Supplemental Digital Content is available in the text.
Background:
Horizontal neck wrinkles are a common aesthetic deficiency but limited treatment options exist and none have been studied with follow-ups of over 2 months.
Methods:
This dual-center, retrospective case series (Apr 2016–Jan 2017) included adult patients receiving CPM-HA to treat horizontal neck wrinkles. Patients were followed up for 40 weeks. CPM-HA treatment efficacy was evaluated through the Horizontal Neck Wrinkle Severity Scale and Global Aesthetic Improvement Scale (GAIS). Adverse events (AEs) and pain on injection, as assessed on the visual analogue scale, were documented.
Results:
Sixty-four lateral neck halves from 32 women (23–61 years) were analyzed. Significant improvement in wrinkle intensity, as assessed by Horizontal Neck Wrinkle Severity Scale, was observed from the second week onwards (1.352 ± 0.682, P<0.05). This improvement was sustained throughout the study duration and remained statistically significant at week 36 (1.423 ± 0.796, P<0.05). Patient GAIS (92.3%–100% through 36 weeks) and physician GAIS (100% through 24 weeks) were both excellent. Visual analogue scale scores revealed more pain associated with blunt cannula use (2.72 ± 1.71) than with sharp needle use (1.75 ± 1.39). AEs included erythema (62.5%), pruritus (43.7%), ecchymosis (43.7%), and localized swelling (25%).
Conclusions:
Subdermal injection of CPM-HA is safe and effective for treating horizontal neck wrinkles. Sustained improvement of wrinkle intensity up to 36 weeks and minimal AEs with no Tyndall effect were observed.
BACKGROUND
An increasing number of patients are seeking neck rejuvenation treatments. Commonly observed aesthetic deficiencies of the neck include horizontal neck wrinkles, crepey skin, sagging of skin with hanging folds, excessive adipose tissue causing double chins and/or poorly defined jawlines, and platysmal bands.1,2
Horizontal neck wrinkles present as linear depressions or furrows which occupy the anterior half of the neck.3 Their etiology differs from that of other facial wrinkles, which are caused by skin aging.4–6 But horizontal neck wrinkles are not entirely caused by aging, as they are often observed in children and young adults.7,8 Importantly, age-related skin laxity can worsen these wrinkles.1,2,7 Frequent bending of the neck to look at cell phones, tablets, or books could also cause wrinkle development in the young.
Various therapies are recommended for neck rejuvenation.9–18 Ablative and nonablative laser treatments, micro-focused ultrasound with visualization, radiofrequency, and plasma resurfacing are all effective at improving skin texture and neck laxity.9–11,16,17 However, while they can tighten the skin, they do not reduce rhytides. Botulinum toxin type A injections effectively reduce platysmal bands,15,18 but only marginally improve horizontal necklines. Skin excision, platysma plication, and liposuction can improve the neck contour,12–14 but again, may have limited effects on horizontal necklines. However, effective treatment for horizontal neck wrinkles is limited.8,19,20
In our clinic, hyaluronic acid (HA) fillers have been used for several years to treat horizontal neck wrinkles with favorable results. Different brands of HA fillers can be used to treat horizontal neck lines, but some produce more lumps and Tyndall effects than others. A different type of HA filler, the Esthelis Basic, is a cohesive polydensified matrix HA (CPM-HA) filler with low elastic modulus, low viscous modulus, and high tan delta.21,22 These rheologic properties translate into a soft and spreadable filler that, although elastic, has a greater component of fluidity, making it easily moldable after implantation, minimizing the risk of lumpiness or irregularities, and thus appropriate for superficial subdermal or even intradermal injection21–23 where tissue integration is desired. The present study therefore aims to evaluate the efficacy and safety of CPM-HA fillers in the treatment of horizontal neck wrinkles.
MATERIALS AND METHODS
We performed a dual-center, retrospective case series study on patients who received CPM-HA for the treatment of horizontal neck wrinkles at the Milano Aesthetic Clinics in Taipei and Taoyuan, Taiwan between April 2016 and January 2017. The study was carried out in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All enrolled patients provided written informed consent.
