Abstract

α-Functionalization of alkyl boronic esters and homologation of aryl boronic esters by regioselective radical C(sp3)–H activation in boron-ate complexes is reported. Reaction of commercial or readily accessed aryl boronic acid pinacol esters with alkyl lithium reagents provides boron-ate complexes. Selective α-C–H abstraction by in situ generated trifluoromethyl radicals leads to radical anions that undergo electron transfer oxidation followed by 1,2-aryl/alkyl migration from boron to carbon to give the α-arylated/alkylated alkyl boronic esters. The valuable boronic ester functionality remains in the products and the cheap trifluoromethyl iodide acts as the oxidant in these C–C couplings. The 1,2-alkyl migration from boron to carbon is highly stereospecific allowing access to stereoisomerically pure boronic esters.
Alkyl boronic esters are valuable reagents in synthesis. They are readily accessed either from commercial sources or by using established chemistry. Such boron compounds engage in various coupling reactions.1 However, the valuable boron moiety is mostly not retained in the product and chemical modification of alkyl boronic esters keeping the boron entity is not well investigated. An established and well investigated route for functionalization of alkyl boronic esters uses prefunctionalized α-halo derivatives as substrates (Scheme 1a). In these Matteson-type rearrangements, the boronic ester is first converted to its boron-ate complex upon reaction with an alkyl or aryl-metal compound. The ate-complex is then activated by a Lewis acid to induce a 1,2-alkyl/aryl migration with concomitant substitution of the halide anion (X) to give an α-alkylated/arylated boronic ester.2 Alternatively, boron-ate complexes bearing an α-anionic leaving group can be generated by α-lithiation of alkyl carbamates/esters and subsequent reaction with boronic esters, as developed by Aggarwal for the preparation of optically active alkyl boronic esters.3
Scheme 1. Chemical Modification of Alkyl Boronic Esters at the α-C-Atom with Organometallic Compounds and Homologation of Aryl Boronic Esters.
α-Halo boronic esters have been used by Fu as substrates in Ni-catalyzed cross couplings to provide α-alkylated boronic esters (Scheme 1b). These formal halide substitution reactions proceed via dialkyl-Ni-complexes as intermediates.4 Both strategies highlighted employ prefunctionalized alkyl boronic esters and correspond to formal substitution reactions. Considering step economy, the direct C–H functionalization5 of alkyl boronic esters would be even more attractive. Herein we disclose our results on radical α-C–H functionalization of various alkyl boronic esters for the preparation of α-arylated and α-alkylated boronic esters (Scheme 1c).
The suggested sequence commences with formation of a boron-ate complex of type I by reacting an alkyl boronic ester with an aryl or alkyl organometallic species (Scheme 1d).6−10 A reactive radical X should then undergo regioselective H-abstraction at the α-position to give the radical anion II. Such radical anions are known to be efficient single electron transfer (SET) reductants6d,7a,8f,10 which deliver in the reaction with a terminal oxidant X-Y the zwitter ion III along with the chain carrying radical X. III will further react in a 1,2-alkyl/aryl migration to provide the targeted α-functionalized boronic ester. However, there are several challenges associated with that reaction design: (a) the terminal oxidant X-Y should be a mild SET-oxidant that does not directly react with the starting boron-ate complex I (Ep/2 = 0.31 V vs SCE in CH3CN);11 (b) X-Y must be readily SET reduced by a radical anion II; (c) radical X derived from X-Y should be an efficient and selective H-abstractor. In the case of the aryl migration process leading to C(sp2)–C(sp3) bond formation, regioselectivity of the H-abstraction step must be controlled, since only α-H-abstraction will lead to a reducing radical anion. For the alkyl migration culminating in C(sp3)–C(sp3) bond formation, along with the intrachain selectivity control, the two alkyl substituents at the boron-ate complex have to be differentiated by radical X.
