Abstract
The complete resection offers the best long-term survival for advanced hepatocellular carcinoma patients. ALPPS as a choice of resection, how is its outcome compared to one-stage resection, liver transplantation and TACE? This retrospective study included 20 ALPPS patients. To minimize the effect of confounding influences of measured covariates, PSM was performed. The overall survival (OS), morbidity, mortality and the increasing rate, KGR were analyzed. The OS in ALPPS group is 27.4 (±3.8 months) moths and the TACE group is 13.5(±1.2 months) (P < .001), LT group is 41.3 (±3.2 months) (P = .048), Resection group is 31.8 (±2.6 months) (P = .368). And the medium increasing volume is 209.5 cm3 (±61.5 cm3) with the increasing ratio 52.4% (+26.9%). The ALPPS is a feasible treatment for HCC patients and it provides a better long-term survival than TACE and it is similar to Resection, less than LT.
Keywords: ALPPS, overall survival, TACE, treatment
1. Introduction
To the advanced hepatocellular carcinoma (HCC) patients, the complete resection offers the best long-term survival.[1] While the main factor of limiting resection is the amount of the future liver remnant (FLR). It is currently considered that the risk of postoperative liver failure is great if its FLR/standard liver volume(SLV) <25% or FLR/body weight (BW) <0.5% for normal livers, and for livers with cirrhosis, FLR/SLV <40% or FLR/body weight <0.8%.[2,3] In China, the HCC patients often have the cirrhosis background with HBV infection.[4] So the PHLF is feared after resection because the insufficient FLR.
To increase the resectability, PVE have been performed in the past years to increase the FLR,[5,6] and the FLR would increase up to 10% to 46% after 4 to 8 weeks.[7] However, some patients would not get the operation for the absence of increasing FLR or the progression of the tumor.[8]
In recent year, a novel method, Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) was debated all over the world, because this method will induce the FLR increasing rapidly and obviously (40%–160% within 1 to 2 week).[9–12] However, the high morbidity and mortality have been reported.[13,14] Till date, these reports are most about patients with colorectal liver metastases (CRLM).[15] As for HCC patients, the ALPPS is recommend to perform carefully, and some studies suggested that the HCC is the relative contraindication.[16] But the studies of the ALPPS for HCC are case reports in majority. There is still lack of evidence.
The therapies for HCC are liver transplantation (LT), resection, ablation, TACE and sorafenib. LT is the best therapy for HCC if it fulfilled the standard, then it is the resection. TACE as a therapy to HCC, however, has a low long-term rate.[17] sorafenib is a palliative care therapy. For patients with ALPPS, the main factor of choice is the lack of FLR. Prior to this, the treatment that this group of patients could receive was TACE. So, is this part of the patients who should have been treated with TACE improved the long-term survival rate after receiving ALPPS? Is there a difference between the long-term survival rate of ALPPS surgery and the other treatments? In this study, the main purpose is to answer these questions.
2. Method
2.1. Patients
This study retrospect patients from August 2014 to August 2018 in West China Hospital. There are 20 ALPPS patients, 985 one-stage resection patients and 128 LT patients and 1105 TACE patients. All patients were told that the results might be applied to clinical studies, and the patients were referred to the current treatment of primary liver cancer, including LT, hepatectomy, radiofrequency ablation, TACE, sorafenib drug therapy, etc. The patient makes the final choice for treatment. ALPPS patients were also informed in detail about the benefits and risks of ALPPS surgery and signed informed consent.
All the ALPPS patients are diagnosed HBV infection with HBV surface antigen or/and HBV-DNA positive. The patients underwent a routine preoperative evaluation, including computed tomography (CT) or magnetic resonance (MRI) to calculate the FLR, transient elastography ultrasound to evaluate the fibrosis, blood test, tumor marker test and so on. Postoperative complications were defined according to the Clavien–Dindo classification and severe complications were defined as grade IIIb or higher.[18] The International Study Group of Liver Surgery (ISGLS) was applied to define the PHLF.[19] The routine hematoxylin & eosin (HE) and immunohistochemical staining were reviewed. The degree of liver fibrosis was considered the Ishak score, and it was gauged as: 0 (no fibrosis), 1 to 2 (mild fibrosis), 3 to 4 (moderate fibrosis), 5 (sever fibrosis), 6 (cirrhosis).[20]
And to minimize the effect of confounding influences of measured covariates, PSM was performed. The three 1:1 matched study groups were created by matching as closely as possible using the following variables: HCC, age, gender, HBV-DNA, tumor size, tumor number, Child- Pugh Score, MELD, and a caliper of 0.02 multiplied by the standard deviation of the logit of the propensity score was used.
2.2. Volumetric assessment
The FLR was measured by the software IQQA-Liver; EDDA Technology Inc, Princeton, NJ. The SLV was calculated by the formula: SLV = 706.2 × BSA + 24.[21] Meanwhile, the Kinetic growth rates (KGR) was calculated too. When the FLR/SLV >40%, the second step was considered, for those patients all have the cirrhosis background, and the ratio >25% for those patients without.