Patient Selection
Adult patients with horizontal neck wrinkles who received treatment with CPM-HA were included in the study. Patients with a known allergy to CPM-HA filler components, immunocompromised status, coagulopathy, or active infection, inflammation and malignancy of the neck, were excluded. Patients who were pregnant and breastfeeding were also excluded.
Study Design
Patients received injection of CPM-HA (Esthelis Basic, 22.5% HA, Anteis SA, Geneva, Switzerland), and only 1 touch-up was allowed within a month of receiving the first dose. Patients were followed up for up to 40 weeks to evaluate the effect of treatment on wrinkles and the occurrence of any adverse events (AEs). To avoid overestimation of the duration of effects, results were grouped according to the periods in which the data was collected and reported as stated in Table 1. Briefly, patients were followed up for up to 40 weeks after treatment, but as not every patient was punctual and to avoid overestimation of the duration of effect, follow-up results gathered from week 4 to 7 were grouped and reported as week-4 visit, week 8 to 11 were grouped in week-8 visit, week 12 to 17 were grouped in week-12 visit, week 18 to 23 were grouped in week-18 visit, week 24 to 29 were grouped in week-24 visit, week 30 to 35 were grouped in week-30 visit, and week 36 to 40 were grouped in week-36 visit.
Table 1.
Grouping of Treatment Data According to Collection Periods
| Date of Data Gathering (Weeks Posttreatment) | Group |
|---|---|
| 4–7 | Week 4 visit |
| 8–11 | Week 8 visit |
| 12–17 | Week 12 visit |
| 18–23 | Week 18 visit |
| 24–29 | Week 24 visit |
| 30–35 | Week 30 visit |
| 36–40 | Week 36 visit |
Photographic Methods
High-resolution photographs were taken before treatment and at each follow-up visit. The photography room, lighting, camera settings (Canon EOS650D, Canon corp., Tokyo, Japan), and camera distance from patients remained consistent for each patient during all follow-up visits. Photos of patients’ necks were taken with their chins up and necks semiextended in the upright sitting position.
Injection Technique
Topical anesthetic cream (2.5% lidocaine, 2.5% prilocaine; CBC Biotechnological & Pharmaceutical Co., Ltd., New Taipei City, Taiwan) was applied to the area to be treated 30 minutes before the procedure. The cream was wiped off just before the procedure, and the area was sterilized with 75% ethanol. Patients were placed in a semisupine position to expose the anterior neck and wrinkles. Each 1-mL syringe of CPM-HA was mixed with 0.1–0.4 mL of 2% xylocaine with 1:50000 epinephrine (Oriental Chemical Works Inc., New Taipei City, Taiwan) to reduce pain and bruising due to injection. The treatment was initiated with a higher dilution ratio (0.3–0.4 mL of 2% xylocaine mixed with 1 mL of CPM-HA) in the first few patients to decrease the likelihood of irregularities; however, the dilution ratio was later reduced to 0.1 mL of 2% xylocaine with 1 mL of CPM-HA, which was determined to be adequate for pain reduction and hemostasis while maintaining the desired result. Thirty-gauge sharp needles were used to deliver CPM-HA to treat finer, more superficial wrinkles with a serial puncture technique, while 25-gauge blunt cannulas were used to deliver CPM-HA to treat wider, deeper furrows in a retrograde linear fashion. Both sharp needles and blunt cannulas were directed almost parallel to the skin (at an angle <10°), and the injection depth was superficial in the immediate subdermal plane. The amount of fillers used varied according to the depth, length, and number of wrinkles. The endpoint of treatment was an immediate and visible flattening of the wrinkles. After injection, ice packs were applied to the treated area for 15 minutes, followed by antibiotic ointment (See Video [online], which displays the injection of the horizontal neck wrinkle technique).
Video.
