Abstract
Introduction
Patients with chronic atrophic gastritis (CAG) and intestinal metaplasia (IM) are at risk of developing gastric adenocarcinoma. Their diagnosis and management currently rely on histopathological guidance after random endoscopic biopsy sampling (Sydney biopsy strategy). This approach has significant flaws such as under-diagnosis, poor reproducibility and poor correlation between endoscopy and histology. This prospective, international multicentre study aims to establish whether endoscopy-led risk stratification accurately and reproducibly predicts CAG and IM extent and disease stage.
Methods and analysis
Patients with CAG and/or IM on standard white light endoscopy (WLE) will be prospectively identified and invited to undergo a second endoscopy performed by an expert endoscopist using enhanced endoscopic imaging techniques with virtual chromoendoscopy. Extent of CAG/IM will be endoscopically staged with enhanced imaging and compared with standard WLE. Histopathological risk stratification through targeted biopsies will be compared with endoscopic disease staging and to random biopsy staging on WLE as a reference. At least 234 patients are required to show a 10 % difference in sensitivity and accuracy between enhanced imaging endoscopy-led staging and the current biopsy-led staging protocol of gastric atrophy with a power (beta) of 80 % and a 0.05 probability of a type I error (alpha).
Ethics and dissemination
The study was approved by the respective Institutional Review Boards (Netherlands: MEC-2018-078; UK: 19/LO/0089). The findings will be published in peer-reviewed journals and presented at scientific meetings.
Trial registration number
NTR7661; Pre-results.
Keywords: endoscopy, gastrointestinal tumours, preventive medicine
Strengths and limitations of this study.
This is the first study to compare endoscopy-led risk stratification of premalignant gastric lesions using advanced imaging and targeted biopsies with white light endoscopy and random biopsies as a reference, performed at separate occasions with the endoscopist blinded to prior results.
This study will additionally provide biobank biopsy and serum material for future biomarker analysis.
A possible limitation of this study is that all procedures will be performed by expert endoscopists in teaching hospitals, therefore external reproducibility will be evaluated using interobserver variability of disease staging through video recordings.
The same limitation holds for the histopathological evaluation of the biopsy samples for which a proportion of the samples will be reviewed and rescored by a blinded second expert gastrointestinal histopathologist.
Introduction
Gastric adenocarcinoma remains a major cause of cancer mortality and is the most commonly diagnosed malignant condition of the gastrointestinal (GI) tract.1–4 Although incidence rates had previously been declining, recent studies demonstrated this differs among population subgroups. For example, an increasing incidence of gastric adenocarcinoma among young white cohorts in Western countries was objectified. This may be due to an increasing prevalence of gastric cancer precursors among younger adults, in particular chronic atrophic gastritis (CAG), intestinal metaplasia (IM) and dysplasia.5 6 These studies suggest that gastric cancer incidence rates may plateau or even increase again in the upcoming years. Importantly, with the exception of Japan and Korea, the majority of gastric cancers worldwide are diagnosed at later stage. This results in a poor prognosis with less than 30% 5 year survival.1 2 7 Japan’s earlier stage of diagnosis and superior 5 year survival highlight the need for earlier recognition and treatment.8
Endoscopic recognition of the premalignant stomach has long been problematic and limited by the ability of endoscopist and the imaging tools. A previous study demonstrated that 22% of high-grade dysplastic lesions and early gastric cancers were missed.9 10 A meta-analysis and systematic review of endoscopy follow-up studies confirmed that a marked proportion of early gastric cancers are missed at endoscopy.10 Therefore, current practice uses histology-based staging.11 12 However, endoscopic imaging has significantly improved with high-definition endoscopes and imaging enhancement technologies now routinely available. Some recent studies already suggested that accurate endoscopic staging of CAG and gastric intestinal metaplasia (GIM) is achievable and can robustly predict gastric adenocarcinoma risk. Importantly, the interobserver and intraobserver reproducibility characteristics of endoscopic CAG and GIM severity assessment are in experienced hands moderate to excellent.13–18 These marked improvements in endoscopic technology and the shift towards an endoscopy-led approach will empower the endoscopist to risk stratify individuals with greater accuracy and decrease the already huge burden placed on our endoscopy and histopathology departments. Therefore, the aim of this study is to evaluate if enhanced endoscopic imaging, including high-definition white light endoscopy (WLE) and virtual chromoendoscopy, alongside targeted biopsies, provides an accurate and reproducible assessment of CAG and IM disease extent and staging, when compared with the current practice of WLE and random biopsies through the Sydney protocol biopsy strategy.