2.3. ALPPS surgical technique
2.3.1. First step
The patient was placed in the dorsal decubitus position, then we performed a “J” incision below the right costal margin. We did the surgery using the anterior approach. An intraoperative ultrasound (IOUS) examination was required to reconfirm the resectability by checking the size of the tumor, the number of tumors, any affected vessels, the presence of cancer embolus and the free FLR, as well as to determine the scission line. If there were several tumors, local excision and radiofrequency ablation were considered. Routine cholecystectomy was performed. We first found the hepatic pedicle, dissected the left and right portal veins, artery and bile duct, then the right portal vein was ligated. A tape was left around the right hepatic artery to identify it during the second step. After that, we dissected the secondary porta of the liver and identified the right, middle and left hepatic veins, also leaving tape around the right hepatic vein to make it easy to identify during the second step. With an ultrasonic dissector, a liver transection was performed according to the scission line determined during the IOUS examination. The liver was split to the level of the inferior vena cava. We used 5 to 0 or 4 to 0 Prolene sutures and metal clips to ensure hemostasis and biliostasis. Two abdominal drains were left at the cut surface of the split liver and the foramen of Winslow, then the abdomen was closed without leaving a plastic bag around the liver or a biomembrane at the cut surface.
2.3.2. Second step
When the radiological evaluation showed that the FLR was large enough to fit the criteria, the second step was performed the next day. We performed a relaparotomy through the previous incision. To dissect the adhesion, we first found the markers left during the first step to identify the right portal vein, bile duct, artery and the right and middle hepatic veins. These were ligated and sectioned in all patients. Finally, we removed the diseased liver from the abdominal cavity after carefully checking the cut surface to make sure there was no bleeding or bile leakage. Two abdominal drains were left at the cut surface and the foramen of Winslow, then we closed the abdomen.
The patients who are judged not to have enough FLR if resected in one step, the TACE is performed to induce the FLR.
2.4. Statistics
All data were analyzed with SPSS 23.0 software (SPSS Inc, Chicago IL). Data were expressed as mean ± SD and median (range values). The Kaplan–Meier method was used to calculate patient survival rate. The significance of difference between the 2 groups was determined by the log-rank test. P < .05 was considered to be statistically significant.
3. Result
3.1. Preoperative information
Twenty patients with ALPPS included 19 males, 1 female, and 20 patients were HCC patients with HBV infection. The average age was 48.6 ± 8.5 years old, and the average tumor size was 10.1 + 4.2 cm. The MELD score averaged 5.8. Nineteen patients were Child-Pugh grade A and one was Child-Pugh grade B. According to BCLC criterion, there are 11 patients with Stage A, 4 patients with Stage B and 5 patients with stage C. All the patients with Stage A are single huge HCC which the FLR is insufficient for surgery.
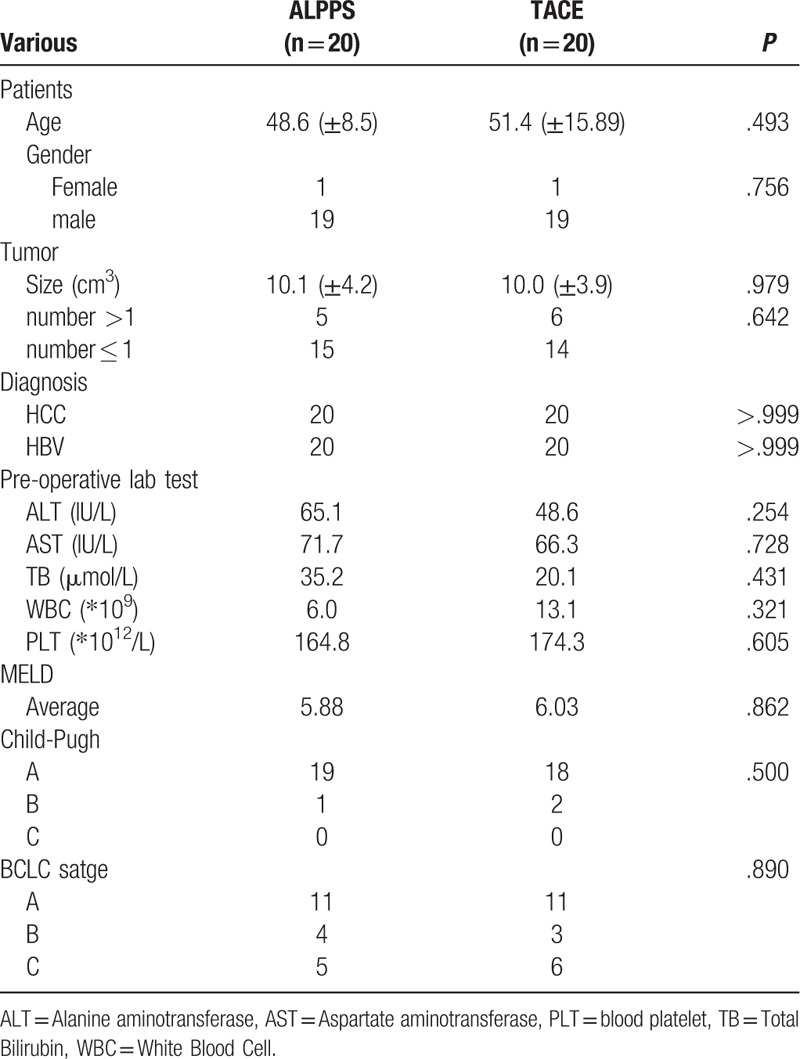
Among the 20 patients with TACE matched by PSM, including 19 males, 1 female, and 20 patients were HBV-infected HCC. The average age was 51.4 ± 15.89 years old, the average tumor size was 10.0 + 3.9 cm. There are 11 patients with Stage A, 3 patients with Stage B and 6 patients with Stage C. The average MELD score was 6.03. Eighteen patients were Child-Pugh A grade, 2 were Child-Pugh grade B. The patients with Stage A are single huge HCC which the FLR is insufficient for surgery. Table 1
Table 1.
