Abstract
Chronic methamphetamine (meth) abuse often turns into a compulsive drug-taking disorder accompanied by persistent cognitive deficits and re-occurring psychosis. Possible common neurobiological substrates underlying meth-induced deficits and schizophrenia remain poorly understood. Serotonin 2A (5-HT2A) and metabotropic glutamate 2 (mGlu2) receptors coregulate psychosis-like behaviors and cognitive function in animals. Therefore, in the present study we examined the effects of chronic exposure to three different drugs known to produce persistent deficits in sensorimotor gating and cognition [meth, phencyclidine (PCP) and MK-801] on the expression of 5-HT2A and mGlu2 within the rat medial prefrontal cortex (PFC), dorsal hippocampus (dHPC) and perirhinal cortex (PRh). Adult male rats underwent 14 days of: (a) meth self-administration (6 hr/day), (b) phencyclidine (PCP; 5 mg/kg, twice/day) administration, or (c) MK-801 (0.3 mg/kg, twice/day) administration. Seven days after the discontinuation of drug administration, tissues of interest were collected for protein expression analysis. We found that despite different pharmacological mechanism of action, chronic meth, PCP, and MK-801 similarly dysregulated 5-HT2A and mGlu2, as indicated by an increase in the 5-HT2A/mGlu2 expression ratio in the mPFC (all three tested drugs), PRh (meth and PCP), and dHPC (MK-801 only). Complementary changes in G-protein expression (increase in Gαq and decrease in Gαi were also observed in the mPFC of meth animals. Finally, we found that 5-HT2A/mGlu2 cooperation can be mediated in part by the formation of the receptor heteromer in some, but not all cortical regions. In summary, these data suggest that a shift towards increased availability (and G-protein coupling) of cortical 5-HT2A vs. mGlu2 receptors may represent a common neurobiological mechanism underlying the emergence of psychosis and cognitive deficits observed in subjects with meth use disorder and schizophrenia.
Keywords: Methamphetamine, Phencyclidine, MK-801, Heterocomplex, mGlu2, 5-HT2A
1. Introduction
Methamphetamine (meth) is the most commonly abused synthetic drug worldwide (United Nations Office on Drugs and Crime, 2016). A significant proportion of meth users develop a pattern of uncontrollable, compulsive drug use that can be classified as meth use disorder (MUD, Paulus et al., 2017). The treatment and recovery of individuals with MUD is complicated by high rates of relapse, and persistent meth-induced psychopathologies. In this respect, memory impairments are among the most pronounced and persistent cognitive complications in MUD (Scott et al., 2007). A number of clinical studies reported deficits in working and episodic memory that can persist weeks or months into the meth-free abstinence (Bechara and Martin, 2004; Gonzalez et al., 2007; Morgan et al., 2012; Simon et al., 2004; Woods et al., 2005). Another pervasive complication in MUD that affects almost 40% of meth users is the emergence of psychotic symptoms (Grant et al., 2012). Psychotic episodes can be triggered acutely by recent meth use, or spontaneously re-appear even after prolonged periods of abstinence (Glasner-Edwards and Mooney, 2014; McKetin et al., 2006; Zweben et al., 2004). While meth can precipitate psychotic symptoms in individuals with no history of a primary psychotic disorder, it can also exacerbate psychotic symptoms in schizophrenia patients, hinting at potential commonalities in the underlying neurobiology of these two conditions (Chen et al., 2003; McKetin et al., 2006). Progress in identifying neurobiological substrates underlying cognitive complications and psychosis in MUD is critical, as they can collectively contribute to poor treatment outcomes in MUD (Paulus et al., 2017).
A well-accepted animal model that recapitulates many complications of MUD is the self-administration paradigm, in which animals are allowed to have extended access to self-administered meth for a period of two or more weeks. Specifically, studies by our lab and others have shown that extended-access meth self-administration results in: escalated meth use (Rogers et al., 2008), increased reinstatement of meth-seeking (Rogers et al., 2008; Schwendt et al., 2009), reduced cognitive flexibility (Cox et al., 2016; Parsegian et al., 2011), as well as impaired episodic (object recognition) memory (Reichel et al., 2012a; Schwendt et al., 2011) and working memory (Recinto et al., 2012). Extended access to self-administered meth also produces deficits in sensorimotor gating akin to deficits observed in schizophrenia patients (Hadamitzky et al., 2011), or across various animal models of schizophrenia (Braff, 1990).
Both cognitive impairments and psychotic symptoms produced by chronic meth are thought to arise from the dysregulation of several neurotransmitter systems within the frontostriatal circuit. Targeting aberrant dopamine transmission using D2 receptor antagonists is a standard-approach therapy of schizophrenia and more recently, also meth psychosis (Samiei et al., 2016; Verachai et al., 2014). However, D2 receptor antagonists do not typically improve cognitive dysfunction, and in some cases can even impair learning and memory performance in humans (Lustig and Meck, 2005; Saeedi et al., 2006; Xu, 2017) and animals (Abdel-Salam et al., 2012; Hou et al., 2006; Hutchings et al., 2013). Alternatively, recent evidence suggests that simultaneous targeting of serotonin and glutamate neurotransmission, namely 5-HT2A and mGlu2(3) receptors, might produce an anti-psychotic effect comparable to that of D2 receptor antagonists (González-Maeso et al., 2008; Moreno et al., 2016). The validity of this approach is based on findings that demonstrate the: a) possible association of mGlu2(3) and 5-HT2A gene polymorphisms with schizophrenia and/or meth-induced psychosis (e.g. Harrison et al., 2008; Tsunoka et al., 2010), b) synergistic antipsychotic properties of 5-HT2A antagonists and mGlu2/3 receptor agonists, c) co-localization of both receptors across several cortical brain regions, and d) close physical (heteroreceptor complex) and functional (signaling cross-talk) interactions between 5-HT2A and mGlu2 receptors (for review see: Wischhof and Koch, 2016). Moreover, targeting 5-HT2A and/or mGlu2 receptors has been shown to improve cognitive function across a variety of psychopathologies, including MUD and schizophrenia (Wischhof and Koch, 2016; Zhang and Stackman, 2015). As such, enhancing mGlu2 receptor function, or inhibiting 5-HT2A receptor activity ameliorated cognitive deficits in pharmacological models of schizophrenia based on NMDA receptor inhibition by drugs like MK-801 (also known as dizocilpine), or phencyclidine (PCP; Griebel et al., 2016; Snigdha et al., 2010). Finally, our data demonstrate that administration of mGlu2 positive allosteric modulator (PAM) reverses object recognition memory deficits in rats with a history of chronic meth self-administration (Schwendt et al., 2011).
While the pharmacological and gene-linkage studies suggest that the mGlu2 and 5-HT2A receptors play a role in manifestation of common MUD and schizophrenia symptoms, (co)regulation of these receptors in either psychopathology is inadequately understood. Postmortem analysis of brain tissue from schizophrenia patients provided contradictory evidence regarding the changes in mGlu2(3) and/or 5-HT2A expression in the forebrain (Ghose et al., 2008; González-Maeso et al., 2008; Muguruza et al., 2016; Rasmussen et al., 2010), while changes in either receptor expression have not been evaluated in MUD. Only a single study analyzed co-regulation of mGlu2 and 5-HT2A following repeated non-contingent administration of meth in mice, showing increased 5-HT2A and decreased mGlu2 receptor expression in the mPFC (Chiu et al., 2014). Despite similarities in the clinical manifestation of MUD and schizophrenia, to this date no studies have concurrently evaluated mGlu2 and 5-HT2A receptor expression in commonly used animal models of these two psychopathologies. The current study aimed to address this knowledge gap by first, validating the tools (antibodies) suitable for the detection of mGlu2 and 5-HT2A proteins in rodent brain tissue. Second, we evaluated the possibility that functional cross-talk occurs via direct physical interaction between these two receptors in the rat brain tissue, and third, we analyzed protein expression of said receptors and their partner G-proteins following the chronic exposure to meth or NMDA receptor antagonists (MK-801 and PCP) within the rat brain regions implicated in psychosis-like behavior (Lewis and Sweet, 2009) and episodic (object recognition) memory, as characterized by: (Warburton and Brown, 2015, 2010). Finally, we focused on the analysis of possible mGlu2-5-HT2A co-regulation at a withdrawal time (7 days), when many of the post-meth, -PCP and -MK-801 behavioral deficits have been previously observed by our laboratory, as well as others (see above).
2. Materials and Methods
2.1. Animals
Male Long-Evans rats (Charles River Laboratories, Wilmington, MA, USA) and male Wistar rats (Velaz, Kolec u Kladna, Czech Republic) weighing 250-300 g at the time of delivery were individually housed in a temperature- and humidity-controlled vivarium on a reversed 12 h light-dark cycle. Rats received ad libitum water and standard rat chow (Harlan, Indianapolis, IN, USA (Long-Evans) or Velaz, Czech Republic (Wistar) unless noted otherwise. Rats that were assigned to the self-administration study received 25 g of chow daily (to maintain their weight at ~85 % of the free-feeding weight). After the completion of the self-administration regimen, rats were switched to ad libitum food access. Mouse cortical tissue used for the validation of 5-HT2A and mGlu2 antibodies was obtained from adult male mice with the following background: 129S6/Sv mice (control tissue), Htr2a−/− and GRM2−/− mice backcrossed for at least 10 generations onto the 129S6/Sv background (5-HT2A and mGlu2 knock-out tissue respectively). For more information see: (González-Maeso et al., 2003; Moreno et al., 2011). Adult male C57BL/6 mice were used to prepare cortical lysates for the 5-HT2A deglycosylation experiment.