Methods and analysis
Aims
The primary aim of this study is to assess the diagnostic accuracy for the endoscopic diagnosis of IM in Sydney biopsy locations comparing standard endoscopic staging with random biopsies with enhanced imaging with biopsies targeted to GIM.19 The Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines were followed.20 Study sites are located in the Netherlands and the UK. Secondary objectives are to evaluate (a) reproducibility of endoscopic staging after expert review, (b) reproducibility of histopathology for detection of IM, (c) the number of dysplastic or neoplastic lesions detected and (d) effects of inspection time of gastric mucosa on diagnostic accuracy.
Design
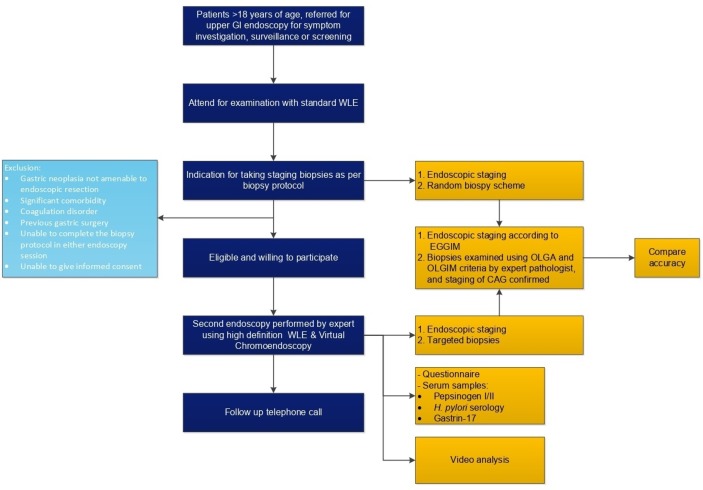
This is a prospective, multicentre registry study on the accuracy and reproducibility of enhanced endoscopic imaging, including high-definition WLE and virtual chromoendoscopy, for the staging of CAG and IM. Two upper endoscopies will be performed on two separate occasions (6–12 months in between) using standard white-light endoscopy plus random biopsies (current diagnostic strategy) at the first endoscopy and enhanced endoscopic imaging with targeted biopsies (proposed diagnostic strategy) at the second endoscopy. We will compare both approaches using histopathology as a reference and assess the accuracy and reproducibility of enhanced endoscopic imaging (figure 1).
Figure 1.
Flowchart of the study design. CAG, chronic atrophic gastritis; EGGIM, endoscopic grading of gastric intestinal metaplasia; GI, gastrointestinal; H. pylori, Helicobacter pylori; OLGA, operative link for gastritis assessment; OLGIM, operative link for gastric intestinal metaplasia assessment; WLE, white light endoscopy.
Patient and public involvement
We maintained close links with patient alliances and interest groups, both in the Netherlands as well as in the UK. This close relationship informs our practice and is the basis for the current study design. We will engage closely with patient interest groups to communicate research findings and ensure that our deliverables are fit for purpose.