The baseline of the patients in ALPPS vs TACE group.
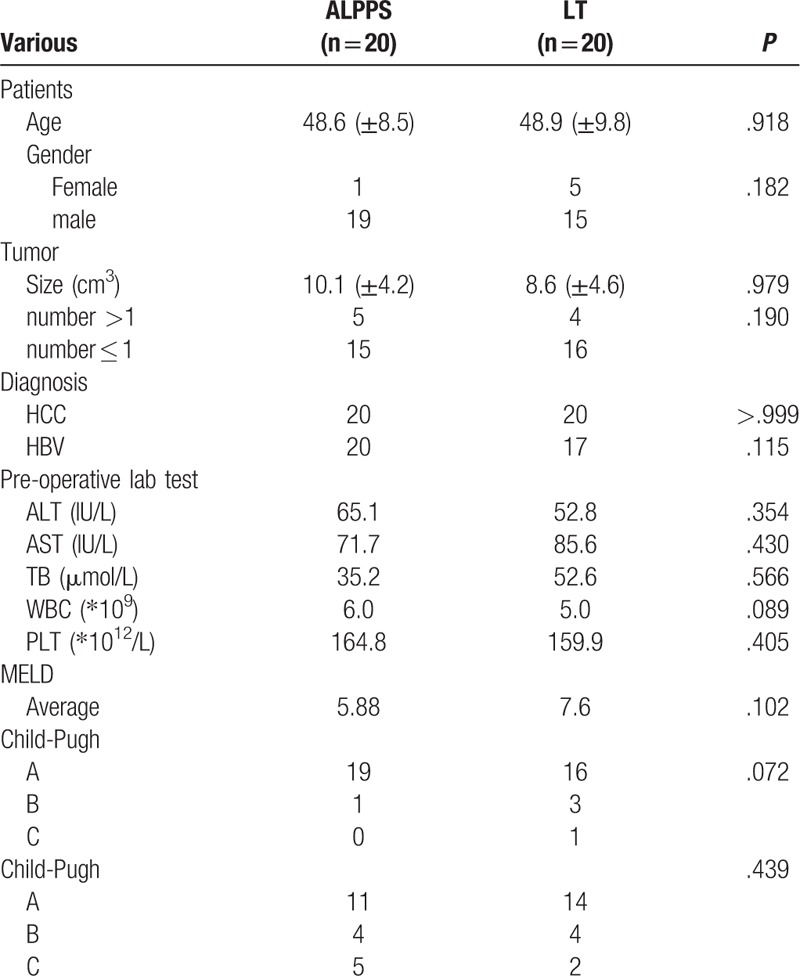
Among the 20 patients with LT who were matched by PSM, including 15 males and 5 females, 17 patients were HBV-infected HCC, and 3 patients were non-HBV-infected HCC. The mean age was 48.9 + 9.8 years, the average tumor size was 8.6 ± 4.6 cm. There are 14 patients with Stage A, 4 patients with Stage B and 2 patients with Stage C. The average MELD score was 7.6. 16 patients were Child-Pugh Class A, 3 were Child-Pugh Class B, and 1 was Child-pugh Class C. Table 2
Table 2.
The baseline of the patients in ALPPS vs LT group.
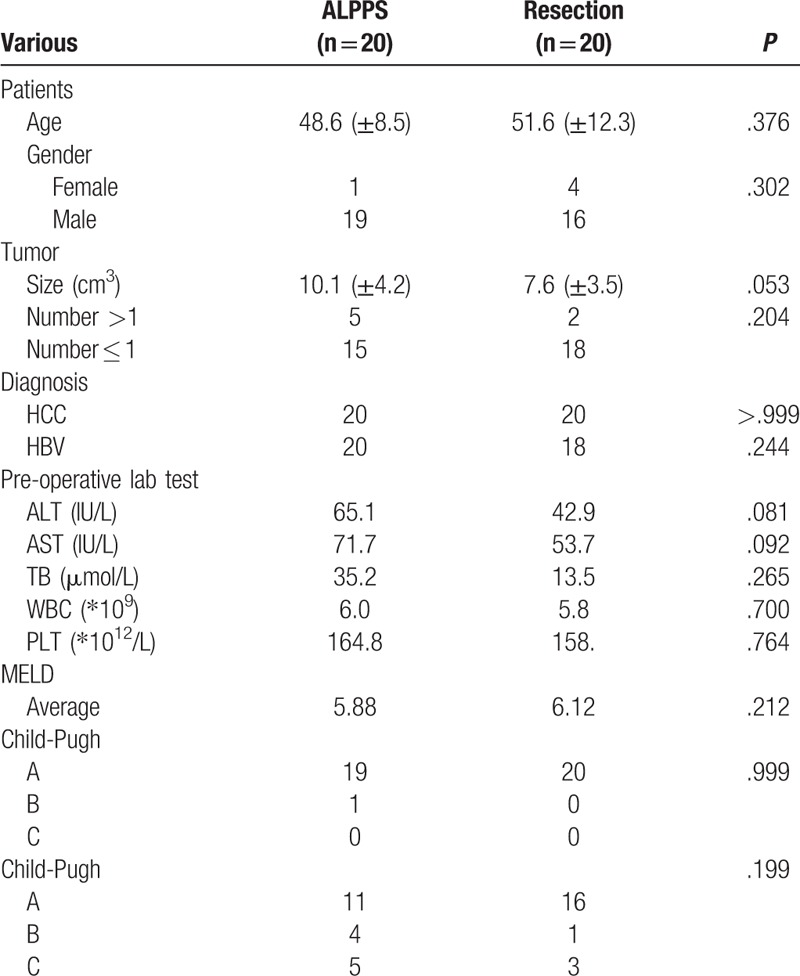
Among the 20 routinely resected patients with PSM, including 16 males and 4 females, 18 patients with HBV-infected HCC, and 2 patients with non-HBV-infected HCC. The average age was 51.6 ± 12.3 years old, the average tumor size was 7.6 + 3.5 cm. There are 16 patients with Stage A, 1 patient with Stage B and 3 patients with Stage C. Table 3
Table 3.