All animal procedures were approved by the Institutional Animal Care and Use Committees of the Medical University of South Carolina, or the Expert Committee for Protection of Experimental Animals of the Prague Psychiatric Center and performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) or with the Guidelines of the European Union Council (86/609/EU).
2.2. Drugs
(+)-Methamphetamine hydrochloride (meth) was obtained from Sigma Aldrich (St. Louis, MO, USA), dissolved in saline (0.9% NaCl) to 0.4 mg/ml concentration and used for intravenous self-administration. MK-801 ((5R,10S)-(+)-5-methyl-10,11-dihydro-5H dibenzo [a,d] cyclohepten-5,10-imine hydrogen maleate; Sigma-Aldrich, Czech Republic), was dissolved in saline and administered intraperitoneally (IP) at 0.3 mg/kg dose (1 ml/kg b.w.). Phencyclidine (1-(1-Phenylcyclohexyl) piperidine; Sigma-Aldrich, Czech Republic) was dissolved in saline and administered IP at 5 mg/kg dose (1 ml/kg b.w.). The control animals self-administered saline (meth rat controls) or received equivalent volumes of IP saline (MK-801 and PCP controls). The doses of each drug were based on previously published studies (Jentsch et al., 1997; Li et al., 2011; Schwendt et al., 2012; Stefani and Moghaddam, 2002; Vales et al., 2006).
2.3. Immunohistochemistry
For the immunohistochemical analysis of 5-HT2A receptor expression, rats were anesthetized and transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS) (in mM: NaCl 137, KCl 2.7, Na2HPO4 4.3, KH2PO4 1.47, pH 7.4). Brains were removed and post-fixed for 4 h and saturated in 30% sucrose/PBS solution. After embedding and freezing, 30μm sections (spanning the rat prefrontal cortex) were cut on a freezing microtome and stored in PBS. Next, the sections were washed in PBS, permeabilized in 0.3% Triton-X-100/1× PBS and blocked in 0.3% Triton-X-100/1× PBS/10% normal goat serum (NGS)/2% bovine serum albumin (BSA) for 2 hours. To detect 5-HT2A receptor signal, PFC sections were incubated with affinity purified rabbit anti-5-HT2A receptor antibody (#24288, ImmunoStar, Hudson, WI) diluted to 1:500 in 0.3% Triton-X-100/l× PBS/2% NGS/2% BSA as previously described (Brownfield et al., 1998). After washing in PBS, sections were incubated with a secondary anti-rabbit antibody conjugated to Alexa Fluor 594 (Life Technologies, Carlsbad, CA), washed again, mounted onto Superfrost slides (Thermo-Fisher, Waltham, MA) and coverslipped with an antifade medium (ProLong™ Gold, Life Technologies, Carlsbad, CA). The staining was visualized and photographed using a BX-51 Olympus microscope equipped with a high-resolution digital camera.
2.4. Protein deglycosylation and analysis
Mouse (C57BL/6) cortical lysate (containing 80 μg of protein) was treated with 1U of Peptide-N-glycosidase F (PNGase F; Roche, Mannheim, Germany) in incubation buffer (39 mM sodium phosphate buffer pH 7.2, 8 mM EDTA, 0.8% NP-40, 0.1% SDS, 1% 2-mercaptoethanol) containing 1 mM PMSF, 7.5 μg/ml aprotinin, 38 mM NaF and protease (cOmplete) and phosphatase (PhosSTOP) inhibitor cocktails (Roche Applied Science, Mannheim, Germany) at 37°C for 3 h. Mouse cortical lysate (80 μg of protein) without PNGaseF was used as a control. The mouse samples and, for a comparison, the lysate from the rat cerebral cortex (40 μg of the protein) were mixed with protein loading buffer (Roti-Load 1, Carl Roth, Karlsruhe, Germany), quickly chilled on ice and immediately separated on a 10% SDS–polyacrylamide gel and transferred onto a PVDF membrane. Next, the membrane was blocked for 1h at room temperature with Odyssey blocking buffer (LI-COR Biotechnology, Bad Homburg, Germany) and incubated for 21h at 4°C with a solution of cold rabbit anti-5-HT2A receptor antibody (#24288; ImmunoStar, Hudson, WI, USA) diluted at 1:500 in Tris-buffered saline solution pH 7.5/0.05% Tween 20 (TBS-T). After washing with TBS-T, the membrane was incubated for 1h at room temperature with secondary donkey anti-rabbit antibody (800CW; LI-COR Biotechnology) diluted at 1:10000 in TBS-T. After washing with TBS-T, the membrane was washed for 10 min at room temperature with TBS and then the detection was performed using Odyssey imaging system (Odyssey CLx; LI-COR Biotechnology).
2.5. Co-immunoprecipitation and immunoblotting
For the co-immunoprecipitation, rats were euthanized by rapid decapitation. Brains were extracted and 2 mm-thick forebrain coronal slices were harvested using a chilled rat brain matrix (ASI instruments, Warren, MI, USA). Next, mPFC, dHPC and PRh were hand-dissected from these acute brain slices (Figure 1) and cut into 250μm thick sections using McIlwain tissue chopper (Ted Pella, Redding, CA, USA). Tissue proteins were immediately crosslinked by incubating the sections with 2.5mM dithiobis-(succinimidyl propionate) (or DSP) in PBS for 2hrs. Crosslinking conditions, such as incubation time and DSP concentration, were based on the manufacturer’s recommendations (#22585, Thermo-Fisher) and series of pilot slice experiments (data not shown). Cross-linking reaction was terminated with 50mM Tris-HCl buffer, pH = 7.5. Tissue sections were transferred to a RIPA lysis buffer (Thermo-Fisher) containing protease (cOmplete Mini; Roche Applied Science, Mannheim, Germany) and phosphatase inhibitors (Halt, Thermo-Fisher) and rapidly homogenized by sonication. Following the solubilization in ice-cold RIPA buffer, insoluble debris was removed by centrifugation and protein concentration in each sample was determined using the BCA assay. Equivalent amount of the lysate protein (500 μg) was used for immunoprecipitation experiments with either rabbit polyclonal anti-mGlu2 antibody (#07-261-I; EMD Millipore, Billerica, MA, USA), or with rabbit anti-PLCα1 antibody (#sc-9050, Santa Cruz Biotechnology; negative control). Antibody-protein complexes were captured with protein-G magnetic beads (SureBeads; Bio-Rad Laboratories, Hercules, CA, USA) according to manufacturer’s instructions. Bound proteins were then stripped off the beads by heating in the high-stringency elution buffer (Laemmli sample buffer; Bio-Rad). Immunoprecipitated proteins were analyzed by immunoblotting as described below.
Fig. 1.
Outlines of the rat brain coronal sections adopted from (Paxinos and Watson, 2006) with highlighted locations used for tissue dissection of the medial prefrontal cortex (mPFC), dorsal hippocampus (dHPC) and the perirhinal cortex (PRh). Numbers above each section indicate distance (in mm) from Bregma.
For immunoblotting analysis, total protein was extracted from the mouse frontal cortex, or rat mPFC, dHPC and PRh tissues as previously described (Schwendt et al., 2006) with few modifications. Protein samples were lysed in RIPA buffer and analyzed under low stringency conditions (heat < 37 °C, dithiothreitol < 20mM) to preserve native receptor oligomers (Copani et al., 2000). Equal protein amounts (15-25 μg) were separated by SDS-PAGE (4–15% polyacrylamide) and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 1 h in 5% milk/Tris-buffered saline and probed overnight at 4 °C with a primary antibody diluted in 5% milk/Tris-buffered saline with 0.1% Tween 20. The following primary 5-HT2A antibodies were used: goat anti-5-HT2A (1:500, #sc-15074), goat anti-5-HT2A (1:500, #sc-15073), goat anti-5-HT2A (1:500, #sc-32538) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti-5-HT2A receptor (1:2500, #Ab16028), rabbit anti-5-HT2A receptor (1:500, #Ab173545) (Abcam Biochemical, Cambridge, MA, USA); rabbit anti-5-HT2A (1:500, #LS-C159876; LifeSpan BioSciences, Seattle, WA, USA) and rabbit anti-5-HT2A receptor (1:500, #24288; ImmunoStar, Hudson, WI, USA). For mGlu2 detection, rabbit anti-mGlu2 (1:1500, #07-261-I; EMD Millipore, Billerica, MA, USA) was used. Tissue G-proteins were detected using the following antibodies: mouse anti-Gαi (1:10000, #26003; NewEast Biosciences, King of Prussia, PA, USA), rabbit anti-Gαo (#sc-387; 1:25000; Santa Cruz Biotechnology), rabbit anti-Gαq/11α (1:5000, #AB1643; EMD Millipore (Billerica, MA, USA). After the incubation with an appropriate HRP-conjugated secondary antisera (Jackson Immuno Research, West Grove, PA), immunoreactive bands on the membranes were detected by ECL+ chemiluminescence reagents on a high performance chemiluminescence film (GE Healthcare, Piscataway, NJ). Equal loading and transfer of proteins were confirmed by stripping and reprobing of the same membranes for the housekeeping protein calnexin (1:20,000, #ADI-SPA-860, Enzo Life Sciences, Farmingdale, NY, USA). The integrated band density of each protein sample was measured using Gel-Pro Analyzer software (Media Cybernetics, Rockville, MD, USA).
