Abstract
The aim of this study was to optimize and characterize Flavourzyme hydrolysis conditions for the preparation of antioxidant peptides from duck meat, using response surface methodology. The results indicated that optimal Flavourzyme hydrolysis conditions for preparation of antioxidant peptides from duck protein were a temperature of 50.19°C, pH 5.45, and a reaction time of 1.03 h. Compared to non-hydrolyzed duck meat, Flavourzyme hydrolysis significantly improved the hydroxyl-radical scavenging, DPPH radical-scavenging, ferrous ion-chelating, reducing, and ABTS radical cation-scavenging activities of duck meat. Therefore, Flavourzyme can be regarded as an effective hydrolytic enzyme for the preparation of antioxidant peptides from duck meat.
Keywords: antioxidant peptides, duck meat, Flavourzyme, hydrolysis, response surface methodology
Introduction
Free radicals are metabolites that are usually counter-balanced by biological antioxidants. (Al Ghouleh et al., 2011). Breakage of this critical balance results in oxidative stress, which may lead to diseases such as cancer, coronary heart disease, diabetes, arthritis, atherosclerosis, and Alzheimer's disease (Qian et al., 2008; Zhang et al., 2018). Free radicals, the main causes of lipid peroxidation, can also shorten shelf life, produce undesired taste and rancid flavor, and generate potentially toxic reaction products (Qian et al., 2008). Therefore, it is imperative to inhibit oxidation in food to prevent its deterioration, and in living organisms to protect the body from serious diseases (Wang et al., 2015). Increasing studies have explored and developed natural and safe antioxidant compounds, such as antioxidant peptides (Liu et al., 2016).
Meat protein, rich in essential amino acids such as methylhistidine and hydroxymethyllysine that are not ordinarily found in plant protein, is a good source of antioxidant peptides. In the muscle tissues, antioxidant peptides, such as carnosine and anserine, can act as metal-ion chelators and free-radical scavengers (Kang et al., 2002). Duck meat is thought to be a good protein source for humans and it is rich in iron, niacin, and selenium, while it is lower in calories than beef (Adzitey et al., 2012). Duck meat and its products are highly appreciated by customers worldwide (Kim et al., 2016). Moreover, duck meat and its products have gained increased consumer interest since it is recommended to reduce the intake of red meat, which has been related to cardiovascular disease (Adzitey et al., 2012; Witak, 2008; Zou et al., 2017b). The consumption of duck meat and its products has increased about twofold in China between 2000 and 2011 (Wang et al., 2013, 2014, 2016). Therefore, the development of new products, preferentially containing antioxidant peptides, is necessary.
Enzymatic hydrolysisis an important process in generating meat with antioxidant peptides. Enzymatic hydrolysis is influenced by various factors , including enzyme type, pH, temperature, and time, which together affect enzyme activity, and thus, allow for controlling the process (Jamil et al., 2016). In our previous study, we found that Flavourzyme is one of the most effective hydrolytic enzymes; however, information on the optimal hydrolysis condition is limited.
The aims of this study were: 1) to optimize Flavourzyme hydrolysis conditions (including pH, temperature, and time) to yield high DPPH radical scavenging activity, using response surface methodology (RSM), and 2) to assess other important antioxidative activity indexes(e.g., ABTS radical cation scavenging, ferrous ion chelating, hydroxyl radical scavenging, and reducing power) under the optimal hydrolysis conditions.
Materials and Methods
Sample Preparation
Thirty-six ducks weighing approximately 2.0 kg, were slaughtered in a local commercial poultry meat processing company (Furun Company, Jiangsu, China). Biceps femoris muscles were collected from the duck carcasses and removed of all connective tissues and visible subcutaneous fat. The duck meat sample was rinsed using distilled water and homogenized. The homogenate was mixed with isopropanol was mixed at a ratio of 1:5 (w/v) and stirred for 5.0 h at 25°C. Isopropanol was changed every 2.5 h. After centrifugation at 5000×g for 20 min at 4°C, the precipitate was collected and freeze-dried. The defatted duck protein powder was stored at −20°C (Chi et al., 2015).
