Abstract
Background:
Laser therapy is widely used for exercise-induced fatigue, while the effect among different studies remains controversial. The present study was to summary available randomized controlled trials (RCTs) to evaluate the effect of laser therapy in subjects with exercise-induced fatigue.
Methods:
PubMed, Embase, and Cochrane Library were searched to identify the potential RCTs from inception to October 2017. The weighted mean difference (WMD) with 95% confidence intervals (CIs) was calculated using a random-effects model.
Results:
Twenty RCTs involving a total of 394 individuals were included in final analysis. No significant differences were observed between the laser therapy and control for the outcomes of lactate (WMD: −0.19; 95%CI: −0.52 to 0.13; P = .244), repetitions (WMD: 4.44; 95%CI: −1.43 to 10.32; P = .138), work load (WMD: 3.38; 95%CI: −1.15 to 7.91; P = .144), time taken to perform the exercise tests (WMD: 4.42; 95%CI: −2.33 to 11.17; P = .199), creatine kinase (WMD: −41.80; 95%CI: −168.78 to 85.17; P = .519), maximum voluntary contraction (WMD: 23.83; 95%CI: −7.41 to 55.07; P = .135), mean peak forces (WMD: 2.87; 95%CI: −1.01 to 6.76; P = = .147), and visual analog scale (VAS) (WMD: −1.91; 95%CI: −42.89 to 39.08; P = = .927). The results of sensitivity analysis suggested that laser therapy might play an important role on the levels of lactate (WMD: −0.30; 95%CI: −0.59 to −0.01; P = = .040), maximum voluntary contraction (WMD: 33.54; 95%CI: 1.95 to 65.12; P = = .037), and VAS (WMD: −21.00; 95%CI: −40.78 to −1.22; P = = .037). The results of subgroup analyses indicated no significant differences between the laser therapy and placebo for lactate and repetitions when stratified by study design, mean age, gender, and study quality.
Conclusions:
The findings of this meta-analysis did not indicate any significant differences between the laser therapy and placebo.
Keywords: exercise-induced fatigue, laser therapy, meta-analysis
1. Introduction
A continuous decrease in muscle strength was associated with the progression of skeletal muscle fatigue.[1] Although the mechanisms of muscle fatigue are not yet elucidated, the most common manifestations include the inability of spontaneous generation and maintenance of strength in a synchronized and efficient manner for a specific period.[2] Several factors including the types, duration, the intensity of exercise, the muscle groups involved, and the local physical and biochemical environment were correlated with progressive fatigue.[3] Furthermore, age, gender, motivation, and adaptation to contract the skeletal muscle withstand the development of fatigue.[4,5] As stated above, the progression of muscle fatigue involved physiological, biomechanical, and psychological elements.[3,5–9]
A previous study illustrated that low power laser radiation exerted physiological therapeutic effects with an increase in cellular metabolism for the synthesis of protein, which could prevent fatigue and improve the recovery of skeletal muscle.[10] In addition, the radiation treatment on skeletal muscle could affect energy metabolism including the mitochondrial level, oxi-reduction of the cells, and transport of electrons in the respiratory chain. Another previous study demonstrated that subjects, who underwent pre- or post-exercise radiation treatment exhibited diverse effects. The pre-exercise phototherapy could enhance strength gains by reducing fatigue and catabolic effect, while the post-exercise phototherapy could prevent an exaggerated inflammatory response caused by muscle damage.[11–13]
In the previous meta-analysis, the date of included trials was through 2012, which was deemed as an early date, nowadays, more and more studies shown that laser therapy might play a critical role in the regulation of the levels of lactate and VAS. Moreover, the prospective retrieved data were limited to the number of repetitions and time until exhaustion with respect to muscle performance and creatine kinase of low-level laser therapy.[14] Therefore, we conducted an update of the previous study in order to evaluate the effect of laser therapy in the treatment of exercise-induced fatigue.
2. Methods
2.1. Experimental approach to the problem
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009.[15] The studies with randomized controlled design and evaluation of the effect of laser therapy in subjects with exercise-induced fatigue were eligible for the current study. Herein, we systematically searched PubMed, Embase, and the Cochrane Library up to October 2017 for potential studies with the terms “laser” and “fatigue.” Furthermore, the reference lists of all the retrieved studies and relevant reviews were searched manually to identify additional eligible articles. Variables such as the study design, subjects’ status, intervention, control, and desirable outcome were employed to select the included studies for analysis.
The literature search was independently undertaken by 2 investigators using a standardized approach, and any discrepancies were settled by a group discussion to achieve a consensus. A study was eligible for inclusion if the following criteria were fulfilled:
-
1.
the study was a randomized controlled design (parallel or crossover);
-
2.
the study investigated the effect of laser therapy in subjects with exercise-induced fatigue;
-
3.
the study report at least 1 of following outcomes: lactate, repetitions, workload, time taken to perform the exercise tests, creatine kinase, maximum voluntary contraction, mean peak forces, and visual analog scale (VAS).
All the observational studies were excluded as the various confounding factors could bias the results.