Regarding the C(sp2)–C(sp3) bond formation, such a sequence can also be achieved upon starting with an aryl boronic ester in combination with an alkyl lithium species, since the same boron-ate complex is generated as an intermediate (Scheme 1e). This important fact allows the scientist to choose the starting materials/reagents depending on their availability and costs. By selecting aryl boronic esters as starting compounds, the sequence corresponds to an unprecedented homologation12 using an alkyl lithium reagent. Considering the prerequisites of our design, we chose trifluoromethyl iodide as oxidant, since it is a weak oxidant (Ep = −1.52 V vs SCE in dimethylformamide)13 that is SET reduced by various radical anions. Moreover, the CF3-radical is reactive14 and should allow for exothermic H atom abstractions from boron-ate complexes.
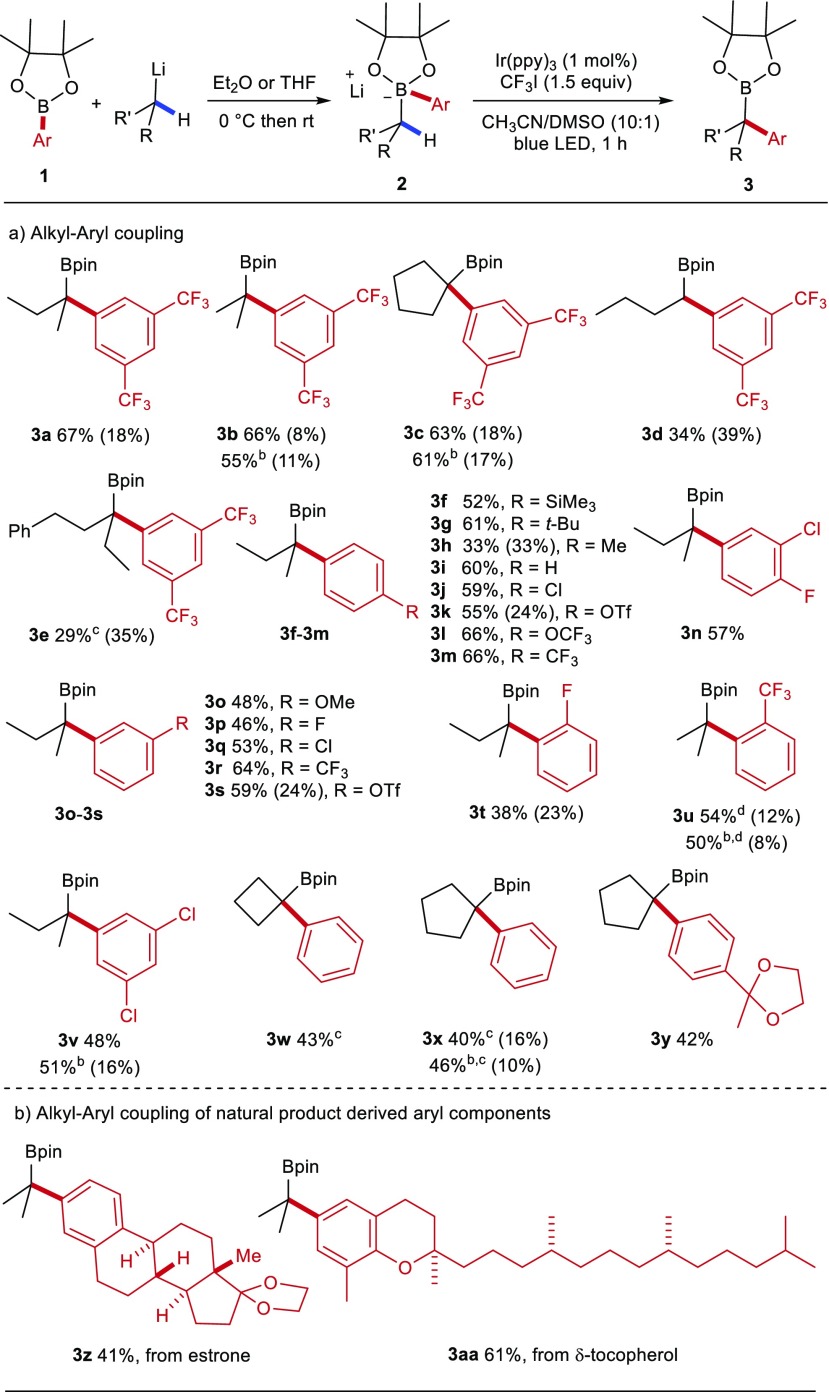
We first studied radical-induced migration in boron-ate complexes bearing an alkyl and an aryl substituent (Scheme 1e) and commenced with aryl boronic esters 1 (arylBpin) in combination with alkyl lithium reagents targeting the homologated boronic esters 3. Reaction optimization was conducted with 3,5-bistrifluoromethylphenylboronic acid pinacol ester and sec-butyl lithium (Supporting Information). The boron-ate complex 2 was generated by addition of sec-butyl lithium in diethyl ether at 0 °C. The solvent was removed and crude 2 was redissolved in acetonitrile. CF3I (1.5 equiv) was added as a solution in dimethyl sulfoxide. Different radical initiators were screened and we found that the cascade works best with tris[2-phenylpyridinato-C2,N]iridium(III) (Ir(ppy)3, 1 mol %) as a smart15 photoinitiator. Blue light irradiation for 1 h at room temperature provided 3a in 67% yield (Scheme 2a). As a side product, aryl boronic ester 1a was identified (18%), likely resulting from competing 2-butyl radical fragmentation of the SET-oxidized boron-ate complex 2a. Initiation can also be achieved with Eosin Y, Rose Bengal and Rhodamine B base or with Ru(bpy)3Cl2 (Supporting Information) and for selected cases, we showed that initiation also works without