The baseline of the patients in ALPPS vs resection group.
3.2. The post-operative information
Twenty patients with ALPPS were right hepatic tumors, who underwent right hepatic or expanded right hepatectomy. One patient had a nodule of 1 cm in size in the left liver and was locally resected in the first operation.
One patient who was judged not to have enough FLR after stage 1, the TACE was performed and he met the standard at the 30th day after stage one. The hospital days are 29.4 days in ALPPS group, while the interval days between stage 1 and stage 2 are 15.3 days (range from 7–30 days).
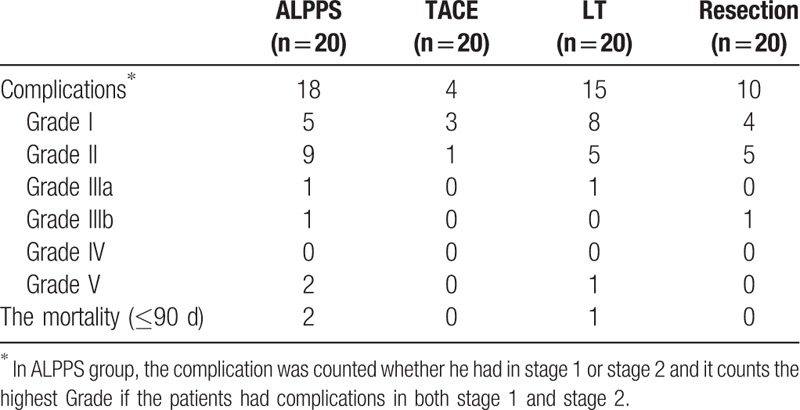
According to the Clavien–Dindo standard, at the ALPPS group, there are 3 severe complications including Grade IIIb, n = 1, and Grade V, n = 2 in ALPPS group. No severe complications happened in TACE group. There is 1 severe complication which is Grade V in LT group and there is also 1 severe complication happened in Resection group with Grade IIIb. Tables 4 and 5.
Table 4.
The complications after ALPPS stage 1 and 2.
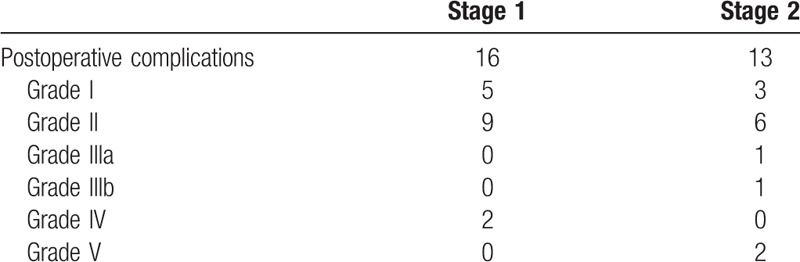
Table 5.
The post-operative information between ALPPS and TACE group.