Participants
Sample size
For estimation of sample size, we assume that the diagnosis of CAG or IM on enhanced imaging and targeted biopsies must be set with at least a 90% sensitivity with WLE and random biopsies as a reference.15 A power (beta) of 80% and a probability of type I error (alpha) of 0.05 will be handled. That purpose requires at least 234 patients to be recruited to show a 10 % difference in sensitivity between enhanced endoscopy-led staging and the standard WLE.
Recruitment
All patients (>18 years of age) referred to the endoscopy department for routine diagnostic upper GI endoscopy and diagnosed with CAG or IM between November 2018 and June 2020 are eligible for inclusion if able to give informed consent. Patients are excluded when having (1) gastric neoplasia not amenable to endoscopic resection, (2) no indication for Sydney biopsy staging on standard WLE, (3) significant comorbidity, (4) a coagulation disorder, (5) previous gastric surgery or (6) are unable to complete the biopsy protocol in either endoscopy session.
Interventions
Baseline characteristics
All patients are asked to complete a questionnaire on lifestyle factors, medical history, past interventions, medication use and family history of gastric cancer.
White light endoscopy
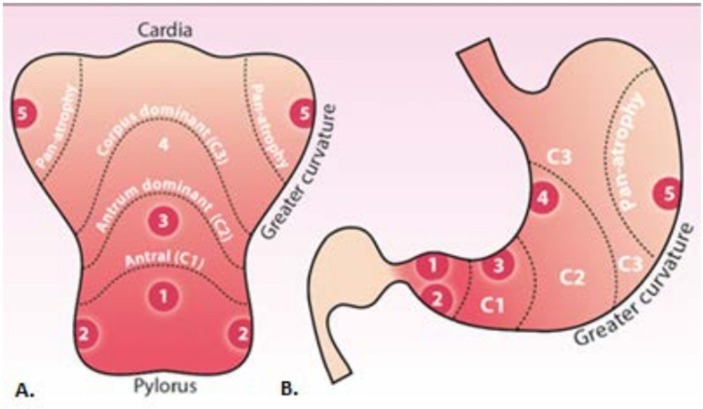
Patients referred to the endoscopy department for upper GI endoscopy for investigation of symptoms or for surveillance of a known condition will undergo their procedure on a standard diagnostic gastroscopy list. Patients found to have CAG or IM will be prospectively identified. During the initial procedure, patients will receive the current recommended practice. Current practice is to initially identify if gastric atrophy is present and to inspect the gastric mucosa for areas suspicious for dysplasia or malignancy. Any mucosal abnormalities suspicious for dysplasia or malignancy are biopsied with tissue biopsies placed in separate containers. Following this, 10 random biopsies are taken according to the Sydney protocol (see also figure 2): 4 quadrant biopsies of the antrum, 2 biopsies from the incisura and 4 biopsies from the body of the stomach, respectively, 2 from the lesser curve, and 2 from the greater curve.
Figure 2.
Biopsy strategy. (A) Sydney protocol biopsy sites in the opened stomach along the greater curvature; (B) biopsy sites in the anatomical view.
Enhanced imaging endoscopy
Patients who opt to be recruited to the study will be invited for a second endoscopy at 6–12 months interval. This will be performed by one of the experts on this protocol. The endoscopists will be blinded to any previous endoscopy or biopsy results. This second endoscopy will be recorded and performed using enhanced endoscopic imaging. Given that these patients will have recently undergone a complete upper GI endoscopy, while all anatomical landmarks will be viewed, the focus of this examination will be on the gastric mucosa. The endoscopist will record (1) the extent of gastric atrophy, (2) the presence and extent of IM in each of the aforementioned areas. This will be done using our simplified endoscopic metaplasia scoring system (GRAHAM Score) (table 1).
Table 1.
Simplified endoscopic gastric intestinal metaplasia staging system: ‘GRAHAM Score’
| Focal/minimal metaplasia (<1/3 of surface coverage) |
Moderate/extensive metaplasia (>1/3 surface coverage) |
|
| Antrum and incisura | 1 | 2 |
| Lesser curve | 1 | 2 |
| Greater curve | 1 | 2 |
Biopsies will then be taken in the following manner: (1) areas of IM found in any of the Sydney protocol areas, (2) Sydney areas negative for GIM will be randomly biopsied, as control, to complete the assessment and (3) lesions suspicious for dysplasia or malignancy.