2.6. Chronic methamphetamine self-administration and withdrawal.
Fifteen Long-Evans rats were first surgically implanted with jugular vein catheters as described previously (Knackstedt et al., 2014). Following at least 5 days of recovery from surgery, rats were randomly assigned to either meth or yoked-saline groups and underwent meth self-administration. Self-administration procedures were conducted in standard operant conditioning chambers (30×20×20 cm, Med Associates, East Fairfield, VT) housed inside sound-attenuating cubicles containing a fan. The chambers were equipped with a house light, two retractable levers, two stimulus lights, and a tone generator. As shown in Figure 3A, animals were first trained to self-administer meth (Sigma, St. Louis, MO) in 7 daily 1 hr-long sessions, followed by 14 days of extended (6-hr) access to intravenous (IV) meth. During each session, a response on the active lever resulted in activation of the pump for a 2-sec meth infusion (20 μg/50 μl bolus infusion) and presentation of a 5-sec tone (78 dB, 4.5 kHz) and a white stimulus light over the active lever, followed by a 20-sec time out. Yoked controls received a 50 μl bolus of saline when the matched subject received the meth infusion. During self-administration, catheter patency was periodically verified with IV methohexital sodium (10 mg/ml dissolved in 0.9% physiological saline; Eli Lilly, Indianapolis, IN, USA). Out of the 15 rats that were implanted with jugular vein catheters, three were removed from the study during self-administration due to catheter patency issues, or other health complications. Following self-administration, rats underwent 7 days of withdrawal in their home cage with daily handling. This experimental design was based on our previous studies indicating that meth-induced behavioral and neurochemical changes can be detected at this exact withdrawal interval (Reichel et al., 2012b; Scofield et al., 2015).
Fig. 3.
Co-immunoprecipitation of mGlu2 and 5-HT2A in the rat brain. (A) Validation of the mGlu2 (07-261) antibody. Under the low reducing conditions this antibody primarily detected mGlu2 receptor dimer (◁; ~220 kDa) in cortical lysates from rat (R) and wild-type mice (WT) that was absent in mGlu2 knock-out (ko) mice. (B) A band corresponding to mature rat 5-HT2A receptors (◀; ~75 kDa) as detected in the PRh lysates immunoprecipitated with mGlu2 (07-261) antibody. This band was not detected in the mPFC and dHPC (not shown) tissue lysates. mGlu2xx – cross-linked mGlu2 receptors. IgG HC – heavy chain of the denatured mGlu2 antibody used for immunoprecipitation.
2.7. Chronic phencyclidine, MK-801 administration and withdrawal.
Thirty-two Wistar rats were given IP injections (1.0 ml/kg) of saline, PCP (5 mg/kg) or MK-801 (0.3 mg/kg) twice a day (approximately 08:30 and 16:30 h) for a period of 14 days, followed by 7 days of withdrawal. At the end of the withdrawal period, animals were euthanized and brain tissues collected. Two brains were lost during the storage and shipping therefore they could not be included in the tissue analysis. Doses of PCP and MK-801 used in this study were selected on the basis of their comparable NMDA receptor inhibitory potency (Boireau et al., 1996). The drug administration regimen was designed to correspond with the length of extended meth access and was also based on the previously published studies (for review see: Mouri et al., 2007). The length of the withdrawal period was chosen to correspond with meth experiment and to mimic the time-course of PCP- and MK-801-induced behavioral deficits (Li et al., 2011; Santini et al., 2013; Snigdha et al., 2010). Non-contingent administration of PCP and MK-801 was utilized, as achieving stable self-administration rates of PCP or MK-801 has proven to be difficult in rodents [e.g. (Marquis et al., 1989)].
2.8. Statistical analysis
A Student’s t-test was used to compare meth intake (mg/kg) averaged over the first three (d1-d3) vs. the final three (d12-d14) days of extended access. Immunoblotting data, represented by integrated density of individual bands, were normalized to the density of calnexin immunoreactivity within the same sample, analyzed by a Student’s t-test, and expressed as the percentage of the saline-treated animals. GraphPad Prism v7.0 software (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis of all data. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Validation of antibodies for the detection of 5-HT2A receptors in rodent brain tissue.
Since the specificity of several antibodies used for 5-HT2A receptor immunodetection have been recently challenged (e.g. Weber and Andrade, 2010), we decided first to validate the most frequently used commercial anti-5-HT2A antibodies. To unambiguously determine antibody specificity, immunoreactivity of all antibodies was tested in cortical lysates obtained from rats, wild type and knockout (KO) mice (see Materials and Methods section for the complete list of antibodies tested). As shown in Figure 2A, only one anti-5-HT2A receptor antibody (Imunostar, cat. no. 24288) detected a band (~75 kDa) in rat and mouse cortical lysates that was not present in the lysates from 5-HT2A receptor KO mice. This band is heavier than the predicted molecular size of the rat 5-HT2A receptor (~52 kDa), because it corresponds to an N-glycosylated form of the receptor (Maginnis et al., 2010). To confirm this, we treated mouse cortical lysate with PNGase F (enzyme that cleaves N-linked glycans from proteins) and observed a corresponding shift in the electrophoretic mobility of 5-HT2A receptor from ~75 to ~52 kDa (Figure 2B). We have further validated 5-HT2A (24288) antibody by preadsorption with its respective immunizing peptide. Immunohistochemical analysis of the rat mPFC using this antibody revealed laminar staining [corresponding to a typical distribution of 5-HT2A receptors in this brain region (Hamada et al., 1998; Weber and Andrade, 2010)] that was completely absent in tissues incubated with 5-HT2A (24288) antiserum preadsorbed with its immunogenic peptide (Figure 2C). In a separate immunoblotting experiment, we observed a loss of the 75 kDa 5-HT2A band in cortical lysates that were incubated with the preadsorbed 5-HT2A (#24288) antibody (Figure 2C).
Fig. 2.
Identification of antibodies suitable for the detection of 5-HT2A and mGlu2 receptor proteins in the rat brain tissue. (A) Validation of six different commercially-available antibodies in the frontal cortex of rats (R), wild-type mice (WT) and 5-HT2A knockout mice (KO). Only one antibody (Immunostar #24288) detected a ~75kDa band (◀) corresponding to a mature, glycosylated rat 5-HT2A receptor that was not present in 5-HT2A KO mice. (B) Deglycosylation of the wild type (WT) mouse cortical lysate (lanes 2 and 3) by PNGase F increased electrophoretic mobility of the ~75 kDa (◀) band to yield ~52 kDa (◁) band (detected by Immunostar #24288 5-HT2A receptor antibody) that corresponds to the sequenced-based molecular weight of the rat 5-HT2A receptor. For a comparison, the 5-HT2A receptor detection (Immunostar #24288) in the rat (R) cerebral cortex has been shown (lane 1). (C) Immunostaining of rat cortical sections with 5-HT2A #24288 antibody produced a laminar staining pattern consistent with the known 5-HT2A distribution in the mPFC (as shown under 10× magnification). This signal was not present in the mPFC slices, or in the mPFC tissue homogenates incubated with the 5-HT2A antiserum preadsorbed with its immunogenic peptide.
3.2. Co-immunoprecipitation of mGlu2 and 5-HT2A receptors in the rat brain.
Recent evidence suggests that behavioral and functional interaction between mGlu2 and 5-HT2A is mediated in part by a direct physical interaction between these two receptors, as detected in the mouse and human cortical tissue using co-immunoprecipitation (Fribourg et al., 2011; González-Maeso et al., 2008). Here we analyzed the presence of 5-HT2A signal in a protein fraction prepared from the three preselected brain regions (mPFC, dHPC and PRh) and immunoprecipitated with anti-mGlu2 antibody. Specificity of the mGlu2 antibody was first validated in cortical samples from mGlu2 KO mice. Figure 3A shows that under low stringent conditions, mGlu2 receptors in the cortex can be detected almost exclusively as dimers (~220 kDa) that are thought to represent the active form of the receptor (El Moustaine et al., 2012). mGlu2 dimer was not detected in the mGlu2 KO samples, confirming the specificity of the mGlu2 antibody (Figure 3A, also see: Sanger et al., 2013; Wood et al., 2017). In the co-immunoprecipitation experiment, mGlu2/5-HT2A receptor interaction was detected in the PRh samples, but not in the samples prepared from the mPFC (both, Figure 3B) or dHPC (data not shown). A lack of 5-HT2A signal in PRh samples precipitated with an unrelated antibody (IP-*Ab; Figure 3B) confirms the specificity of the mGlu2/5-HT2A receptor complex detection.
3.3. Extended access to self-administered meth: behavior and protein expression changes.
Figure 4A depicts the timeline of the meth self-administration experiment. Rats assigned to the meth group rapidly acquired self-administration and displayed a clear discrimination between active and passive lever responses throughout the whole study (≥300 % and ≥900 % active lever discrimination during the limited and extended access period respectively; Figure 4B). Yoked-saline rats indiscriminately displayed low levels of responding on both levers. During the 14 days of extended access to meth, escalation of meth intake was observed (Figure 4C). Specifically, the average daily meth intake during the last three days (days 12-14) of extended access to meth was significantly higher than on the first three days (days 1-3; [t(4) = 2.93, p<0.05]). Cumulative meth intake ranged from 52.4–76.3 mg/kg and was comparable to meth intake observed in our previous studies (Reichel et al., 2012b; Schwendt et al., 2012). There was no difference in weight gain between yoked-saline and meth groups (data not shown).
