Abstract
In Japan, the majority of chicken meat is obtained from fast-growing broiler chickens. Because most Japanese chicken breeds have a low meat yield and egg production, many of these breeds are endangered. Recently, the palatability of meat and eggs of native chickens has been reevaluated in the Japanese market. Jidori, which means chicken from the local, is an indigenous local chicken that is more delicious, firmer in texture, and more expensive than the broiler chickens. Most Japanese consumers recognize that the meat of Jidori chicken is richer in flavor than that of the broiler chicken. However, the reason for this rich flavor of the meat of Jidori chicken has not been elucidated. Recently, we found that arachidonic acid (AA) (C20:4n-6), a polyunsaturated fatty acid, is associated with the rich flavor of the meat and eggs of Jidori chicken. The present paper summarizes the discovery of the involvement of AA in the flavor characteristic of the meat and eggs of chicken, and also the genetic regulation of the AA content in the meat and eggs of Jidori chicken.
Keywords: arachidonic acid, chicken, egg, flavor, genetics, meat
Introduction
Globally, the majority of chicken meat is obtained from a limited number of fast-growing broiler strains supplied by commercial breeding companies that employ intensive fattening strategies to ensure high meat yield. However, some consumers are willing to pay a higher price for a better quality of chicken meat, especially for the meat of Jidori chicken. Most Jidori chickens were initially bred by crossing the native Japanese breeds with selected lines having rapid growth rate. For example, Hinai-jidori chicken, a cross between Hinai-dori (a chicken breed native to Akita prefecture, Japan) sires and Rhode Island Red dams, is a popular variety of Jidori chicken (Rikimaru and Takahashi, 2007). As Jidori chickens require a relatively long growing period with a considerably high cost of production, their selling price can be 2–5 times more than that of the broilers.
Most Japanese consumers recognize that the meat of Jidori chicken has a richer flavor than that of the broiler chickens. However, the underlying reason for this rich flavor has not been elucidated. Meat texture is an important factor, and most Japanese consumers believe that Jidori meat is characteristically tough (Ito et al., 1996; Matsuishi et al., 2005). Other key factors include the presence of free amino acids (FAA), including glutamic acid (Glu) and purine compounds, such as inosine 5′-monophosphate (IMP). Because Glu and IMP are well known active components of umami taste (the taste of l-glutamate), their salts have been widely used as flavor enhancers of food (Yamaguchi and Ninomiya, 2000). Studies conducted in the 1980s and 1990s have compared the contents of FAA, including Glu and IMP, in the meat of Jidori and broiler chickens. Karasawa et al. (1989) reported that the contents of Glu and total FAA in the leg muscle (gastrocnemius muscle) of three types of Jidori chicken was significantly higher than those in the leg muscle of the broilers. However, subsequent studies have reported no significant difference in the content of FAA between Jidori and broiler chickens (Fujimura et al., 1994, 1996; Ito et al., 1996). Fujimura et al. (1996) reported that the content of IMP in the meat of Hinai-jidori chicken is significantly higher than that in the meat of the broiler chicken. Other studies have not shown a significant difference in the IMP content in the meat of Jidori and broiler chickens (Fukunaga et al., 1989; Karasawa et al., 1989; Ito et al., 1996). Most Japanese consumers recognize that the meat of Jidori chicken is more delicious than that of the broilers; however, it has not been verified if the difference in the contents of FAA, Glu, and IMP is actually correlated with the flavor of Jidori chicken. Based on earlier studies, we conclude that to determine the flavor of chicken meat, it is necessary to characterize the active substances related to the flavor of broiler and Jidori chicken meat.
Arachidonic Acid Effectively Improves the Flavor of Chicken Meat
To identify the substances influencing the flavor of chicken meat, we quantitatively analyzed the general biochemical components, such as the FAAs, including Glu, IMP, and fatty acids, in thigh meat of Hinai-jidori and broiler chickens (Rikimaru and Takahashi, 2010). Since nearly 100% of Hinai-jidori chickens, sold commercially, are female, we compared the females belonging to the two strains. Female chicks, which hatched on the same day, were reared under identical environmental conditions for the same duration. The results showed that relatively high contents of AA and docosahexaenoic acid (DHA, C22: 6) is a characteristic feature of Hinai-jidori chicken (Table 1). As described elsewhere (Rikimaru and Takahashi, 2010), the total FAA content, in 8-wk-old broiler, was significantly higher than those in 22-wk-old broiler and 22-wk-old Hinai-jidori chicken. The content of Glu was the highest in the 8-wk-old broiler and lowest in the 22-wk-old broiler. The content of IMP in the 22-wk-old Hinai-jidori chicken was significantly higher than that in the 8-wk-old broiler, whereas there was no significant difference in the IMP content between the 22-wk-old broiler and 22-wk-old Hinai-jidori chicken. These data suggest that 1) FAA content decreases with age, 2) IMP content increases with age, and 3) difference in the FAA and IMP contents, observed in the broiler and Hinai-jidori chickens, reflects their age at the time of slaughter.
Table 1. Arachidonic acid and docosahexaenoic acid contents (percent of total analyzed fatty acids) in the thigh meat of the broiler and Hinai-jidori chickens (mean±SD).
| Broiler, female | Broiler, female | Hinai-jidori, female | |
|---|---|---|---|
| Age (wk) | 8 | 22 | 22 |
| N | 5 | 5 | 5 |
| AA | 1.42±0.27b | 1.26±0.33b | 1.92±0.04a |
| DHA | 0.20±0.07b | 0.24±0.11ab | 0.38±0.04a |
Means within a row without a common superscript are significantly different (P<0.05).
