Abstract
This study determined the effects of pro-inflammatory cytokines (interleukin (IL)-1β and IL-6) on the expression of eggshell mineralization-related ion transporters in the hen uterus mucosa. Uterine mucosal tissues collected from White Leghorn laying hens were cultured for 1.5 or 3 h in TCM-199 medium with or without 100 ng/mL recombinant chicken IL-1β or IL-6. Total RNA and protein were extracted from the cultured tissues for real-time polymerase chain reaction (PCR) and western blot analyses and some tissues were processed into paraffin sections for immunostaining with calcium-binding protein D28K (CaBP-D28K) antibody. The gene expression of CaBP-D28K, PMCA1, PMCA2 (plasma membrane calcium-transporting ATPase 1 and 2; calcium pumps), CA2 (carbonic anhydrase 2), and SLC26A9 (solute carrier family 26 member 9; HCO3− transporter) was analyzed by real-time PCR and protein density of CaBP-D28K by western blotting. Expression of CaBP-D28K, PMCA1, PMCA2, CA2, and SLC26A9 was significantly higher in the tissues treated with IL-1β and IL-6 than in the control group at 1.5 h of incubation. Immunoreactive CaBP-D28K was localized in the uterine tubular gland cells in all groups, but its level was significantly lower in the tissues incubated for 1.5 h with IL-1β and IL-6 than in the control group. No significant differences were observed in the expression of all tested genes and CaBP-D28k content between the cytokine-treated and control groups at 3 h of incubation. These results suggest that IL-1β and IL-6 may not suppress the expression of genes related to Ca2+ and HCO3− transportation for eggshell formation, while CaBP-D28K protein content in uterine glandular cells was reduced by these cytokines during the early exposure phase. Thus, IL-1β and IL-6 induced by infections may disrupt the transportation of Ca2+ for eggshell formation through decreased CaBP-D28K content in the uterus.
Keywords: CaBP-D28K, eggshell, pro-inflammatory cytokine, hen, uterus
Introduction
In hens, eggshell is formed in the uterine segment of the oviduct. The lamina propria in the uterus mucosa of laying hens is filled with tubular gland cells (Nii et al., 2014). The calcium-binding protein D28K (CaBP-D28K) acts as a Ca2+ transporter in the glandular cells for eggshell formation (Ohira et al., 1998; Bar, 2009; Ebeid et al., 2012). Plasma membrane calcium-transporting ATPase (PMCA) 1 and PMCA 2 are among the calcium pumps responsible for intracellular Ca2+ release into the uterine lumen (Strehler and Zacharias, 2001; Bouillon et al., 2003; Nys et al., 2004). Carbonic anhydrase (CA) 2 dehydrates H2CO3 to HCO3−, which is released into the uterine lumen through HCO3− transporters, such as solute carrier family 26 member 9 (SLC26A9) (Common, 1941; Xu et al., 2005; Dorwart et al., 2008). HCO3− is further dehydrated to CO32− by CA. Ca2+ and CO32− are released from the mucosal tissue to form calcium carbonates that precipitate on the surface of the eggshell membrane (Lörcher and Hodges, 1969; Brionne et al., 2014). It has been reported that the expression of the genes involved in eggshell formation is dependent on the laying cycle (Jonchere et al., 2012).
Avian infectious bronchitis (IB) virus belongs to the Coronaviridae family and infects the hen oviduct. IB virus infection in the hen oviduct leads to the impairment of eggshell formation and disorder of egg production (Cavanagh and Naqi, 2003). Interleukin (IL)-1β and IL-6 are pro-inflammatory cytokines, which not only enhance inflammation but also increase antibody production by B cells. In addition, these inflammatory cytokines promote hematopoiesis and protein degradation (Akira et al., 1990; Ebisui et al., 1995; Narsale and Carson, 2014). Our previous study (Nii et al., 2014) showed upregulated IL-1β and IL-6 and downregulated CaBP-D28K expression in response to stimulation with attenuated IB virus antigen in the uterine mucosa of laying hens. Ebeid et al. (2012) reported the detection of CaBP-D28K protein in the glandular cells in uterus. Therefore, it is hypothesized that IL-1β and IL-6 produced in response to infection may affect the functions of the uterine glandular cells, which play essential roles in calcification. However, the direct effects of IL-1β and IL-6 on the expression of ion transporters involved in eggshell mineralization in the tubular gland cells of the uterine mucosa are unknown.