2.2. Procedures
The following data from each study were extracted by 2 investigators independently: first author's name, publication year, country, study design, sample size, mean age, percentage of males, intervention, control, and reported outcomes. Any disagreement was resolved by discussion until a consensus was reached. The Jadad scale was used to assess the methodological quality, which is comprehensive and has been partially validated for evaluating the quality of randomized controlled trials (RCTs) in a meta-analysis.[16] The Jadad scale is based on the following 5 subscales: randomization (1 or 0), concealment of the treatment allocation (1 or 0), blinding (1 or 0), completeness of follow-up (1 or 0), and the use of intention-to-treat analysis (1 or 0). Thus, we developed a scoring system ranging from 0 to 5 for quality assessment. In the current study, we considered the study with a score 4 or 5 as high quality. The quality was assessed by the 2 investigators independently.
2.3. Statistical analyses
Weighted mean difference (WMD) was used as a summary statistic for all the investigated outcomes based on the mean, standard deviation, and sample size in each group. A random-effects model was employed to calculate the summary effects, and the WMD was significant if the 95% confidence interval (CI) did not include 0.[17,18] The potential heterogeneity across studies was examined via Cochrane Q-statistic and I2 statistic test.[19,20] The P value for heterogeneity <.05 or I2 > 50% indicated that the heterogeneity was statistically significant. A sensitivity analysis was conducted by removing each trial from the meta-analysis sequentially.[21] The subgroup analysis for lactate and repetitions was performed according to the study design, mean age, gender, and study quality. The funnel plots for lactate and repetitions were qualitatively assessed the publication bias, and the Egger[22] and Begg tests[23] quantitatively evaluated the publication bias for lactate and repetitions. All the reported P values were two-sided and values <.05 were considered as significant for all the included trials. Statistical analyses were performed using STATA software (version 10.0 StataCorp, Texas, USA).
3. Results
The study selection process is illustrated in Figure 1. A total of 769 potentially relevant articles were identified after a systematic search of electronic databases. After reviewing the title or abstract, 739 articles were excluded, and 30 articles were subjected to full-text review. Ten articles were discarded at the stage of full-text review, and 20 studies were finally identified and included in the analysis of the efficacy of laser therapy in adult patients with exercise-induced fatigue.[24–43] Studies were excluded for the following reasons: if the subjects received other therapies, the outcome of interest was unavailable, and the study reported same populations. A manual search of the reference lists of these trials did not yield any new eligible trials. The general characteristics of the included studies are presented in Table 1.
Figure 1.
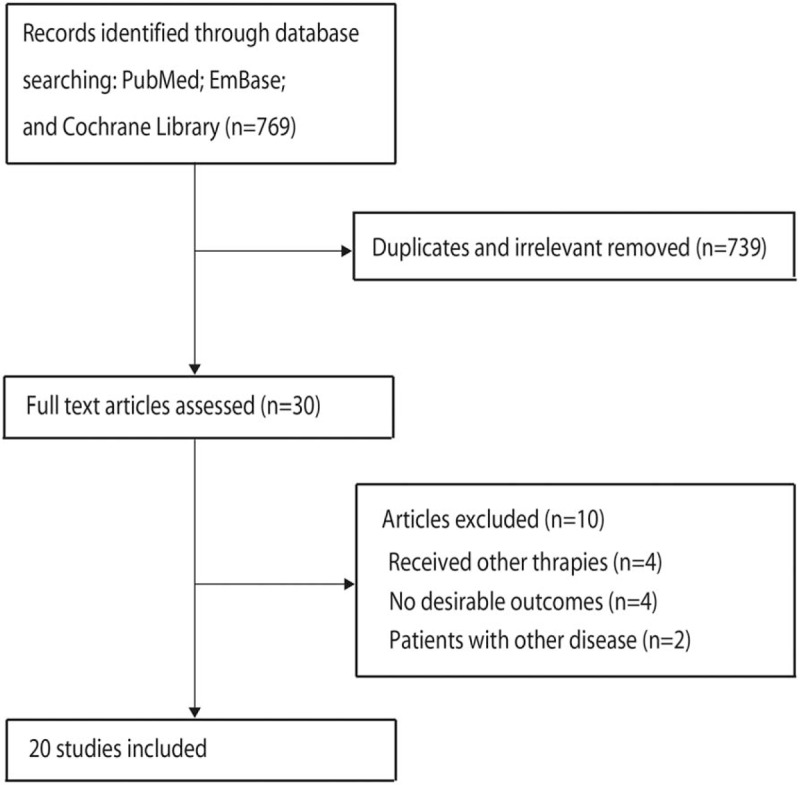
Schematic representation of the literature search and trial selection process.
Table 1.
Baseline characteristic of the included studies.