Creation of Horizontal Neck Wrinkles Severity Scale
No classification scale to grade the severity of horizontal neck wrinkles was available during the treatment, although Jones et al.7 had developed a photonumeric scale of transverse neck lines after the present study was initiated. To objectively measure this study’s outcomes and to facilitate future studies of the same indication, the authors developed the Horizontal Neck Wrinkles Severity Scale (HNWS) based on the depth and appearance of the horizontal neck wrinkles (Fig. 1). Each side of the anterior neck was evaluated separately, from a vertical line drawn across the earlobe to the midline of the neck. Since the posterior neck is usually obscured by hair, is less accessible when patients are supine on treatment beds, and is aesthetically less important, only the anterior neck was evaluated.
Fig. 1.
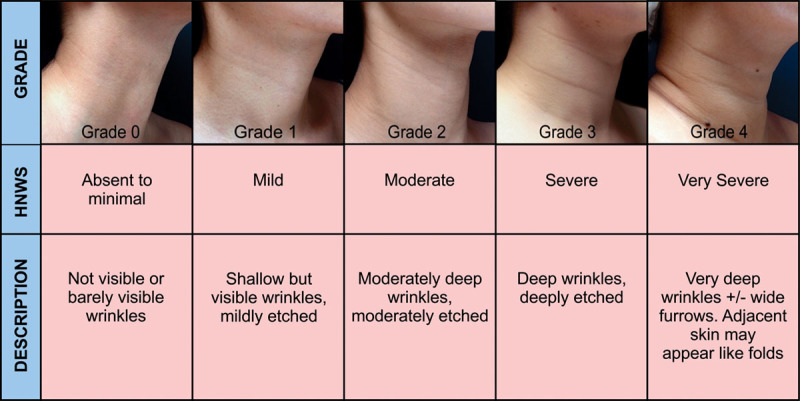
HNWS Scale description.
Study Variables
The primary objective was to evaluate the effectiveness of CPM-HA on horizontal neck wrinkles through the Horizontal Neck Wrinkle Severity Scale (HNWS). One of the authors (F.W.T.) and a second, independent, trained physician were provided with all pretreatment and posttreatment patient photos, in random order, to assign HNWS grades (Grade 0: wrinkle absent or minimal to Grade 4: very severe wrinkles, detailed scaling presented in Figure 1) at every follow-up visit.
The secondary objective was to evaluate the effectiveness of CPM-HA on horizontal neck lines using the Global Aesthetic Improvement Scale (GAIS)24 which included patient- and physician-reported scores. GAIS categorized the patients based on the extent of improvement (very much improved, much improved, improved, and no improvement). The grading was documented at every follow follow-up visit.
Pain on injection was evaluated using the visual analogue scale (VAS, 0: no pain and 10: worst pain). All AEs observed with the treatment were documented at each follow-up visit to evaluate safety. Patients were examined to evaluate the occurrence of ecchymosis, swelling, nodules, pruritus, erythema, mild pain, Tyndall effect, and skin necrosis.
Statistical Analysis
The demographic variables were presented using descriptive statistics. The dose of CPM-HA received by the patients was calculated at baseline (week 0) and at touch-up, and presented as mean and ranges. The HNWS at each follow-up period were compared with that at baseline to determine the extent of improvement. The number of patients who appeared for each follow-up visit was presented as percentages and compared with the number of patients who received CPM-HA at baseline. Inter-rater reliability of the HNWS scale at week 0 and week <4 was calculated using the weighted Kappa as the data do not satisfy the assumption of a normal distribution (See table, Supplemental Digital Content 1, which displays inter-rater reliability of HNWS scale at week 0 and week <4, http://links.lww.com/PRSGO/B172); and the correlation between the HNWS scale measurements for the left and right side is shown in Supplemental Digital Content (see table, Supplemental Digital Content 1, which displays correlation between left and right-side measurements for HNWS scale, http://links.lww.com/PRSGO/B172).
AEs were presented as number and percentages. All analyses were done by considering each side of the neck as a separate entity (ie, 32 patients and 64 lateral halves). All two-tailed paired sample testing and statistical analyses were done using SPSS Inc version 18 (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc). A P-value of less than 0.05 was considered statistically significant.