The goal of this study was to determine whether these two pro-inflammatory cytokines affect the expression of factors in the uterine mucosa involved in eggshell formation. In addition, we analyzed the expression of CaBP-D28K, PMCA1, PMCA2, CA2, and SLC26A9 as well as the localization and content of CaBP-D28K protein in the uterine mucosa stimulated with or without IL-1β or IL-6.
Materials and Methods
Experimental Birds
White Leghorn hens, approximately 180 days old, laying five or more eggs in a clutch, were used in this study. Hens were housed in individual cages under a daily 14-h light/10-h dark regimen and were provided with food and water ad libitum. Birds were euthanized under anesthesia with sodium pentobarbital (Somnopentyl; Kyoritsu Pharmaceutical Co., Tokyo, Japan) and uteri were collected at a predicted time of 3 h after the entrance of the egg into the uterus (8 h after oviposition). The uterus samples were used for in vitro analysis of the effects of pro-inflammatory cytokines on the expression of ion transporters involved in mineralization of the eggshell. This experiment was approved by Hiroshima University Animal Research Committee (No. C15-17).
Tissue Culture of Uterine Mucosa
Mucosal tissues of the uterus were collected and were washed with sterile phosphate-buffered saline (PBS) containing 10 U/mL penicillin and 10 µg/mL streptomycin (Cosmo Bio Co., Ltd., Tokyo, Japan). Pieces of mucosal fold (approximately 2 × 2 × 1 mm) were placed in sterile culture tubes (Greiner Bio-One Ltd., Tokyo, Japan; 10 pieces per tube) containing 2 mL of TCM-199 medium (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) with 10 U/mL penicillin and 10 µg/mL streptomycin. Recombinant chicken IL-1β or IL-6 (Bio-Rad Laboratories Inc., Hercules, CA, USA) was added to the culture medium at a concentration of 100 ng/mL and incubated for 0 (without cytokines), 1.5, and 3 h at 39°C in 5% CO2 and 95% air to analyze the time-course effects of cytokines on the expression of eggshell mineralization-related ion transporters. The concentration of recombinant IL-1β and IL-6 was determined based on a preliminary examination using 10, 100, and 1,000ng/mL concentration (data not shown). Five pieces of uterine mucosal fold were used for RNA extraction, while the remaining pieces were used for protein extraction and histological analysis. The experiments were repeated five times using different birds.
Real-time Polymerase Chain Reaction (PCR) Analysis
Total RNA was extracted from the cultured uterine mucosal tissues using Sepasol RNA I Super (Nacalai Tesque, Kyoto, Japan). The extracted RNA was dried and dissolved in TE buffer (10 mM Tris-HCl [pH 8.0] and 1 mM ethyl-enediaminetetraacetic acid [EDTA]). The RNA samples were treated with 1 U RQ1 RNase-Free DNase (Promega Co., Madison, WI, USA) on a PTC-100 thermal controller (MJ Research, Waltham, MA, USA), programmed at 37°C for 45 min and 65°C for 10 min. The concentration of RNA in each sample was measured using Gene Quant Pro (Amersham Pharmacia Biotech, Cambridge, UK), and RNA was reverse-transcribed using ReverTra Ace (Toyobo Co. Ltd., Osaka, Japan) according to the manufacturer's instructions. The reaction mixture (10 µL) comprised 1 µg total RNA, 1× RT buffer, 1 mM dNTP mixture, 20 U of RNase inhibitor, 0.5 µg of oligo(dT) 20, and 50 U ReverTra Ace. Reverse transcription was carried out at 42°C for 30 min, followed by heat inactivation for 5 min at 99°C, using the PTC-100 programmable thermal controller (MJ Research). Real-time PCR was performed using the Roche LightCycler Nano system (Roche Applied Science, Indianapolis, IN, USA) and a Fast Start Essential DNA Green Master kit (Roche Diagnostics GmbH, Mannheim, Germany). Reaction mixtures (20 µL), composed of 1 µL of cDNA, 10 µL Master Mix (Takara, Tokyo, Japan), 0.25 µM of each primer, and 8.5 µL of ultrapure water, were placed in LightCycler 8-Tube Strips (Roche Diagnostics GmbH). Table 1 shows the primers used for PCR in this study. The cycle parameters for the PCR program used in the analysis of RPS17 comprised denaturation at 95°C for 10 s and annealing at 60°C for 20 s. The PCR program for CA2 included denaturation at 95°C for 10 s, annealing at 60°C for 10 s, and extension at 72°C for 15 s. Annealing temperature was 58°C for CaBP-D28K and 56°C for PMCA1, PMCA2, and SLC26A9. Real-time PCR data were analyzed by the 2-ΔΔCT method (Livak and Schmittgen, 2001). The results were expressed as fold change obtained from the ratio of the expression levels of the samples treated with cytokines to the control samples (incubated without cytokines) for the same incubation times.