Fig. 4.
Chronic meth self-administration. (A) Timeline of the experiment. (B) Mean daily lever responding for meth and yoked-saline animals (a/i – active/inactive lever presses). (C) Mean daily meth intake (mg/kg) during short (1 hr) and extended (6 hr) self-administration sessions. Escalation of meth intake was observed during extended access to meth (average intake on days 12-15 vs. days 1-3; *p < 0.05). n = 5-7 per group.
Next, we analyzed protein levels of mGlu2 and 5-HT2A receptors (and their cognate G-proteins) in the mPFC, dHPC and PRh following 7 days of withdrawal (Figure 5). This interval was based on the previous studies showing that extended access to self-administered meth and 7 days of withdrawal alters synaptic plasticity and mGlu expression in the PRh (Reichel et al., 2011; Scofield et al., 2015), produces PRh/dHPC-dependent memory deficits (Reichel et al., 2011), dysregulates 5-HT levels (Brennan et al., 2010) and glutamate neurophysiology in the PFC (Mishra et al., 2016). As the functional (and behavioral) outcome of the 5-HT2A and mGlu2 interaction depends on the relative amount of each receptor (Baki et al., 2013), the 5-HT2A/mGlu2 protein expression ratio was also calculated. Extended access to meth upregulated 5-HT2A protein levels in the mPFC [t(9) = 2.96, p<0.05] and the PRh [t(9) = 2.61, p<0.05] compared to yoked-saline rats. While mGlu2 expression was not altered by meth, the 5-HT2A/mGlu2 expression ratio was increased in both the mPFC [t(9) = 3.41, p<0.01] and PRh [t(9) = 2.91, p<0.05] regions of the cortex of meth rats. With regard to G-proteins, only changes in Gαq protein subunit were detected in this experiment. Extended access to self-administered meth increased Gαq protein levels in the mPFC [t(9) = 2.46, p<0.05], while reducing it in the PRh region [t(9) = 3.41, p<0.01].
Fig. 5.
Analysis of the protein expression after chronic meth self-administration in the (A) mPFC (B) dHPC and (C) PRh. (A,B,C - top insets): Representative immunoblotting results show the expression of 5-HT2A, mGlu2, Gαq/11, Gαi and Gαo proteins in saline and meth animals. Calnexin - control protein. (A, B, C - bar graphs): Quantitative analysis of the individual protein expression and of the calculated 5-HT2A/mGlu2 protein expression ratio. Data are expressed as the percentage of saline controls, n = 5-7 animals per group. (*p < 0.05, **p < 0.01 vs. yoked-saline controls). 2A/2 ratio - 5-HT2A/mGlu2 protein expression ratio.
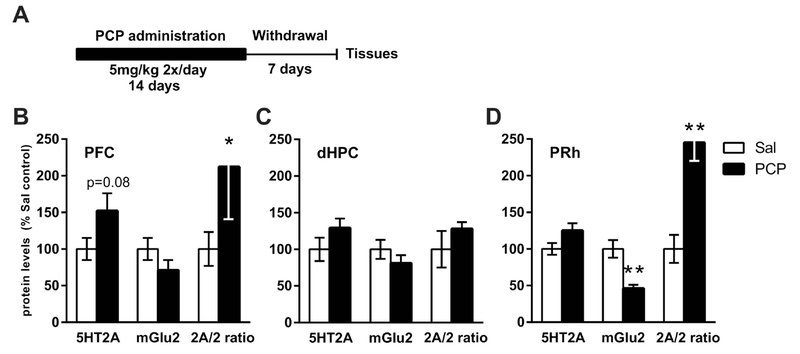
3.4. Chronic PCP administration and protein expression changes.
Figure 6A depicts the timeline of the PCP experiment. Chronic PCP administration (5mg/kg IP, twice a day for 14 days) did not affect body weight gain when compared to the saline group (data not shown). Seven days after the discontinuation of PCP administration, the 5-HT2A/mGlu2 expression ratio was increased in the mPFC [t(14) = 2.31, p<0.05] and the PRh [t(14) = 4.66, p<0.001] regions, but not in the dHPC. PCP-induced changes in individual proteins were limited to a decrease in mGlu2 levels in the PRh [t(14) = 4.15, p<0.001], with no other proteins altered in any of the brain regions analyzed (Figure 6 and Table 1).
Fig. 6.
Analysis of protein expression after chronic PCP administration. (A) Timeline of the experiment. (B, C, D): Quantitative analysis of the individual protein expression and of the calculated 5-HT2A/mGlu2 protein expression ratio in the mPFC (B), dHPC (C) and PRh (D). Data are expressed as the percentage of saline controls. n = 7-8 animals per group. (*p < 0.05, **p < 0.01 vs. saline-treated controls). 2A/2 ratio - 5-HT2A/mGlu2 protein expression ratio.
Table 1:
Protein levels of the selected G-protein subunits following chronic PCP administration.
| PFC | dHPC | PRh | ||||
|---|---|---|---|---|---|---|
| Sal | PCP | Sal | PCP | Sal | PCP | |
| Gq | 100 ± 12.4 | 96 ± 12.9 | 100 ± 4.4 | 103 ± 4.5 | 100 ± 7.6 | 82 ± 5 |
| Gi | 100 ± 18.4 | 108 ± 20.6 | 100 ± 3.7 | 105 ± 3.3 | 100 ± 7.8 | 101 ± 6.6 |
| Go | 100 ± 7.8 | 106 ± 12.3 | 100 ± 6.1 | 105 ± 5.2 | 100 ± 5.2 | 102 ± 5 |
Data represent mean immunoreactivity of each protein normalized to calnexin and expressed as percent of the Sal group ± SEM (n = 8). PFC, prefrontal cortex; dHpc, dorsal hippocampus; PRh, perirhinal cortex; Sal, saline; PCP, phencyclidine.
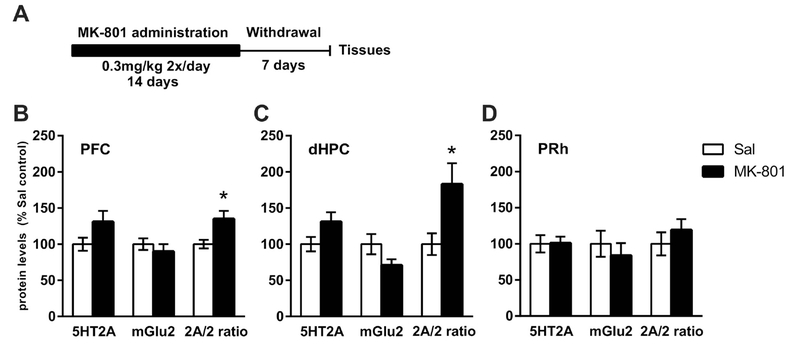
3.5. Chronic MK-801 administration and protein expression changes.
Figure 7A depicts the timeline of the MK-801 experiment. Chronic MK-801 administration (0.3 mg/kg IP, twice a day for 14 days) did not affect body weight when compared to the saline group (data not shown). Seven days after the discontinuation of MK-801 administration, the 5-HT2A/mGlu2 expression ratio was increased in the mPFC [t(14) = 2.25, p<0.05] and dHPC [t(14) = 2.54, p<0.05], but not in the PRh. No changes in the levels of G-protein subunits measured were detected in any brain region analyzed (Table 2).
Fig. 7.
Analysis of protein expression after chronic MK-801 administration. (A) Timeline of the experiment. (B, C, D): Quantitative analysis of the individual protein expression and of the calculated 5-HT2A/mGlu2 protein expression ratio in the mPFC (B), dHPC (C) and PRh (D). Data are expressed as the percentage of saline controls. n = 7- 8 animals per group. (*p < 0.05 vs. saline-treated controls). 2A/2 ratio - 5-HT2A/mGlu2 protein expression ratio.
Table 2:
Protein levels of the selected G-protein subunits following chronic MK-801 administration.
| PFC | dHPC | PRh | ||||
|---|---|---|---|---|---|---|
| Sal | MK | Sal | MK | Sal | MK | |
| Gq | 100 ± 9.2 | 112 ± 15.3 | 100 ± 5.5 | 95 ± 3.4 | 100 ± 6 | 81 ± 7.4 |
| Gi | 100 ± 6.2 | 93 ± 3.3 | 100 ± 5.6 | 95 ± 2.8 | 100 ± 11.2 | 95 ± 9.9 |
| Go | 100 ± 8.3 | 90 ± 5.1 | 100 ± 6.6 | 88 ± 4.1 | 100 ± 11.4 | 110 ± 8.1 |
Data represent mean immunoreactivity of each protein normalized to calnexin and expressed as percent of the Sal group ± SEM (n = 8). PFC, prefrontal cortex; dHpc, dorsal hippocampus; PRh, perirhinal cortex; Sal, saline; MK, dizocilpine.