Histopathological assessment
Each biopsy will be reviewed at the teaching hospital by one of the expert GI histopathologists named on this protocol according to the established operative link for gastritis assessment and operative link for gastric intestinal metaplasia assessment staging systems.21 Histopathologists will be blinded to whether biopsies were directed at areas suspicious for IM and to the biopsy results of WLE staging. A proportion of biopsy samples will be reviewed and rescored by a second expert GI histopathologist, who is blinded to the initial results. This is to ensure interobserver reproducibility for histopathological detection of IM.
Serology assessment
A proportion of the collected serum will be used to assess Helicobacter pylori serology, pepsinogen I/II ratio and gastrin-17. The remaining serum will then be stored for use in future studies exploring the development of molecular biomarkers for gastric atrophy risk stratification.
Data collection and management
All data collected for this study will be recorded in an anonymised format on a centralised, secure web-based platform (OpenClinica). Source data will be recorded in patients’ notes or electronic health records, and hard copies of consent forms will be stored in a secure locked cabinet per site. All study data will be stored in a linked anonymised fashion against a study number, with the registry of study numbers stored separately on an encrypted database.
Statistical analyses
For descriptive statistics, mean (±SD) will be used in case of a normal distribution of variables and median (25–75%) will be used for variables with a skewed distribution. Where appropriate, the Student’s t-test or Mann–Whitney U test will be used.
Diagnostic accuracy of endoscopic diagnosis of CAG and IM is defined as the total number of directed biopsies that confirm the endoscopic impression of the presence or absence of IM divided by the total number of biopsies (accuracy=true positives+true negatives/all biopsies). Results will be compared with the histopathology outcomes using the χ2 test after multiple testing correction as well as kappa values for interobserver agreement among endoscopists and histopathologists.
After study completion, all videos will be collated and anonymised prior to expert panel review and estimation of the severity and extent of atrophy as well as IM. Five expert reviewers will review 50 videos each (kappa 0.4) for the purposes of assessing interobserver reliability. Sensitivity, specificity and global accuracy along with the 95% confidence intervals will be established. Duration of inspection time of gastric mucosa and its relation to diagnostic accuracy will be evaluated using, when appropriate, a paired t-test or Wilcoxon test. All tests will be two-sided.
Ethics and dissemination
Results will be disseminated to potential users in academia and medical industries, through the standard routes of presentations, oral and posters, at local, national and international conferences, undergraduate and graduate teaching and through peer-reviewed publication. Efforts will be made to present work in a timely manner at key international meetings to encourage collaboration with research partners.
Discussion
The recently updated European MAnagement of Precancerous conditions and lesions in the Stomach (MAPS) guidelines recommend surveillance of patients with premalignant gastric mucosal lesions by performing endoscopy (preferably with advanced imaging) and taking random biopsies of the stomach for histopathological assessment. This enables the detection of progression to high-risk lesions and eventually cancer.22 However, various studies indicate that a marked proportion of advanced gastric lesions are missed at a stage when these lesions are potentially still amenable to endoscopic management. This implies that the risk of undertreatment is undeniable.9 The development of high-definition endoscopy and virtual chromoendoscopy has been a main focus of research in the past years and it has revolutionised the endoscopic assessment of the premalignant stomach by being superior to white light imaging.23 The updated MAPS guidelines opt for the use of advanced imaging as the preferred surveillance method. Recently, Esposito et al showed a scoring tool based on endoscopic staging using Endoscopic Grading of Gastric Intestinal Metaplasia (EGGIM) with advanced imaging as a promising decision tool to identify patients at risk of gastric cancer.24 However, currently there are no studies on how the use of advanced endoscopic imaging to detect IM of the stomach can be applied in countries with a low prevalence of IM. Still, histological confirmation is needed through random biopsies. Future steps are to evaluate the possible shift towards an endoscopy-led strategy now these marked improvements in endoscopic technology are within our reach. This prospective study was therefore designed to determine the validity of endoscopy-led staging of the premalignant stomach using advanced imaging and taking targeted biopsies for histological confirmation.