Table 1. Primers used for PCR analysis of the expression of cytokine receptors and eggshell formation-related genes.
| Target genes | Primer sequence (5′-3′) | Genbank accession no. |
|---|---|---|
| CaBP-D28K | F-ATGGATGGGAAGGAGCTACAA | M14230 |
| R-TGGCACCTAAAGAACAACAGGAAAT | ||
| PMCA1 | F-CTGCACTGAAGAAAGCAGATGTTG | XM_416133 |
| R-GCTGTCATATACGTTTCGTCCCC | ||
| PMCA2 | F-TTACTGTACTTGTGGTTGCTGTCCC | XM_001231767 |
| R-GGTTGTTAGCGTCCCTGTTTTG | ||
| CA2 | F-ATCGTCAACAACGGGCACTCCTTC | NM_205317 |
| R-TGCACCAACCTGTAGACTCCATCC | ||
| SLC26A9 | F-GCCTCTTCGATGAGGAGTTTGAG | XM_425821 |
| R-CTGACCCCACCAAGAACATCAG | ||
| IL-6 | F-AGAAATCCCTCCTCGCCAAT | NM_204628.1 |
| R-AAATAGCGAACGGCCCTCA | ||
| RPS17 | F-AAGCTGCAGGAGGAGGAGAGG | NM_204217 |
| R-GGTTGGACAGGCTGCCGAAGT |
F, forward; R, reverse
Immunohistochemistry for CaBP-D28K Protein in Cultured Uterine Tissues
The cultured uterine tissues were fixed with 10% (v/v) formalin in PBS, embedded in paraffin, sectioned (at 4-µm thickness), and stained with Hansen's hematoxylin and eosin for histological observation or used for the identification of CaBP-D28K protein by immunohistochemistry, as described previously (Nii et al., 2014). Briefly, the sections were incubated with 10% (v/v) normal horse serum, followed by overnight incubation with mouse monoclonal antibodies to CaBP-D28K (1:2000 dilution; Swant Swiss antibodies, Marly, Switzerland). The immunoreaction products were detected using a Histofine SAB-PO (M) kit (Nichirei Co., Tokyo, Japan). Immunoprecipitates were visualized by incubating the sections with a reaction mixture of 0.02% (w/v) 3,3′-diaminobenzidine tetrahydrochloride and 0.005% (v/v) hydrogen peroxide in 0.05 M Tris-HCl (pH 7.6). The sections were counterstained with Hansen's hematoxylin. For negative-control staining, primary antibodies were replaced with normal mouse IgG (1 µg/mL).
Western Blot Analysis
Cultured tissues were homogenized in 5× volume homogenization buffer (0.02 M Tris-HCl pH 7.4, 0.15 M NaCl, 5 mM EDTA, 1% [v/v] Triton X-100, 10% [v/v] glycerol, 0.1 % [w/v] sodium dodecyl sulfate [SDS], and 1 mM phenylmethanesulfonyl fluoride) using a Polytron homogenizer (PT 1200 E; Kinematica Ag., Luzern, Switzerland) on ice. The homogenates were centrifuged at 12,000 ×g for 20 min at 4°C. The supernatant was collected and stored at −80°C until analysis. Samples were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE; 15% running and 4% stacking gel) as described by Laemmli (1970). The protein concentration in each sample was analyzed using protein assay reagent (Bio-Rad Laboratories Inc.) by measuring the absorbance at 595 nm, as described by the manufacturer, using a spectrophotometer (U-1100; Hitachi Ltd., Tokyo, Japan). Each protein sample was mixed with sample buffer (30% [v/v] glycerol, 5% [v/v] mercaptoethanol, 4% [w/v] SDS, 150 mM Tris-HCl pH 7.0, and 0.06% [w/v] bromophenol blue) and boiled for 5 min. The samples were loaded onto the gel and run at 80 V in the upper gel and at 120 V in the lower gel. After SDS-PAGE, the samples were electrophoretically transferred onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories