 2011. December;59(6):1111–21. [DOI] [PubMed] [Google Scholar]
- 2.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010. June 1;24(3):349–58. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee D, Katz MH, Rashid A, Estrella JS, Wang H, Varadhachary GR, et al. Pancreatic Intraepithelial Neoplasia and Histologic Changes in Non-neoplastic Pancreas Associated with Neoadjuvant Therapy in Patients with Pancreatic Ductal Adenocarcinoma. Histopathology [Internet] 2013. December [cited 2017 Oct 12];63(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3836824/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017. January;265(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee D, Katz MH, Foo WC, Sundar M, Wang H, Varadhachary GR, et al. Prognostic Significance of New AJCC Tumor Stage in Patients With Pancreatic Ductal Adenocarcinoma Treated With Neoadjuvant Therapy. Am J Surg Pathol 2017. August 1;41(8):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan H-X, Xu J-W, Wu D, Wu Z-Y, Wang L, Hu S-Y, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med 2017. June;6(6):1201–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan R, Gibbons D, Hyland JMP, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005. August;47(2):141–6. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee D, Katz MH, Rashid A, Varadhachary GR, Wolff RA, Wang H, et al. Histologic Grading the Extent of Residual Carcinoma Following Neoadjuvant Chemoradiation in Pancreatic Ductal Adenocarcinoma: A Predictor for Patient Outcome. Cancer 2012. June 15;118(12):3182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SM, Katz MH g, Liu L, Sundar M, Wang H, Varadhachary GR, et al. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am J Surg Pathol 2016. December 1;40(12):1653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washington K, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons P, et al. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. Coll Am Pathol Cap Report Cancer Specim Case Summ Backgr Doc Northfield IL Cap 2011;
- 11.Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg Chic Ill 1960 1992. November;127(11):1335–9. [DOI] [PubMed] [Google Scholar]
- 12.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal 1999. May 28;30(3):253–70. [Google Scholar]
- 13.Strasberg SM, Sanchez LA, Hawkins WG, Fields RC, Linehan DC. Resection of tumors of the neck of the pancreas with venous invasion: the “Whipple at the Splenic Artery (WATSA)” procedure. J Gastrointest Surg Off J Soc Surg Aliment Tract 2012. May;16(5):1048–54. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa O, Ohhigashi H, Sasaki Y, Imaoka S, Iwanaga T, Teshima T, et al. [The histopathological effect of preoperative irradiation in adenocarcinoma of the periampullary region]. Nihon Gan Chiryo Gakkai Shi 1988. March 20;23(3):720–7. [PubMed] [Google Scholar]
- 15.White RR, Xie HB, Gottfried MR, Czito BG, Hurwitz HI, Morse MA, et al. Significance of histological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol 2005. March;12(3):214–21. [DOI] [PubMed] [Google Scholar]
- 16.Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol 2017. July 1;24(7):2023–30. [DOI] [PubMed] [Google Scholar]
- 17.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006. August;13(8):1035–46. [DOI] [PubMed] [Google Scholar]
- 18.Satoi S, Yanagimoto H, Toyokawa H, Takahashi K, Matsui Y, Kitade H, et al. Surgical results after preoperative chemoradiation therapy for patients with pancreatic cancer. Pancreas 2009. April;38(3):282–8. [DOI] [PubMed] [Google Scholar]
- 19.Abbott DE, Tzeng C-WD, Merkow RP, Cantor SB, Chang GJ, Katz MH, et al. The cost-effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma. Ann Surg Oncol 2013. December;20 Suppl 3:S500–508. [DOI] [PubMed] [Google Scholar]