AA, arachidonic acid; DHA, docosahexaenoic acid.
To elucidate the relationship between the content of AA and flavor of Hinai-jidori chicken, we examined the effects of a diet supplemented with palm oil (PO), corn oil (CO), or AA-enriched oil (AAO) (SUNTGA40S; Nippon Suisan Co., Tokyo, Japan) on the fatty acid content and sensory perception of thigh meat (Kiyohara et al., 2011). The oils were individually mixed with silicate at a ratio of 7:3, and 5% of fresh matter was added to finisher diet. Hinai-jidori chickens were fed these diets for 2 wk before slaughter. The AA content in the thigh meat of the AAO group was over 2-times higher than that of the PO and CO groups. The contents of other fatty acids were not significantly different among the groups. Sensory evaluation using chicken soup that included fat and steamed minced meat revealed that the total intensity of taste, umami, kokumi (continuity, mouthfullness, and thickness [Yamamoto et al., 2009]), and aftertaste of the AAO group were significantly improved when compared with those of the PO and CO groups (Table 2). These data suggest that the flavor of chicken meat can be improved by dietary supplementation of arachidonic acid.
Table 2. Sensory evaluation of steamed minced meat from Hinai-jidori chicken fed experimental diets.
| Chicken soup | Steamed minced meat | |||
|---|---|---|---|---|
| Characteristic | PO-AAO1 | CO-AAO2 | PO-AAO1 | CO-AAO2 |
| Total taste intensity | 0.86** | 0.75** | 0.72** | 0.56** |
| Sweetness | 0.27* | 0.27* | −0.03 | 0.34* |
| Sourness | 0.55** | 0.11 | 0.34* | 0.03 |
| Umami | 0.68** | 0.64** | 0.59** | 0.50* |
| Kokumi | 0.73** | 0.77** | 0.66** | 0.75** |
| Aftertaste | 0.86** | 0.61** | 0.69** | 0.50* |
AAO, AA-enriched oil; CO, corn oil; PO, palm oil.
Average AAO score of each subject when PO score was 0.
Average AAO score of each subject when CO score was 0.
statistically significant at P = 0.05;
statistically significant at P = 0.01.
To elucidate the relationship between the AA content and flavor of broiler meat, we evaluated the effects of AAO supplement on the fatty acid content and sensory perception of thigh meat (Takahashi et al., 2012). We formulated four types of oil: CO; a 1:1 mixture of AAO and PO (1/2 AAO); a 1:3 mixture of AAO and PO (1/4 AAO); and a 1:7 mixture of AAO and PO (1/8 AAO). Each type of oil was mixed with silicate at a ratio of 7:3, and 5% of fresh matter was added. Broiler chickens were fed these diets for 1 wk before slaughter. The AA content in the thigh meat of the groups 1/2 AAO and 1/4 AAO was significantly higher than that in the thigh meat of the CO group. Further, the AA content in the thigh meat (y, mg/g) increased linearly with the increasing content of dietary AAO (x, g/100 g of diet) according to the equation y = 0.5674 + 0.4596x (R2 = 0.8454). The content of other fatty acids was not significantly different among the four diet groups. The sensory evaluation revealed that the flavor intensity, umami, kokumi, and aftertaste, of the groups 1/2 AAO and 1/4 AAO were significantly improved when compared with those of the CO group. Furthermore, there were significant positive correlations between the AA content of the thigh meat and flavor intensity, total taste intensity (umami), and aftertaste. These data suggest that the flavor of the broiler and Hinai-jidori chickens can be improved by increased supplementation of dietary AA.
In our previous studies (Kiyohara et al., 2011; Takahashi et al., 2012), we measured the Glu and IMP contents of the chicken samples and calculated the umami intensity of each experimental group. The value was expressed as the content of monosodium glutamate (MSG; mg/100 mL) with respect to the synergistic effect between Glu and IMP according to Yamaguchi (1967). Earlier studies reported that the difference in the intensity of umami among the experimental groups was less than 1%. This suggests that the difference in the intensity of umami between the experimental groups cannot be attributed to the contents of Glu and IMP. Because Yamaguchi (1967) reported that the differential threshold of umami between samples was 21%. Therefore, we conclude that the difference in chicken flavor, observed in our previous studies, was caused by AA and not by water-soluble umami substances, such as Glu and IMP.
Genetic Regulation of Arachidonic Acid Content in Chicken Meat
In the thigh meat of Hinai-jidori chicken, we observed a strong correlation between the contents of AA and DHA (Fig. 1) suggesting that this was due to common molecular pathways. Arachidonic acid originates from dietary sources by the elongation-desaturation process of its precursor, linoleic acid (LA, C18:2n-6). The key enzymes delta (δ)-5 desaturase (D5D) and δ-6 desaturase (D6D) mediate this pathway (Fig. 2) (Malerba et al., 2008). The enzyme D6D catalyzes the conversion of LA to γ-linolenic acid (C18:3n-6), which is then elongated to dihomo-γ-linolenic acid (C20: 3n-6) by elongase 5(EL5 ). Further, C20:3n-6 is desaturated to AA by D5D. The enzymes EL5, D5D, and D6D are also involved in the n-3 fatty acid pathway (Fig. 2), which favors the conversion of α-linolenic acid (ALA) into DHA. The enzymes D5D and D6D are encoded by the genes fatty acid desaturase 1 (FADSI) and 2 (FADS2), respectively. Furthermore, the FADS1 and FADS2 genes are clustered in a back-to-back configuration on chromosome 5 of chicken (Ensembl Genomes, 2017; UCSC Genome Browser Gateway, 2017). Therefore, we speculated that the genes FADS1 and FADS2 control the contents of AA and DHA in chicken meat.