Inc.). The membrane was incubated with 1% (w/v) blocking reagent (Roche Diagnostics GmbH) in TBS-T (200 mM NaCl, 20 mM Tris pH 7.6, and 0.1% Tween-20) for 1.5 h, followed by overnight incubation with monoclonal antibody to CaBP-D28K (diluted to 1:1000 in TBS-T; Swant Swiss antibodies) or anti-chicken β-actin antibody (diluted to 1:2000 in TBS-T; Santa Cruz Biotech., Inc, Santa Cruz, CA, USA) at 4°C. After washing with TBS-T for 15 min (5 min, thrice), the membrane was incubated with peroxidase-labeled anti-mouse IgG antibody (diluted to 1:2500; GE Healthcare UK Ltd., Buckinghamshire, UK) at room temperature for 1 h. After washing with TBS-T for 15 min (5 min, thrice), the immunoprecipitates on the membrane were visualized by treatment with Amersham ELC Western Blotting Detection Reagents (GE Healthcare UK Ltd.), according to the manufacturer's instructions. Images of the bands on the membrane were captured using AE-9150 Ez-Capture II (Atto Co., Tokyo, Japan) and were analyzed using CS Analyzer ver. 3.0 (Atto Co.). Relative CaBP-D28K protein content in each sample was normalized by calculating the ratio of the band density of CaBP-D28K to that of β-actin.
Statistical Analysis
Values were expressed as the mean ± standard error of the mean (SEM). Differences in mRNA expression levels and CaBP-D28K protein contents between the control and cytokine treatment groups were examined by paired t-test (differences were considered significant at P<0.05). Differences among incubation times within the control or cytokine group were examined by paired t-test with Bonferroni correction (differences were considered significant at P<0.017).
Results
Figure 1 shows the effects of IL-1β on the expression of CaBP-D28K, PMCA1, PMCA2, CA2, and SLC26A9 genes in the cultured uterine mucosa. The expression of CaBP-D28K was significantly higher in tissues stimulated with 100 ng/mL IL-1β than in the control group (0 ng/mL) at 1.5 h of incubation (P<0.05) (Fig. 1a). In addition, the expression of PMCA1, PMCA2, CA2, and SLC26A9 was significantly higher in the tissues stimulated with IL-1β for 1.5 h than in the control (P<0.01) (Fig. 1b, c, d, and e). PMCA1 expression in the IL-1β-treated and control groups and CA2 expression in the control group were significantly increased at 3 h of incubation as compared with 0 h (P<0.05) (Fig. 1b and d). PMCA2 expression was lower at 1.5 h than at 0 and 3 h of incubation in the control group (Fig. 1c).
Fig. 1.
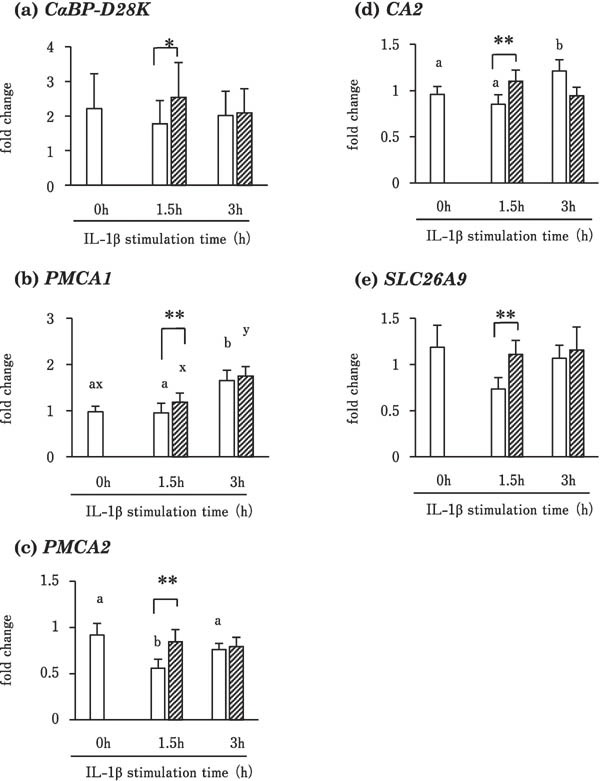
Effects of IL-1β on the expression of CaBP-D28k (a), PMCA1 (b), PMCA2 (c), CA2 (d), and SLC26A9 (e) in cultured uterine mucosal tissues. Tissues were incubated with (striped box) or without (blank box) recombinant chicken IL-1β (100 ng/mL) for 0, 1.5, or 3 h. Values are the mean ± SEM (n = 5) of fold changes in expression. * P<0.05 and ** P<0.01 vs. control tissues (incubated without IL for the same time). Different letters indicate significant differences among different incubation times within control (a and b) and IL-1β-stimulated (x and y) groups (P<0.017 after Bonferroni correction).