RESULTS
Baseline Demographic Characteristics
Overall, 32 women (64 lateral neck halves) between 23 and 61 years of age were included in the study. Not all patients appeared for every follow-up. At baseline, an average of 0.29 cc of CPM-HA was injected per patient (range 0.05–1.2 cc). Twenty-seven patients received an average of 0.24 cc of CPM-HA per patient for touch-up within 1 month (range 0.05–0.55 cc). Not all patients returned for follow-up at all timepoints of the 64 neck halves—the number of neck halves evaluated at each timepoint was (Table 2): 64 (week 2), 40 (week 4), 44 (week 8), 54 (week 12), 36 (week 18), 52 (week 24), 26 (week 30), and 26 (week 36).
Table 2.
HNWS Scores from 2 Trained Physicians at Each Follow-up Visit
| Follow up Visit | No. Neck Halves, n | Mean±SD | P |
|---|---|---|---|
| Week 0 | 64 | 2.484 ± 0.771 | — |
| Week 2 | 64 | 1.352 ± 0.682 | <0.05 |
| Week 4 | 40 | 1.013 ± 0.796 | <0.05 |
| Week 8 | 44 | 1.011 ± 0.735 | <0.05 |
| Week 12 | 54 | 0.991 ± 0.730 | <0.05 |
| Week 18 | 36 | 1.264 ± 0.722 | <0.05 |
| Week 24 | 52 | 1.173 ± 0.797 | <0.05 |
| Week 30 | 26 | 1.346 ± 0.946 | <0.05 |
| Week 36 | 26 | 1.423 ± 0.796 | <0.05 |
HNWS Scales
The HNWS scores from 2 trained physicians showed that the wrinkles began to improve from week 2 onwards (HNWS score [mean±SD]: 1.352 ± 0.682, compared with 2.484 ± 0.771 at baseline). The effect of CPM-HA on horizontal neck lines remained consistent throughout the study until week 36 (HNWS score: 1.423 ± 0.796). Maximum improvement was observed between 4 and 12 weeks, with a mean score of 0.991 ± 0.730. Overall, the improvement from baseline was statistically significant at every follow-up visit (P < 0.05 by two-tailed paired sample t-test, for all follow-up visits, Table 2). The extent of improvement in horizontal neck wrinkles at each follow-up as evaluated using HNWS is illustrated in Figure 2. The extent of improvement observed in 2 random patients with CPM-HA at each follow-up is illustrated in Figure 3.
Fig. 2.
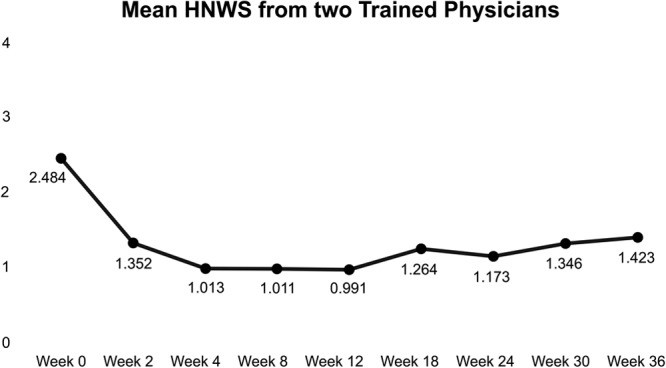
Mean HNWS scores as reported by 2 trained physicians throughout the study period. (Range from 0 – not visible or barely visible horizontal neck wrinkles, to 4 – very deep wrinkles ± wide furrows; adjacent skin may appear as folds).
Fig. 3.
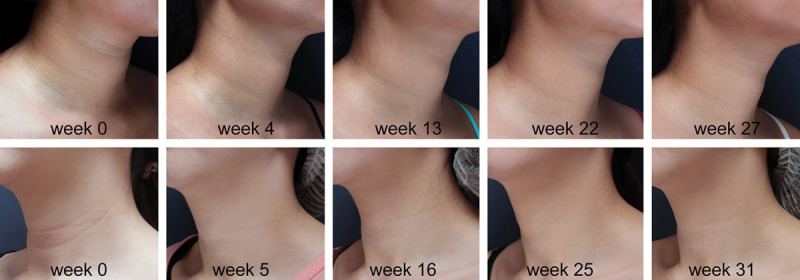
Improvement in horizontal neck wrinkles after receiving CPM-HA in 2 patients at different follow-up timepoints. Patient 1 is shown at week 0 and at each follow-up until week 27. Patient 2 is shown at week 0 and at each follow-up until week 31.