The defatted duck protein powder was dissolved (∼10% w/v) in double-distilled water and homogenized at 8000 rpm, then hydrolyzed with Flavourzyme at an enzyme/substrate ratio of 3% (w/w). For single-factor analysis, hydrolysis was performed at 35–60°C, pH 5.0–7.5, and reaction times of 0.5–3.0 h. On the basis of single-factor experiments, we used the RSM method to estimate the influence of independent variables(temperature, X1; pH, X2; time, X3) on DPPH radical scavenging activity (Y). Box–Behnken design at 3 levels was selected to analyze the effects of these three variables on DPPH radical scavenging activity. Hydrolysis was terminated by heating in boiling water for 12 min. The hydrolysate was cooled to room temperature and centrifuged at 5000×g for at least 20 min. The supernatant was stored at −20°C for further analyses.
DPPH Radical Scavenging Activity
DPPH radical scavenging activity of the hydrolysate was determined according to the method described by Saiga et al. (2003), with modification. Hydrolysate (200 µL, 1.0 mg/mL) was mixed with ethanolic solution of DPPH (0.18 mM, 1.0 mL). The test solution was mixed well and then left in the dark for 15 min. The mixture was subsequently injected into the sample cavity of a Bruker E-scan electron paramagnetic resonance spectrometer (EPR, Bruker Biospin Co., Billerica, MA, USA). Ethanol was used instead of the hydrolysate as a control. The Bruker E-scan operation parameters were set as reported by Saiga et al. (2003).
The DPPH radical scavenging activity of the test sample wasobtained by solving the following equation: DPPH radical scavenging activity (%)=(1−signal intensity for sample/signal intensity for control)×100.
Ferrous Ion Chelating Capacity
The ferrousion chelating capacity of hydrolysate was measured by the method of Wang et al. (2015), with some modification. Hydrolysate of test compounds (1.0 mL, 1.0 mg/mL) was mixed well with a ferrouschloride solution (2 mM, 0.1 mL). To the mixture, ferrozine solution (5 mM, 0.2 mL) was added, and the reaction mixture wasallowed to stand for 20 min for color development. The absorbance of the test sample at 562 nm was determined. A blank without ferrozine was used for each compound, since the antioxidant–Fe2+ complex gives a color that might interfere with the absorbance reading. Ferrous ion chelating capacity was obtained by solving the following equation: ferrous ion chelating capacity (%)=[1−(As−Ab/Ac)]×100, where As is the absorbance of the sample, Ac is absorbance in the absence of hydrolysate, and Ab is the absorbance in the absence of ferrozine.
ABTS Radical Cation Scavenging Capacity
The ABTS (3-ethylbenzothiazoline-6-sulfonate) radical cation scavenging activity of duck protein hydrolysate was determined using the method reported by Karaçelik et al. (2015), with minor modification. AAPH (2.5 mM) and ABTS (2.5 mM) were prepared in phosphate buffer (0.1 M) with 0.15 M sodium chloride (pH 7.4). ABTS solution was prepared by mixing AAPH and ABTS stock solutions at the ratio of 1:1 (v/v). The solution was heated to 60°C for 20 min in the dark. Fifty microliters of sample (1.0 mg/mL) was mixed with ABTS solution (1.95 mL), and the absorbance of the mixture at 734 nm was determined after 6 min. ABTS radical cation scavenging activity was obtained by solving the following equation: ABTS radical cation scavenging activity (%)=(1−(As/Ab)×100, where Asisthe absorbance of the sample and Ab is the absorbance in the absence hydrolysate.
Reducing Power Analysis
The reducing power of duck protein hydrolysate was determined by the method reported by Wu et al. (2003). Two milliliters of sample (1.0 mg/mL) was mixed with phosphate buffer (1.0 mL, 0.2 M, pH 6.6) and 1.0 mL of potassium ferricyanide solution (1%). The mixture was heated at 50°C for 20 min, then 1.0 mL trichloroacetic acid (10%) was added, and the mixture was centrifuged at 1000×g for 10 min. An aliquot (1.0 mL) of the supernatant was transferred to a tube containing distilled water (1.0 mL) and ferric chloride (0.2 mL, 0.1%). The content was mixed well and the absorbance at 700 nm was recorded. The higher the absorbance of the reaction solution, the greater the reducing power.