4. Discussion
The present study characterized co-regulation of 5-HT2A and mGlu2 receptor expression within the rat mPFC, dHPC and PRh cortex following a chronic exposure to drugs known to induce psychotic symptoms and cognitive impairment in humans. Two weeks of extended access to self-administered meth (or administration of PCP or MK-801 over the same period) increased the ratio of 5-HT2A/mGlu2 protein expression in the mPFC. Chronic meth and PCP also increased the 5-HT2A/mGlu2 expression ratio in the PRh, while MK-801 produced the similar change in the dHPC. The rationale behind the parallel analysis of 5-HT2A and mGlu2 receptor expression is based on the evidence of close functional cooperation between these two receptors in triggering psychosis or maintaining cognitive function. This cooperation could be mediated in part by a formation of the 5-HT2A-mGlu2 heterocomplex in the PRh. Given the known psychosis-like effects of 5-HT2A receptor activation and cognitive deficits associated with the loss of mGlu2 function, an increase in the 5-HT2A/mGlu2 expression ratio offers a novel neural mechanism for targeting the pro-psychotic and cognitive-impairing effects of chronic meth, PCP or MK-801 exposure.
Recent reports suggest that alarmingly low reproducibility of published protein analysis data is related to poor specificity of thousands of commercially-available antibodies, often caused by the lack of thorough antibody validation (Berglund et al., 2008; Bradbury and Plückthun, 2015). Since 5-HT2A receptor antibodies have been plagued by specificity issues, a critical part of this study was to identify antibody/antibodies suitable for the detection of this receptor in the rat brain tissue. To that end, we conducted a comparative validation of six commercially-available 5-HT2A antibodies (Figure 2). Surprisingly, only one of the tested antibodies (anti-5-HT2A #24288, currently distributed by Immunostar), was found to be specific. First, this antibody detected a single band corresponding to the molecular weight of the mature (glycosylated) rat 5-HT2A receptors that was not present in cortical samples obtained from the 5-HT2A receptor KO mice. Next, enzymatic deglycosylation increased electrophoretic mobility of the 5-HT2A receptors to yield a band with a molecular weight corresponding to the known rat 5-HT2A receptor amino-acid sequence. And finally, this antibody produced a characteristic laminar immunostaining signal previously seen in the mPFC of transgenic animals expressing a fluorescent marker under the control of the 5-HT2A receptor promoter (Weber and Andrade, 2010).
Next, using this 5-HT2A antibody and the validated mGlu2 antibody (Figure 3; and Sanger et al., 2013; Wood et al., 2017), we sought to determine whether these two receptors physically interact in the rat brain. As described below, the rationale for investigating 5-HT2A-mGlu2 receptor-receptor interaction is the proposed role of this receptor heterocomplex in psychosis and in mediating the effects of hallucinogens, including 5-HT2A receptor agonists (González-Maeso et al., 2008; Moreno et al., 2011). Previously, 5-HT2A-mGlu2 heterocomplex was detected in cell lines overexpressing both receptors, as well as in the mouse and human cortical tissue (Fribourg et al., 2011; González-Maeso et al., 2008). Here, we report for the first time that 5-HT2A co-immunoprecipitates with mGlu2 in the rat brain tissue. Interestingly, under the conditions used in this study, 5-HT2A-mGlu2 receptor-receptor interaction was only detected in the PRh tissue, but not in the PFC or dHPC. There are several possible explanations for this observation. First, unlike previous studies (Fribourg et al., 2011; González-Maeso et al., 2008), we investigated 5-HT2A-mGlu2 interaction in the rat, not mouse, or human brain tissue. We utilized a cross-linking approach to stabilize protein-protein interactions, while other studies have not. While in the majority of cases, protein cross-linking increases the likelihood of heterocomplex detection, in some cases, crosslinker can alter antibody-antigen binding hindering protein detection (Corgiat et al., 2014). Secondly, there may be regional differences in the presence of 5-HT2A-mGlu2 heterocomplexes. Previously, 5-HT2A-mGlu2 heterocomplexes were reported in a broadly-defined area of the frontal cortex, but were not evaluated specifically in the PFC. Further, our finding does not contradict previously observed functional interactions between cortical 5-HT2A and mGlu2 receptors, as they might be mediated indirectly, via integration of downstream cellular signaling (Delille et al., 2013). In the HPC, the expression of 5-HT2A receptors is relatively low (Hamada et al., 1998; Wright et al., 1995), also evidenced by the difficulty to detect 5-HT2A by immunoblotting in our experiments, which might have limited our ability to detect heterocomplexes.
Regardless of the exact mechanism mediating the 5-HT2A-mGlu2 interaction, preclinical and clinical evidence suggest that communication between these two receptors plays a role in pathophysiology of psychosis and cognitive dysfunction (as reviewed by: Wischhof and Koch, 2016). However, almost all the published studies investigated 5-HT2A-mGlu2 interaction in relation to schizophrenia, but not MUD. This is a critical knowledge gap, as meth-dependent subjects frequently develop psychotic symptoms and cognitive deficits (such as decline in memory function), akin to schizophrenia (Grant et al., 2012). Surprisingly, there is a relative paucity of information on: what is the identity of potential common neural substrates (e.g. genes, proteins), and how these substrates are dysregulated in MUD vs. schizophrenia patients or in animal models of either disorder. While two previous studies analyzed the profile of cortical gene expression after acute meth or PCP administration (Ouchi et al., 2005, 2004), no published studies compared the expression of candidate susceptibility proteins such as 5-HT2A and mGlu2 in validated animal models of MUD, and/or schizophrenia. Therefore, the main (and novel) finding of the current study is that chronic meth, PCP or MK-801 produce analogous increases in the 5-HT2A/mGlu2 protein expression ratio in the PFC (and to a lesser extent in the PRh). In the PFC and PRh of meth animals, altered 5-HT2A/mGlu2 expression ratio was primarily driven by an increase in 5-HT2A receptors themselves. Similarly, Chiu et al. (2014) reported that non-contingent meth administration upregulates 5-HT2A receptor expression in the mouse frontal cortex, as well as cellular and behavioral responses to 5-HT2A receptor agonists. It is possible that the mechanism driving upregulation of cortical 5-HT2A receptor is a compensatory response to a serotonin depletion (Heal et al., 1985), as low serotonin levels have been reported in the frontal cortex of rodents following a chronic meth exposure (Brennan et al., 2010; McFadden et al., 2013) and in the forebrain of abstinent meth abusers (Wilson et al., 1996). It is tempting to speculate that meth-induced upregulation of cortical 5-HT2A receptors contributes to an increased likelihood of psychotic episodes in meth-dependent users (Ujike and Sato, 2004). In addition to the change in receptor numbers, upregulation of their cognate G-proteins might also facilitate receptor function. Since 5-HT2A couples through Gαq proteins (Roth et al., 1998), while mGlu2 uses Gαi/o (Kammermeier et al., 2003), increased availability of Gαq (but not Gαi/o) proteins observed in the PFC and PRh of meth animals (Figure 5) can further facilitate cellular activity of 5-HT2A receptors. Indeed, Chiu and colleagues (2014) observed augmented 5-HT2A activity following the non-contingent repeated meth administration, though G-protein expression was not analyzed in that study.
Unlike meth, PCP and MK-801 are primarily non-competitive NMD A receptor antagonists. Still, acute administration of all three investigated drugs has been shown to increase synaptic levels of dopamine, serotonin and glutamate in the PFC; albeit via distinct pharmacological mechanisms (Adams and Moghaddam, 1998; Amargós-Bosch et al., 2006; Halpin et al., 2014; Hondo et al., 1994; Jentsch et al., 1998; López-Gil et al., 2009; Martin et al., 1998; Schmidt and Fadayel, 1996; Zuo et al., 2006). For example, meth increases serotonin in the PFC via canonical mechanisms involving 5-HT transporters, while acute serotonergic effects of MK-801 and PCP depend on direct or indirect activation of local 5-HT2A receptors (Kapur and Seeman, 2002; López-Gil et al., 2009). However, our findings need to be interpreted within the contex of neurochemical consequences produced by the chronic meth, PCP or MK-801 exposure in the PFC. Parsegian et al. reported that chronic meth self-administration (followed by 10-14 days of extinction training) reduced basal glutamate levels in the PFC and increased glutamate efflux during reinstatement of meth-seeking, an observation consistent with a reduced function of mGlu2 receptors in this brain region (Parsegian and See, 2014). However, utilizing the identical meth paradigm we did not observe lasting changes in the overall 5-HT tissue content in the PFC (Schwendt et al., 2009). In a recent study, no significant changes in 5-HT2 receptor binding in the PFC were detected immediately after the cessation of meth self-administration (McFadden et al., 2018). However, neither of these studies evaluated glutamatergic, serotonergic markers in the PFC after a undistrubed withdrawal period (this study). In comparison to meth, serotonergic and glutmatergic consequences of chronic PCP or MK-801 administration are less understood. Studies using shorter administration regimen and lower doses of PCP (subchronic paradigms) reported blunted glutamate release in the PFC (Amitai et al., 2012), but no observable changes in 5-HT2A receptors in the PFC (Choi et al., 2009; Santini et al., 2013).