A previous comparative study between white light and high-definition endoscopy for the diagnosis of premalignant gastric lesions indeed showed a superior diagnostic accuracy of high-definition endoscopy.15 However, one limitation was that WLE and high-definition endoscopy were performed during one occasion, which implied that the endoscopist was not blinded to the WLE results. Within the current protocol, we choose to perform the procedures on two separate occasions with blinding of the expert endoscopist to the previous WLE results.
Over the years, serological markers have shown major promise for predicting the presence and severity of gastric premalignant lesions.25–27 Pepsinogens are serological markers for atrophy in the stomach and can be divided into pepsinogen I and II. A decreased PG I/II ratio indicates the presence of atrophic changes. Gastrin serum levels are indicative for gastric acid output and are increased in the presence of atrophic changes.27 The collection of serum samples was included in our protocol to strengthen risk stratification for progression of premalignant gastric lesions.
A few limitations of the study should be mentioned. All high-definition endoscopies will be performed by expert endoscopists at either site. A potential caveat with this design is the generalisability of the study outcomes to non-expert settings. To test this, we selected a panel of independent endoscopists who will review recorded endoscopy videos in order to assess interobserver variability. The same limitation holds for the histopathological evaluation of the biopsy samples. Therefore, a proportion of the samples will be reviewed and rescored by a blinded second expert GI histopathologist.
In conclusion, prospective validation of endoscopy-led staging of the premalignant stomach will provide the needed evidence for an endoscopy-led risk stratification of patients at risk for gastric adenocarcinoma. This will allow rational design of tiered screening and surveillance protocols to benefit early stage gastric cancer detection within at-risk populations. This will cause major implications for affected patients and general healthcare resource utilisation.
Supplementary Material
Footnotes
Correction notice: This article has been corrected since it was published. The licence has been updated.
Contributors: EJK and MRB conceived the idea for the study, designed the protocol and supervise study execution. MCWS, DG and MJ supervise study execution. SAVN and WW drafted the manuscript. EJK, MRB, DG, MJ, MCWS, SAVN and WW analyse and interpret data. MRJ and KB interpret data. All authors provided critical revision of the manuscript for important intellectual content and approved the final draft of the protocol for submission.
Funding: This work was supported by a grant from the Maag Lever Darm Stichting, Dutch Digestive Foundation grant number D17-22 and the Medical Research Council (clinical research training fellowship) number MR/S022244/1.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Institutional Review Boards of the Erasmus Medical Center (Erasmus MC) and University College London Hospitals (UCLH) (Netherlands: MEC-2018-078; UK: 19/LO/0089).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Stomach cancer incidence statistics, 2015. Available: http://www.cancerresearchuk.org/cancer-info/cancerstats
- 2. Global cancer statistics: World Health organization. Available: https://www.who.int/cancer/en/
- 3. Amiri M, Janssen F, Kunst AE. The decline in stomach cancer mortality: exploration of future trends in seven European countries. Eur J Epidemiol 2011;26:23–8. 10.1007/s10654-010-9522-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol 2014;20:4483–90. 10.3748/wjg.v20.i16.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song H, Held M, Sandin S, et al. Increase in the Prevalence of Atrophic Gastritis Among Adults Age 35 to 44 Years Old in Northern Sweden Between 1990 and 2009. Clin Gastroenterol Hepatol 2015;13:1592–600. 10.1016/j.cgh.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 6. Anderson WF, Camargo MC, Fraumeni JF, Jr. Age-Specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723–8. 10.1001/jama.2010.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maconi G, Manes G, Porro G-B. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol 2008;14:1149–55. 10.3748/wjg.14.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsuda T, Saika K. The 5-year relative survival rate of stomach cancer in the USA, Europe and Japan. Jpn J Clin Oncol 2013;43:1157–8. 10.1093/jjco/hyt166 [DOI] [PubMed] [Google Scholar]