Hydroxyl Radical Scavenging Capacity
The hydroxyl scavenging activity was assayed according to the method reported by You et al. (2010). Briefly, duck protein hydrolysate (1.0 mg/mL, 1.0 mL) was mixed well with 1,10-phenanthroline monohydrate solution (5 mM, 0.6 mL). Then, phosphate buffer (0.2 M, pH 7.4, 0.4 mL), FeSO4 solution (50 mM, 0.6 mL), and EDTA solution (15 mM, 0.6 mL) were added, followed by addition of 0.1% H2O2 solution (0.4 mL). The mixture was incubated at 37°C for 1 h and then, the absorbance at 536 nm was determined. The hydroxyl scavenging activity was obtained by solving the following equation: hydroxyl scavenging activity (%)=[(As−Ad)/(An−Ad)]×100, where As is the absorbance of the sample solution, Ad is the absorbance of the control solution without duck protein hydrolysate, and An is the control solution without H2O2 absorbance.
Statistical Analysis
Single-factor analysis was used to evaluate differences among groups, with SPSS 16.0 software (SPSS Inc. Chicago, IL, USA). Differences were regarded significant when P<0.05. RSM was used for statistical analysis of experimental data, using Design-Expert 8.0.6 software (State-Ease, Inc., Minneapolis MN, USA). RSM was based on multiple linear regression analysis, taking into account the two major and interactive effects, using the following equation:
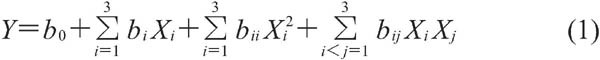 |
where Y is the predicted response variable, and b0, bi, bii, bij are linear, square, and interaction regression coefficients, respectively, and Xi and Xj are the independent variables (i>j). All results were analyzed using ANOVA. The optimum hydrolysis conditions for Flavourzyme were estimated using independent and dependent variables by regression analysis and 3D response surface analysis.
Results and Discussion
Effects of Temperature, pH, and Reaction Time on DPPH Radical Scavenging of Antioxidant Peptides from Duck Protein
We aimed to develop an RSM model to optimize the Flavourzyme hydrolysis condition for duck meat protein in terms of temperature, pH, and reaction time, to achieve high antioxidative activity of antioxidant peptides. Changes in DPPH radical scavenging activity were observed in single factor experiments, the results of which are shown in Fig. 1.
Fig. 1. Effects of temperature (a), pH (b) and reaction time (c) on DPPH radical scavenging of antioxidant peptides from duck protein.
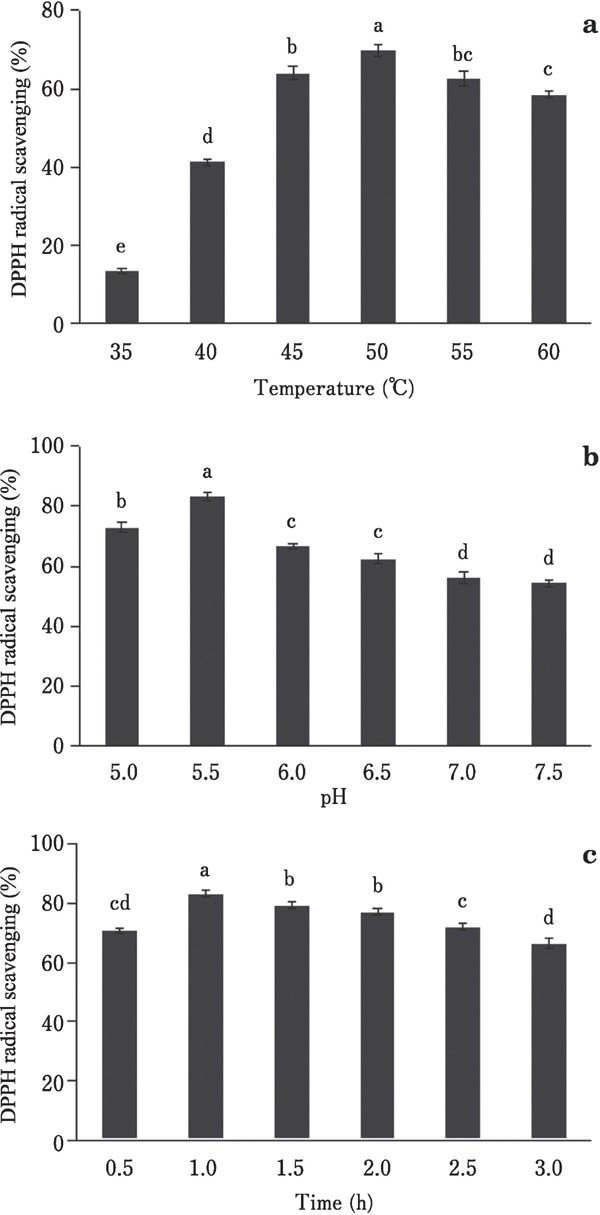
To test the effect of temperature on antioxidant activity, temperature was varied from 35°C to 60°C, while both other parameters were maintained constant at pH 6.5 and a reaction time of 2.0 h. DPPH radical scavenging activity increased significantly (P<0.05) when the processing temperature was increased from 35°C to 50°C, after which it significantly decreased (P<0.05) as the temperature was further elevated to 60°C (Fig. 1a). The low antioxidant activity at high temperature can be explained by protein denaturation, and the low activity at low temperature may be because of the incomplete enzymatic hydrolysis (Roslan et al., 2014), which indicated that temperature is an important factor in duck meat hydrolyzation. Based on these findings, 50°C was selected as the optimum temperature and was therefore used in subsequent experiments.