Fig. 1. Correlation between arachidonic acid (AA) and docosahexaenoic acid (DHA) contents (percent of total analyzed fatty acids) in the thigh meat of Hinai-jidori chicken.
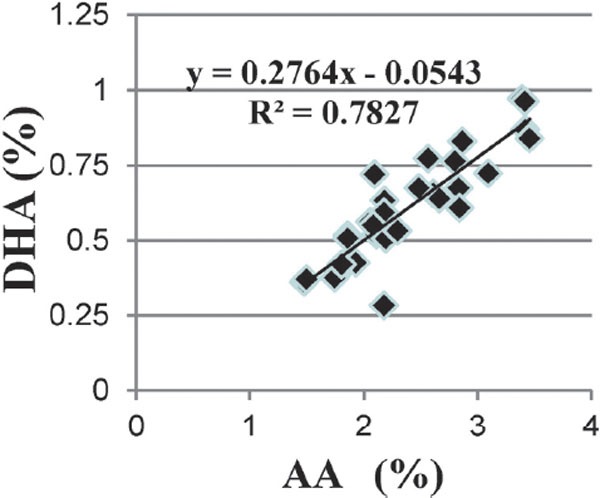
Fig. 2.
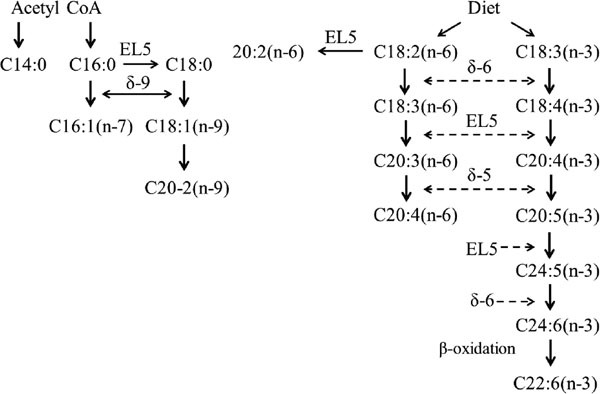
Metabolic pathway of fatty acid synthesis. δ-5, delta-5 desaturase; δ-6, delta-6 desaturase; δ-9, delta-9 desaturase; EL5: elongase 5.
We genotyped the polymorphism of the genes FADS1 and FADS2 and investigated their association with the fatty acid profile of the meat of Hinai-jidori (Rikimaru et al., 2016). The 5′-flanking regions, all exons, and 3′-untranslated regions of the genes FADS1 and FADS2 in three chicken breeds (Hinai-dori, Rhode Island Red, and White Plymouth Rock) were amplified by PCR. Subsequently, their nucleotides were sequenced and single nucleotide polymorphisms (SNPs) were identified. Of the 71 and 46 SNPs found in FADS1 and FADS2 genes, respectively, two SNPs (rs 733003230 (adenine (A) > guanine (G)) and LC060926 (g.25 A > G)) were chosen from each gene. Hinai-jidori female chickens, which hatched on the same day and reared under identical environmental conditions for the same duration, were used for all analyses. In each SNP of the genes FADS1 and FADS2, the compositions of AA and DHA were significantly high in allele G than allele A (Table 3). We also examined the association of the FADS1 and FADS2 haplotypes with the contents of fatty acids. The contents of AA and DHA of the G-G-haplotype were significantly higher than that of the A-A-haplotype (Table 4). Thus, we conclude that the SNPs in the FADS1 and FADS2 gene cluster increase the contents of AA and DHA. Furthermore, this result might help develop strategies for improving the flavor of Hinai-jidori chicken.
Table 3. Effects of single nucleotide polymorphism (SNP) of the genes fatty acid desaturase 1 (FADS1) and 2 (FADS2) on the fatty acid profile of Hinai-jidori thigh meat (mean±SE).
| Gene | FADS1 | FADS2 | ||
|---|---|---|---|---|
| Locus | rs733003230 (A>G) | LC060926 (g.25A>G) | ||
| SNP type | A | G | A | G |
| SNP Frequency | 0.453 | 0.547 | 0.813 | 0.188 |
| Fatty acid % of total analyzed fatty acids | ||||
| AA | 1.01±0.15 | 1.33±0.07* | 1.10±0.07 | 1.55±0.19* |
| DHA | 0.25±0.04 | 0.35±0.02* | 0.28±0.02 | 0.40±0.06* |
statistically significant at P = 0.05;
** statistically significant at P = 0.01.
AA, arachidonic acid; DHA, docosahexaenoic acid; A, adenine; G, guanine.
Table 4. Effects of haplotype of the genes fatty acid desaturase 1 (FADS1) and 2 (FADS2) on the fatty acid profile of Hinai-jidori thigh meat (mean±SE).
| Combined haplotypes of FADS1 and FADS2 | A-A | G-A | G-G |
| Frequencies of plausible haplotypes under linkage equilibrium | 0.453 | 0.359 | 0.188 |
| Fatty acid % of total analyzed fatty acids | |||
| AA | 0.99±0.12b | 1.24±0.15ab | 1.56±0.24a |
| DHA | 0.25±0.04b | 0.32±0.04ab | 0.40±0.07a |
Means within a row without a common superscript letter are significantly different (P<0.05).
AA, arachidonic acid; DHA, docosahexaenoic acid; A, adenine; G, guanine.