Figure 2 shows the effects of IL-6 on CaBP-D28K, PMCA1, PMCA2, CA2, and SLC26A9 gene expression in the uterine mucosa. The expression of CaBP-D28K, PMCA1, PMCA2, CA2, and SLC26A9 was significantly higher in tissues incubated with 100 ng/mL recombinant IL-6 for 1.5 h than in the control (0 ng/mL; Fig. 2). PMCA1 expression in the IL-6-treated and control groups and CA2 expression in the control group were significantly higher at 3 h than at 0 h. (P<0.05; Fig. 2d). PMCA2 expression in the control group was lower at 1.5 h than at 0 and 3 h of incubation (Fig. 2c).
Fig. 2.
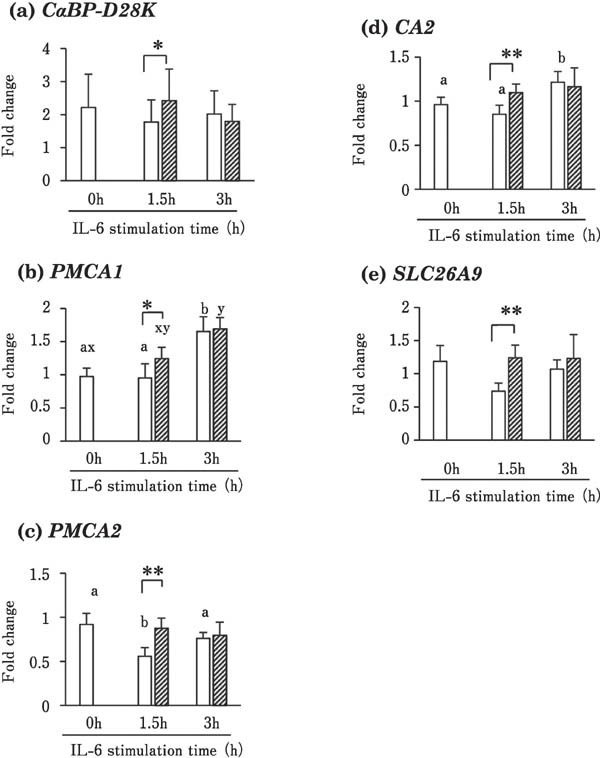
Effects of IL-6 on the expression of CaBP-D28k (a), PMCA1 (b), PMCA2 (c), CA2 (d), and SLC26A9 (e) in cultured uterine mucosal tissues. Tissues were incubated with (striped box) or without (blank box) 100 ng/mL recombinant chicken IL-6 for 1.5 or 3 h. Values are the mean ± SEM (n = 5) of fold change in expression. * P<0.05 vs. control tissues (incubated without IL for the same time) Different letters indicate significant differences among different incubation times within control (a and b) and IL-1β-stimulated (x and y) groups (P<0.017 after Bonferroni correction).
Immunoreactive CaBP-D28K protein was detected by immunohistochemistry and western blot analysis in uterine tissues incubated with or without IL-1β and IL-6 (Fig. 3 and 4). CaBP-D28K protein was localized in the tubular gland cells of the uterine tissues in all experimental groups incubated with or without IL-1β and IL-6 (only tissue incubated for 1.5 h with IL-6 (Fig. 3a) and without IL-6 (Fig. 3b) are shown). No signal was detected in the negative staining controls (Fig. 3c: incubated with control IgG). Specific immunoreactive bands corresponding to CaBP-D28k were observed in western blot analysis in all samples treated with or without IL-1β and IL-6 (Fig. 4a). The concentration of immunoreactive CaBP-D28K was significantly lower in tissues incubated with IL-1β than in the control group (without IL-1β) at 1.5-h incubation (P<0.05; Fig. 4b). In addition, the content of immunoreactive CaBP-D28K was significantly lower in tissues from the IL-6 treatment group than in those of the control (without IL-6) at 1.5-h incubation (P<0.01; Fig. 4b).
Fig. 3.