any photocatalyst by simple light emitting diode (LED) irradiation (365 nm). As expected for a radical process, the cascade was fully inhibited in the presence of 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (2 equiv). Reaction proceeds also well on isopropyl boron-ate complexes (3b) but cyclic (3c,w–y), sterically more demanding secondary-alkyl (3e) and linear alkyl systems (3d) showed lower yields. Problems are direct SET-oxidation of the boron-ate complex 2 leading to alkyl radical fragmentation to give starting ester 1 that was identified as a side product (yields in bracket). More challenging C–H activation due to stronger and sterically more shielded C–H bonds also leads to a lowering of the yield. Trifluoromethylation at sterically accessible aryl rings was also observed (see Supporting Information for 3h, 3x) which suggested the generation of a CF3 radical in the reaction.
Scheme 2. Substrate Scope for C(sp2)–C(sp3) Coupling.
Conducted at 0.2 mmol scale. Arylboronic ester identified as side product (GC-yield in bracket).
Conducted under 365 nm LED irradiation without photocatalyst.
The boron-ate complexes were formed from the corresponding alkylboronic esters and aryl lithium reagents.
Isolated as alcohol after H2O2/NaOH oxidative workup.
Keeping sec-butyl lithium, we showed that differently substituted (ortho, meta and para) aryl boronic esters engage in the homologation and the esters 3f–3v were isolated in 33–66% yields. Various functionalities such as trimethylsilyl, chloro, fluoro, trifluoromethylsulfonyl, trifluoromethyl, trifluoromethoxy and acetal are tolerated. The method is also applicable to the homologation of more complex natural product derived aryl boronic esters, as documented by the synthesis of the estrone derived 3z and the δ-tocopherol derivative 3aa (Scheme 2b). The tocopherol system bears three additional activated methine C–H bonds and a benzylic methylene moiety that all did not react, showing that H-abstraction with the CF3-radical occurs with excellent regioselectivity. Along these lines, the estrone derivative carries a benzylic methine H atom that is not interfering.