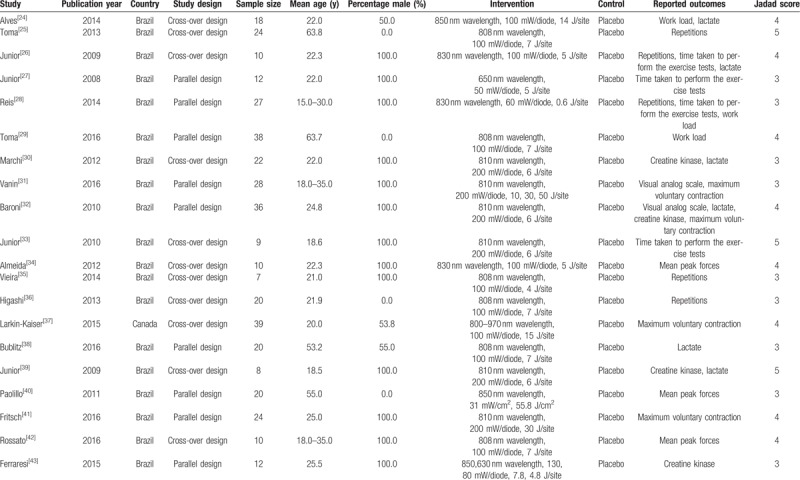
Of the 20 included trials, 11 studies were crossover design and the remaining 9 trials were parallel design. The sample sizes of each trial varied from 7 to 39 subjects, and the mean age of the subjects ranged from 18 to 63.8 years. Furthermore, 13 included trials comprised of males, 4 trials included female, and the remaining 3 trials included both males and females. Of these 20 trials, the study quality was evaluated using the Jadad scale. Overall, 3 trials presented a score of 5,[25,33,39] 8 trials had a score of 4,[24,26,29,32,34,37,41,42] and the remaining 9 trials had a score of 3.[27,28,30,31,35,36,38,40,43]
A total of 6 trials displayed data for the effect of laser therapy on the level of lactate. The pooled WMD showed a 0.19 mmol/L reduction in lactate level, while this reduction was not statistically significant (WMD: −0.19; 95% CI: −0.52–0.13; P = = .244; Fig. 2). In addition, significant heterogeneity was evident across the included trials (I2: 54.7%; P = = .050). According to the sensitivity analysis, we excluded the study by Marchi et al[30] and concluded that subjects received laser therapy were associated with a significantly reduced lactate level (WMD: −0.30; 95% CI: −0.59 to 0.01; P = .040; Table 2). In addition, a subgroup analysis was conducted for analyzing the lactate level according to the study design, mean age, gender, and study quality in order to evaluate the effect of laser therapy in specific subsets. We noted laser therapy did not yield a significant effect on lactate level in all the subsets (Table 3).
Figure 2.
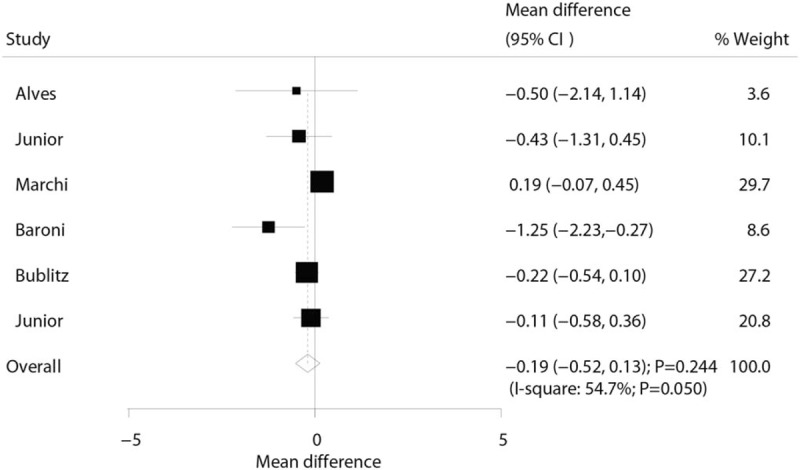
Effect of the laser therapy on lactate.
Table 2.
Sensitivity analysis.
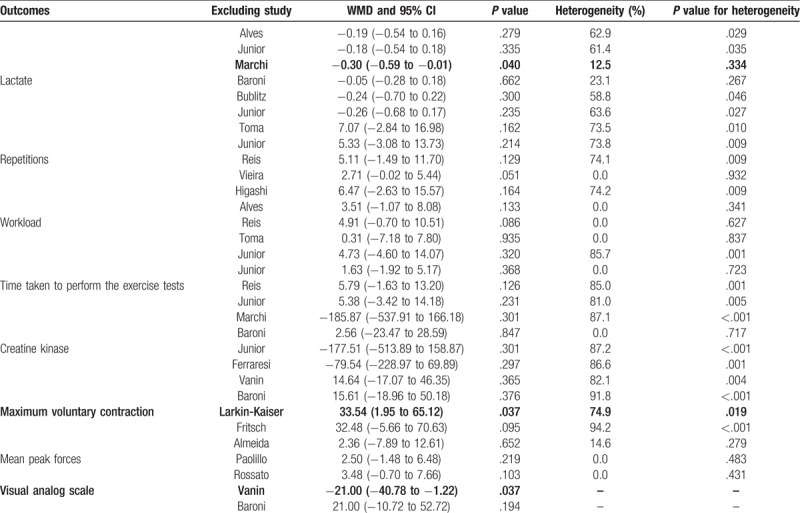
Table 3.
Subgroup analysis for lactate and repetitions.