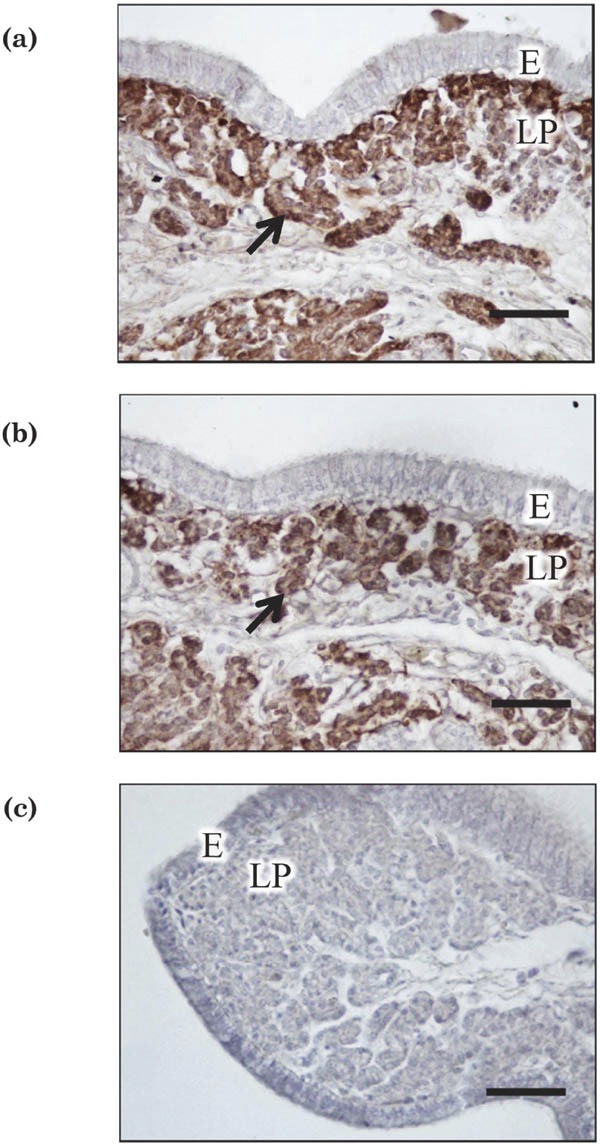
Immunostaining for CabP-D28k in uterine tissues incubated with (a) or without (b) IL-6 (100 ng/mL) for 1.5 h. Arrows indicate the presence of immunoreaction products in tubular gland cells. Negative-control staining (using normal mouse IgG instead of primary antibody) did not yield a positive reaction (c). E = mucosal epithelium, LP = lamina propria. Scale bars = 50 µm.
Fig. 4.
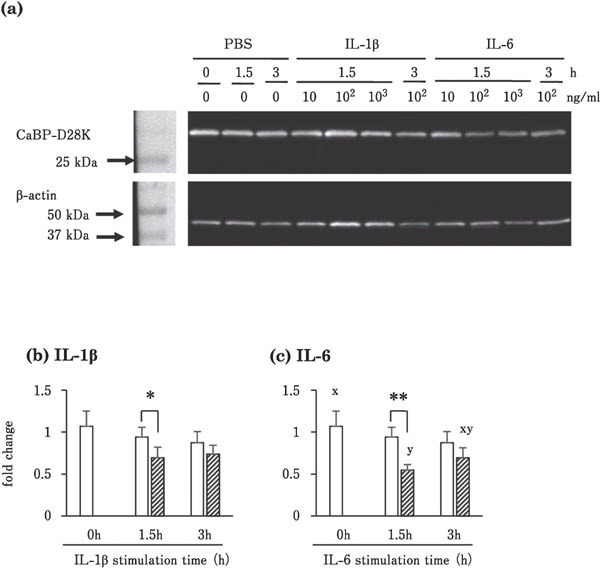
Effects of IL-1β and IL-6 on CaBP-D28k protein levels in the uterine mucosa. (a) Western blot of CaBP-D28k and β-actin in cultured uterine mucosal tissues incubated with 100 ng/mL IL-1β or IL-6 for 1.5 h. (b) Effects of IL-1β and IL-6 on CaBP-D28k protein content in cultured uterine mucosal tissues. Values are the mean ± SEM (n = 5) of the ratio of CaBP-D28k to β-actin densities. * P<0.05 and ** P<0.01 vs. control tissues (incubated without IL for the same time). Different letters indicate significant differences among different incubation times within the IL-1β-treated group (P<0.017 after Bonferroni correction).