We next investigated the more challenging αC–H alkylation of alkyl boronic esters 4 (Scheme 3) first focusing on symmetrical dialkyl boron-ate complexes 5, where the two alkyl substituents do not need to be differentiated. Pleasingly, α-butylation of butyl boronic ester with butyl lithium worked and 6a was isolated in 49% yield. Moderate to good yields were noted for cyclic and noncyclic α-branched alkyl boronic esters (6b–6f, 35–77% yield). For unsymmetrical dialkyl boron-ate complexes 5, in selected cases, excellent site selectivity was achieved. For the cyclopentyl-butyl ate complex, H-abstraction occurred with a 9:1 selectivity at the cyclopentyl moiety (66%, 6h). The selectivity is understood considering that the secondary-alkyl C–H bond is weaker than the methylene C–H bond, as also observed for 6i (74%, rr = 12:1). Cyclopropane has stronger C–H bonds than other alkanes.16 This reactivity trend is well reflected by the regioselectivities obtained, where the three-membered ring does preferably act as the migrating moiety (6k,l). Considering secondary-alkyl C–H activation, the nonstrained and sterically least hindered isopropyl group in most cases gets selectively activated (6m,n,o). To our surprise, the isopropyl substituent outcompeted an α-methoxy-alkyl group and an α-ethylthiyl-alkyl group, although the methoxy and ethylthiyl groups are known to stabilize C-radicals (6p, 72%; 6q, 63%). However, for the 5-membered ring analogue, H-abstraction by the CF3-radical occurred preferably next to the N atom (6r, 76%, rr = 4:1; 6s, 58%, rr = 32:1). For the sterically hindered primary alkylboronic ester 4v, H-abstraction selectivity at the isopropyl group was exclusive (6v, rr > 99:1). These results indicate, that along with C–H bond strength, conformational effects and steric shielding play an important role. α-Functionalization also works with tert-alkyl groups as migrating substituents (6t,u).
Scheme 3. Substrate Scope for C(sp3)–C(sp3) Coupling.
Conducted at 0.2 mmol scale. Alkylboronic ester identified as a side product (GC-yield in bracket).
Conducted under 365 nm LED irradiation without a photocatalyst.
Studies were continued by investigating stereospecific reactions (Scheme 3b). With readily accessed17 enantioenriched tertiary alkyl boronic esters, isopropyl insertion was achieved in good yields and excellent stereospecificity (es = 97%) under retention of absolute configuration (6w). tert-Butyloxycarbonyl (Boc)-protected piperidine was stereoselectively α-borylated using an established methodology.18 The corresponding isopropyl lithium derived ate complex reacts with the CF3-radical with high chemoselectivity (rr = 10:1) at the isopropyl group. Subsequent stereospecific migration affords 6x in excellent stereoselectivity (44%, es > 99%). Enantiopure boronic esters can also be prepared from chiral alkenes via diastereoselective hydroboration or borylation of a chiral alkyl halide. Boron-ate complex formation with isopropyl lithium followed by radical-mediated α-C–H-activation leads to the homologation products conserving the initial stereochemistry, as documented for natural product derived terpenes and steroids (48–70%, 6y–6ab). For 6y–6ab, regioselectivity was complete.
To better understand the regioselectivities for the α-C–H abstraction, density functional theory (DFT) calculations were conducted for two representative compounds. Energies were obtained with the PWPB95-D3 double hybrid functional,19 and take solvent effects implicitly into account with the CPCM model20 (Figure 1). The thermodynamic driving force of H-abstraction from the benzylic position of ethylbenzene is larger by 5.9 kcal/mol than for the boron-ate complex. However, the free energy barrier of H-transfer from the anionic complex is lower by 4 kcal/mol (13.2 kcal/mol) which corresponds to a selectivity of around 103:1 over ethylbenzene. Due to the electrophilicity of the CF3-radical, polar effects operate. This confirms the both extremely facile and regioselective reaction of the CF3-radical next to the B atom. We have already shown7a that electron transfer from the radical anion II (see Scheme 1d) to CF3I is highly exothermic. In the complex, the σ*-orbital of CF3I overlaps with the SOMO of the radical anion II and hence a nearly barrierless SET is expected generating the zwitter ion III which rearranges without barrier to the product IV.7a
Figure 1.

DFT studies. Transition states, activation barriers and free energies of hydrogen atom transfer of phenyl isopropyl boron-ate complex (left) and ethylbenzene (right) with the CF3-radical.