3.3. FLR volume increasing
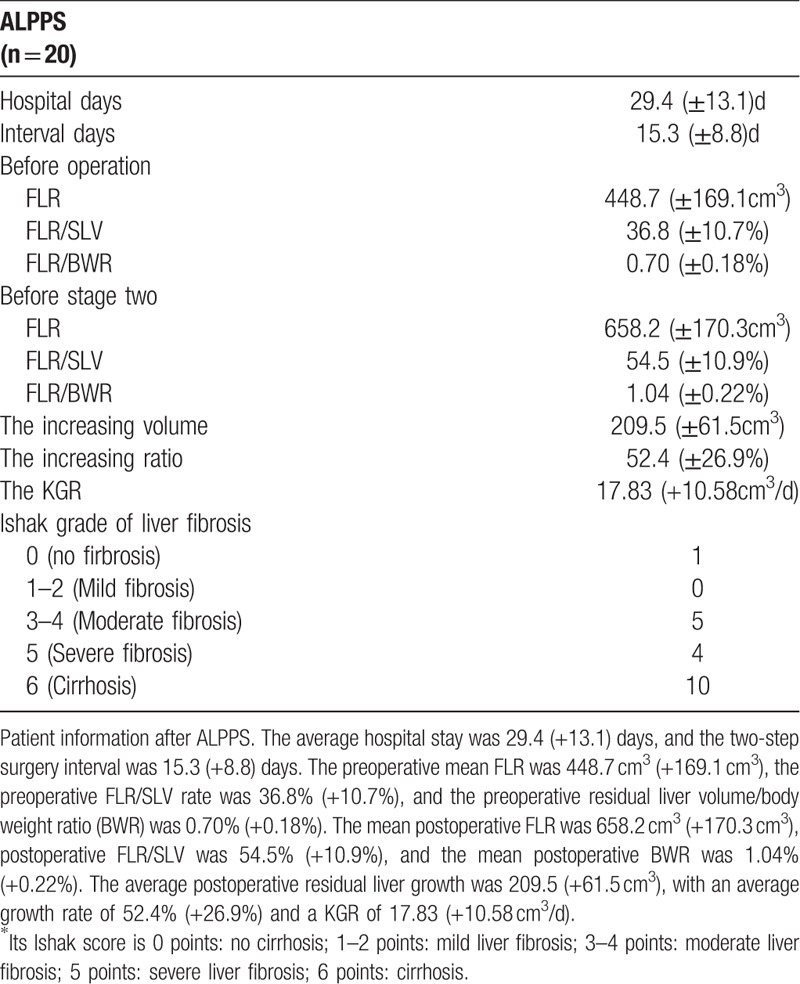
The medium pre-opreative FLR is 448.7cm3 (±169.1 cm3), and the medium pre-operative FLR/SLV is 36.8% (±10.7%), the medium pre-operative FLR to body weight ratio (BWR) is 0.70% (±0.18%). While the medium post-operative FLR is 658.2 cm3 (±170.3 cm3) and the medium Post-operative FLR/SLV is 54.5% (±10.9%), the medium post-operative FLR to BWR is 1.04% (±0.22%).
So, the medium increasing volume is 209.5 cm3 (±61.5 cm3) with the increasing ratio 52.4% (+26.9%). The KGR is 17.83 (±10.58 cm3/day).
And the Ishak score were as follows: score 0 (no fibrosis), n = 1, score 1–2 (Mild fibrosis), n = 0, score 3 to 4 (Moderate fibrosis), n = 5, score 5 (Severe fibrosis), n = 4, score 6 (Cirrhosis), n = 10. Interestingly, the patients with score 0 has a better volume increasing tendency than others even the statistic could not be applied. These are showed in Table 6.
Table 6.
The information for ALPPS group.
3.4. The follow-up information
All the patients have been well followed-up. The medium survival in ALPPS group is 27.4 (±3.8 months) moths and the TACE group is 13.5 (±1.2 months) (P < .001). The 1 and 3 years of OS is 69.6% and 58.9% in ALPPS group vs 55% and 5% in TACE group, respectively (P < .001). The medium survival in LT group is 41.3 (±3.2 months), the 1 and 3 years of OS is 90% and 77.9% (P = .048 compared to ALPPS group). And the medium survival is 31.8 (±2.6 months) in Resection group, the 1 and 3 years of OS is 85% and 61.1%(P = .368 compared to ALPPS group). Those are showed in Figures 1–3.
Figure 1.
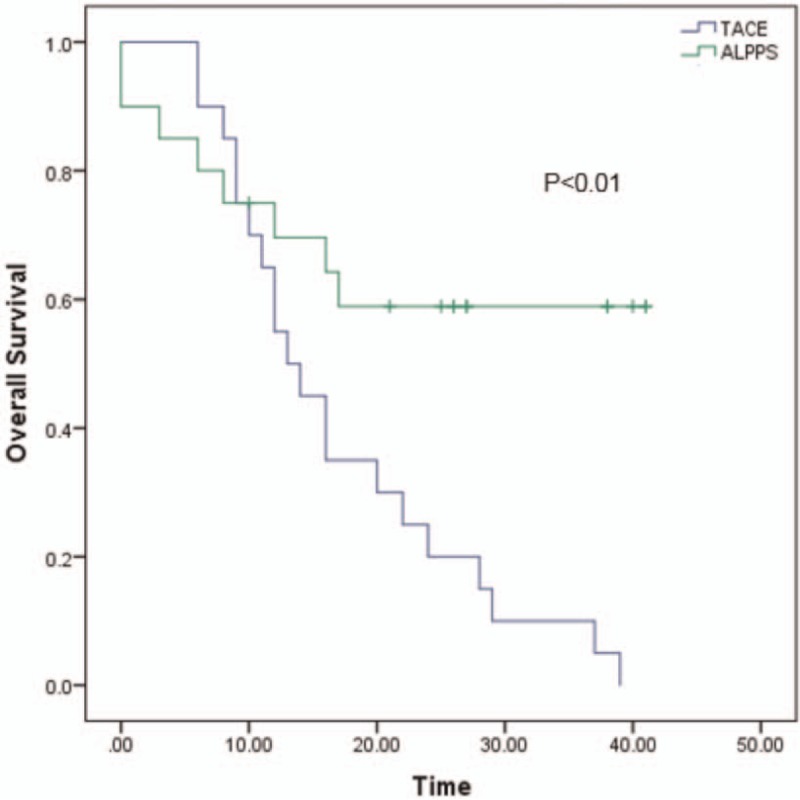
The overall survival between ALPPS and TACE. The 1 and 3 years of overall survival is 69.6% and 58.9% in ALPPS group vs 55% and 5% in TACE group, respectively (P < .001). The blue line represents TACE and the green line represents ALPPS. ALPPS = Associating liver partition and portal vein ligation for staged hepatectomy.