GAIS Scores
Patient Reported GAIS
Patients (92.3%–100%) demonstrated some level of improvement (“very much improved,” “much improved,” and “improved”) throughout the study. Only 5% of patients showed no improvement at week 4 and 7.6% at week 36. The combined proportion of patients who judged themselves to be “very much improved” and “much improved” was highest during the first 18 weeks (77.8%–86.3%), and gradually decreased to 53.8% (week 36) by the end of the study. (Fig. 4).
Fig. 4.
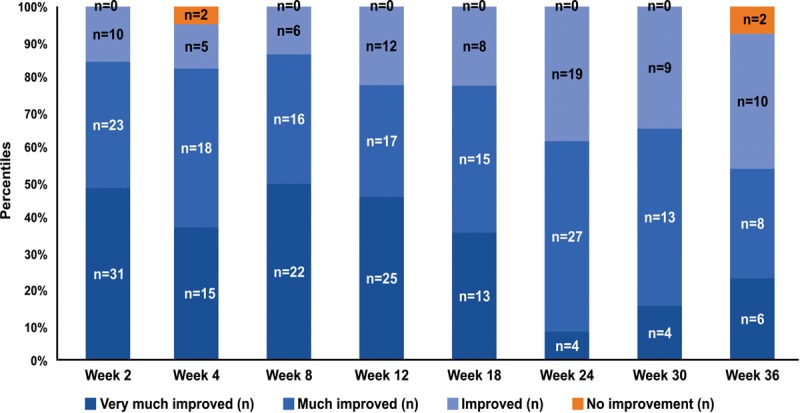
Distribution of patients throughout the study as per the patient-reported GAIS scores.
Physician-Reported GAIS
Patients (100%) were rated as having some level of improvement (“very much improved,” “much improved,” and “improved”) from weeks 2 to 24, and this gradually decreased to 84.6% at the end of study. Only 3.8% of patients at week 30 and 15.4% at week 36 were rated as having no improvement. Similar to patient reported GAIS, the combined proportion of patients rated as “very much improved” and “much improved” were highest from week 4 (95%) to 18 (83.3%), and gradually decreased to 61.5% at the end of this study (Fig. 5).
Fig. 5.
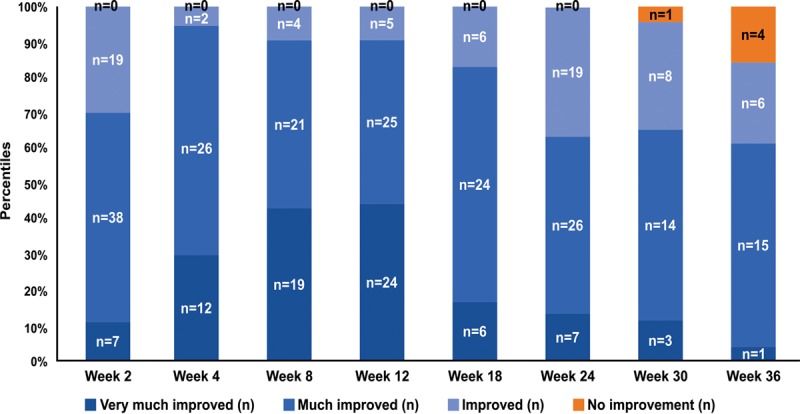
Distribution of patients throughout the study as per the physician-reported GAIS scores.
Safety Results
VAS Scores
The VAS score for pain (0, no pain and 10, worst pain possible) during CPM-HA injection at week 0 was recorded separately for blunt cannulas and sharp needles. Patients experienced slightly more intense pain with blunt cannulas (2.72 ± 1.71) than with sharp needles (1.75 ± 1.39). Overall discomfort was rated as mild for most patients.