We further addressed the regioselectivity of the H-abstraction for the cyclopentyl isopropyl complex 5j and the cyclohexyl isopropyl homologue 5m where a surprising reversal of the regioselectivity was experimentally observed. In agreement with the experiment, calculations revealed the H-abstraction in 5m at the isopropyl group to be favored by 3 kcal/mol. For the ate complex 5j, where the experiment showed a slight preference for H-abstraction at the cyclopentyl group, calculations revealed similar activation barriers for the two competing processes with a barrier leading to the preferred product which is 0.3 kcal/mol lower (see Supporting Information). Of note, intermolecular H-transfer reactions to C-radicals are rarely used in organic chemistry, since such HAT-processes are generally too slow. Due to its high reactivity as H-abstracting species, the CF3-radical is unique in that sense.21
Finally, we studied the initiation step of the cascade and found the trifluoromethyl iodide to be efficiently reduced by the photoexcited Ir-complex, as analyzed by Stern–Volmer quenching (Supporting Information). As an alternative initiation step, the boron-ate complex can be oxidized by the photoexcited Ir-complex, albeit less efficiently. The quantum yield of the process22 was determined to be 8.8, showing that the Ir-complex mainly acts to initiate the radical chain (see Scheme 1d) and is best described as a smart initiator.15 This is in line with the observation that various organic and inorganic redox systems initiate the chain with similar efficiency and that initiation also proceeds in the absence of any photocatalyst. For reactions run without any smart redox initiator, initiation likely proceeds by direct reduction of the CF3I/dimethyl sulfoxide complex with the boron-ate complex upon irradiation.
We are confident that the herein introduced radical C–C couplings will significantly enlarge the portfolio of boron chemistry. The starting materials are easily accessed, and special equipment is not required to run these valuable sequences.
Acknowledgments
We thank the Alexander von Humboldt foundation (postdoctoral fellowship to D.W.) and the European Research Council ERC (advanced grant agreement No. 692640) for supporting this work.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b07960.
Experimental details and characterization data, NMR spectrum of new compounds, DFT calculations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]; b Suzuki A. Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct C-C Bonds (Nobel Lecture). Angew. Chem., Int. Ed. 2011, 50, 6722–6737. 10.1002/anie.201101379. [DOI] [PubMed] [Google Scholar]
- Matteson D. S.; Mah R. W. H. Neighboring Boron in Nucleophilic Displacement. J. Am. Chem. Soc. 1963, 85, 2599–2603. 10.1021/ja00900a017. [DOI] [Google Scholar]
- Leonori D.; Aggarwal V. K. Lithiation–Borylation Methodology and Its Application in Synthesis. Acc. Chem. Res. 2014, 47, 3174–3183. 10.1021/ar5002473. [DOI] [PubMed] [Google Scholar]
- Schmidt J.; Choi J.; Liu A. T.; Slusarczyk M.; Fu G. C. A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters. Science 2016, 354, 1265–1269. 10.1126/science.aai8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Newhouse T.; Baran P. S. If C–H Bonds Could Talk: Selective C-H Bond Oxidation. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yamaguchi J.; Yamaguchi A. D.; Itami K. C-H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; c White M. C. Adding Aliphatic C–H Bond Oxidations to Synthesis. Science 2012, 335, 807–809. 10.1126/science.1207661. [DOI] [PubMed] [Google Scholar]