Figure 3.
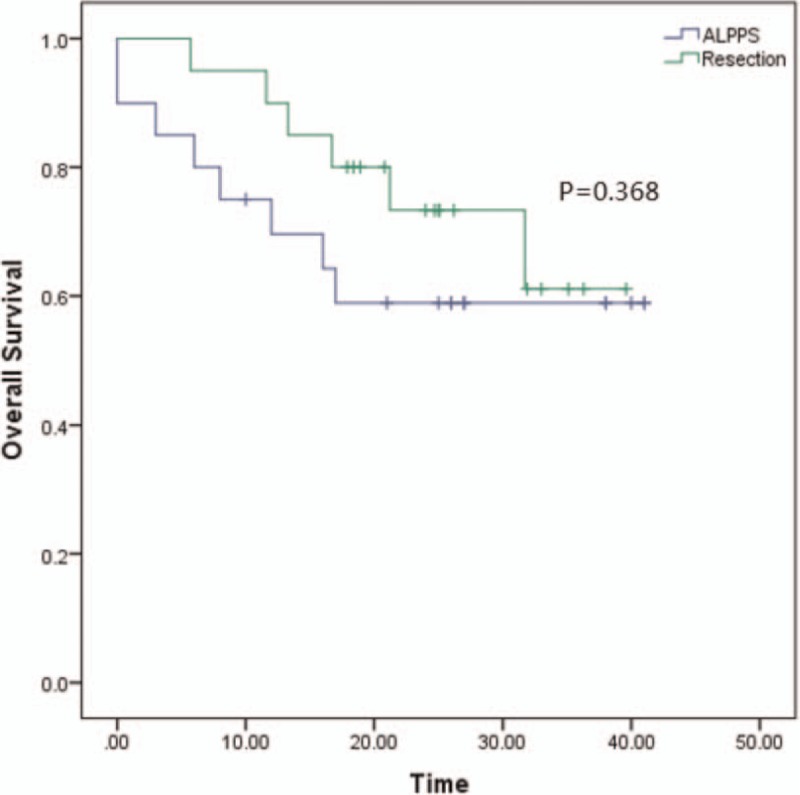
The overall survival between ALPPS and Resection. The 1 and 3 years of OS is 69.6% and 58.9% in ALPPS group vs 85% and 61.1% in LT group, respectively (P = .368). The blue line represents ALPPS and the green line represents resection. ALPPS = Associating liver partition and portal vein ligation for staged hepatectomy.
Figure 2.
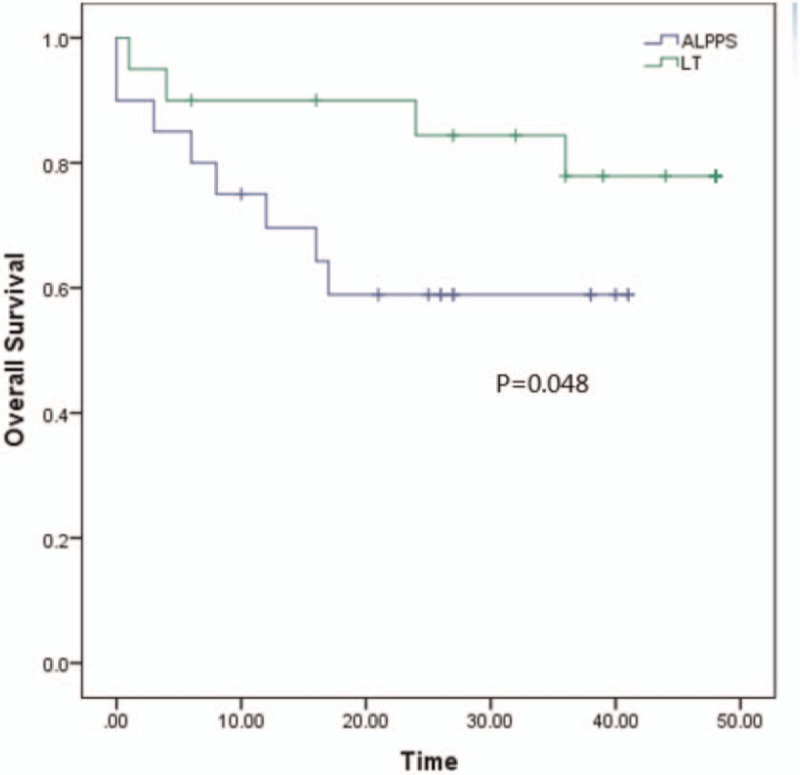
The overall survival between ALPPS and LT. The 1 and 3 years of OS is 69.6% and 58.9% in ALPPS group vs 90% and 77.9% in LT group, respectively (P = .048). The blue line represents ALPPS and the green line represents LT. ALPPS = Associating liver partition and portal vein ligation for staged hepatectomy.