- 9. Ren W, Yu J, Zhang Z-M, et al. Missed diagnosis of early gastric cancer or high-grade intraepithelial neoplasia. World J Gastroenterol 2013;19:2092–6. 10.3748/wjg.v19.i13.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc Int Open 2014;02:E46–E50. 10.1055/s-0034-1365524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (maps): guideline from the European Society of gastrointestinal endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012;44:74–94. 10.1055/s-0031-1291491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2018;21:579–87. 10.1007/s10120-018-0812-3 [DOI] [PubMed] [Google Scholar]
- 13. Miwata T, Quach DT, Hiyama T, et al. Interobserver and intraobserver agreement for gastric mucosa atrophy. BMC Gastroenterol 2015;15:95 10.1186/s12876-015-0327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toyoshima O, Yamaji Y, Yoshida S, et al. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc 2017;31:2140–8. 10.1007/s00464-016-5211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pimentel-Nunes P, Libânio D, Lage J, et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016;48:723–30. 10.1055/s-0042-108435 [DOI] [PubMed] [Google Scholar]
- 16. Xirouchakis E, Laoudi F, Tsartsali L, et al. Screening for gastric premalignant lesions with narrow band imaging, white light and updated Sydney protocol or both? Dig Dis Sci 2013;58:1084–90. 10.1007/s10620-012-2431-x [DOI] [PubMed] [Google Scholar]
- 17. Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy 2006;38:819–24. 10.1055/s-2006-944632 [DOI] [PubMed] [Google Scholar]
- 18. Banks M, Graham D, Jansen M, et al. British Society of gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019;68:1545 10.1136/gutjnl-2018-318126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 20. Cohen JF, Korevaar DA, Altman DG, et al. Stard 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6:e012799 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cho S-J, Choi IJ, Kook M-C, et al. Staging of intestinal- and diffuse-type gastric cancers with the OLGA and OLGIM staging systems. Aliment Pharmacol Ther 2013;38:1292–302. 10.1111/apt.12515 [DOI] [PubMed] [Google Scholar]
- 22. Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (maps II): European Society of gastrointestinal endoscopy (ESGE), European Helicobacter and microbiota Study Group (EHMSG), European Society of pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019. [DOI] [PubMed] [Google Scholar]
- 23. East J, Vleugels J, Roelandt P, et al. Advanced endoscopic imaging: European Society of gastrointestinal endoscopy (ESGE) technology review. Endoscopy 2016;48:1029–45. 10.1055/s-0042-118087 [DOI] [PubMed] [Google Scholar]
- 24. Esposito G, Pimentel-Nunes P, Angeletti S, et al. Endoscopic grading of gastric intestinal metaplasia (EGGIM): a multicenter validation study. Endoscopy 2019;51:515–21. 10.1055/a-0808-3186 [DOI] [PubMed] [Google Scholar]
- 25. den Hollander WJ, Holster IL, den Hoed CM, et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 2018. [DOI] [PubMed] [Google Scholar]
- 26. Huang Y-kai, Yu J-chun, Kang W-ming, et al. Significance of serum pepsinogens as a biomarker for gastric cancer and atrophic gastritis screening: a systematic review and meta-analysis. PLoS One 2015;10:e0142080 10.1371/journal.pone.0142080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leja M, Kupcinskas L, Funka K, et al. Value of gastrin-17 in detecting antral atrophy. Adv Med Sci 2011;56:145–50. 10.2478/v10039-011-0040-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.