- a Aparece M. D.; Gao C.; Lovinger G. J.; Morken J. P. Vinylidenation of Organoboronic Esters Enabled by a Pd-Catalyzed Metallate Shift. Angew. Chem., Int. Ed. 2019, 58, 592–595. 10.1002/anie.201811782. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Myhill J. A.; Zhang L.; Lovinger G. J.; Morken J. P. Enantioselective Construction of Tertiary Boronic Esters by Conjunctive Cross-Coupling. Angew. Chem., Int. Ed. 2018, 57, 12799–12803. 10.1002/anie.201807775. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Myhill J. A.; Wilhelmsen C. A.; Zhang L.; Morken J. P. Diastereoselective and Enantioselective Conjunctive Cross-Coupling Enabled by Boron Ligand Design. J. Am. Chem. Soc. 2018, 140, 15181–15185. 10.1021/jacs.8b09909. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lovinger G. J.; Morken J. P. Ni-Catalyzed Enantioselective Conjunctive Coupling with C(sp3) Electrophiles: A Radical-Ionic Mechanistic Dichotomy. J. Am. Chem. Soc. 2017, 139, 17293–17296. 10.1021/jacs.7b10519. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Lovinger G. J.; Aparece M. D.; Morken J. P. Pd-Catalyzed Conjunctive Cross-Coupling between Grignard-Derived Boron “Ate” Complexes and C(sp2) Halides or Triflates: NaOTf as a Grignard Activator and Halide Scavenger. J. Am. Chem. Soc. 2017, 139, 3153–3160. 10.1021/jacs.6b12663. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Edelstein E. K.; Namirembe S.; Morken J. P. Enantioselective Conjunctive Cross-Coupling of Bis(alkenyl)borates: A General Synthesis of Chiral Allylboron Reagents. J. Am. Chem. Soc. 2017, 139, 5027–5030. 10.1021/jacs.7b01774. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Chierchia M.; Law C.; Morken J. P. Nickel-Catalyzed Enantioselective Conjunctive Cross-Coupling of 9-BBN Borates. Angew. Chem., Int. Ed. 2017, 56, 11870–11874. 10.1002/anie.201706719. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Zhang L.; Lovinger G. J.; Edelstein E. K.; Szymaniak A. A.; Chierchia M. P.; Morken J. P. Catalytic conjunctive cross-coupling enabled by metal-induced metallate rearrangement. Science 2016, 351, 70–74. 10.1126/science.aad6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kischkewitz M.; Okamoto K.; Mück-Lichtenfeld C.; Studer A. Radical-polar crossover reactions of vinylboron ate complexes. Science 2017, 355, 936–938. 10.1126/science.aal3803. [DOI] [PubMed] [Google Scholar]; b Das S.; Daniliuc C. G.; Studer A. Lewis Acid Catalyzed Stereoselective Dearomative Coupling of Indolylboron Ate Complexes with Donor–Acceptor Cyclopropanes and Alkyl Halides. Angew. Chem., Int. Ed. 2018, 57, 4053–4057. 10.1002/anie.201711923. [DOI] [PubMed] [Google Scholar]; c Gerleve C.; Kischkewitz M.; Studer A. Synthesis of alpha-Chiral Ketones and Chiral Alkanes Using Radical Polar Crossover Reactions of Vinyl Boron Ate Complexes. Angew. Chem., Int. Ed. 2018, 57, 2441–2444. 10.1002/anie.201711390. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kischkewitz M.; Gerleve C.; Studer A. Radical-Polar Crossover Reactions of Dienylboronate Complexes: Synthesis of Functionalized Allylboronic Esters. Org. Lett. 2018, 20, 3666–3669. 10.1021/acs.orglett.8b01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Stymiest J. L.; Bagutski V.; French R. M.; Aggarwal V. K. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 2008, 456, 778. 10.1038/nature07592. [DOI] [PubMed] [Google Scholar]; b Burns M.; Essafi S.; Bame J. R.; Bull S. P.; Webster M. P.; Balieu S.; Dale J. W.; Butts C. P.; Harvey J. N.; Aggarwal V. K. Assembly-line synthesis of organic molecules with tailored shapes. Nature 2014, 513, 183. 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rasappan R.; Aggarwal V. K. Synthesis of hydroxyphthioceranic acid using a traceless lithiation–borylation–protodeboronation strategy. Nat. Chem. 2014, 6, 810. 10.1038/nchem.2010. [DOI] [PubMed] [Google Scholar]; d Sandford C.; Rasappan R.; Aggarwal V. K. Synthesis of Enantioenriched Alkylfluorides by the Fluorination of Boronate Complexes. J. Am. Chem. Soc. 2015, 137, 10100–10103. 