The effect of pH on DPPH radical scavenging activity was analyzed at the suitable range of 5.0–7.5, and hydrolyzation wascarried out at 50°C for 2.0 h (Fig. 1b). The DPPH radical scavenging activity significantly increased (P<0.05) when the pH was increased from 5.0 to 5.5, and significantly decreased at pH 5.5 to 6.0 (Fig. 1b). There was no significant decrease in DPPH radical scavenging activity (P>0.05) when the pH was further increased from 6.0 to 6.5 and from 7.0 to 7.5. In brief, the antioxidant activity increased at a pH of around 5.5 and decreased thereafter. In a previous report, Flavourzyme was active at pH 5.0 to 7.0, with a pH of 7.0 found to be optimal (Charoensiddhi et al., 2016). The discrepancy may be caused by the fact that this study used a different substrate. The influence of pH on duck meat hydrolysis was significant (P<0.05). Based on our findings, a pH of 5.5 was chosen as the RSM central point.
The effect of processing time on the antioxidative activity was tested using a range of 0.5–3.0 h, and hydrolyzation was carried out at 50°C and pH 5.5. DPPH radical scavenging activity increased from 70.94% to 83.22% when the reaction time was increased from 0.5 to 1 h (Fig. 1c). Between 1.0 h and 1.5 h, the change in processing time resulted in a significant decline in the scavenging activity (P<0.05). Between 1.5 h and 2.0 h, although the scavenging activity of DPPH radical still decreased, the change was not significant. Between 2.0 h and 3.0 h, the scavenging activity decreased significantly when again (P<0.05). Thus, during the initial stage of enzymatic hydrolysis, the hydrolysis degree increased, and the content of antioxidant peptides and the DPPH radical scavenging activity also increased. At the later stage of enzymatic hydrolysis, some long-chain antioxidant peptides can be hydrolyzed, which might have caused the decrease in DPPH radical scavenging activity (Chel-Guerrero et al., 2016). These findings indicated that time is also an important factor in the hydrolyzation of duck meat. Thus, 1 h was selected as the optimum reaction time for development of the RSM model.
Optimization of the Hydrolysis Conditions by RSM
RSM is a statistical method widely used in food science and research in recent years to simulate and optimize the response of a system to one or more factors (Zou et al., 2017a; Hu et al., 2014; Wang et al., 2007; Kim et al., 2013). In our present study, temperature, pH, and reaction time were selected as independent variables for further optimization of the Flavourzyme hydrolysis conditions because of their obvious effects on antioxidant activity. DPPH radical scavenging activities evaluated in a 3-level and 3-factor factorial design, which was adopted to optimize the hydrolysis conditions, are shown in Table 1. Based on the limited number of experimental results, the statistical model was built, and the relationships between DPPH radical scavenging activity and the test variables were determined to be as follows:
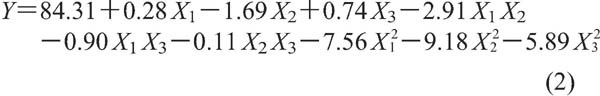 |
Table 1. Box–Behnken design and experimental results of RSM.