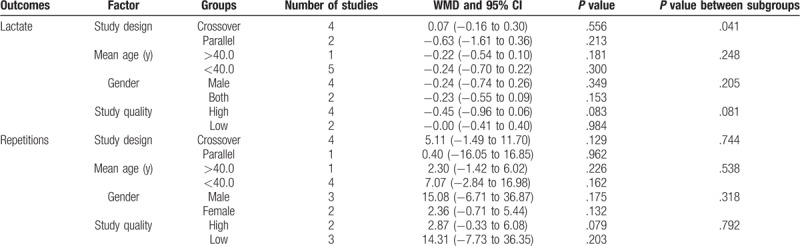
The data for the effect of laser therapy were available from the number of repetitions from 5 trials. However, no significant difference was observed between laser therapy and placebo for the number of repetitions (WMD: 4.44; 95% CI: −1.43 to 10.32; P = .138; Fig. 3), and substantial heterogeneity was observed (I2: 65.8%; P = .020). Next, we then conducted sensitivity analysis and found that the conclusion was not affected by the exclusion of any individual trial (Table 2). Similarly, subgroup analysis suggested no significant differences between the laser therapy and placebo in all the subsets (Table 3).
Figure 3.
Effect of the laser therapy on repetitions.
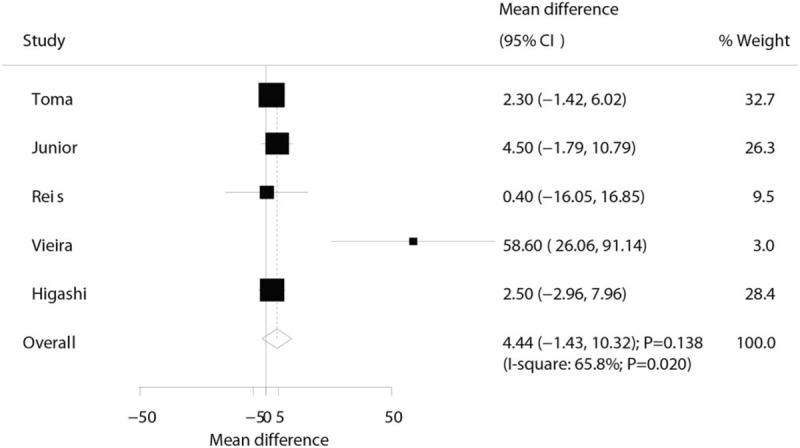
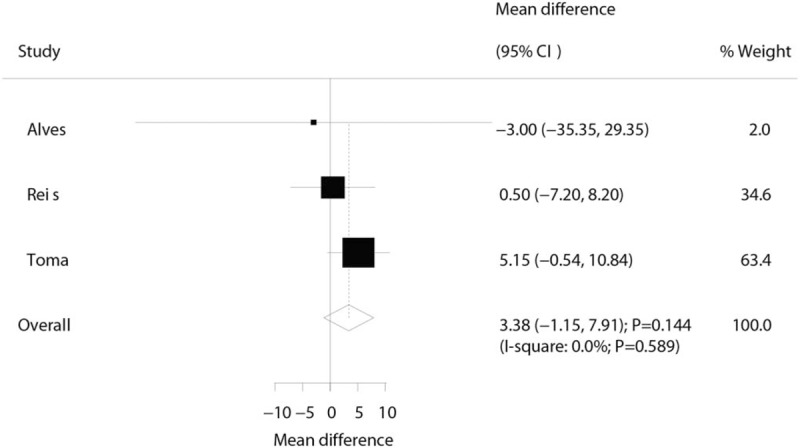
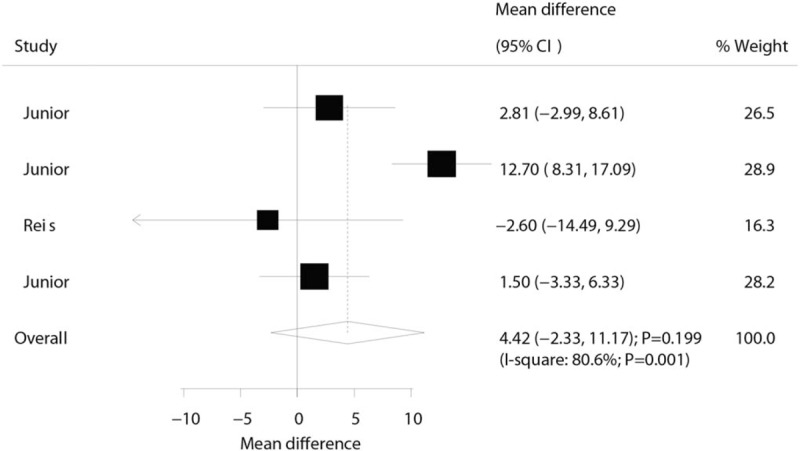
Three trials reported the effect of laser therapy on workload; however, no significant effect of laser therapy was observed (WMD: 3.38; 95% CI: −1.15 to 7.91; P = .144; no evidence of heterogeneity; Fig. 4). The results of sensitivity analysis indicated the conclusion was not altered by excluding any individual trial (Table 2). Subsequently, 4 trials reported the effect of laser therapy on the duration required to perform the exercise tests and did not find any significant effect (WMD: 4.42; 95% CI: −2.33 to 11.17; P = .199; Fig. 5). Substantial heterogeneity was detected among the included trials. Next, we conducted sensitivity analysis and found that the conclusion was not affected by the exclusion of any individual trial (Table 2). Third, 4 trials reported the effect of laser therapy on the level of creatine kinase and indicated no significant effect of laser therapy (WMD: −41.80; 95% CI: −168.78 to 85.17; P = .519; Fig. 6). Moreover, substantial heterogeneity was observed, and the results of sensitivity analysis remained unaltered (Table 2). Finally, no significant differences were observed between laser therapy and placebo regarding the outcomes of maximum voluntary contraction (WMD: 23.83; 95% CI: −7.41 to 55.07; P = .135; substantial heterogeneity; Fig. 7), mean peak forces (WMD: 2.87; 95% CI: −1.01 to 6.76; P = .147; no evidence of heterogeneity; Fig. 8), and VAS (WMD: −1.91; 95% CI: −42.89 to 39.08; P = .927; substantial heterogeneity; Fig. 9). The conclusion of sensitivity analysis was not altered for mean peak forces (Table 2). In addition, the sensitivity analysis for maximum voluntary contraction and VAS indicated the conclusions were changed after sequentially excluding individual trials (Table 2).