In the present study, chronic meth, PCP, and MK-801 administration all increased the 5-HT2A/mGlu2 ratio in the PFC, with an additional increase detected in the PRh of PCP-treated animals and in the dHPC of animals treated with MK-801. In comparison to chronic meth, increases in 5-HT2A/mGlu2 ratios were driven by more subtle changes in 5-HT2A and mGlu2 receptor protein expression. Despite this, we argue that the receptor ratio (as calculated in this study) is not an artificial construct, rather it reflects the balance between 5-HT2A and mGlu2 receptor signaling (as described below), and is predictive of the psychoactive properties of their respective receptor ligands (Baki et al., 2013; Fribourg et al., 2011). For example, Santini and colleagues (2013) reported that in mice, chronic PCP treatment increases behavioral and cellular effects of 5-HT2A agonists, but not the total protein levels of cortical 5-HT2A receptors (Santini et al., 2013). This suggests that a history of PCP administration altered one of the 5-HT2A partner proteins (such as mGlu2) which in turn altered coupling, trafficking, or surface availability of 5-HT2A receptors to produce the observed effect. In the present study, chronic PCP significantly reduced mGlu2 protein levels in the PRh. It is not clear what the cause is for this brain region-selective effect of this drug, though in one previously published study, repeated (14 day-long) PCP administration produced a downregulation of mGlu2 mRNA levels selectively only in some, but not all regions of the rat frontal cortex (Abe et al., 2001). It should be noted that in general, the scale and/or the character of neuroadaptations produced by chronic drug exposure may depend on the contingency of drug delivery. Here we compared the contingent (meth) vs. non-contingent (PCP, MK-801) drug administration. This was due to practical reasons (e.g. difficulty and low rates of PCP or MK-801 self-administration in rodents), as well as our goal to employ drug administration paradigms with well-characterized behavioral outcomes (e.g. object recognition and working memory deficits, sensorimotor gating deficits). Interestingly, we observed a similar increase in PFC 5-HT2A receptors as shown in mice after non-contingent binge meth administration (Chiu et al., 2014), suggesting that contingency of drug delivery did not affect this finding.
A number of gene-association and postmortem studies evaluated the link between 5-HT2A and/or mGlu2 receptors and schizophrenia, or psychosis in general (for review see: Wischhof and Koch, 2016). Unfortunately, discrepancies exist on the direction (increase/decrease) or the presence of 5-HT2A and/or mGlu2 receptor changes. The lack of consensus is likely due to variations in clinical and demographic factors (such as a history of antipsychotic treatment, gender and age). A single study that evaluated postmortem levels of 5-HT2A and mGlu2 receptors in the same population of unmedicated schizophrenia subjects reported that upregulation of 5-HT2A receptors is accompanied by downregulation of mGlu2 receptors in the frontal cortex (González-Maeso et al., 2008).
The majority of available evidence suggests that the interaction between cortical 5-HT2A and mGlu2 receptors has an antagonistic character. At the cellular level, this is evidenced by the suppressive effects of mGlu2 receptor agonists on excitatory postsynaptic potentials (Marek et al., 2000) and immediate early gene activation (Moreno et al., 2011; Zhai et al., 2003) evoked by 5-HT2A receptor agonists in the PFC. At the behavioral level, the frequency of a typical ‘head-twitch’ response induced in rodents by hallucinogenic 5-HT2A receptor agonists (administered systemically or directly into the PFC) is suppressed by pharmacological activation of mGlu2/3 receptors (Gewirtz and Marek, 2000; Klodzinska et al., 2002; Moreno et al., 2011). Therefore, it could be predicted that upregulation of the 5-HT2A/mGlu2 ratio in the PFC, a common consequence of chronic meth, PCP, and MK-801 exposure observed in the present study, augments 5-HT2A receptor function and/or reduces inhibitory control of mGlu2 receptor over 5-HT2A receptor activity. To confirm this, future studies will investigate meth/PCP/MK-801-induced changes in 5-HT2A receptor function within the PFC and PRh as assessed by the efficacy of 5-HT2A receptor agonists to induce downstream cellular signaling (tissue PLC activity; (Shi et al., 2006), or to activate immediate early genes (e.g. c-fos or Arc; González-Maeso et al., 2003). Changes in the ability of mGlu2 receptor agonists to suppress 5-HT2A receptor activation will be also evaluated to gain an insight on altered cooperation of 5-HT2A and mGlu2 receptors in the brain regions of interest.
5. Conclusions.
The present study demonstrated that chronic exposure to either meth, PCP, or MK-801 produces complementary changes in 5-HT2A and mGlu2 receptors in the PFC that can be commonly described as an increase in the 5-HT2A/mGlu2 protein expression ratio. Increase in this ratio was also observed in the PRh (meth and PCP) and dHPC (MK-801) tissues. As cortical 5-HT2A and mGlu2 receptors closely cooperate in regulating sensorimotor and cognitive processing, an altered 5-HT2A/mGlu2 ratio could represent a common neurobiological mechanism underlying the emergence of psychosis and cognitive disruption observed with the illicit use of meth or NMDA-receptor antagonists (such as PCP and MK-801). Further, some (though not all) studies suggest that dysregulation of 5-HT2A and mGlu2 receptor expression also occurs in the brains of subjects with schizophrenia diagnosis. Therefore, future studies will need to address not only cellular, but also brain-wide functional and behavioral consequences of disrupted 5-HT2A/mGlu2 balance in translational animal models of MUD and schizophrenia.
Highlights.
5-HT2A and mGlu2 receptors co-immunoprecipitate in the rat cortical tissue.
Chronic meth increased the 5-HT2A/mGlu2 expression ratio in the mPFC and PRh
Chronic PCP and MK-801 also increased the 5-HT2A/mGlu2 expression ratio in the mPFC
Chronic meth increased 5-HT2A partner G-protein (Gαq) in the mPFC
Acknowledgements
The authors would like to thank Rebecca Madell for the assistance with surgeries and meth self-administration and Jenna Bilodeau for the help with tissue analysis. Authors would also like to thank Dr. González-Maeso (Department of Psychiatry, Mount Sinai Medical Center, New York) for providing mGlu2 and 5-HT2A KO mice tissues. The current research was supported in part by the NARSAD Young Investigator Award and NIDA DA025846 (both awarded to MS), Ministry of Science and Higher Education (Warszawa, grant no. 632/MOB/2011/0) (awarded to MZ) and the project Nr. LO1611 with a financial support from the MEYS CR under the NPU I program, and Ministry of Health of the Czech Republic, grant AZV nr. 15-29370A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- Abdel-Salam OME, El-Shamarka MES, Salem NA, El-Mosallamy AEMK, Sleem AA, 2012. Amelioration of the haloperidol-induced memory impairment and brain oxidative stress by cinnarizine. EXCLI J. 11, 517–530. [PMC free article] [PubMed] [Google Scholar]
- Abe S, Suzuki T, Ito T, Yamaguchi M, Baba A, Hori T, Kurita H, Shiraishi H, Okado N, 2001. Effects of single and repeated phencyclidine administration on the expression of metabotropic glutamate receptor subtype mRNAs in rat brain. Neuropsychopharmacology 25 (2), 173–184. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B, 1998. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J. Neurosci. 18 (4), 5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargós-Bosch M, López-Gil X, Artigas F, Adell A, 2006. Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int. J. Neuropsychopharmacol. 9 (5), 565–573. [DOI] [PubMed] [Google Scholar]
- Amitai N, Kuczenski R, Behrens MM, Markou A, 2012. Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats. Neuropharmacology 62 (3), 1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Eltit JM, Fribourg M, Younkin J, Park G, Vysotskaya Z, Sealfon SC, Liapakis G, González-Maeso J, Logothetis DE, 2013. Functional Crosstalk between mGluR2 and 5-HT2A Depends on their Expression Ratios. Biophys. J. 104 (2), 116a. [Google Scholar]
- Bechara A, Martin EM, 2004. Impaired Decision Making Related to Working Memory Deficits in Individuals with Substance Addictions. Neuropsychology 18(1), 152–162 [DOI] [PubMed] [Google Scholar]
- Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, Al-Khalili Szigyarto C, Persson A, Ottosson J, Wernérus H, Nilsson P, Lundberg E, Sivertsson Å, Navani S, Wester K, Kampf C, Hober S, Pontén F, Uhlén M, 2008. A Genecentric Human Protein Atlas for Expression Profiles Based on Antibodies. Mol. Cell. Proteomics 7 (10), 2019–2027. [DOI] [PubMed] [Google Scholar]
- Boireau A, Bordier F, Durand G, Doble A, 1996. The antidepressant metapramine is a low-affinity antagonist at N-methyl-D-aspartic acid receptors. Neuropharmacology 35 (12), 1703–1707. [DOI] [PubMed] [Google Scholar]
- Bradbury A, Plückthun A, 2015. Reproducibility: Standardize antibodies used in research. Nature. 518 (7537), 27–29. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, 1990. Sensorimotor Gating and Schizophrenia. Arch. Gen. Psychiatry 47 (2), 181–188. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S, 2010. Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res. 1317, 137–146. [DOI] [PubMed] [Google Scholar]
- Brownfield MS, Yracheta J, Chu F, Lorenz D, Diaz A, 1998. Functional chemical neuroanatomy of serotonergic neurons and their targets: Antibody production and immunohistochemistry (IHC) for 5-HT, its precursor (5-HTP) and metabolite (5-HIAA), biosynthetic enzyme (TPH), transporter (SERT), and three receptors. Ann. N. Y. Acad. Sci. 861 (1), 232–233. [DOI] [PubMed] [Google Scholar]
- Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC, Chiang YL, Ree SC, Lee CH, Murray RM, 2003. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol. Med. 33 (8), 1407–1414. [DOI] [PubMed] [Google Scholar]
- Chiu H-Y, Chan M-H, Lee M-Y, Chen S-T, Zhan Z-Y, Chen H-H, 2014. Long-lasting alterations in 5-HT2A receptor after a binge regimen of methamphetamine in mice. Int. J. Neuropsychopharmacol. 17 (10), 1647–1658. [DOI] [PubMed] [Google Scholar]
- Choi YK, Snigdha S, Shahid M, Neill JC, Tarazi FI, 2009. Subchronic effects of phencyclidine on dopamine and serotonin receptors: Implications for schizophrenia. J. Mol. Neurosci. 38 (3), 227–235. [DOI] [PubMed] [Google Scholar]