| Run | Independent variablesa | Dependent variablesb | ||
|---|---|---|---|---|
| Temperature (°C) | pH | Time (h) | DPPH radical scavenging (%) | |
| 1 | 50.0 | 5.5 | 1.0 | 83.23 |
| 2 | 50.0 | 5.0 | 0.5 | 70.04 |
| 3 | 50.0 | 5.5 | 1.0 | 84.46 |
| 4 | 50.0 | 6.0 | 1.5 | 68.20 |
| 5 | 55.0 | 6.0 | 1.0 | 63.05 |
| 6 | 45.0 | 6.0 | 1.0 | 67.73 |
| 7 | 50.0 | 6.0 | 0.5 | 67.84 |
| 8 | 45.0 | 5.0 | 1.0 | 66.26 |
| 9 | 50.0 | 5.5 | 1.0 | 84.10 |
| 10 | 50.0 | 5.5 | 1.0 | 84.65 |
| 11 | 55.0 | 5.0 | 1.0 | 73.21 |
| 12 | 55.0 | 5.5 | 0.5 | 69.75 |
| 13 | 55.0 | 5.5 | 1.5 | 71.95 |
| 14 | 50.0 | 5.5 | 1.0 | 85.10 |
| 15 | 45.0 | 5.5 | 1.5 | 72.13 |
| 16 | 45.0 | 5.5 | 0.5 | 69.58 |
| 17 | 50.0 | 5.0 | 1.5 | 70.86 |
Independent variables: X1, temperature; X2, pH; X3, time.
Dependent variables: Y, DPPH radical scavenging; data expressed as the mean (n=6).
The significance of each of the model coefficients was determined by ANOVA, the results of which are summarized in Table 2. X2, X1X2, X21, X22, and X23 were significant parameters (P<0.05), while the coefficients of other terms were not significant (P>0.05). The determination coefficient (R2=0.9932) of ANOVA of the quadratic regression model showed that the model explained 99.32% of total variation. The adjusted coefficient of decision (RAdj2=0.9845) confirmed that the model was highly significant. At the same time, the coefficient of variation was 1. 26, which indicated good reliability of the experimental data.
Table 2. ANOVA for response surface quadratic model: estimated regression model of relationships between dependent variables and independent variables.
| Source | Sum of squares | DF | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 887.45 | 9 | 98.61 | 113.86 | <0.0001 |
| X1 -Temperature | 0.64 | 1 | 0.64 | 0.74 | 0.4175 |
| X2 - pH | 22.94 | 1 | 22.94 | 26.49 | 0.0013 |
| X3 - Time | 4.42 | 1 | 4,42 | 5.10 | 0.0585 |
| X1X2 | 33.79 | 1 | 33.79 | 39.02 | 0.0004 |
| X1X3 | 0.032 | 1 | 0.032 | 0.037 | 0.8524 |
| X2X3 | 0.052 | 1 | 0.052 | 0.061 | 0.8128 |
| X 21 | 240.87 | 1 | 240.87 | 278.13 | <0.0001 |
| X 22 | 355.07 | 1 | 355.07 | 410.00 | <0.0001 |
| X 23 | 146.15 | 1 | 146.15 | 168.76 | <0.0001 |
| Residual | 6.06 | 7 | 0.87 | ||
| Lack of fit | 4.09 | 3 | 1.36 | 2.76 | 0.1762 |
| Pure error | 1.98 | 4 | 0.49 | ||
| Cor total | 893.51 | 16 | |||
| R2=0.9932 RAdj2=0.9845 RPred2=0.9234 C.V.%=1.26 | |||||
The interactions of independent variables and their interactions with antioxidant activity can be observed from 3D response surfaces of multiple nonlinear regression models (Basu and Basu, 2015; Zou et al., 2017a). Using the complete model established by Eq. (2), 3D response surface and contour maps were generated to predict the relationships between the independent and the dependent variables. The interaction effects of pH (X2) and temperature (X1) on DPPH radical scavenging activity are shown in Fig. 2a and 2a1, while pH wasfixed at 5.5. With the increase in temperature, the scavenging activity began to increase, and then decreased sharply. When the temperature reached the optimum, the scavenging rate of DPPH radical reached 84.38%. As shown in the response surface plot and the corresponding counter plot, temperature had no significant effect on