4. Discussion
The complete resection is the best choice to the advanced HCC patients.[1] The main factor to limit resection is the FLR which is likely to be PHLF if the FLR is not enough to maintain the liver function especially for the patients with cirrhosis. For those unresectable patients, TACE is recommend.[22] While to increase the resectability and the FLR, PVE is widely performed with the FLR increasing up to 10% to 46% within 4 to 8 weeks.[7]However, recent years ALPPS has been performed and debated over the world for its rapid FLR increasing time and obviously FLR increasing rate.[9–12,23]
For PVE, the current reports show that the median time from PVE to feasible hepatectomy is 28 days which is much longer than the two-step interval of ALPPS surgery 15.3 days.[24] Shindoh et al also support this view, suggesting that ALPPS surgery has a shorter interval than post-PVE resection and has a lower rate of tumor progression than post-PVE resection.[25] At the same time, for CRLM patients, multicenter clinical randomized trials show that ALPPS improves resectability compared with PVE.[26] Reviewing these reports, the patients are most CRLM and the surgery for HCC patients was not investigated well. Is ALPPS available for those patients? In our study, the medium post-operative FLR is 658.2 cm3 (±170.3 cm3) and the medium Post-operative FLR/SLV ratio is 54.5% (±10.9%). The medium increasing volume is 209.5 cm3 (±61.5 cm3) and the medium increasing ratio is up to 52.4% (+26.9%). The increasing ratio may be less than those studies reported,[23,27] but it is do enough for our cirrhosis patients to endure the surgery. So, we may have the same view with the Chan.A. C reported that ALPPS was available for HCC patients.[28]
The medium interval day with 15.3 (±8.8) days was observed in our study which is much less than 4 to 8 weeks in PVE,[7,24] and the other studies have the similar result with ours.[12,23,29]
After PSM matching, there was no statistical difference in baseline between the ALPPS group and other LT groups, Resection group, and TACE group. Compared the overall survival time of ALPPS group and the TACE group, a significantly difference was observed with 27.4 (±3.8 months) moths in ALPPS group vs 13.5 (±1.2 months) in TACE group (P < .001). The TACE group has a similar OS with those studies.[30–32] In the Resection group, the medium survival is 31.8 (±2.6months) and the OS compared to ALPPS is no significant (P = .368). While in the LT group, the medium survival is 41.3 (±3.2months), the OS is significant compared to ALPPS (P = .048). We may conclude that the ALPPS group has a better long-term survival than the TACE group, less than LT group, and there is no significant with Resection group according to our study. Especially for those patients who received TACE treatment for the insufficient FLR to surgery, the ALPPS could improve their OS. Recent report has a similar conclusion with ours.[33] In addition there is a case report for ALPPS to save the unsuccessful TACE.[34]
The high morbidity and mortality in ALPPS is the outstanding shortcomings.[13,14] In our study the mortality is 10% and the severe complication rate is 15% in ALPPS group. However, the ALPPS Registry reported 31% mortality while other studies have similar mortality (0%–12%) with ours.[28,35–38] This might because ALPPS is a mature surgery now, doctors would try their best to reduce the complications. However, there is a small sample, the statistic needs to be examed by a large sample. Analyzing the 2 death cases in ALPPS, we found 1 case is because the ligation of the common bile duct and another is for the liver failure with the high TB before stage 2. As the Clavien said, the TB is a risk factor to influence the ALPPS procedure.[16,39] The age, degree of fibrosis, MELD score, Child-Pugh score, are also considered the risk factors for the poor outcome of ALPPS. So, the high selected patients are necessary for HCC patients especially those who have cirrhosis background.
This study also has some limitations. First, this is a retrospective study, the evidenced is not well. Second, the number of the sample are small, the PSM could not eliminate the basis completely. we still collect the adaptable patients prospectively the future study is ongoing. Third this is a single center's experience, the multiple RCT should be conduct in future.
5. Conclusion
According our study, the ALPPS is a feasible treatment for HCC patients and it provides a better long-term survival than TACE, and has a similar long-term survival with Resection, while it is worse than LT. So, the ALPPS could be performed to those patients who are considered unresectable tumors conventionally, and the patient should be high selected.
Author contributions
Resources: Chang Liu.
Supervision: Tianfu Wen.
Writing – original draft: Chihan Peng.
Writing – review & editing: Chuan Li, Tianfu Wen, Jiayin Yang, Bo Li, Lvnan Yan.
Chihan Peng orcid: 0000-0001-6278-3598.
Footnotes
Abbreviations: ALPPS = associating liver partition and portal vein ligation for staged hepatectomy, CRLM = colorectal liver metastases, FLR = future liver remnant, HCC = hepatocellular carcinoma, IOUS = intraoperative ultrasound, ISGLS = International Study Group of Liver Surgery, LT = liver transplantation, OS = overall survival, PSM = Propensity Score Matching, SLV = standard liver volume.
How to cite this article: Peng C, Li C, Liu C, Wen T, Yang J, Li B, Yan L. The outcome of the HCC patients underwent ALPPS. Medicine. 2019;98:38(e17182).