- Copani A, Romano C, Di Giorgi Gerevini V, Nicosia A, Casabona G, Storto M, Mutel V, Nicoletti F, 2000. Reducing conditions differentially affect the functional and structural properties of group-I and -II metabotropic glutamate receptors. Brain Res. 867 (1-2), 165–172. [DOI] [PubMed] [Google Scholar]
- Corgiat BA, Nordman JC, Kabbani N, 2014. Chemical crosslinkers enhance detection of receptor interactomes. Front. Pharmacol. 4, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Cope ZA, Parsegian A, Floresco SB, Aston-Jones G, See RE, 2016. Chronic methamphetamine self-administration alters cognitive flexibility in male rats. Psychopharmacology (Berl). 233 (12), 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delille HK, Mezler M, Marek GJ, 2013. The two faces of the pharmacological interaction of mGlu2 and 5-HT2A-Relevance of receptor heterocomplexes and interaction through functional brain pathways. Neuropharmacology 70, 296–305. [DOI] [PubMed] [Google Scholar]
- El Moustaine D, Granier S, Doumazane E, Scholler P, Rahmeh R, Bron P, Mouillac B, Baneres J-L, Rondard P, Pin J-P, 2012. Distinct roles of metabotropic glutamate receptor dimerization in agonist activation and G-protein coupling. Proc. Natl. Acad. Sci. 109 (40), 16342–16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell AD, Brezina V, Sealfon SC, Filizola M, González-Maeso J, Logothetis DE, 2011. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147 (5), 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ, 2000. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23 (5), 569–576. [DOI] [PubMed] [Google Scholar]
- Ghose S, Crook JM, Bartus CL, Sherman TG, Herman MM, Hyde TM, Kleinman JE, Akil M, 2008. Metabotropic glutamate receptor 2 and 3 gene expression in the human prefrontal cortex and mesencephalon in schizophrenia. Int. J. Neurosci. 118 (11), 1609–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, 2014. Methamphetamine psychosis: Epidemiology and management. CNS Drugs 28 (12), 1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC, 2008. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452 (7183), 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC, 2003. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 23 (26), 8836–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM, 2007. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: Preliminary observations. J. Clin. Exp. Neuropsychol. 29 (2), 155–159. [DOI] [PubMed] [Google Scholar]
- Grant KM, LeVan TD, Wells SM, Li M, Stoltenberg SF, Gendelman HE, Carlo G, Bevins RA, 2012. Methamphetamine-associated psychosis. J. Neuroimmune Pharmacol. 7 (1), 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Pichat P, Boulay D, Naimoli V, Potestio L, Featherstone R, Sahni S, Defex H, Desvignes C, Slowinski F, Vigé X, Bergis OE, Sher R, Kosley R, Kongsamut S, Black MD, Varty GB, 2016. The mGluR2 positive allosteric modulator, SAR218645, improves memory and attention deficits in translational models of cognitive symptoms associated with schizophrenia. Sci. Rep. 6, 35320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, Markou A, Kuczenski R, 2011. Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav. Brain Res. 217 (2), 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK, 2014. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 97 (1), 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Senzaki K, Hamaguchi-Hamada K, Tabuchi K, Yamamoto EL, Yamamoto T, Yoshikawa S, Okano H, Okado N, 1998. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Mol. Brain Res. 54 (2), 199–211. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PWJ, Lane TA, 2008. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): Expression, function and involvement in schizophrenia. J. Psychopharmacol. 22 (3), 308–322. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Philpot J, Molyneux SG, Metz A, 1985. Intracerebroventricular administration of 5,7-dihydroxytryptamine to mice increases both head-twitch response and the number of cortical 5-HT2receptors. Neuropharmacology 24 (12), 1201–1205. [DOI] [PubMed] [Google Scholar]
- Hondo H, Yonezawa Y, Nakahara T, Nakamura K, Hirano M, Uchimura H, Tashiro N, 1994. Effect of phencyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Res. 633 (1-2), 337–342. [DOI] [PubMed] [Google Scholar]
- Hou Y, Wu CF, Yang JY, Guo T, 2006. Differential effects of haloperidol, clozapine and olanzapine on learning and memory functions in mice. Prog. Neuro-Psychopharmacology Biol. Psychiatry 30 (8), 1486–1495. [DOI] [PubMed] [Google Scholar]
- Hutchings EJ, Waller JL, Terry AV, 2013 Differential long-term effects of haloperidol and risperidone on the acquisition and performance of tasks of spatial working and short-term memory and sustained attention in rats. J. Pharmacol. Exp. Ther. 347 (3), 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Taylor JR, Roth RH, 1998. Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: Behavioral and neurochemical evidence. Psychopharmacology (Berl). 138 (1), 89–95. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH, 1997. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology 17 (2), 92–99. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P, 2002. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D2 and serotonin 5-HT2 receptors - Implications for models of schizophrenia. Mol. Psychiatry 7 (8), 837–844. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Davis MI, Ikeda SR, 2003. Specificity of Metabotropic Glutamate Receptor 2 Coupling to G Proteins. Mol. Pharmacol. 63 (1), 183–191. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Bijak M, Tokarski K, Pile A, 2002. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol. Biochem. Behav. 73 (2), 327–332. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M, 2014. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict. Biol. 19(1), 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA, 2009. Schizophrenia from a neural circuitry perspective: Advancing toward rational pharmacological therapies. J. Clin. Invest. 119 (4), 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JT, Su YA, Guo CM, Feng Y, Yang Y, Huang RH, Si TM, 2011. Persisting cognitive deficits induced by low-dose, subchronic treatment with MK-801 in adolescent rats. Eur. J. Pharmacol. 652 (1–3), 65–72. [DOI] [PubMed] [Google Scholar]
- López-Gil X, Artigas F, Adell A, 2009. Role of different monoamine receptors controlling MK-801-induced release of serotonin and glutamate in the medial prefrontal cortex: Relevance for antipsychotic action. Int. J. Neuropsychopharmacol. 12 (4), 487–499. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meek WH, 2005. Chronic treatment with haloperidol induces deficits in working memory and feedback effects of interval timing. Brain Cogn. 58 (1), 9–16. [DOI] [PubMed] [Google Scholar]
- Maginnis MS, Haley SA, Gee GV, Atwood WJ, 2010. Role of N-linked glycosylation of the 5-HT2A receptor in JC virus infection. J. Virol. 84 (19), 9677–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK, 2000. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 292 (1), 76–87. [PubMed] [Google Scholar]
- Marquis KL, Webb MG, Moreton JE, 1989. Effects of fixed ratio size and dose on phencyclidine self-administration by rats. Psychopharmacology (Berl). 97 (2), 179–182. [DOI] [PubMed] [Google Scholar]
- Martin P, Carlsson ML, Hjorth S, 1998. Systemic PCP treatment elevates brain extracellular 5-HT: A microdialysis study in awake rats. Neuroreport 9 (13), 2985–2988. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Hanson GR, Fleckenstein AE, 2013. The effects of methamphetamine selfadministration on cortical monoaminergic deficits induced by subsequent high-dose methamphetamine administrations. Synapse 67 (12), 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Cordie R, Livermont T, Johansen A, 2018. Behavioral and Serotonergic Changes in the Frontal Cortex Following Methamphetamine Self-Administration. Int. J. Neuropsychopharmacol. 21 (8), 758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, McLaren J, Lubman DI, Hides L, 2006. The prevalence of psychotic symptoms among methamphetamine users. Addiction 101 (10), 1473–1478. [DOI] [PubMed] [Google Scholar]
- Mishra D, Pena-Bravo JI, Leong KC, Lavin A, Reichel CM, 2016. Methamphetamine self-administration modulates glutamate neurophysiology. Brain Struct. Funct. 222 (5), 2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J, 2011. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 493 (3), 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A, Ben-Ezra A, Voloudakis G, Fakira AK, Baki L, Ge Y, Georgakopoulos A, Moron JA, Milligan G, Lopez-Gimenez JF, Robakis NK, Logothetis DE, Meana JJ, González-Maeso J, 2016. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci. Signal. 9 (410), ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Poquette AJ, Vigil O, Heaton RK, Grant I, 2012. Visual memory in methamphetamine-dependent individuals: Deficient strategic control of encoding and retrieval. Aust. N. Z. J. Psychiatry 46 (2), 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T, 2007. Phencyclidine animal models of schizophrenia: Approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem. Int. 51 (2–4), 173–184. [DOI] [PubMed] [Google Scholar]
- Muguruza C, Meana JJ, Callado LF, 2016. Group II Metabotropic Glutamate Receptors as Targets for Novel Antipsychotic Drugs. Front. Pharmacol. 7, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the Care and Use of Laboratory Animals, 8th ed. The National Academies Press, Washington, D.C. [Google Scholar]
- Ouchi Y, Kubota Y, Ito C, 2004. Serial analysis of gene expression in methamphetamine- and phencyclidine-treated rodent cerebral cortices: Are there common mechanisms? Ann. N. Y. Acad. Sci. 1025 (1), 57–61. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Kubota Y, Kuramasu A, Watanabe T, Ito C, 2005. Gene expression profiling in whole cerebral cortices of phencyclidine- or methamphetamine-treated rats. Mol. Brain Res. 140 (1–2), 142–149. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See RE, 2011. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol. Psychiatry 69 (3), 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, See RE, 2014. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology 39 (4), 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M, Saxon AJ, Hermann R, 2017. Methamphetamine use disorder: Epidemiology, clinical manifestations, course, assessment, and diagnosis - UpToDate [WWW Document], https://www.uptodate.com/contents/methamphetamine-use-disorder-epidemiology-clinical-manifestations-course-assessment-and-diagnosis.