Figure 4.
Effect of the laser therapy on workload.
Figure 5.
Effect of the laser therapy on the time taken to perform the exercise tests.
Figure 6.
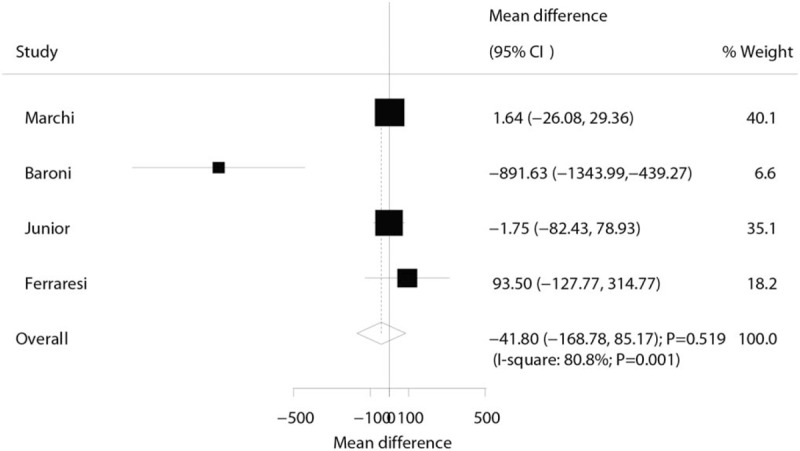
Effect of the laser therapy on creatine kinase.
Figure 7.
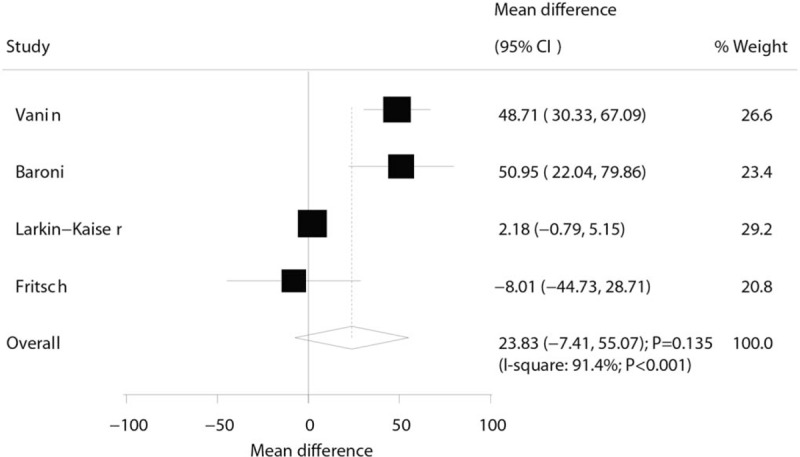
Effect of the laser therapy on maximum voluntary contraction.
Figure 8.
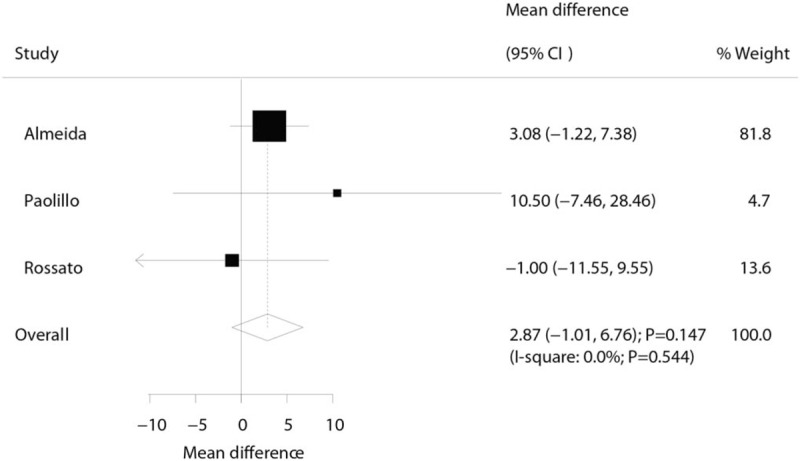
Effect of the laser therapy on mean peak forces.