This research was supported by grants from Scientific and Technological Support Project of Sichuan Province 17PJ393. And this study was supported by grants from the “1.3.5 project for discipline of excellence, West China Hospital, Sichuan University (ZY2017308)
The authors have no conflicts of interests to disclose.
References
- [1].Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg 2011;253:656–65. [DOI] [PubMed] [Google Scholar]
- [2].Rahbari NN, Reissfelder C, Koch M, et al. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol 2011;18:3640–9. [DOI] [PubMed] [Google Scholar]
- [3].Tucker ON, Heaton N. The 'small for size’ liver syndrome. Curr Opin Crit Care 2005;11:150–5. [DOI] [PubMed] [Google Scholar]
- [4].El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–73. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521–7. [PubMed] [Google Scholar]
- [6].Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386–94. [DOI] [PubMed] [Google Scholar]
- [7].Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg 2009;197:686–90. [DOI] [PubMed] [Google Scholar]
- [8].Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49–57. [DOI] [PubMed] [Google Scholar]
- [9].Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405–14. [DOI] [PubMed] [Google Scholar]
- [10].Alvarez FA, Ardiles V, Sanchez Claria R, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg 2013;17:814–21. [DOI] [PubMed] [Google Scholar]
- [11].Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, et al. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery 2015;157:194–201. [DOI] [PubMed] [Google Scholar]
- [12].Knoefel WT, Gabor I, Rehders A, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg 2013;100:388–94. [DOI] [PubMed] [Google Scholar]
- [13].Sala S, Ardiles V, Ulla M, et al. Our initial experience with ALPPS technique: encouraging results. Updat Surg 2012;64:167–72. [DOI] [PubMed] [Google Scholar]
- [14].Nadalin S, Capobianco I, Li J, et al. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol 2014;52:35–42. [DOI] [PubMed] [Google Scholar]
- [15].Lau WY, Lai EC, Lau SH. Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development. Hepatobiliary Pancreat Dis Int 2017;16:17–26. [DOI] [PubMed] [Google Scholar]
- [16].Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829–36. discussion 36–8. [DOI] [PubMed] [Google Scholar]
- [17].Lee S, Kim BK, Song K, et al. Subclassification of Barcelona Clinic Liver Cancer B and C hepatocellular carcinoma: A cohort study of the multicenter registry database. J Gastroenterol Hepatol 2016;31:842–7. [DOI] [PubMed] [Google Scholar]
- [18].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–24. [DOI] [PubMed] [Google Scholar]
- [20].Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- [21].Urata K, Hashikura Y, Ikegami T, et al. Standard liver volume in adults. Transplant Proc 2000;32:2093–4. [DOI] [PubMed] [Google Scholar]
- [22].Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Croome KP, Hernandez-Alejandro R, Parker M, et al. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB 2015;17:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Glantzounis GK, Tokidis E, Basourakos SP, et al. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur J Surg Oncol 2017;43:32–41. [DOI] [PubMed] [Google Scholar]
- [25].Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126–33. discussion 33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sandstrom P, Rosok BI, Sparrelid E, et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a scandinavian multicenter randomized controlled trial (LIGRO Trial). Ann Surg 2018;267:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Matsuo K, Murakami T, Kawaguchi D, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016;159:1289–98. [DOI] [PubMed] [Google Scholar]
- [28].Chan AC, Poon RT, Chan C, et al. Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann Surg 2016;263:e14–6. doi: 10.1097/SLA.0000000000001272. [DOI] [PubMed] [Google Scholar]
- [29].Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg 2018;42:806–15. [DOI] [PubMed] [Google Scholar]
- [30].Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429–42. [DOI] [PubMed] [Google Scholar]
- [31].Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734–9. [DOI] [PubMed] [Google Scholar]
- [32].Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164–71. [DOI] [PubMed] [Google Scholar]
- [33].Wang Z, Peng Y, Hu J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg 2018;doi: 10.1097/SLA.0000000000002942. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [34].Romic B, Romic I, Mance M, et al. Successful associating liver partition and portal vein ligation after unsuccessful double TACE procedure complicated with sepsis and pancreatitis. Klin Onkol 2016;29:59–62. [DOI] [PubMed] [Google Scholar]
- [35].D’Haese JG, Neumann J, Weniger M, et al. Should ALPPS be Used for Liver Resection in Intermediate-Stage HCC? (1534-4681 (Electronic)). [DOI] [PubMed] [Google Scholar]
- [36].de Santibanes E, Alvarez FA, Ardiles V, et al. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini-ALPPS technique. Langenbeck's Arch Surg 2016;401:557–63. [DOI] [PubMed] [Google Scholar]
- [37].Rosok BI, Bjornsson B, Sparrelid E, et al. Scandinavian multicenter study on the safety and feasibility of the associating liver partition and portal vein ligation for staged hepatectomy procedure. Surgery 2016;159:1279–86. [DOI] [PubMed] [Google Scholar]
- [38].Chan AC, Poon RT, Lo CM. Modified anterior approach for the ALPPS procedure: how we do it. World J Surg 2015;39:2831–5. [DOI] [PubMed] [Google Scholar]
- [39].Wanis KN, Buac S, Linecker M, et al. Patient survival after simultaneous ALPPS and colorectal resection. World J Surg 2017;41:1119–25. [DOI] [PubMed] [Google Scholar]