- Paxinos G, Watson C, 2006. The Rat Brain in Stereotaxic Coordinates: 6th Edition. London: Academic Press. [Google Scholar]
- Rasmussen H, Erritzoe D, Andersen R, Ebdrup BH, Aggernaes B, Oranje B, Kalbitzer J, Madsen J, Pinborg LH, Baare W, Svarer C, Lublin H, Knudsen GM, Glenthoj B, 2010. Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch. Gen. Psychiatry 67 (1), 9–16. [DOI] [PubMed] [Google Scholar]
- Recinto P, Samant ARH, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD, 2012. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology 37 (5), 1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE, 2012. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology 62 (2), 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE, 2011. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36 (4), 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE, 2008. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl). 199 (4), 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Berry SA, Kroeze WK, Willins DL, Kristiansen K, 1998. Serotonin 5-HT2A receptors: molecular biology and mechanisms of regulation. Crit. Rev. Neurobiol. 12 (4), 319–338. [DOI] [PubMed] [Google Scholar]
- Saeedi EL, Remington G, Christensen BK, 2006. Impact of haloperidol, a dopamine D2antagonist, on cognition and mood. Schizophr. Res. 85 (1–3), 222–231. [DOI] [PubMed] [Google Scholar]
- Samiei M, Vahidi M, Rezaee O, Yaraghchi A, Daneshmand R, 2016. Methamphetamine-Associated Psychosis and Treatment With Haloperidol and Risperidone: A Pilot Study. Iran. J. psychiatry Behav. Sci. 10 (3), e7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger H, Hanna L, Colvin EM, Grubisha O, Ursu D, Heinz BA, Findlay JD, Vivier RG, Sher E, Lodge D, Monn JA, Broad LM, 2013. Pharmacological profiling of native group II metabotropic glutamate receptors in primary cortical neuronal cultures using a FLIPR. Neuropharmacology 66, 264–273. [DOI] [PubMed] [Google Scholar]
- Santini MA, Ratner C, Aznar S, Klein AB, Knudsen GM, Mikkelsen JD, 2013. Enhanced prefrontal serotonin 2A receptor signaling in the subchronic phencyclidine mouse model of schizophrenia. J. Neurosci. Res. 91 (5), 634–641. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Fadayel GM, 1996. Regional effects of MK-801 on dopamine release: effects of competitive NMDA or 5-HT2A receptor blockade. J. Pharmacol. Exp. Ther. 277 (3), 1541–1549. [PubMed] [Google Scholar]
- Schwendt M, Gold SJ, McGinty JF, 2006. Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D i and D 2 dopamine receptors. J. Neurochem. 96 (6), 1606–1615. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Reichel CM, Madell RL, See RE, 2011. Extended access to methamphetamine results in dysregulation of mGluR2/3 receptors in the rat prefrontal cortex and cognitive deficits: rescue by mGluR2 PAM., in: Neuroscience Meeting Planner Washington, DC: Society for Neuroscience, Online. Program No. 797.21. [Google Scholar]
- Schwendt M, Reichel CM, See RE, 2012. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW, 2009. Extended Methamphetamine Self-Administration in Rats Results in a Selective Reduction of Dopamine Transporter Levels in the Prefrontal Cortex and Dorsal Striatum Not Accompanied by Marked Monoaminergic Depletion. J. Pharmacol. Exp. Ther. 331 (2), 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Trantham-Davidson EL, Schwendt M, Leong KC, Peters J, See RE, Reichel CM, 2015. Failure to Recognize Novelty after Extended Methamphetamine Self-Administration Results from Loss of Long-Term Depression in the Perirhinal Cortex. Neuropsychopharmacology 40 (11), 2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I, 2007. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol. Rev. 17 (3), 275–297. [DOI] [PubMed] [Google Scholar]
- Shi J, Damjanoska KJ, Zemaitaitis BW, Garcia F, Carrasco G, Sullivan NR, She Y, Young KH, Battaglia G, Van De Kar LD, Howland DS, Muma NA, 2006. Alterations in 5-HT2Areceptor signaling in male and female transgenic rats over-expressing either Gq or RGS-insensitive Gq protein. Neuropharmacology 51 (3), 524–535. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dacey J, Glynn S, Rawson R, Ling W, 2004. The effect of relapse on cognition in abstinent methamphetamine abusers. J. Subst. Abuse Treat. 27 (1), 59–66. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Horiguchi M, Huang M, Li Z, Shahid M, Neill JC, Meltzer HY, 2010. Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J. Pharmacol. Exp. Ther. 332 (2), 622–631. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B, 2002. Effects of repeated treatment with amphetamine or phencyclidine on working memory in the rat. Behav. Brain Res. 134 (1–2), 267–274. [DOI] [PubMed] [Google Scholar]
- Tsunoka T, Kishi T, Kitajima T, Okochi T, Okumura T, Yamanouchi Y, Kinoshita Y, Kawashima K, Naitoh H, Inada T, Ujike H, Yamada M, Uchimura N, Sora I, Iyo M, Ozaki N, Iwata N, 2010. Association analysis of GRM2 and HTR2A with methamphetamine-induced psychosis and schizophrenia in the Japanese population. Prog. Neuro-Psychopharmacology Biol. Psychiatry 34 (4), 639–644. [DOI] [PubMed] [Google Scholar]
- Ujike H, Sato M, 2004. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis, in: Annals of the New York Academy of Sciences. 1025 (1), 279–287. [DOI] [PubMed] [Google Scholar]
- UNODC United Nations Office on Drugs and Crime, 2016. World Drug Report (2016) [WWW Document]. http://www.unodc.org/wdr2016/
- Vales K, Bubenikova-Valesova V, Klement D, Stuchlik A, 2006. Analysis of sensitivity to MK-801 treatment in a novel active allothetic place avoidance task and in the working memory version of the Morris water maze reveals differences between Long-Evans and Wistar rats. Neurosci. Res. 55 (4), 383–388. [DOI] [PubMed] [Google Scholar]
- Verachai V, Rukngan W, Chawanakrasaesin K, Nilaban S, Suwanmajo S, Thanateerabunjong R, Kaewkungwal J, Kalayasiri R, 2014. Treatment of methamphetamine-induced psychosis: a double-blind randomized controlled trial comparing haloperidol and quetiapine. Psychopharmacology (Berl). 231 (16), 3099–3108. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW, 2010. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia 48 (8), 2262–2272. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW, 2015. Neural circuitry for rat recognition memory. Behav. Brain Res. 285, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ET, Andrade R, 2010. Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front. Neurosci. 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AF, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ, 1996. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 2 (6), 699–703. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Koch M, 2016. 5-HT2Aand mGlu2/3 receptor interactions: On their relevance to cognitive function and psychosis. Behav. Pharmacol. 27 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- Wood CM, Nicolas CS, Choi SL, Roman E, Nylander I, Fernandez-Teruel A, Kiianmaa K, Bienkowski P, de Jong TR, Colombo G, Chastagnier D, Wafford KA, Collingridge GL, Wildt SJ, Conway-Campbell BL, Robinson ESJ, Lodge D, 2017. Prevalence and influence of cys407* Grm2 mutation in Hannover-derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour. Neuropharmacology 115, 128–138. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant L, Atkinson JH, McCutchan JA, Marcotte TD, Wallace MR, Ellis RJ, Letendre S, Schrier R, Sadek J, Jemigan T, Hesselink J, Taylor MJ, Masliah E, Langford D, Masys DR, Frybarger M, Abramson I, Lazzaretto D, 2005. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology 19 (1), 35–43. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L, 1995. Comparative localization of serotoninl A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 351 (3), 357–373. [DOI] [PubMed] [Google Scholar]
- Xu H, 2017. Chronic Administration of Haloperidol and Working Memory : Roles of Caudate Putamen Dopamine Receptors. Curr Neurobiol 8 (2), 27–33. [Google Scholar]
- Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK, 2003. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology 28 (1), 45–52. [DOI] [PubMed] [Google Scholar]
- Zhang G, Stackman RWJ, 2015. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 6, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo DY, Zhang YH, Cao Y, Wu CF, Tanaka M, Wu YL, 2006. Effect of acute and chronic MK-801 administration on extracellular glutamate and ascorbic acid release in the prefrontal cortex of freely moving mice on line with open-field behavior. Life Sci. 78 (19), 2172–2178 [DOI] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M, 2004. Psychiatric Symptoms in Methamphetamine Users. Am. J. Addict. 13 (2), 181–190. [DOI] [PubMed] [Google Scholar]