In the present study, we will discuss the effects of sex, and the genes FADS1 and FADS2 on the fatty acid profile of chicken meat. Sirri et al. (2011) compared the fatty acid profile of breast and thigh meat of fast- (Cobb 700), medium-(Naked Neck Kabir), and slow- (Brown Classic Lohman) growing chicken strains slaughtered at the age of 81 d. The contents of stearic acid (SA, C18:0), AA, and DHA in the slow-growing strain were significantly higher than those of the fast- and medium-growing strains. However, the contents of myristic (C14:0), palmitoleic (C16:1), and oleic (C18:1) acids in the slow-growing strain were significantly lower than those of the fast- and medium-growing strains. Jayasena et al. (2014) reported that 100-d-old Korean native chickens exhibited significantly higher contents of LA, AA, and DHA than those of the 32-d-old broilers. Boschetti et al. (2016) reported that the medium-growing (Kabir Red) and in particular slow-growing (Hyline W36) strains showed higher expression of the genes FADS1 and FADS2 in the hepatic tissue than that in the fast-growing line (Cobb 500) at the age of 81 days. However, in the present study, we did not assess the association between the polymorphism of the genes FADS1 and FADS2 and fatty acid profile of the meat. These reports suggest that there is a significant difference in the fatty acid profile of the meat between strains. However, these studies did not verify if the differences in the samples were caused by the difference in the sex of the examined chickens. Rikimaru and Takahashi (2010) used Hinai-jidori females, while Sirri et al. (2011), Jayasena et al. (2014), and Boschetti et al. (2015) used males. Furthermore, Sirri et al. (2009) reported that the contents of AA, DHA, and docosapentaenoic acid (C22:5n-3) in the breast and thigh meat of cocks were significantly higher than those in 180-d-old capons. Further, the difference between the cocks and capons was attributed to the testosterone affecting the activity of D6D. Clejan et al. (1982) demonstrated that the decreased contents of AA and DHA in castrated rats was due to the lack of testosterone, and the administration of testosterone elevated the content of AA to normal level. Testosterone exists in plasma before the onset of puberty in cockerels (Sharp et al., 1977; Tanabe et al., 1981; Rikimaru et al., 2009). The differences in the fatty acid profile observed among 81-d-old Cobb 700, Naked Neck Kabir, and Brown Classic Lohman (Sirri et al., 2011), and between 100-d-old Korean native chicken and 32-d-old broiler (Jayasena et al., 2014) might reflect the concentration of plasma testosterone of each strain at the age of slaughter. In our previous study (Rikimaru et al., 2016) we assessed the effects of the genes FADS1 and FADS2 on the fatty acid profile of chicken meat. The effects of testosterone and environmental factors on fatty acid profile were negligible, because we used female chickens that hatched on the same day and were reared under identical environmental conditions for the same duration.
Genetic Regulation of Fatty Acid Profile and Flavor of Eggs
Most Japanese consumers recognize that the eggs of Jidori chickens have a rich kokumi flavor. We speculated that: 1) AA might be the substance related to the flavor of the eggs of Jidori chicken, and 2) FADS1 and FADS2 are the key genes that control the AA content in the eggs. We verified if the polymorphism of the genes FADS1 and FADS2 affected the fatty acid profile of the eggs of Suruga-shamo—a Japanese chicken breed (Matsui and Takahashi, 2017). An SNP in the FADS1 gene was significantly associated with the AA content. The n-6/n-3 polyunsaturated fatty acid ratio in yolk showed that the SNP allele, which was associated with high AA content, had a low n-6/n-3 ratio (Table 5). Further, we found that adding trace amounts of AA corresponding to the difference in the SNP genotype enhanced the flavor intensity and continuity of the eggs (Table 6). We concluded that the SNP of the FADS1 gene can be used to develop strategies to improve egg flavor and decrease the n-6/n-3 ratio in the yolk.
Table 5. Effects of single nucleotide polymorphism (SNP) of the enzyme fatty acid desaturase 1 (FADS1) on the fatty acid profile of yolk in Suruga-shamo chicken eggs (mean±SE).
| SNP type | G | A |
| SNP Frequency | 0.802 | 0.198 |
| Fatty acid % of total analyzed fatty acids | ||
| AA | 0.977±0.010 | 0.913±0.031* |
| n-6/n-3 PUFA | 3.698±0.069 | 4.093±0.193* |
statistically significant at P=0.05.
AA, arachidonic acid; PUFA, polyunsaturated fatty acids; G, guanine; A, adenine.
Table 6. Sensory evaluation of yolk seasoned with soy sauce.
Average score of yolk with arachidonic acid-enriched oil (AAO) when the yolk without the AAO score was 0.
statistically significant at P=0.05;
statistically significant at P=0.01.
Because consumers, worldwide, are growing more health conscious, the reduction in the n-6/n-3 ratio is a special concern for the poultry and egg industry. Feed additives, such as flaxseed and fish oil, are used for this purpose (Fraeye et al., 2012). However, the increased amount of n-3 polyunsaturated fatty acids (PUFA) in the egg yolk is proportional to the decreased amount of n-6 PUFAs, in particular AA (Bean and Leeson, 2003; Hayat et al., 2009). The organoleptic quality of n-3 PUFA-enriched eggs is similar to that of the regular eggs; however, occasionally, off-flavored eggs can be detected (Elswyk et al., 1992; Caston et al., 1994). In contrast, we found that in the FADS1 gene, the allele exhibiting a high AA also has a low n-6/n-3 ratio. A synonymous observation in the FADS2 gene with respect to the n-6/n-3 ratio has been reported in Japanese quail by Khang et al. (2007). These data suggest that the n-6/n-3 ratio in the eggs is genetically changeable by manipulating the gene polymorphism of the enzymes mediating the LA—AA pathway. This approach might render the use of feed additives unnecessary.