AEs
The most common patient-reported AEs included erythema (62.5%), pruritus (43.7%), ecchymosis (43.7%), and localized swelling (25%, Table 3). All AEs resolved spontaneously within 14 days and did not require intervention. No AEs were reported at subsequent follow-up visits. None of the patients experienced tenderness, infection, infarction, or Tyndall effect throughout the study period.
Table 3.
Most Common Patient-reported AEs
| AE | Patients, n (%) |
|---|---|
| Redness | 20 (62.5) |
| Itching | 14 (43.7) |
| Bruising | 14 (43.7) |
| Localized swelling | 8 (25) |
DISCUSSION
Although different procedures are used for neck rejuvenation,3 treatments for horizontal neck wrinkles, though successful, are limited. These include using diluted calcium hydroxyapatite filler for horizontal neck lines,19 injecting noncrosslinked HA solutions with a subdermal, minimally-invasive technology,20 and injecting Restylane Vital (Q-Med AB, Uppsala, Sweden) and Belotero Balance (Merz Aesthetics, Raleigh, NC).8 To our knowledge, this is the first report of a safe and effective procedure to improve the appearance of horizontal neck wrinkles with an extended, long-term follow-up duration of up to 36 weeks. According to our HNWS, our patients showed substantial reduction in neck wrinkle depth from that seen at the first posttreatment follow-up (HNWS = 1.352 ± 0.682; P < 0.05). After the single touch-up allowed within 1 month of treatment, the HNWS reduction significantly peaked at week 4 and week 8 (P < 0.05 for both). After this, although the HNWS increased slightly, as anticipated due to filler resorption, improvement remained statistically significant at every timepoint up to 36 weeks (P < 0.05).
At the study initiation, no classification scales existed for the objective grading of HNWS. The investigators therefore developed the HNWS scale for its evaluation. After the study initiation, Jones et al.7 developed the Allergan transverse neck line scale, the descriptors of which were similar to the HNWS scales, with grade 0 representing no transverse neck lines and grade 4 representing noneffaceable transverse neck furrows with redundant neck skin. The similarities between these scales confirm the similarities in horizontal neck wrinkles observed by physicians in Asia and the West. However, Allergan’s transverse neck line scale requires a distinction between “effaceable” and “noneffaceable” neck lines, while the HNWS scale only considers wrinkle depth and the presence of adjacent skin folds. Both scales are useful for assessing neck rejuvenation treatment effectiveness and can be applied in daily practice and clinical studies.
Nearly all patients and physicians in our study observed improvements in neck wrinkles (92.3%–100% and 84.6%–100%, respectively). The best ratings occurred between weeks 4 and 18 (77.8%–95%). These improvements gradually declined by week 36 (53.8% for patients and 61.5% for physicians), although it was sustained in most patients until the end of the study.
Unlike facial wrinkles, horizontal neck wrinkles are less associated with aging because they are observed in children and young adults.7,8 Treating horizontal neck wrinkles with energy-based skin tightening devices such as micro-focused ultrasound, radiofrequency, and lasers is also ineffective. In clinical practice, we observed that horizontal neck wrinkles are likely caused by a relative deficiency of linearly-distributed subdermal adipose tissue thickness. This stems from observations that overweight patients have deeper horizontal neck wrinkles, with skin rolls adjacent to these wrinkles appearing rich in subcutaneous fat. As a patient gains weight and subcutaneous adipose tissue around the neck increases, the overall girth of the neck increases because the skin rolls between the horizontal wrinkles thicken, while horizontal wrinkles deepen, suggesting that the skin overlying the wrinkles may be attached to deeper, fibro-muscular structures. This supports using dermal fillers such as HA to treat horizontal neck wrinkles.
Thin skin and a relative lack of adipose tissue in the anterior neck increase the risk of lumps, irregularities, and the Tyndall effect when HA fillers are injected here. Esthelis Basic was chosen due to its ideal filler rheology and to avoid the Tyndall effect.25,26 To achieve a smooth surface when fillers are placed superficially under thin neck skin, minimal amounts of fillers must be injected precisely and just under the wrinkles. In addition, choosing fillers with suitable rheology is crucial. Ideal fillers are soft and easily molded, such as Esthelis Basic’s CPM-HA. Here, 25% of patients reported transient and localized swelling that resolved spontaneously within a week, and no patient presented lumps or nodules in subsequent visits.