DPPH radical scavenging activity, which was in accordance with the data in Table 2. However, mutual interactions between temperature (X1) and pH (X2) were found (Fig. 2a1), which were also in accordance with the results in Table 2. Fig. 2b and 2b1 presents the interaction effect of temperature (X1) and time (X3) on the scavenging activity under an experimental reaction of 1.0 h. At medium levels of temperature and time, maximum DPPH radical scavenging activity of 84.33% was achieved, while increases in temperature and time did not lead to increased scavenging activity. The effect of pH (X2) and time (X3) on the scavenging activity is shown in Fig. 2c. With increasing pH value and treatment time at the fixed temperature of 50°C, the DPPH radical scavenging activity began to increase, and then decreased significantly. Under the optimum conditions, the scavenging rate was the highest, with 84.40%. The contour map in Fig. 2c1 shows that reaction time does not affect the DPPH radical scavenging activity significantly. The results also showed that the optimal Flavourzyme hydrolysis conditions for producing antioxidant peptides from duck meat protein were at 50.19°C, pH 5.45, a reaction time of 1.03 h.
Fig. 2.
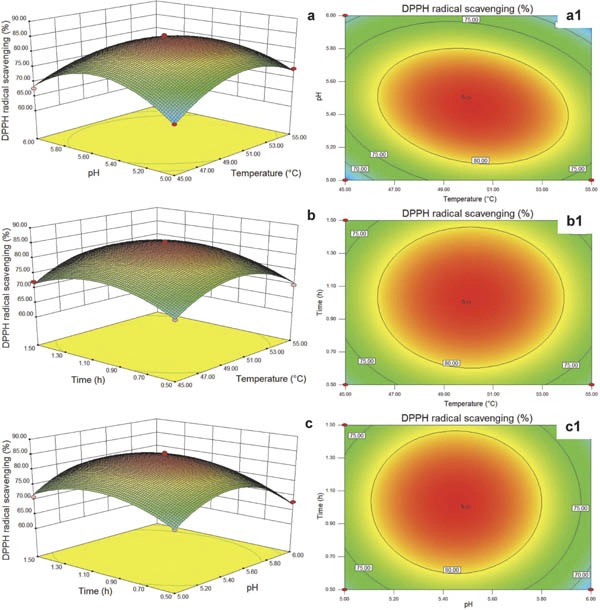
Response surface plots and contour plots of the DPPH radical scavenging (%) in response to changes in temperature (°C), pH, and time (h). a, a1: temperature and pH; b, b1: temperature and time; c, c1: pH and time.
These theoretically determined optimum hydrolysis parameters were validated experimentally. A mean DPPH radical scavenging activity of 84.36% (n=6) was achieved, demonstrating the experimental results were very close to values predicted by the regression model. Therefore, the RSM model can be effectively applied to the prediction of Flavourzyme hydrolysis conditions.
Comparison of Flavourzyme Hydrolysis with Control Duck Protein
Table 3 shows that Flavourzyme hydrolysis notably increased DPPH radical scavenging, ferrous ion chelation, reducing power, ABTS radical cation scavenging, and hydroxyl radical scavenging, as compared to control. Determination of antioxidant capacity is an important step in the validation of functional food properties. For decades, methods for testing the antioxidant activity of food compounds and biological samples have been developed, and they have been comprehensively reviewed in some papers (Magalhães et al., 2008; Antolovich et al., 2002). To date, there is no distinguished way to describe the overall antioxidant potential of proteolyzed, partially purified hydrolytic peptides. The most widely used detection methods based on hydrogen atoms include oxygen radical absorption capacity (ORAC) and total free radical capture antioxidant parameters(TRAP). Therefore, DPPH radical scavenging, ferrous ion chelation, ABTS radical scavenging, oxidation of hydroxyl radicals, and reducing power are widely used in the assessment of antioxidant capacity. Taken together, hydrolysates of duck meat had a high antioxidant activity, demonstrating their potential use as food ingredients with antioxidative function. Other effects related to beneficial health effects of such hydrolysates and identification of their amino acid sequence remain to be explored.