A Proposed Mechanism to Explain the Effect of Arachidonic Acid on the Enhancement of Food Flavor
The addition of AAO to cooked foods improves its flavor; this is widely recognized in Japan. For instance, when food, such as vegetable soup, croquettes, and fried rice, are cooked in vegetable oil containing AAO, their palatability index increases (Kiyohara et al., 2009). Arachidonic acid-enriched cooking and frying oils, Bimi-Tokutoku, are commercially available from J-OIL MILLS, Inc. (Yokohama, Japan) in the Japanese market. However, the mechanism through which AA enhances food flavor has not been clearly elucidated.
Fig. 3.
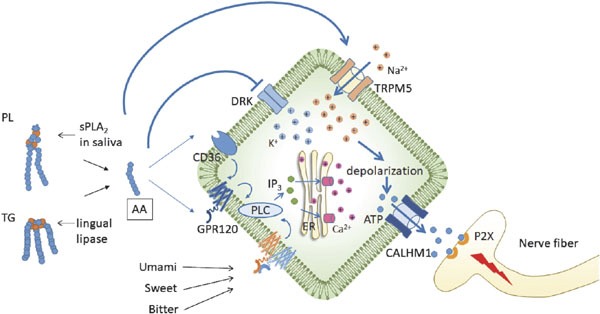
Proposed signaling pathway in type II receptor cells. AA inhibits the DRK channel and activates the TRPM5 channel. Intracellular K+ ions and influx of Na+ ions, through the TRPM5 channel, cause depolarization of the cells and firing of action potentials. AA, arachidonic acid; Ca2+, calcium ion; CALHM1, calcium homeostasis modulator 1; CD36, cluster of differentiation 36; DRK, delayed rectifying K+ channel; ER, endoplasmic reticulum; GPR120, G-protein coupled receptor 120; IP3, type 3 isoform of inositol 1,4,5-trisphosphate; K+, potassium ion; Na+, sodium ion; P2X, purinergic receptors; PL, phospholipids; PLC, phospholipase C; sPLA2, secreted phospholipase A2; TG, triglycerides.
Dietary fats, including AAO, are predominantly in the form of triglycerides, which are not effective at stimulating taste. Kawai and Fushiki (2003) propose that lingual lipase produces FFA from triglycerides rapidly enough to enable detection by a fat sensor on the surface of the tongue. Recently, cluster of differentiation 36 (CD36) and G-protein coupled receptors (GPR), such as GPR120 and GPR40, have been identified as putative FFA taste receptors (Cartoni et al., 2010; Laugerette et al., 2005). Because CD36 is expressed in some type II (sweet, bitter, and umami) receptor cells in mouse taste buds (Laugerette et al., 2005) and GPR120 and GPR40 are mainly expressed in type II and type I (salty) receptor cells, respectively (Cartoni et al., 2010), FFA might affect the taste perception of sweet, bitter, umami, and salty flavors based on the distribution of taste receptors. However, the presence of GPR40 has not been confirmed in the gustatory papillae of humans (Galindo et al., 2012). Gilbertson et al. (1997) reported that PUFAs, especially LA, AA, DHA, and eicosapentaenoic acid, might inhibit the delayed rectifying K+ (DRK) channels. Because K+ is a major intracellular monovalent cation, the inhibition of DRK channels might elicit a rapid cell depolarization response caused by the transient accumulation of positive charges in taste bud cells. Oike et al. (2006) reported that AA activates transient receptor potential cation channel subfamily M member 5 (TRPM5) channel, which is a component of the sweet, bitter, and umami taste pathways in the type II receptor cells. Additionally, TRPM5-null mice showed no licking response to a sweet tastant, a diminished preference ratio for sweet and umami tastants, and a reduced response to bitter taste (Damak et al., 2006). These data suggest that by modulating the DRK and TRPM5 channels, AA might serve as a flavor enhancer for the type II receptor cells. A model of fatty acid signal transduction in the type II receptor cells has been proposed in our previous report (Figure 2; Matsui and Takahashi, 2017).
Apart from cholesterol, triglycerides and phospholipids constitute approximately 2/3 and 1/3 of the total lipid content in egg yolk, respectively (Awad et al., 1997). In the yolk, AA is present almost entirely in the second carbon group of glycerol of phospholipids (Gładkowski et al., 2011). Therefore, phospholipase A2 (sPLA2), secreted in saliva, is required to release AA from the second carbon group of glycerol in phospholipids. The activity of sPLA2 has been documented in acinar cells and a fraction of apical plasma membrane acquired from the salivary gland of rat (Mizuno-Kamiya et al., 2001; Takuma and Ichida, 1997). Komiyama et al. (2009) reported that type V sPLA2 (sPLA2-V) is expressed in the human parotid and submandibular glands under disease-free conditions. We hypothesize that the yolk phospholipids are digested by the salivary sPLA2-V, releasing free AA, which affects the taste perception of the yolk. We conclude that the free AA, generated in the oral cavity, is one of the keys that enhance the flavor of the egg.