The Tyndall effect, a bluish hue appearing after superficial HA filler implantation,27,28 was not observed in any patient, as supported by other studies with Esthelis Basic.23,29 Previous histologic studies have attributed this to CPM-HA’s homogeneous intradermal distribution,30,31 which does not accumulate in discrete or optically-isolated pools,30,31 thus preventing the scattering of blue light26,27 and minimizing the risk of the Tyndall effect. This is valuable when filler is placed superficially under thin skin in the periorbital area, fine wrinkles and the neck.
This study is limited by its small sample size and short duration, and future studies should investigate longer-term safety and effectiveness. We also acknowledge the lack of quantification of outcomes, as technologies to do so were unavailable to our clinical practice, however we have sought to provide some quantification of treatment outcomes by using 2 scales for aesthetic improvements (the HNWS and GAIS). Also, one of the investigators who injected the patients also graded the patient photographs, but to reduce potential bias, the sequence of pre- and posttreatment photos were randomized during grading. AEs reported here were expected, and all were transient, injection site-related reactions of mild intensity, including erythema, pruritus, ecchymosis, and localized swelling. All AEs resolved spontaneously within 2 weeks without intervention. More severe AEs including allergic or hypersensitivity reactions, infections, granulomas, skin necrosis, or Tyndall effect, were not reported. Filler injection to the neck risks lumps and Tyndall effects because of skin thinness and subcutaneous fat and can be avoided with accurate subdermal injections just under the wrinkles, using small amounts of filler per pass of the cannula or needle, and choosing rheologically appropriate fillers for superficial injections.
CONCLUSIONS
This retrospective case series demonstrates that the subdermal injection of CPM-HA is safe and effective for the treatment of horizontal neck wrinkles, with improvement being sustained for up to 36 weeks. AEs were limited to transient injection site reactions and no Tyndall effect was observed even though CPM-HA was injected superficially. The HNWS scale developed by the investigators may serve as a useful tool for assessing the outcomes of neck rejuvenation treatments in daily clinical practice or future clinical studies.
ACKNOWLEDGMENTS
The authors acknowledge Shawna Tan, Medical Writers Asia, for editorial assistance, and Turacoz Healthcare Solutions (www.Turacoz.com), Gurugram, India for statistical analysis and editorial assistance.
Footnotes
Published online 19 August 2019.
This case series was performed at the Milano Aesthetic Clinic in Taipei and Taoyuan, Taiwan.
Dr. FW Tseng has been a speaker for Merz, Galderma, Allergan, Cynosure, and Solta/Valeant. Dr. HE Yu has been a speaker for Solta/Valeant. Funding and products for the present study were provided by Merz Pharmaceuticals GmbH.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Shadfar S, Perkins SW. Anatomy and physiology of the aging neck. Facial Plast Surg Clin North Am. 2014;22:161–170. [DOI] [PubMed] [Google Scholar]
- 2.Lemperle G, Holmes RE, Cohen SR, et al. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108:1735–1750; discussion 1751. [DOI] [PubMed] [Google Scholar]
- 3.Dibernardo BE. The aging neck: a diagnostic approach to surgical and nonsurgical options. J Cosmet Laser Ther. 2013;15:56–64. [DOI] [PubMed] [Google Scholar]
- 4.Silva SAM, Michniak-Kohn B, Leonardi GR. An overview about oxidation in clinical practice of skin aging. An Bras Dermatol. 2017;92:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31–38. [PMC free article] [PubMed] [Google Scholar]
- 6.Puri P, Nandar SK, Kathuria S, et al. Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol. 2017;83:415–423. [DOI] [PubMed] [Google Scholar]