Discussion
We examined the effects of stimulation with pro-inflammatory cytokines (IL-1β and IL-6) on the expression of ion transporters involved in eggshell mineralization in hen uterine tissues. We observed increases in the expression levels of eggshell-related genes (CaBP-D28K, PMCA1, PMCA2, CA2, and SLC26A9) in uterine tissues upon treatment with IL-1β or IL-6 as compared with untreated control tissues at 1.5 h of incubation. No difference was observed in the expression of these genes at 3 h. In contrast, the expression of CaBP-D28K protein, which was localized in the tubular gland cells, was significantly downregulated following stimulation with IL-1β and IL-6. CaBP-D28K is a calcium-binding protein that plays roles in intracellular Ca2+ transportation in the uterus. PMCA1 and PMCA2 are calcium pump molecules in shell gland cells. CA2 is an enzyme that produces HCO3−, whereas SLC26A9 transports HCO3− from the inside of the cells to the outside (Nys et al., 2004; Bar, 2009). A recent report showed that the expression of CaBP-D28K, PMCA1 (ATP2B1), PMCA2 (ATP2B2), CA2, and SLC26A9 in the uterus is significantly increased during calcification as compared to the non-calcification phase, suggestive of their role in eggshell formation (Jonchere et al., 2012). The current study confirmed the expression of these genes in the uterine mucosa, as shown in a previous report (Jonchere et al., 2012). In addition, we observed that the stimulation of uterine tissues and cells with IL-1β or IL-6 resulted in the transient upregulation of the expression of genes involved in the Ca2+ transporting pathway (CaBP-D28K, PMCA1, and PMCA2) and HCO3− transporting pathway (CA2 and SLC26A9) at 1.5 h of incubation. The physiological significance of the transient upregulation of these genes following IL-1β or IL-6 stimulation in the uterine mucosa is unknown. However, these cytokines may not downregulate the expression of these genes within 3 h of incubation.
Although IL-1β and IL-6 temporarily upregulated the gene expression of CaBP-D28K, we observed downregulation of CaBP-D28K protein mediated by these cytokines in the cultured uterine mucosal tissues at 1.5 h of incubation. Studies have suggested protein degradation mediated by IL-6 in muscle cells through the enhancement of proteolytic factors in mammals (Tsujinaka et al., 1996; Zoico and Roubenoff, 2002; Narsale and Carson, 2014). Thus, IL-6 was thought to enhance CaBP-D28K protein degradation or to disrupt CaBP-D28K protein synthesis from the mRNA in the tubular gland cells of the uterus. No significant differences were found in uterine CaBP-D28k content between the groups treated with or without IL-1β and IL-6 at 3 h of incubation. It is possible that the protein density decreased even in the control group with a longer time of culture. Although the reason why the changes in the mRNA expression did not correlate with the protein levels of CaBP-D28K is not known, we speculate that CaBP-D28K protein was decreased by cytokine stimulation through degradation of protein or inhibition of post-transcriptional processes. Post-transcriptional regulation, such as translation from mRNA to protein, is known to account for discordant mRNA and protein levels (Greenbaum et al., 2003). Further studies are necessary to determine this mechanism.
Attenuated IB virus stimulation in the chicken oviduct leads to an increase in IL-1β and IL-6 expression in the uterine mucosa and production of soft-shelled or shell-less eggs, suggesting that IB virus disrupts eggshell formation through increased pro-inflammatory cytokines (Nii et al., 2014). A recent report showed that H9N2 avian influenza virus infection disturbs the formation of eggshell and shell membrane through decreased expression of CaBP-D28K and reduced Ca2+ contents in the oviductal uterus of laying hens (Qi et al., 2016). Furthermore, H9N2 virus infection enhances the gene expression of pro-inflammatory cytokines in the uterus (Wang et al., 2015). These reports indicate that microbial infections in the uterus induce the expression of proinflammatory cytokines and are responsible for the malformation of eggshell. This process may be accompanied with a decrease in CaBP-D28k content in the uterus by proinflammatory cytokines, as observed in the current study.
In conclusion, IL-1β and IL-6 may not suppress the gene expression of molecules related to Ca2+ and HCO3− transportation in eggshell formation, whereas CaBP-D28K protein levels in the uterine glandular cells may be reduced by proinflammatory cytokines at the early exposure phase. Thus, it is assumed that IL-1β and IL-6 induced by infections may disrupt the transportation of Ca2+ for eggshell formation via decreased CaBP-D28K protein content in the uterus.