Table 3. Antioxidant activities of antioxidant peptides from duck protein (1.0 mg/mL).
| Control | Antioxidant peptides | |
|---|---|---|
| DPPH radical scavenging (%) | 11.13±0.43b | 84.36±2.55a |
| Ferrousion chelating (%) | 4.05±0.22b | 16.27±2.84a |
| ABTS radical cation scavenging (%) | 8.66±0.17b | 77.79±2.28a |
| Reducing power (A700) | 0.15±0.02b | 1.53±0.59a |
| Hydroxyl radical scavenging (%) | 5.24±0.35b | 38.65±0.63a |
Meansin the same row with different lettersdiffer significantly (P<0.05); data expressed asthe mean±SD (n=6).
Acknowledgments
This study was supported by Jiangsu Province Natural Science Foundation Program (BK20161378) and Jiangsu Province Innovation of Agricultural Science and Technology (CX(15)1008).
References
- Adzitey F, Huda N and Rahmat Ali GR. Prevalence and antibiotic resistance of Campylobacter, Salmonella, and L. monocytogenes in ducks: a review. Foodborne pathogens and disease, 9: 498-505. 2012. [DOI] [PubMed] [Google Scholar]
- Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM and Darley-Usmar V. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radical Biology and Medicine, 51: 1271-1288. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolovich M, Prenzler PD, Patsalides E, McDonald S and Robards K. Methods for testing antioxidant activity. Analyst, 127: 183-198. 2002. [DOI] [PubMed] [Google Scholar]
- Basu A, Basu S, Bandyopadhyay S and Chowdhury R, Optimization of evaporative extraction of natural emulsifier cum surfactant from Sapindus mukorossi-Characterization and cost analysis. Industrial Crops and Products, 77: 920-931. 2015. [Google Scholar]
- Charoensiddhi S, Lorbeer AJ, Lahnstein J, Bulone V, Franco CM and Zhang W. Enzyme-assisted extraction of carbohydrates from the brown alga Ecklonia radiata: Effect of enzyme type, pH and buffer on sugar yield and molecular weight profiles. Process Biochemistry, 51: 1503-1510. 2016. [Google Scholar]
- Chel-Guerrero L, Galicia-Martínez S, Acevedo-Fernández JJ, Santaolalla-Tapia J and Betancur-Ancona D. Evaluation of Hypotensive and Antihypertensive Effects of Velvet Bean (Mucuna pruriens L.) Hydrolysates. Journal of Medicinal Food, 19: 1511-1517. 2016. [DOI] [PubMed] [Google Scholar]
- Chi CF, Hu FY, Wang B, Li ZR and Luo HY. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (katsuwonus pelamis) dark muscle. Marine Drugs, 13: 2580-2601. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YQ, Yu HX, Dong KC, Yang SB, Ye XQ and Chen SG. Analysis of the tenderisation of jumbo squid (Dosidicus gigas) meat by ultrasonic treatment using response surface methodology. Food Chemistry, 160: 219-225. 2014. [DOI] [PubMed] [Google Scholar]
- Jamil NH, Halim NR and Sarbon NM. Optimization of enzymatic hydrolysis condition and functional properties of eel (Monopterus sp.) protein using response surface methodology (RSM). International Food Research Journal, 23: 1-9. 2016. [Google Scholar]
- Kang JH, Kim KS, Choi SY, Kwon HY, Won MH and Kang TC. Carnosine and related dipeptides protect human ceruloplasmin against peroxyl radical-mediated modification. Molecules and Cells, 13: 498-502. 2002. [PubMed] [Google Scholar]
- Karaçelik AA, Küçük M, İskefiyeli Z, Aydemir S, De Smet S, Miserez B and Sandra P. Antioxidant components of Viburnum opulus L. determined by on-line HPLC–UV–ABTS radical scavenging and LC–UV–ESI-MS methods. Food Chemistry, 175: 106-114. 2015. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Yong HI, Lee HJ, Jung S, Kwon JH, Heo KN and Jo C. Identification of Microorganisms in Duck Meat Products Available in Korea and the Effect of High Hydrostatic Pressure. Korean Journal for Food Science of Animal Resources, 36: 283-288. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GD, Jung TC, Jung EY, Jeong JY, Yang HS and Joo ST. Optimization of processing conditions for meat paper from beef semimembranosus muscle using response surface methodology. LWT-Food Science and Technology, 50: 326-330. 2013. [Google Scholar]