Other Chemical Substances Affect the Umami Characteristic of Chicken Meat
As mentioned earlier, Glu and IMP are the typical active taste components of umami. Based on recent reports, comparing the quality of meat between broiler and Jidori chickens, it appears that the broiler has a higher Glu content than that of Jidori chicken, when the comparison was made at the marketable age of each strain (Goda, 2003; Matsuishi et al., 2005; Rikimaru and Takahashi, 2010; Yamada and Yamada, 2013). Because the content of Glu decreases with age in broiler chicken (Chow and Jacobson, 1968; Rikimaru and Takahashi, 2010; Chae et al., 2012), the difference in the content of Glu between broiler and Jidori chicken might be a reflection of their marketing age. Further, Wattanachant et al. (2004) reported that Thai indigenous chicken had higher Glu content than the broilers. Jayasena et al. (2014) reported that there was no difference in Glu content between Korean native and broiler chickens. These data suggest that the Glu content varies among different genotypes of chicken. Furthermore, the FAA content in the chicken meat increased with postmortem aging, and is responsible for improving the taste of the meat (Nishimura et al., 1988). Watanabe et al. (2017) reported that a reduction in dietary lysine increases the content of free Glu in the broiler meat and improves its taste. An elevation in dietary lysine also increases the content of free Glu in the broiler meat, thus improving its taste (Watanabe et al., 2015).
Jidori chicken exhibited a higher IMP content than that of the broiler when their meat was compared at the marketing age of each strain (Goda, 2003; Rikimaru and Takahashi, 2010). Similar results were obtained when the meat of the broiler chicken was compared with three Chinese native breeds (Tang et al., 2009). Because there was no significant difference in the IMP content between 22-wk-old broiler and 22-wk-old Hinai-jidori chicken (Rikimaru and Takahashi, 2010), the difference in the IMP content between broiler and Jidori chicken might reflect their marketing age. Furthermore, the IMP content can be increased by dietary supplementation of IMP (Zhang et al., 2008), purine nucleotides, betaine, soybean isoflavones, and combinations thereof (Wang et al., 2014). Terasaki et al. (1965) reported that the IMP content of broiler breast meat reached maximal level at 8 h after slaughter, and then decreased gradually when the meat was stored at 4°C. Further, the flavor of the chicken was more pleasant at 8 h after slaughter than that of the chicken immediately after slaughter. Postmortem aging might increase the content of IMP more effectively than that by the slaughter age and feed additives.
In conclusion, we have shown that the content of AA in chicken meat can be manipulated by dietary supplementation of AA and by genetic selection exploiting the polymorphism of the genes of FADS1 and FADS2 as selection markers. These approaches improve the flavor of chicken meat. Furthermore, we have shown the possibility of using the FADS1 gene polymorphism as a selection marker to improve the fatty acid profile, in particular the content of AA and n- 6/n-3 ratio, thereby improving the flavor of chicken eggs. We will be conducting studies on improving the flavor of Jidori meat and eggs, using molecular breeding and marker-assisted selection techniques in the near future.
Acknowledgments
This work was conducted in collaboration with the Akita Prefectural Livestock Experiment Station (Daisen, Japan); Oils and Fats Fundamental Technology Laboratory, J-OIL MILLS, Inc. (Yokohama, Japan); and Swine and Poultry Research Center, Shizuoka Prefectural Research Institute of Animal Industry (Kikugawa, Japan). The review contains a part of studies awarded 2014 Scientist Prize of Japan Poultry Science Association.
References
- Awad AC, Bennink MR and Smith DM. Composition and functional properties of cholesterol reduced egg yolk. Poultry Science, 76: 649-653. 1997. [DOI] [PubMed] [Google Scholar]
- Bean LD and Leeson S. Long-term effects of feeding flaxseed on performance and egg fatty acid composition of brown and white hens. Poultry Science, 82: 388-394. 2003. [DOI] [PubMed] [Google Scholar]
- Besnard P, Passilly-Degrace P and Khan NA. Taste of fat: a sixth taste modality? Physiological Reviews, 96: 151-176. 2016. [DOI] [PubMed] [Google Scholar]
- Boschetti E, Bordoni A, Meluzzi A, Castellini C, Dal Bosco A and Sirri F. Fatty acid composition of chicken breast meat is dependent on genotype-related variation of FADS1 and FADS2 gene expression and desaturating activity. Animal, 10: 700-708. 2016. [DOI] [PubMed] [Google Scholar]
- Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N and Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. Journal of Neuroscience, 30: 8376-8382. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caston LJ, Squires EJ and Leeson S. Hen performance, egg quality, and the sensory evaluation of eggs from SCWL hens fed dietary flax. Canadian Journal of Animal Science, 74: 347-353. 1994. [Google Scholar]
- Chae HS, Choi HC, Na JC, Kim MJ, Kang HK, Kim DW, Kim JH, Jo SH, Kang GH and Seo OS. Effect of raising periods on amino acids and fatty acids properties of chicken meat. Korean Journal of Poultry Science, 39: 77-85. 2012. (in Korean) [Google Scholar]
- Chow I and Jacobson M. Inosine monophosphate, inosine, and hypoxanthine in meat from broilers 5, 7, and 9 weeks of age. Poultry Science, 47: 604-608. 1968. [Google Scholar]