Figure 9.
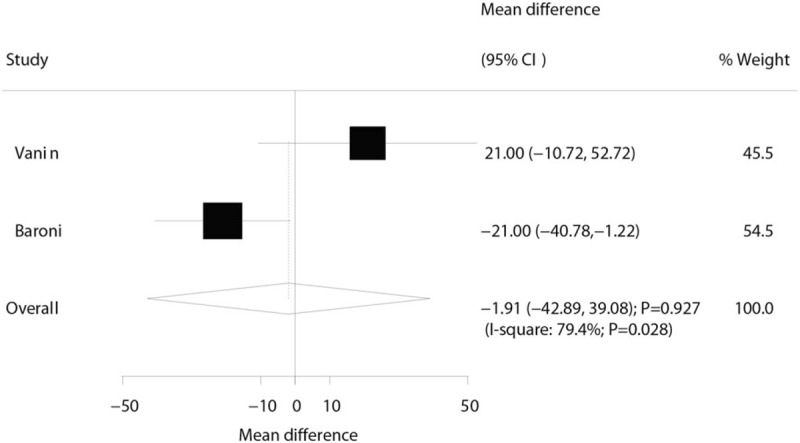
Effect of the laser therapy on the VAS.
The review of funnel plots did not reveal any significant publication bias for lactate level and the number of repetitions (Fig. 10). Also, the Egger and Begg tests did not present any evidence of publication bias for lactate level (P value for Egger: 0.102; P-value for Begg: 0.452) and the number of repetitions (P value for Egger: 0.182; P value for Begg: 0.221).
Figure 10.
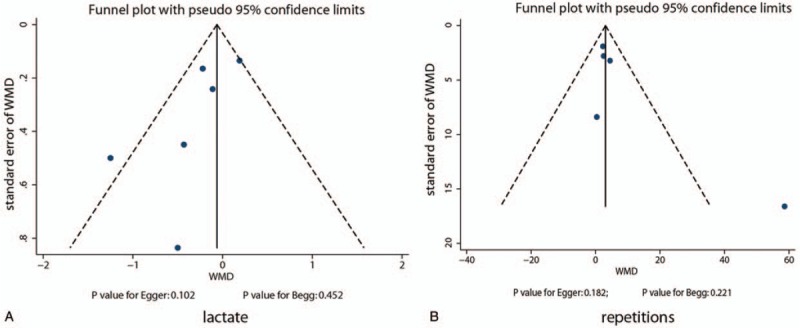
Funnel plots for lactate and repetitions.
4. Discussion
The present meta-analysis determined the effect of laser therapy in adults with exercise-induced fatigue. A total of 20 trials were identified that encompassed 394 individuals. The findings of this study indicated no significant differences between laser therapy and placebo for the outcomes of lactate, repetitions, workload, time taken to perform the exercise tests, creatine kinase, maximum voluntary contraction, mean peak forces, and VAS. In addition, the sensitivity analyses showed that laser therapy played a major role in lactate, maximum voluntary contraction and VAS. However, no significant difference was observed for lactate and repetitions when stratified by study design, mean age, gender, and study quality. These observations might better define the effect of laser therapy in adults with exercise-induced fatigue.
A previous meta-analysis included 13 RCTs and suggested that the laser therapy was associated with high levels of exhaustion (WMD: 4.12; 95% CI: 1.21–7.02; P < .005) and the number of repetitions (WMD: 5.47; 95% CI: 2.35–8.59; P < .001). Also, the study pointed out that laser therapy improved the muscle performance and accelerated the recovery if used before exercise.[14] However, the effects on other outcomes were not investigated, and the analysis was not further stratified by factors that could affect the treatment effects of laser therapy. In the present study, the overall analyses were inconsistent, and subgroup analysis yielded similar conclusions as compared to a previous meta-analysis; this phenomenon might be attributed to the need for updated data from additional trials.
The current findings did not reveal any significant difference in the investigated outcomes among all groups. However, several studies reported inconsistent results. Baroni et al indicated that laser therapy before exercise significantly attenuated the increased muscle proteins and decreased the muscle force, which in turn, could reduce the levels of lactate, creatine kinase, and VAS and increase the maximum voluntary contraction.[32] Vieira et al suggested that laser therapy increased the number of repetitions, with a small electromyography fatigue index in vastus medialis (P = .004) and rectus femoris.[35] Junior et al suggested that the laser therapy delayed the onset of muscle fatigue and exhaustion.[27] Vanin et al demonstrated that laser therapy significantly increased the maximum voluntary contraction immediately after exercise up to 24 hours.[31] These observations could be attributed to the interaction between laser therapy and biological tissues, especially in mitochondria and blood flow.[44] The effects of laser therapy were similar to aerobic training on muscle cells, thereby positively affecting the fatigue and exercise capacity.[30,45]
Although no significant differences were noted in all the investigated outcomes, the results of sensitivity analysis indicated that laser therapy might play a critical role on the lactate level, maximum voluntary contraction and VAS. This might be effectuated based on the numerous studies designed with other outcomes as a primary endpoint, and their sample sizes were not sufficient to detect the potential clinical differences among the investigated outcomes. In addition, the differences between the laser therapy and placebo for lactate and repetitions were not observed in all the subsets, while these results were variable due to a small number of studies included.