10.1021/jacs.5b05848. [DOI] [PubMed] [Google Scholar]; e Bootwicha T.; Feilner J. M.; Myers E. L.; Aggarwal V. K. Iterative assembly line synthesis of polypropionates with full stereocontrol. Nat. Chem. 2017, 9, 896. 10.1038/nchem.2757. [DOI] [PubMed] [Google Scholar]; f Silvi M.; Sandford C.; Aggarwal V. K. Merging Photoredox with 1,2-Metallate Rearrangements: The Photochemical Alkylation of Vinyl Boronate Complexes. J. Am. Chem. Soc. 2017, 139, 5736–5739. 10.1021/jacs.7b02569. [DOI] [PubMed] [Google Scholar]; g Fawcett A.; Biberger T.; Aggarwal V. K. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem. 2019, 11, 117–122. 10.1038/s41557-018-0181-x. [DOI] [PubMed] [Google Scholar]
- a Panda S.; Ready J. M. Palladium Catalyzed Asymmetric Three-Component Coupling of Boronic Esters, Indoles, and Allylic Acetates. J. Am. Chem. Soc. 2017, 139, 6038–6041. 10.1021/jacs.7b01410. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Panda S.; Ready J. M. Tandem Allylation/1,2-Boronate Rearrangement for the Asymmetric Synthesis of Indolines with Adjacent Quaternary Stereocenters. J. Am. Chem. Soc. 2018, 140, 13242–13252. 10.1021/jacs.8b06629. [DOI] [PubMed] [Google Scholar]
- Tappin N. D. C.; Gnägi-Lux M.; Renaud P. Radical-Triggered Three-Component Coupling Reaction of Alkenylboronates, α-Halocarbonyl Compounds, and Organolithium Reagents: The Inverse Ylid Mechanism. Chem. - Eur. J. 2018, 24, 11498–11502. 10.1002/chem.201802384. [DOI] [PubMed] [Google Scholar]
- Shu C.; Noble A.; Aggarwal V. K. Photoredox-Catalyzed Cyclobutane Synthesis by a Deboronative Radical Addition–Polar Cyclization Cascade. Angew. Chem., Int. Ed. 2019, 58, 3870–3874. 10.1002/anie.201813917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. S. Boronic Esters in Asymmetric Synthesis. J. Org. Chem. 2013, 78, 10009–10023. 10.1021/jo4013942. [DOI] [PubMed] [Google Scholar]
- Andrieux C. P.; Gelis L.; Medebielle M.; Pinson J.; Saveant J. M. Outer-sphere dissociative electron transfer to organic molecules: a source of radicals or carbanions? Direct and indirect electrochemistry of perfluoroalkyl bromides and iodides. J. Am. Chem. Soc. 1990, 112, 3509–3520. 10.1021/ja00165a040. [DOI] [Google Scholar]
- Studer A. A “Renaissance” in Radical Trifluoromethylation. Angew. Chem., Int. Ed. 2012, 51, 8950–8958. 10.1002/anie.201202624. [DOI] [PubMed] [Google Scholar]
- Studer A.; Curran D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem., Int. Ed. 2016, 55, 58–102. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- Tian Z.; Fattahi A.; Lis L.; Kass S. R. Cycloalkane and Cycloalkene C–H Bond Dissociation Energies. J. Am. Chem. Soc. 2006, 128, 17087–17092. 10.1021/ja065348u. [DOI] [PubMed] [Google Scholar]
- Pulis A. P.; Blair D. J.; Torres E.; Aggarwal V. K. Synthesis of Enantioenriched Tertiary Boronic Esters by the Lithiation/Borylation of Secondary Alkyl Benzoates. J. Am. Chem. Soc. 2013, 135, 16054–16057. 10.1021/ja409100y. [DOI] [PubMed] [Google Scholar]
- Varela A.; Garve L. K. B.; Leonori D.; Aggarwal V. K. Stereocontrolled Total Synthesis of (−)-Stemaphylline. Angew. Chem., Int. Ed. 2017, 56, 2127–2131. 10.1002/anie.201611273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerigk L.; Grimme S. Efficient and Accurate Double-Hybrid-Meta-GGA Density Functionals—Evaluation with the Extended GMTKN30 Database for General Main Group Thermochemistry, Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2011, 7, 291–309. 10.1021/ct100466k. [DOI] [PubMed] [Google Scholar]
- Barone V.; Cossi M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. 10.1021/jp9716997. [DOI] [Google Scholar]
- Gong J.; Fuchs P. L. Alkynylation of C–H Bonds via Reaction with Acetylenic Triflones. J. Am. Chem. Soc. 1996, 118, 4486–4487. 10.1021/ja953518p. [DOI] [Google Scholar]
- Cismesia M. A.; Yoon T. P. Characterizing chain processes in visible light photoredox catalysis. Chem. Sci. 2015, 6, 5426–5434. 10.1039/C5SC02185E. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.