- Clejan S, Castro-Magana M, Platon J, Collipp EJ and Vaddanahally TM. Effects of zinc deficiency and castration on fatty acid composition and desaturation in rats. Lipids, 17: 129-135. 1982. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N and Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chemical Senses, 31: 253-264. 2006. [DOI] [PubMed] [Google Scholar]
- Elswyk ME, Sams AR and Hargis PS. Composition, functionality, and sensory evaluation of eggs from hens fed dietary menhaden oil. Journal of Food Science, 57: 342-344. 1992. [Google Scholar]
- Ensembl Genomes. http://ensemblgenomes.org/. Accessed on July 28, 2017. [Google Scholar]
- Fraeye I, Bruneel C, Lemahieu C, Buyse J, Muylaert K and Foubert I. Dietary enrichment of eggs with omega-3 fatty acids: A review. Food Research International, 48: 961-969. 2012. [Google Scholar]
- Fujimura S, Muramoto T, Katsukawa M, Hatano T and Ishibashi T. Chemical analysis and sensory evaluation of free amino acids and 5′-inosinic acid in meat of Hinai-dori, Japanese native chicken: Comparison with broilers and layer pullets. Animal Science and Technology, 65: 610-618. 1994. [Google Scholar]
- Fujimura S, Koga H, Takeda H, Tone N, Kadowaki M and Ishibashi T. Chemical compositions of pectoral meat of Japanese native chicken, Hinai-jidori, and broiler of the same and marketing age. Animal Science and Technology, 67: 541-548. 1996. [Google Scholar]
- Fukunaga T, Koga K, Maita Y and Matsuoka S. Free amino acid, carnosine and 5′-inosinic acid contents in the breast and leg meats from the cross and triplecross chickens of Satsuma native fowl. Bulletin of the Faculty of Agriculture, Kagoshima University, 39: 223-232. 1989. (in Japanese) [Google Scholar]
- Galindo MM, Voigt N, Stein J, van Lengerich J, Raguse JD, Hofmann T and Behrens M. G protein-coupled receptors in human fat taste perception. Chemical Senses, 37: 123-139. 2012. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Fontenot DT, Liu L, Zhang H and Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. American Journal of Physiology - Cell Physiology, 272: C1203-C1210. 1997. [DOI] [PubMed] [Google Scholar]
- Gładkowski W, Kiełbowicz G, Chojnacka A, Gil M, Trziszka T, Dobrzański Z and Wawrzeńczyk C. Fatty acid composition of egg yolk phospholipid fractions following feed supplementation of Lohmann Brown hens with humic-fat preparations. Food Chemistry, 126: 1013-1018. 2011. [Google Scholar]
- Goda Y. 2003. Japanese Game cross and local chicken. Journal for the Integrated Study of Dietary Habits, 14: 93-96. (in Japanese) [Google Scholar]
- Hayat Z, Cherian G, Pasha TN, Khattak FM and Jabbar MA. Effect of feeding flax and two types of antioxidants on egg production, egg quality, and lipid composition of eggs. Journal of Applied Poultry Research, 18: 541-551. 2009. [Google Scholar]
- Ito H, Ozeki N, Yoshida Y, Kato S, Kawamura T, Tsubouchi R, Yoshino M and Shin S. Food histological characteristics of chicken meat in Nagoya Cochin (Nagoya breed) (part 3): Relationship between histological structure and structural comparisons in cooked dark meat of Nagoya Cochin. Journal of Cookery Science of Japan, 29: 168-177. 1996. (in Japanese) [Google Scholar]
- Jayasena DD, Kim SH, Lee HJ, Jung S, Lee JH, Park HB and Jo C. Comparison of the amounts of taste-related compounds in raw and cooked meats from broilers and Korean native chickens. Poultry Science, 93: 3163-3170. 2014. [DOI] [PubMed] [Google Scholar]
- Karasawa Y, Aoki K and Hirakata A. Free amino acids and purine compounds in leg and breast muscles from broiler, Satsuma, Satsuma cross and Kukin cross. Japanese Poultry Science, 26: 29-34. 1989. (in Japanese) [Google Scholar]
- Kawai T and Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology, 285: R447-R454. 2003. [DOI] [PubMed] [Google Scholar]
- Khang NT, Jennen DGJ, Tholen E, Tesfaye D, Mennicken L, Hoelker M and Wimmers K. Association of the FADS2 Gene with ω -6 and ω -3 PUFA concentration in the egg yolk of Japanese Quail. Animal Biotechnology, 18: 189-201. 2007. [DOI] [PubMed] [Google Scholar]
- Kiyohara R, Yamaguchi S, Ushio H, Shimomura M and Ichikawa T. Effect of adding arachidonic acid to cooked foods. Journal of Cookery Science of Japan, 42: 294-299. 2009. (in Japanese). [Google Scholar]
- Kiyohara R, Yamaguchi S, Rikimaru K and Takahashi H. Supplemental arachidonic acid-enriched oil improves the taste of thigh meat of Hinai-jidori chickens. Poultry Science, 90: 1817-1822. 2011. [DOI] [PubMed] [Google Scholar]
- Komiyama K, Tsuruta T, Mukae S, Amano Y, Okazaki Y, Matsumoto M and Ishii T. In vivo localization of secretory type V phospholipase A2 (sPLA2-V) in human salivary glands under normal and pathological conditions. Oral Medicine & Pathology, 13: 99-104. 2009. [Google Scholar]
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP and Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. Journal of Clinical Investigation, 115: 3177-3184. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba G, Schaeffer L, Xumerle L, Klopp N, Trabetti E, Biscuola M, Cavallari U, Galavotti R, Martinelli N, Guarini P, Girelli D, Olivieri O, Corrocher R, Heinrich J, Pignatti PF and Illig T. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids, 43: 289-299. 2008. [DOI] [PubMed] [Google Scholar]
- Matsui S and Takahashi H. Is egg flavour changeable by chicken breeding? Association of chicken fatty acid desaturase 1 gene single-nucleotide polymorphisms with egg fatty acid profiles and flavour in a Japanese hybrid chicken. Cogent Food & Agriculture, 3: 1287812. 2017. [Google Scholar]