- 7.Jones D, Carruthers A, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of transverse neck lines. Dermatol Surg. 2016;42(Suppl 1):S235–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SK, Kim HS. Correction of horizontal neck lines: our preliminary experience with hyaluronic acid fillers. J Cosmet Dermatol. 2018;17:590–595. [DOI] [PubMed] [Google Scholar]
- 9.Duplechain JK. Neck skin rejuvenation. Facial Plast Surg Clin N Am. 2014;22:203–216. [DOI] [PubMed] [Google Scholar]
- 10.Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099–1110. [DOI] [PubMed] [Google Scholar]
- 11.Oram Y, Akkaya AD. Neck rejuvenation with fractional CO2 laser: long-term results. J Clin Aesthet Dermatol. 2014;7:23–29. [PMC free article] [PubMed] [Google Scholar]
- 12.Adamson PA, Litner JA. Surgical management of the aging neck. Facial Plast Surg. 2005;21:11–20. [DOI] [PubMed] [Google Scholar]
- 13.Knize DM. Limited incision submental lipectomy and platysmaplasty. Plast Reconstr Surg. 1998;101:473–481. [DOI] [PubMed] [Google Scholar]
- 14.Feldman JJ. Corset platysmaplasty. Plast Reconstr Surg. 1990;85:333–343. [DOI] [PubMed] [Google Scholar]
- 15.Brandt FS, Boker A. Botulinum toxin for the treatment of neck lines and neck bands. Dermatol Clin. 2004;22:159–166. [DOI] [PubMed] [Google Scholar]
- 16.Alster TS, Konda S. Plasma skin resurfacing for regeneration of neck, chest, and hands: investigation of a novel device. Dermatol Surg. 2007;33:1315–1321. [DOI] [PubMed] [Google Scholar]
- 17.Sadick NS, Trelles MA. Nonablative wrinkle treatment of the face and neck using a combined diode laser and radiofrequency technology. Dermatol Surg. 2005;31:1695–1699. [DOI] [PubMed] [Google Scholar]
- 18.Brandt FS, Bellman B. Cosmetic use of botulinum A exotoxin for the aging neck. Dermatol Surg. 1998;24:1232–1234. [DOI] [PubMed] [Google Scholar]
- 19.Chao YY, Chiu HH, Howell DJ. A novel injection technique for horizontal neck lines correction using calcium hydroxylapatite. Dermatol Surg. 2011;37:1542–1545. [DOI] [PubMed] [Google Scholar]
- 20.Han TY, Lee JW, Lee JH, et al. Subdermal minimal surgery with hyaluronic acid as an effective treatment for neck wrinkles. Dermatol Surg. 2011;37:1291–1296. [DOI] [PubMed] [Google Scholar]
- 21.Santoro S, Russo L, Argenzio V, et al. Rheological properties of cross-linked hyaluronic acid dermal fillers. J Appl Biomater Biomech. 2011;9:127–136. [DOI] [PubMed] [Google Scholar]
- 22.Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Suppl 1):302–312. [DOI] [PubMed] [Google Scholar]
- 23.Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and drug administration-approved fillers. Plast Reconstr Surg. 2015;136:678–686. [DOI] [PubMed] [Google Scholar]
- 24.Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of restylane versus zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. [DOI] [PubMed] [Google Scholar]
- 25.Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015;41(Suppl 1):S120–S126. [DOI] [PubMed] [Google Scholar]
- 26.Micheels P, Sarazin D, Besse S, et al. A blanching technique for intradermal injection of the hyaluronic acid belotero. Plast Reconstr Surg. 2013;132(4 Suppl 2):59S–68S. [DOI] [PubMed] [Google Scholar]
- 27.Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4 Suppl 2):5S–21S. [DOI] [PubMed] [Google Scholar]
- 28.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kühne U, Imhof M, Kirchmeir M, et al. Five-year retrospective review of safety, injected volumes, and longevity of the hyaluronic acid belotero basic for facial treatments in 317 patients. J Drugs Dermatol. 2012;11:1032–1035. [PubMed] [Google Scholar]
- 30.Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637–643. [DOI] [PubMed] [Google Scholar]
- 31.Tran C, Carraux P, Micheels P, et al. In vivo bio-integration of three hyaluronic acid fillers in human skin: a histological study. Dermatology. 2014;228:47–54. [DOI] [PubMed] [Google Scholar]