Nevertheless, the present meta-analysis has 3 highlighted. First, only RCTs were included, which should eliminate the bias of confounders as compared to other observational studies. Second, the summary results suggested that laser therapy could improve the lactate level and VAS. Third, a large sample size was included, which strengthened the result of the present study than any individual trial. The limitations of the present meta-analysis stated that substantial heterogeneity was not addressed using sensitivity and subgroup analysis. The data and the consequently introduced potential bias might be attributed to the difference in subjects’ characteristics, intervention, and study design of the included trials. Finally, the findings of subgroup analyses might be unreliable and variable due to the small number of studies included.
Despite the limitations, our findings exhibit significant clinical implications since systematic reviews and meta-analyses are the most powerful tools for evaluating inconsistencies. The findings of this meta-analysis did not indicate any significant differences between the laser therapy and placebo for lactate, repetitions, workload, the time taken to perform the exercise tests, creatine kinase, maximum voluntary contraction, mean peak forces, and VAS. Thus, the treatment effect between laser therapy and placebo requires further investigations in large-scale RCTs.
Author contributions
Conceptualization: Dongmei Wang, Xingtong Wang.
Data curation: Dongmei Wang, Xingtong Wang.
Formal analysis: Dongmei Wang.
Investigation: Dongmei Wang, Xingtong Wang.
Methodology: Xingtong Wang.
Resources: Dongmei Wang.
Software: Dongmei Wang, Xingtong Wang.
Validation: Xingtong Wang.
Visualization: Xingtong Wang.
Writing – original draft: Dongmei Wang.
Writing – review & editing: Xingtong Wang.
Footnotes
Abbreviations: CI = confidence interval, CIs = confidence intervals, RCTs= randomized controlled trials, VAS = visual analog scale, WMD = weighted mean difference.
How to cite this article: Wang D, Wang X. Efficacy of laser therapy for exercise-induced fatigue. Medicine. 2019;98:38(e17201).
This study was supported by grants from the National Science Foundation of Shandong Province (grant number ZR2016CL12)
Ethical approval and informed consent is not applicable in this study.
The authors declare that they have no conflict of interest.
References
- [1].Green S, Langberg H, Skovgaard D, et al. Interstitial and arterial-venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J Physiol 2000;529(Pt 3):849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol (1985) 1992;72:1631–48. [DOI] [PubMed] [Google Scholar]
- [3].Weir JP, Beck TW, Cramer JT, et al. Is fatigue all in your head? A critical review of the central governor model. Br J Sports Med 2006;40:573–86. discussion 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hurley BF. Age, gender, and muscular strength. J Gerontol A Biol Sci Med Sci 1995;50:41–4. [DOI] [PubMed] [Google Scholar]
- [5].Szubski C, Burtscher M, Loscher WN. Neuromuscular fatigue during sustained contractions performed in short-term hypoxia. Med Sci Sports Exerc 2007;39:948–54. [DOI] [PubMed] [Google Scholar]
- [6].Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 2008;88:287–332. [DOI] [PubMed] [Google Scholar]
- [7].Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol (1985) 2008;104:551–8. [DOI] [PubMed] [Google Scholar]
- [8].Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 2008;586:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cairns SP, Knicker AJ, Thompson MW, et al. Evaluation of models used to study neuromuscular fatigue. Exerc Sport Sci Rev 2005;33:9–16. [PubMed] [Google Scholar]
- [10].Manteifel V, Bakeeva L, Karu T. Ultrastructural changes in chondriome of human lymphocytes after irradiation with He-Ne laser: appearance of giant mitochondria. J Photochem Photobiol B 1997;38:25–30. [DOI] [PubMed] [Google Scholar]
- [11].Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 2007;37:145–68. [DOI] [PubMed] [Google Scholar]
- [12].Leal-Junior EC. Photobiomodulation therapy in skeletal muscle: from exercise performance to muscular dystrophies. Photomed Laser Surg 2015;33:53–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ziemann E, Zembron-Lacny A, Kasperska A, et al. Exercise training-induced changes in inflammatory mediators and heat shock proteins in young tennis players. J Sports Sci Med 2013;12:282–9. [PMC free article] [PubMed] [Google Scholar]
- [14].Leal-Junior EC, Vanin AA, Miranda EF, et al. Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci 2015;30:925–39. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [18].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [19].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull 1999;47:15–7. [Google Scholar]