- Matsuishi M, Kato A, Ishige N, Hori T, Ishida Y, Kaneko S, Takenonaka M, Miyamura Y, Iwata T and Okitani A. Comparison of meat palatability factors of Nagoya Cochin with broiler and Aigamo. Nihon Chikusan Gakkaiho, 76: 423-430. 2005. (in Japanese) [Google Scholar]
- Mizuno-Kamiya M, Inokuchi H, Kameyama Y, Yashiro K and Fujita A. Ca2+-independent phospholipase A2 activity in apical plasma membranes from the rat parotid gland. Archives of Oral Biology, 46: 789-799. 2001. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Rhue MR, Okitani A and Kato H. Components contributing to the improvement of meat taste during storage. Agricultural and Biological Chemistry, 52: 2323-2330. 1988. [Google Scholar]
- Oike H, Wakamori M, Mori Y, Nakanishi H, Taguchi R, Misaka T and Abe K. Arachidonic acid can function as a signaling modulator by activating the TRPM5 cation channel in taste receptor cells. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1761: 1078-1084. 2006. [DOI] [PubMed] [Google Scholar]
- Rikimaru K and Takahashi H. A method for discriminating a Japanese brand of chicken, the Hinai-jidori, using microsatellite markers. Poultry Science, 86: 1881-1886. 2007. [DOI] [PubMed] [Google Scholar]
- Rikimaru K, Yasuda M, Komatsu M and Ishizuka J. Effects of canonization on growth performance and carcass characteristics in Hinai-jidori. Journal of Poultry Science, 46: 351-355. 2009. [Google Scholar]
- Rikimaru K and Takahashi H. Evaluation of the meat from Hinai-jidori chickens and broilers: Analysis of general biochemical components, free amino acids, inosine 5′-monophosphate, and fatty acids. Journal of Applied Poultry Research, 19: 327-333. 2010. [Google Scholar]
- Rikimaru K, Egawa Y, Yamaguchi S and Takahashi H. Association of chicken fatty acid desaturase 1 and 2 gene single-nucleotide polymorphisms with the fatty acid composition of thigh meat in Japanese Hinai-dori crossbred chickens. Journal of Fisheries & Livestock Production, 4: 202. 2016. [Google Scholar]
- Sharp PJ, Culbert J and Wells JW. Variations in stored and plasma concentrations of androgens and luteinizing hormone during sexual development in the cockerel. Journal of Endocrinology, 74: 467-476. 1977. [DOI] [PubMed] [Google Scholar]
- Sirri F, Bianchi M, Petracci M and Meluzzi A. Influence of partial and complete caponization on chicken meat quality. Poultry Science, 88: 1466-1473. 2009. [DOI] [PubMed] [Google Scholar]
- Sirri F, Castellini C, Bianchi M, Petracci M, Meluzzi A and Franchini A. Effect of fast-, medium- and slow-growing strains on meat quality of chickens reared under the organic farming method. Animal, 5: 312-319. 2011. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Rikimaru K, Kiyohara R and Yamaguchi S. Effect of arachidonic acid-enriched oil diet supplementation on the taste of broiler meat. Asian Australasian Journal of Animal Sciences, 25: 845-851. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma T and Ichida T. Role of Ca2+-independent phospholipase A2 in exocytosis of amylase from parotid acinar cells. Journal of Biochemistry, 121: 1018-1024. 1997. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Nakamura T, Tanase H and Doi O. Comparisons of plasma LH, progesterone, testosterone and estradiol concentrations in male and female chickens (Gallus domesticus) from 28 to 1141 days of age. Endocrinologia Japonica, 28: 605-613. 1981. [DOI] [PubMed] [Google Scholar]
- Tang H, Gong YZ, Wu CX, Jiang J, Wang Y and Li K. Variation of meat quality traits among five genotypes of chicken. Poultry Science, 88: 2212-2218. 2009. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Kajikawa M, Fujita E and Ishii K. Studies on the flavor of meats. Agricultural and Biological Chemistry, 29: 208-215. 1965. [Google Scholar]
- UCSC Genome Browser Gateway. http://genome.ucsc.edu/. Accessed on July 28, 2017. [Google Scholar]
- Wang XF, Liu GH, Cai HY, Chang WH, Ma JS, Zheng AJ and Zhang S. Attempts to increase inosinic acid in broiler meat by using feed additives. Poultry Science, 93: 2802-2808. 2014. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Kobayashi H, Shibata M, Kubota M, Kadowaki M and Fujimura S. Regulation of free glutamate content in meat by dietary lysine in broilers. Animal Science Journal, 86: 435-442. 2015. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Kobayashi H, Shibata M, Kubota M, Kadowaki M and Fujimura S. Reduction of dietary lysine increases free glutamate content in chicken meat and improves its taste. Animal Science Journal, 88: 300-305. 2017. [DOI] [PubMed] [Google Scholar]
- Wattanachant S, Benjakul S and Ledward DA. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poultry Science, 83: 123-128. 2004. [DOI] [PubMed] [Google Scholar]
- Yamada M and Yamada K. Characteristics of free amino acids and fatty acids composition in the marketing meat of Aizu Jidori. Journal for the Integrated Study of Dietary Habits, 24: 177-182. 2013. (in Japanese) [Google Scholar]
- Yamaguchi S. The synergistic taste effect of monosodium glutamate and disodium 5′-inosinate. Journal of Food Science, 32: 473-478. 1967. [Google Scholar]
- Yamaguchi S and Ninomiya K. Umami and food palatability. Journal of Nutrition, 130: 921S-926S. 2000. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Watanabe U, Fujimoto M and Sako N. Taste preference and nerve response to 5′-inosine monophosphate are enhanced by glutathione in mice. Chemical Senses, 34: 809-818, 2009. [DOI] [PubMed] [Google Scholar]
- Zhang GQ, Ma QG and Ji C. Effects of dietary inosinic acid on carcass characteristics, meat quality, and deposition of inosinic acid in broilers. Poultry Science, 87: 1364-1369. 2008. [DOI] [PubMed] [Google Scholar]