Acknowledgments
This work was supported by a Grant-in-Aid for JSPS Fellows (No. 13J02100) to T.N. and Grant-in-Aid for Scientific Research from JSPS (No. 25292161) to Y.Y.
References
- Akira S, Hirano T, Taga T and Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). The Journal of the Federation of American Societies for Experimental Biology, 4: 2860-2867. 1990. [PubMed] [Google Scholar]
- Bar A. Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 152: 447-469. 2009. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Van Cromphaut S and Carmeliet G. Intestinal calcium absorption: molecular vitamin D mediated mechanisms. Journal of Cellular Biochemistry, 88: 332-339. 2003. [DOI] [PubMed] [Google Scholar]
- Brionne A, Nys Y, Hennequet-Antier C and Gautron J. Hen uterine gene expression profiling during eggshell formation reveals putative proteins involved in the supply of minerals or in the shell mineralization process. BMC Genomics, 15: 220. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D and Naqi S. Infectious bronchitis. In: Diseases of Poultry. (Saif Y, Barnes H, Glisson J, Fadly A, McDougald L, and Swayne D, eds.). Vol. 11. pp. 101-119. Iowa State University Press. Ames. 2003. [Google Scholar]
- Common R. The carbonic anhydrase activity of the hen's oviduct. The Journal of Agricultural Science, 31: 412-414. 1941. [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D and Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology, 23: 104-114. 2008. [DOI] [PubMed] [Google Scholar]
- Ebeid T, Suzuki T and Sugiyama T. High ambient temperature influences eggshell quality and CaBP-D28K-D28k localization of eggshell gland and all intestinal segments of laying hens. Poultry Science, 91: 2282-2287. 2012. [DOI] [PubMed] [Google Scholar]
- Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K and Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clinical Science, 89: 431-439. 1995. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K and Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biology, 4: 117. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonchere V, Brionne A, Gautron J and Nys Y. Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiology, 12: 10. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25: 402-408. 2001. [DOI] [PubMed] [Google Scholar]
- Lörcher K and Hodges RD. Some possible mechanisms of formation of the carbonate fraction of egg shell calcium carbonate. Comparative Biochemistry and Physiology, 28: 119-128. 1969. [DOI] [PubMed] [Google Scholar]
- Narsale AA and Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Current Opinion in Supportive and Palliative Care, 8: 321-327. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T, Isobe N and Yoshimura, Y. Effects of avian infectious bronchi-tis virus antigen on eggshell formation and immunoreaction in henoviduct. Theriogenology, 81: 1129-1138. 2014. [DOI] [PubMed] [Google Scholar]
- Nys Y, Gautron J, Garcia-Ruiz JM and Hincke MT. Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. Comptes Rendus Palevol, 3: 549-562. 2004. [Google Scholar]
- Ohira H, Yoshimura Y and Tamura T. Increase in calcium binding protein-D28K contents in the shell gland by an injection of 1, 25-dihydroxyvitamin D3 into the shell gland lumen in laying hens. Japanese Poultry Science, 35: 99-107. 1998. [Google Scholar]
- Qi X, Tan D, Wu C, Tang C, Li T, Han X, Wang J, Liu C, Li R and Wang J. Deterioration of eggshell quality in laying hens experimentally infected with H9N2 avian influenza virus. Veterinary Research, 47: 35. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler EE and Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiological Reviews, 81: 21-50. 2001. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y and Shiozaki H. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. Journal of Clinical Investigation, 97: 244-249. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Tang C, Wang QZ, Wang RQ, Chen ZL, Han XY, Wang J and Xu XG. Apoptosis induction and release of inflammatory cytokines in the oviduct of egg-laying hens experimentally infection with H9N2 avian influenza virus. Veterinary Microbiology, 177: 302-314. 2015. [DOI] [PubMed] [Google Scholar]
- Xu J, Henriksnäs J, Barone S, Witte D, Shull GE, Forte JG, Holm L and Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl−/HCO3− exchange, and is inhibited by NH4+. American Journal of Physiology-Cell Physiology, 289: C493-C505. 2005. [DOI] [PubMed] [Google Scholar]
- Zoico E and Roubenoff R. The role of cytokines in regulating protein metabolism and muscle function. Nutrition Reviews, 60: 39-51. 2002. [DOI] [PubMed] [Google Scholar]