- [22].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [24].da Silva Alves MA, Pinfildi CE, Neto LN, et al. Acute effects of low-level laser therapy on physiologic and electromyographic responses to the cardiopulmonary exercise testing in healthy untrained adults. Lasers Med Sci 2014;29:1945–51. [DOI] [PubMed] [Google Scholar]
- [25].Toma RL, Tucci HT, Antunes HK, et al. Effect of 808nm low-level laser therapy in exercise-induced skeletal muscle fatigue in elderly women. Lasers Med Sci 2013;28:1375–82. [DOI] [PubMed] [Google Scholar]
- [26].Leal Junior EC, Lopes-Martins RA, Vanin AA, et al. Effect of 830nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med Sci 2009;24:425–31. [DOI] [PubMed] [Google Scholar]
- [27].Leal Junior EC, Lopes-Martins RA, Dalan F, et al. Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg 2008;26:419–24. [DOI] [PubMed] [Google Scholar]
- [28].Dos Reis FA, da Silva BA, Laraia EM, et al. Effects of pre- or post-exercise low-level laser therapy (830nm) on skeletal muscle fatigue and biochemical markers of recovery in humans: double-blind placebo-controlled trial. Photomed Laser Surg 2014;32:106–12. [DOI] [PubMed] [Google Scholar]
- [29].Toma RL, Vassao PG, Assis L, et al. Low level laser therapy associated with a strength training program on muscle performance in elderly women: a randomized double blind control study. Lasers Med Sci 2016;31:1219–29. [DOI] [PubMed] [Google Scholar]
- [30].De Marchi T, Leal Junior EC, Bortoli C, et al. Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci 2012;27:231–6. [DOI] [PubMed] [Google Scholar]
- [31].Aver Vanin A, De Marchi T, Tomazoni SS, et al. Pre-exercise infrared low-level laser therapy (810nm) in skeletal muscle performance and postexercise recovery in humans, what is the optimal dose? A randomized, double-blind. Placebo-Controlled Clinical Trial Photomed Laser Surg 2016;34:473–82. [DOI] [PubMed] [Google Scholar]
- [32].Baroni BM, Leal Junior EC, De Marchi T, et al. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol 2010;110:789–96. [DOI] [PubMed] [Google Scholar]
- [33].Leal Junior EC, Lopes-Martins RA, Frigo L, et al. Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports Phys Ther 2010;40:524–32. [DOI] [PubMed] [Google Scholar]
- [34].de Almeida P, Lopes-Martins RA, De Marchi T, et al. Red (660nm) and infrared (830nm) low-level laser therapy in skeletal muscle fatigue in humans: what is better? Lasers Med Sci 2012;27:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].de Brito Vieira WH, Bezerra RM, Queiroz RA, et al. Use of low-level laser therapy (808nm) to muscle fatigue resistance: a randomized double-blind crossover trial. Photomed Laser Surg 2014;32:678–85. [DOI] [PubMed] [Google Scholar]
- [36].Higashi RH, Toma RL, Tucci HT, et al. Effects of low-level laser therapy on biceps braquialis muscle fatigue in young women. Photomed Laser Surg 2013;31:586–94. [DOI] [PubMed] [Google Scholar]
- [37].Larkin-Kaiser KA, Christou E, Tillman M, et al. Near-infrared light therapy to attenuate strength loss after strenuous resistance exercise. J Athl Train 2015;50:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bublitz C, Renno AC, Ramos RS, et al. Acute effects of low-level laser therapy irradiation on blood lactate and muscle fatigue perception in hospitalized patients with heart failure-a pilot study. Lasers Med Sci 2016;31:1203–9. [DOI] [PubMed] [Google Scholar]
- [39].Leal Junior EC, Lopes-Martins RA, Baroni BM, et al. Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg 2009;27:617–23. [DOI] [PubMed] [Google Scholar]
- [40].Paolillo FR, Milan JC, Aniceto IV, et al. Effects of infrared-LED illumination applied during high-intensity treadmill training in postmenopausal women. Photomed Laser Surg 2011;29:639–45. [DOI] [PubMed] [Google Scholar]
- [41].Fritsch CG, Dornelles MP, Severo-Silveira L, et al. Effects of low-level laser therapy applied before or after plyometric exercise on muscle damage markers: randomized, double-blind, placebo-controlled trial. Lasers Med Sci 2016;31:1935–42. [DOI] [PubMed] [Google Scholar]
- [42].Rossato M, Dellagrana RA, Lanferdini FJ, et al. Effect of pre-exercise phototherapy applied with different cluster probe sizes on elbow flexor muscle fatigue. Lasers Med Sci 2016;31:1237–44. [DOI] [PubMed] [Google Scholar]
- [43].Ferraresi C, Dos Santos RV, Marques G, et al. Light-emitting diode therapy (LEDT) before matches prevents increase in creatine kinase with a light dose response in volleyball players. Lasers Med Sci 2015;30:1281–7. [DOI] [PubMed] [Google Scholar]
- [44].Baroni BM, Leal Junior EC, Geremia JM, et al. Effect of light-emitting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomed Laser Surg 2010;28:653–8. [DOI] [PubMed] [Google Scholar]
- [45].Leal Junior EC, Lopes-Martins RA, de Almeida P, et al. Effect of low-level laser therapy (GaAs 904nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol 2010;108:1083–8. [DOI] [PubMed] [Google Scholar]