- Liu R, Xing L, Fu Q, Zhou GH and Zhang WG. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products, Antioxidants. 5: 1-15. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães LM, Segundo MA, Reis S and Lima JL. Methodological aspects about in vitro evaluation of antioxidant properties. Analytica Chimica Acta, 613: 1-9. 2008. [DOI] [PubMed] [Google Scholar]
- Qian ZJ, Jung WK, Byun HG and Kim SK. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresource Technology, 99: 3365-3371. 2008. [DOI] [PubMed] [Google Scholar]
- Roslan J, Kamal SMM, Yunos KFM and Abdullah N. Optimization of enzymatic hydrolysis of tilapia muscle (Oreochromis niloticus) using response surface methodology (RSM). Sains Malaysiana, 43: 1715-1723. 2014. [Google Scholar]
- Saiga AI, Tanabe S and Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. Journal of Agricultural and Food Chemistry, 51: 3661-3667. 2003. [DOI] [PubMed] [Google Scholar]
- Wang DY, Deng SY, Zhang MH, Geng ZM, Sun C, Bian H, Liu F, Zhu YZ and Xu WM. Optimization of the Tenderization of Duck Breast Meat by Adenosine 5′-Monophosphate (AMP) using Response Surface Methodology. Journal of Poultry Science, 53: 93-101. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Dong H, Zhang MH, Liu F, Bian H, Zhu YZ and Xu WM. Changes in actomyosin dissociation and endogenous enzyme activities during heating and their relationship with duck meat tenderness. Food Chemistry, 141: 675-679. 2013. [DOI] [PubMed] [Google Scholar]
- Wang DY, Zhang MH, Xu WM, Bian H, Liu F, Geng ZM, Zhu YZ and Xu XL. Changes in chemical-physical index and microstructure during dry-cured duck processing. Journal of Poultry Science, 51: 220-226. 2014. [Google Scholar]
- Wang LS, Huang JC, Chen YL, Huang M and Zhou GH. Identification and characterization of antioxidant peptides from enzymatic hydrolysates of duck meat. Journal of Agricultural and Food Chemistry, 63: 3437-3344. 2015. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Chen F, Wu JH, Wang ZF, Liao XJ and Hu XS. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. Journal of Food Engineering, 78: 693-700. 2007. [Google Scholar]
- Witak BA. Tissue composition of carcass, meat quality and fatty acid content of ducks of a commercial breeding line at different age. Archiv Fur Tierzucht, 51: 266-275. 2008. [Google Scholar]
- Wu HC, Chen HM and Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Research International, 36: 949-957. 2003. [Google Scholar]
- You L, Zhao M, Regenstein JM and Ren J. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chemistry, 20: 810-816. 2010. [Google Scholar]
- Zou Y, Bian H, Li PP, Sun ZL, Sun C, Zhang MH, Geng ZM, Xu WM, Xu XL and Wang DY. Optimization and physicochemical properties of nutritional protein isolate from pork liver with ultrasound-assisted alkaline extraction. Animal Science Journal, DOI: 10.1111/asj.12930.2017a. [DOI] [PubMed] [Google Scholar]
- Zou Y, Wang L, Li PP, Cai PP, Zhang MH, Sun ZL, Sun C, Geng ZM, Xu WM, Xu XL and Wang DY. Effects of ultrasound assisted extraction on the physiochemical, structural and functional characteristics of duck liver protein isolate. Process Biochemistry, 52: 174-182. 2017b. [DOI] [PubMed] [Google Scholar]
- Zhang MH, Wang DY, Geng ZM, Li PP, Sun ZL, and Xu WM. Effect of heat shock protein 90 against ROS-induced phospholipid oxidation. Food Chemistry, 240: 642-647. 2018. [DOI] [PubMed] [Google Scholar]


